A Review on Phytochemicals of the Genus Maytenus and Their Bioactive Studies
Abstract
1. Introduction
2. Chemical Constituents of Maytenus
2.1. Triterpenoids
2.1.1. Friedelane Friterpenoids
2.1.2. Lupane Triterpenes
2.1.3. Oleanane Triterpenes
2.1.4. Other Triterpenes
2.2. Sesqiterpenoids
2.3. Alkaloids
2.3.1. Sesquiterpene Pyridine Alkaloids
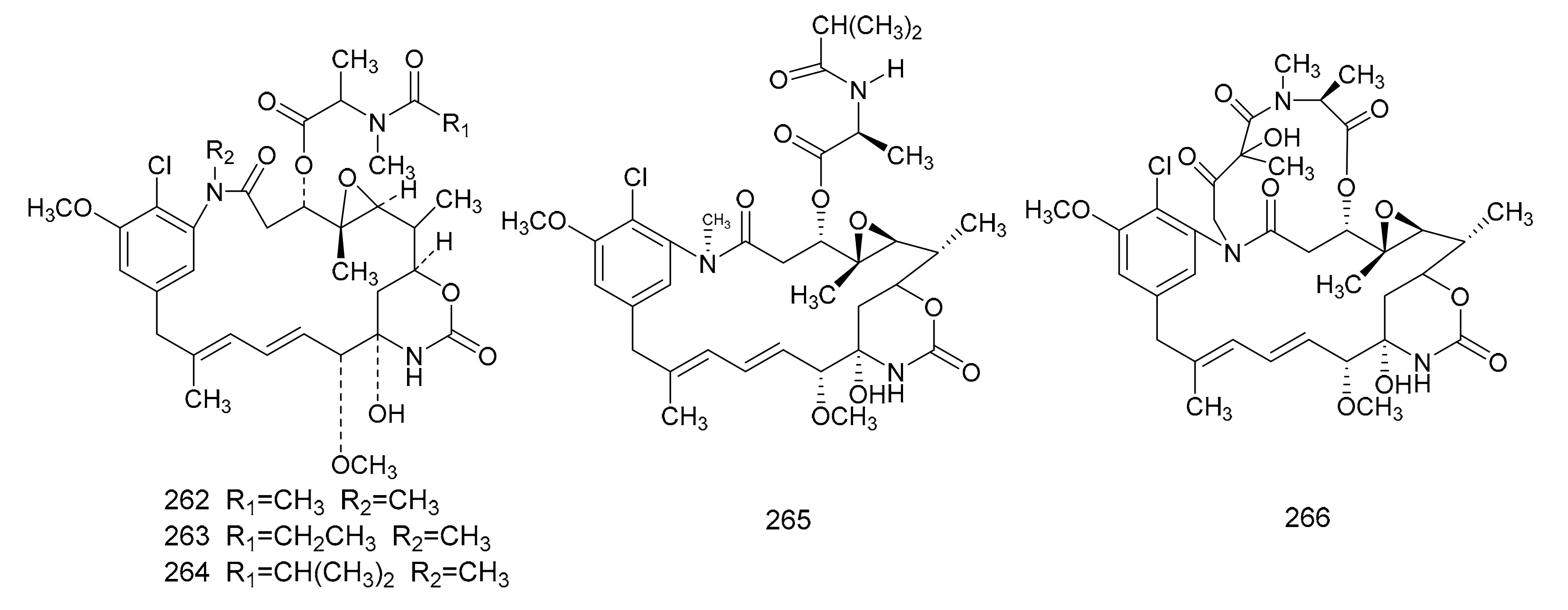
2.3.2. Maytansinoids
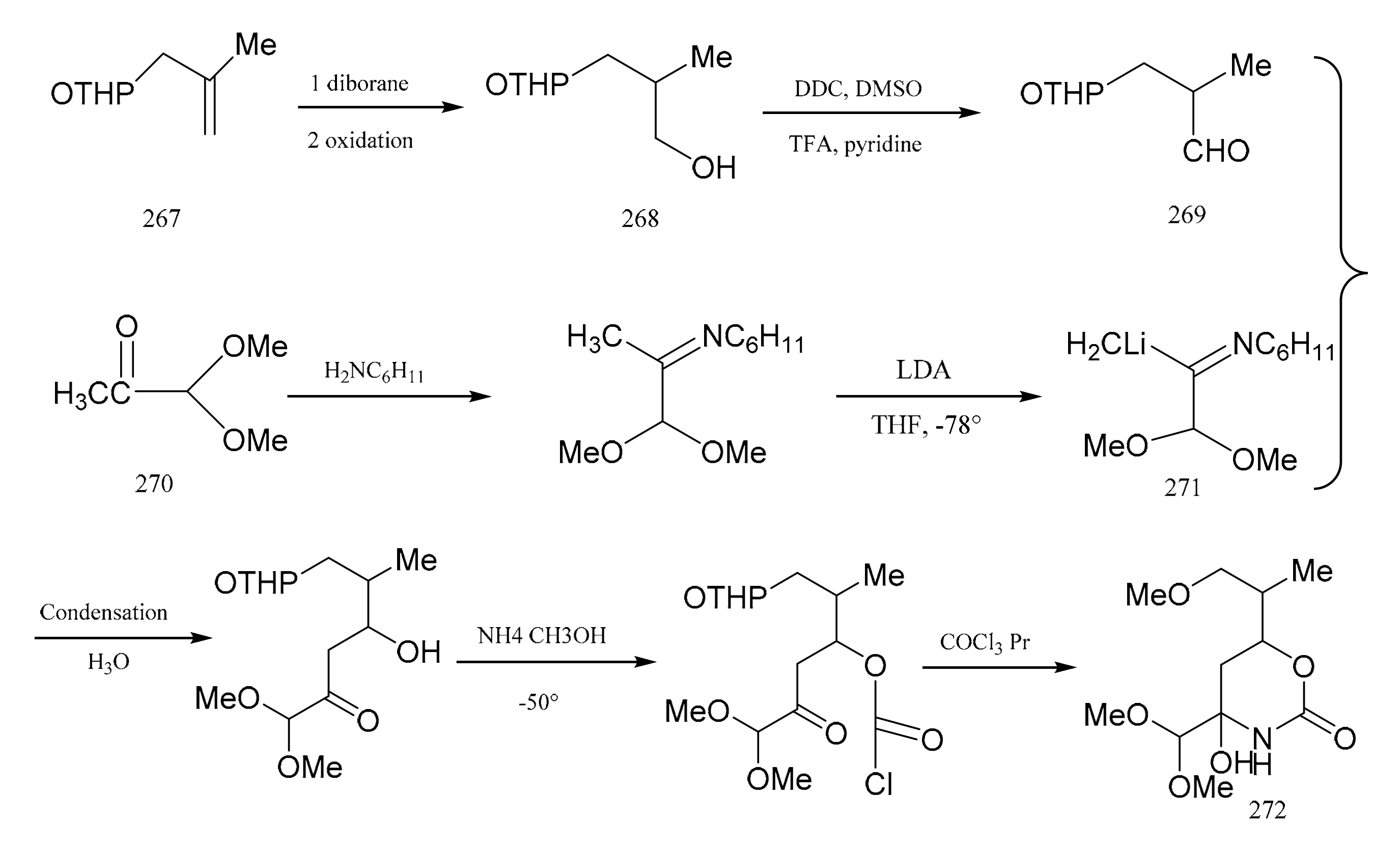
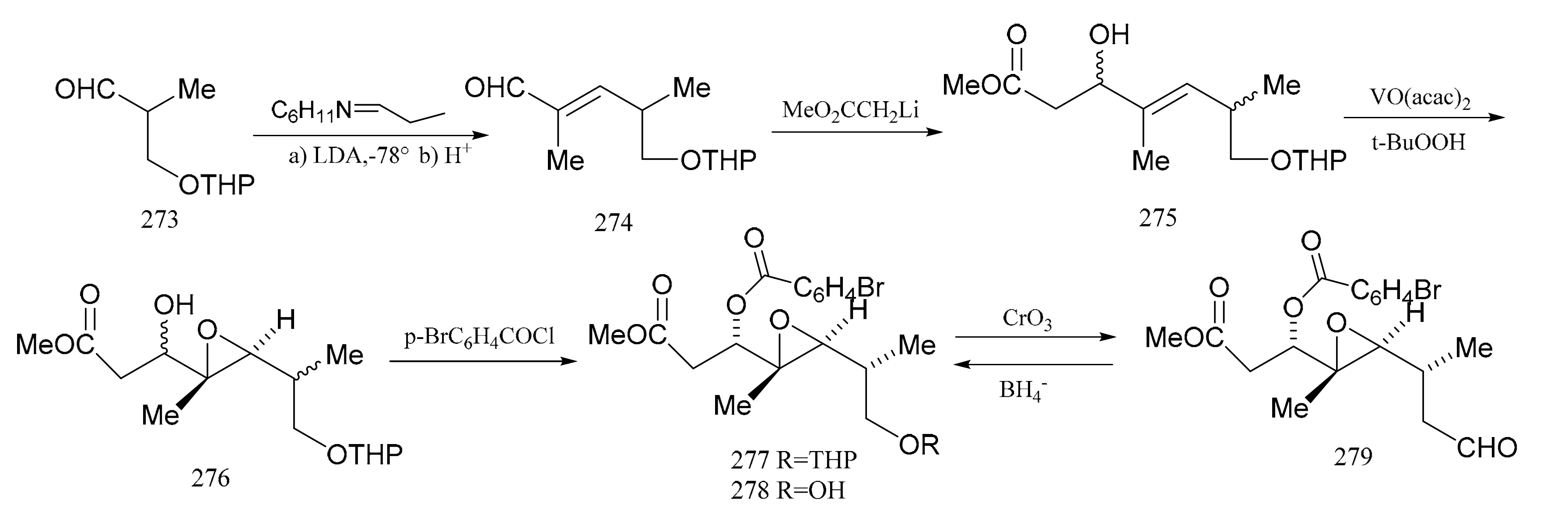
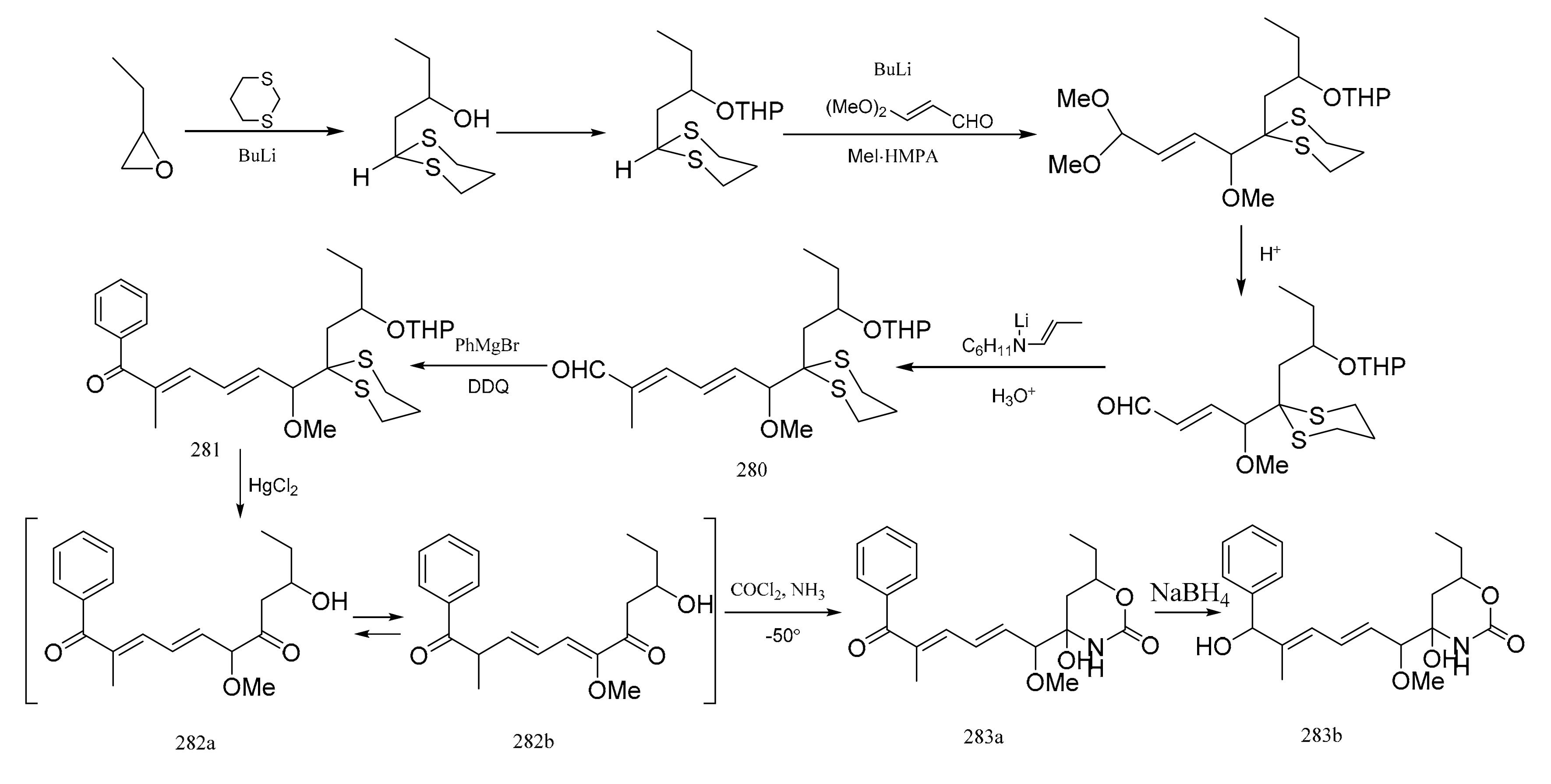
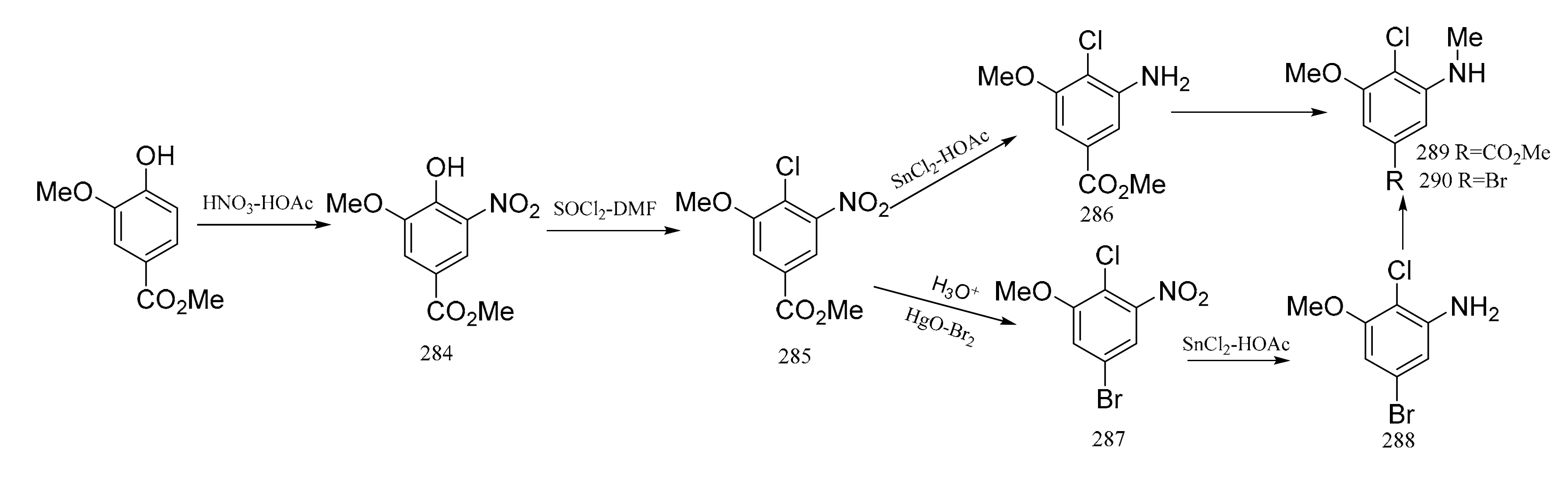
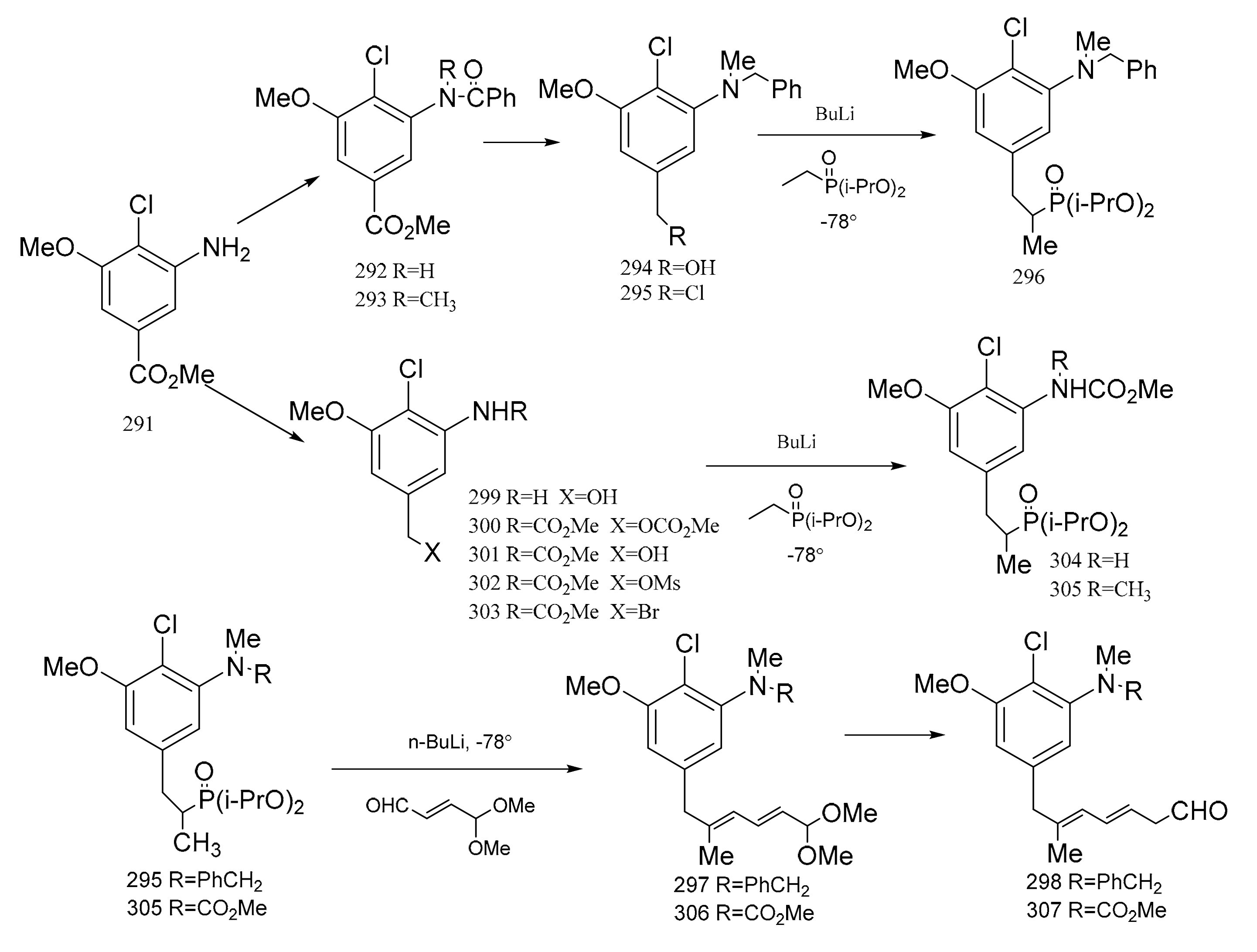
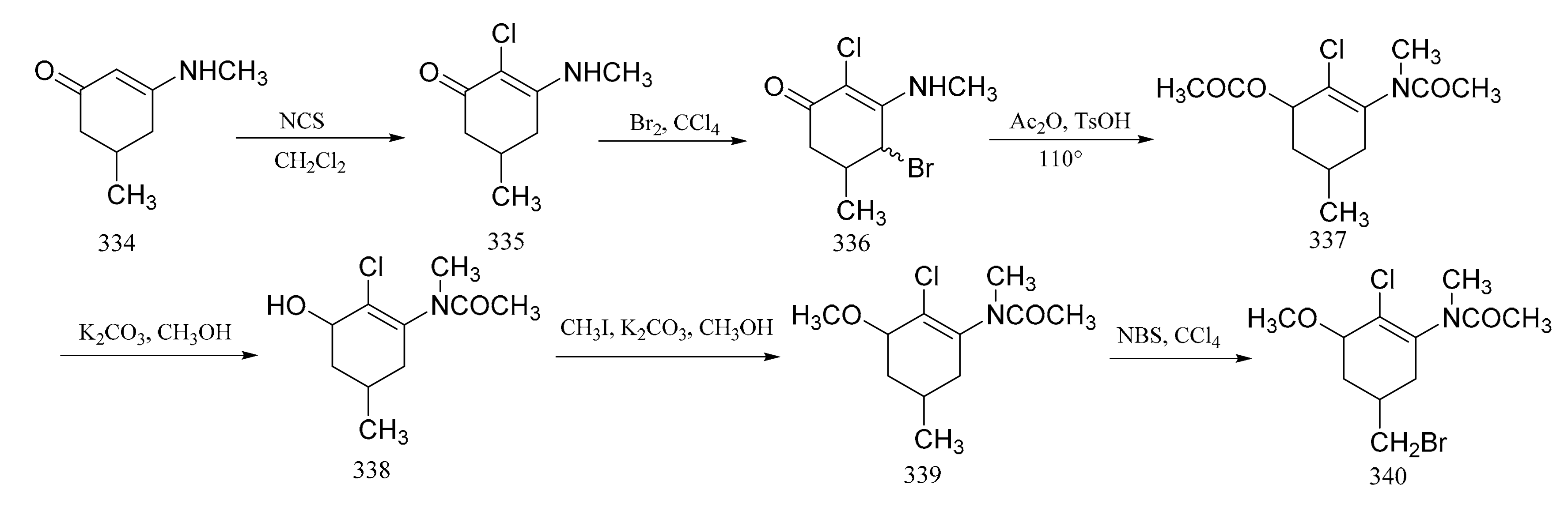
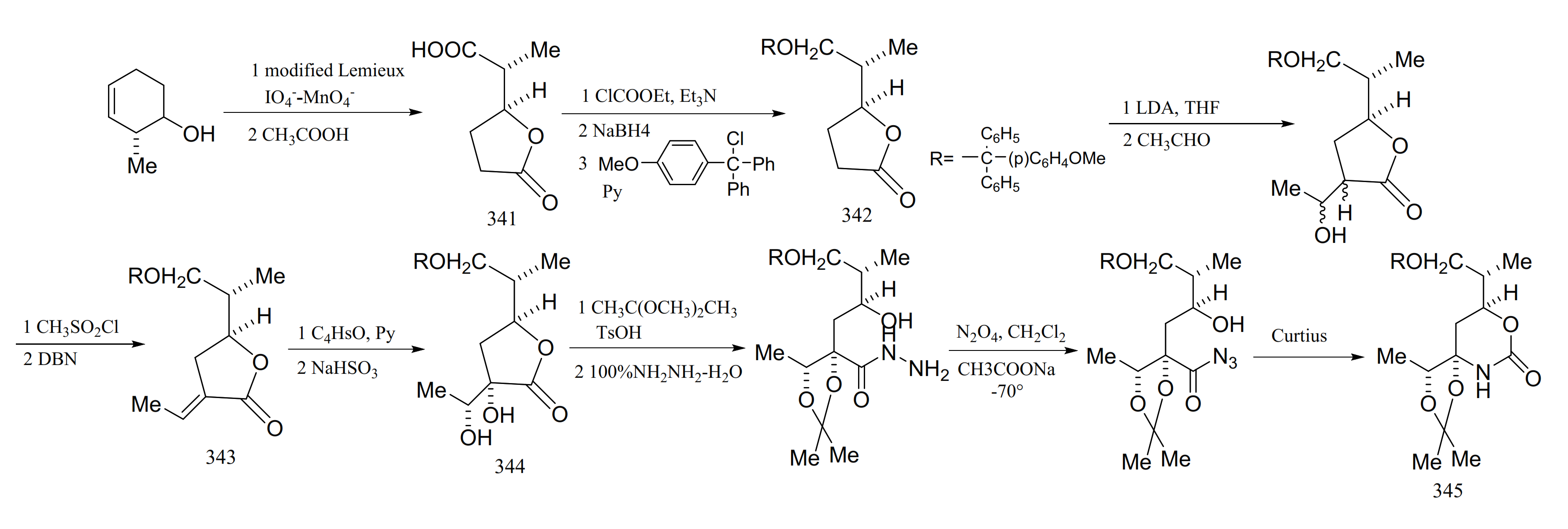
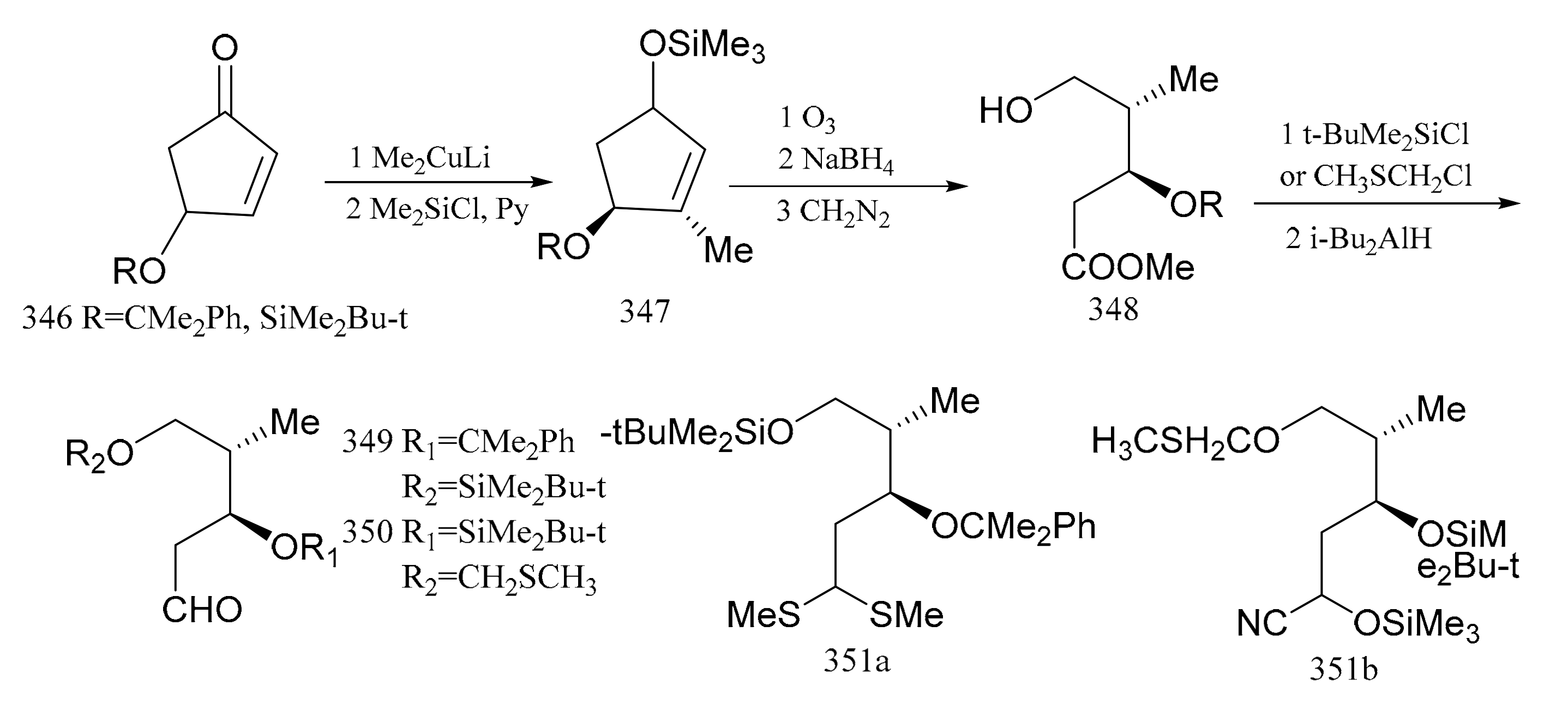
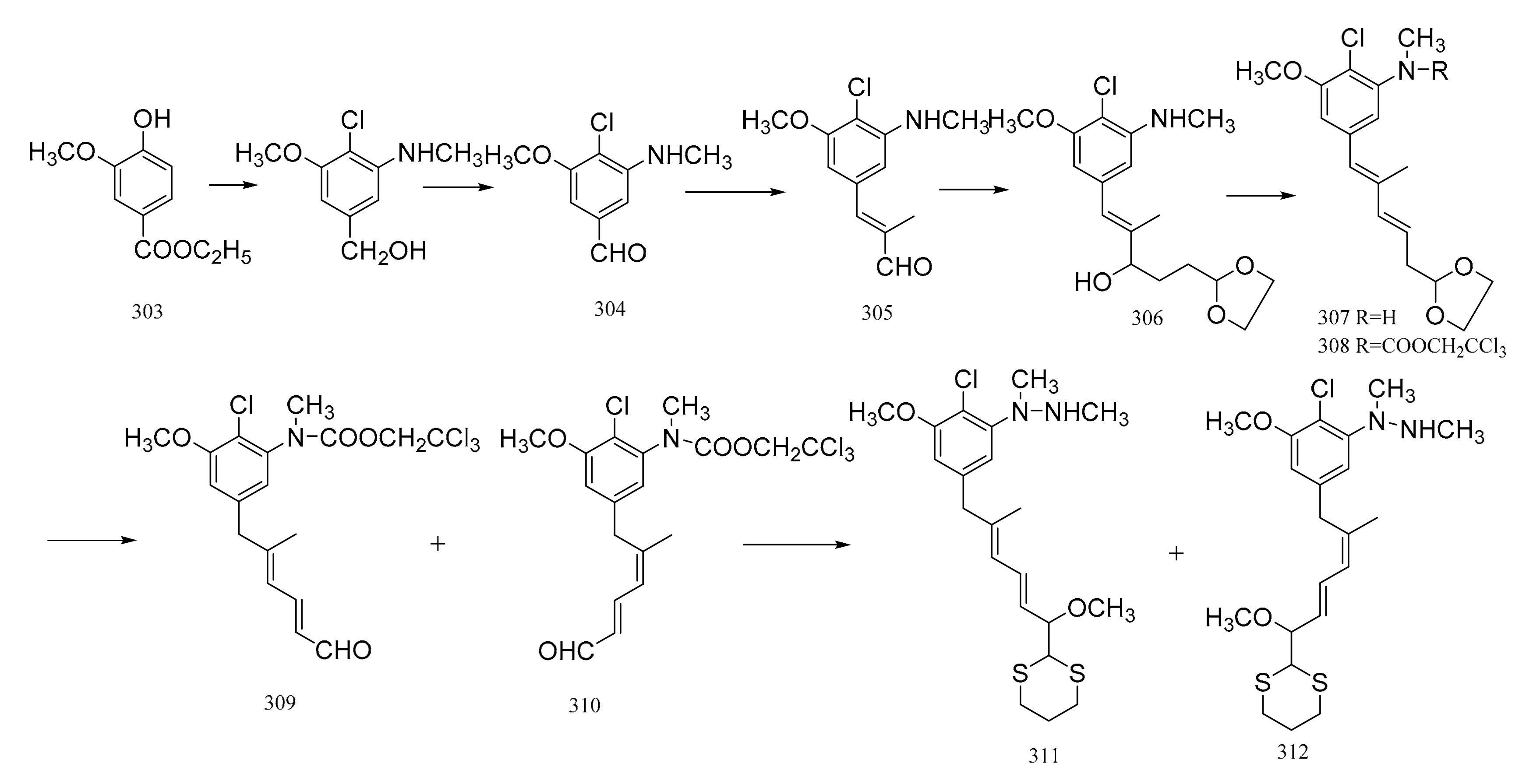
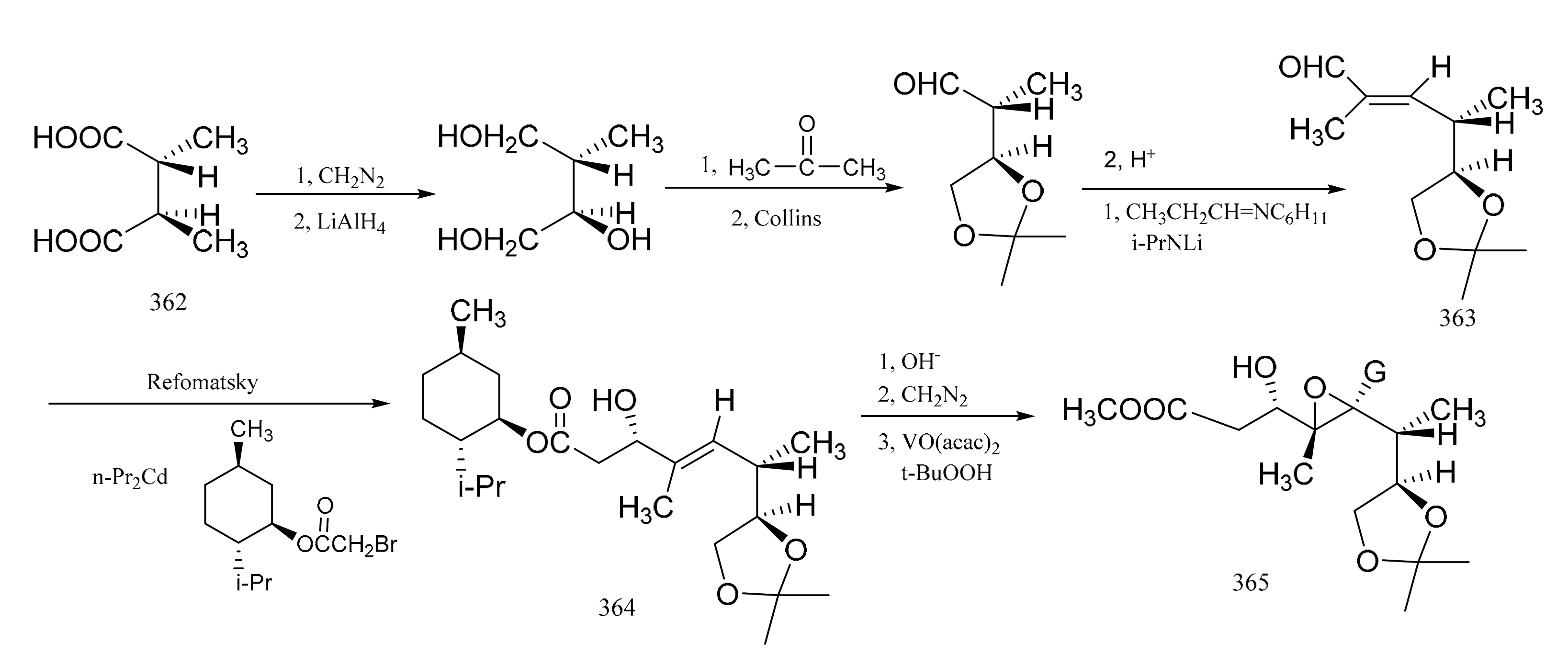
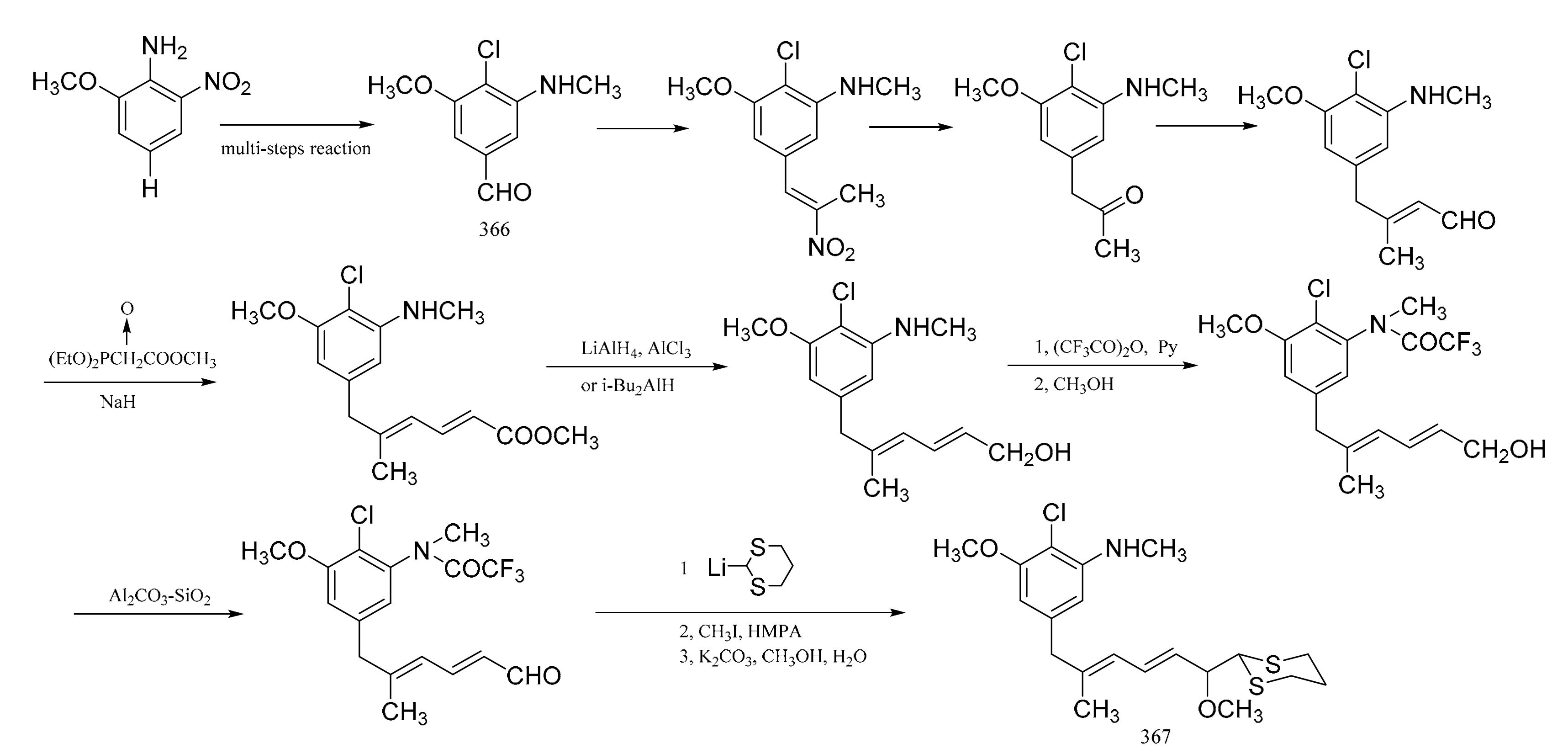
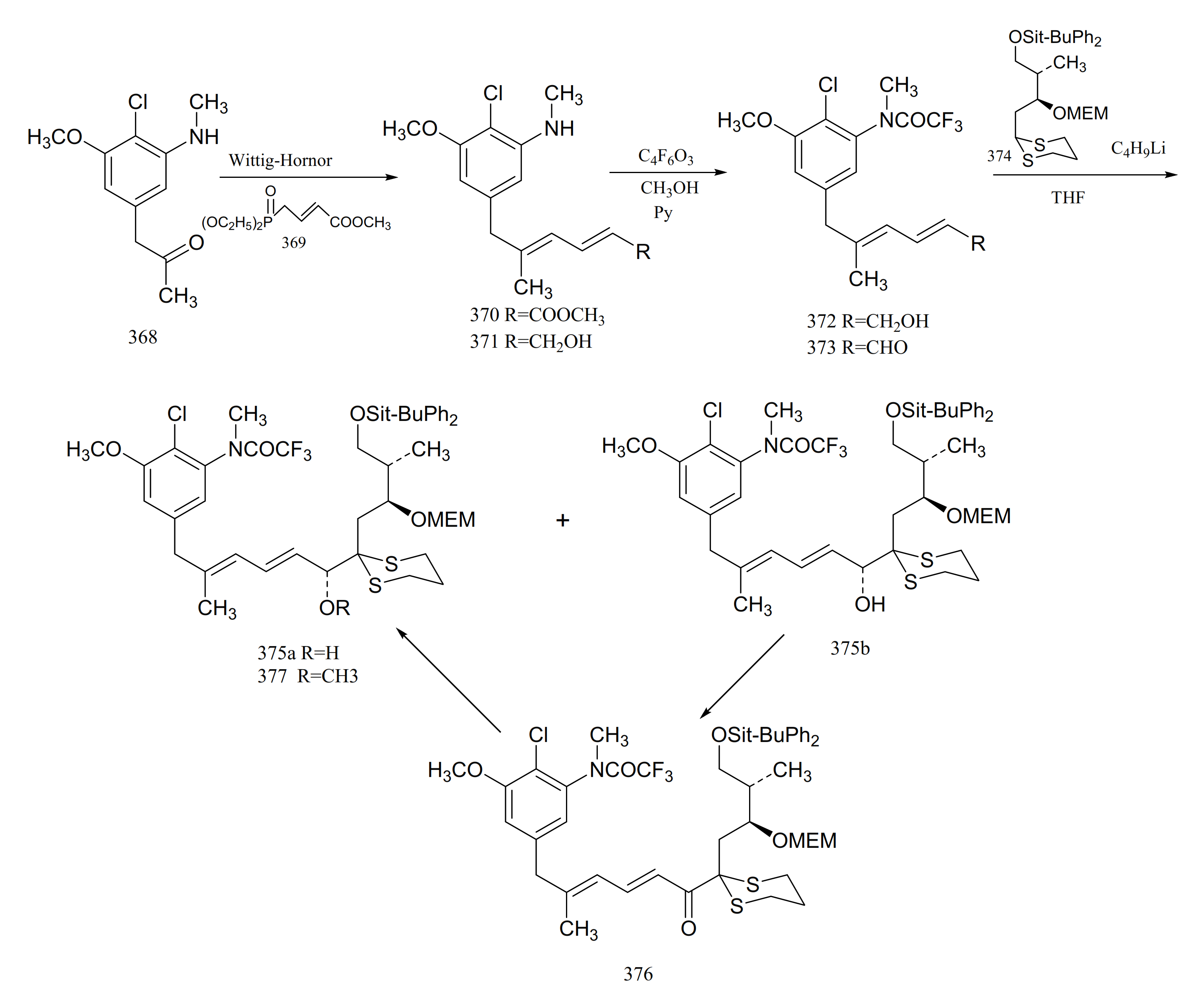
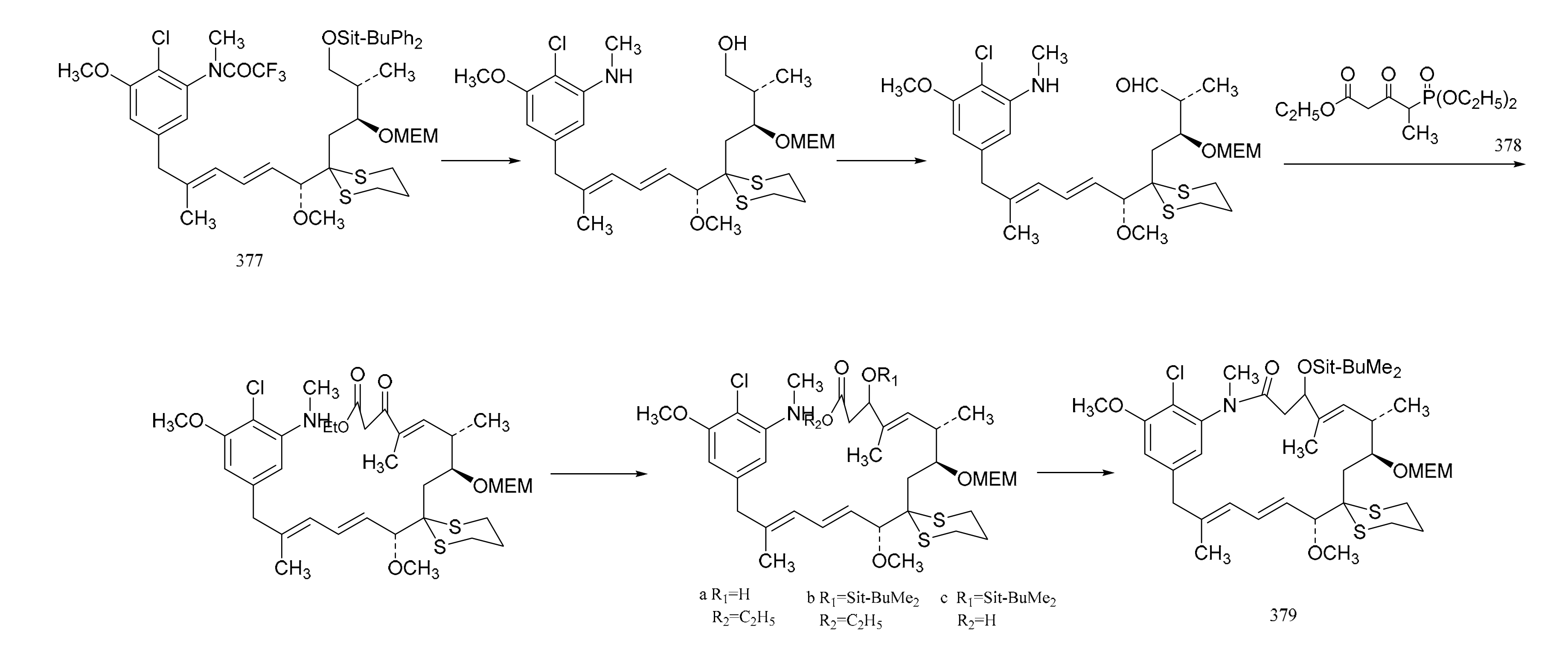
3. Chemical Synthesis of Maytansine and Maytansine Fragments
4. Pharmacological Activities
4.1. Antitumor Activities
4.2. Anti-Bacterial Activities
4.3. Other Pharmacological Activities
5. Toxic Effects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Editorial Committee of Flora of China. Flora of China (Forty-Fifth Volume); Science Press: Beijing, China, 1999. [Google Scholar]
- Ghazanfar, S.A. Handbook of Arabian Medicinal Plants; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Gonzalez, J.G.; Delle, M.G.; Delle Monache, F.; Marini-Bettolò, G.B. Chuchuhuasha-a drug used in folk medicine in the Amazonian and Andean areas, a chemical study of Maytenus laevis. J. Ethnopharmacol. 1982, 5, 73–75. [Google Scholar] [CrossRef]
- Veloso, C.C.; Oliveira, M.C.; Rodrigues, V.G.; Oliveira, C.C.; Duarte, L.P.; Teixeira, M.M.; Ferreira, A.V.M.; Perez, A.C. Evaluation of the effects of extracts of Maytenus imbricata (Celastraceae) on the treatment of inflammatory and metabolic dysfunction induced by high-refined carbohydrate diet. Inflammopharmacology 2019, 27, 539–548. [Google Scholar] [CrossRef]
- Elmer, L.C.; Henry, M.S.; Alexander, M.V.; Karola, M.C.; María, M.R.; Zaida, L.C.; Carlos, P.M.; Alberto, S.G. Anti-Inflammatory and Neurobehavioral Effects of the Leaves from Maytenus macrocarpa (Ruiz and Pavón) Briquet in Mice. Pharmacogn. J. 2019, 11, 75–80. [Google Scholar] [CrossRef]
- Tang, H.; Li, F.; Wei, X.; Li, H.; Huang, X.Y. Advance of researches on medicnal plants of maytenus. Hubei Agric. Sci. 2009, 48, 2275–2277. [Google Scholar]
- Kupchan, S.M.; Komoda, Y.; Branfman, A.R.; Sneden, A.T.; Court, W.A.; Thomas, G.J.; Hintz, H.P.; Smith, R.M.; Karim, A.; Howie, G.A.; et al. The maytansinoids, isolation, structural elucidation and chemical interrelation of novel anse macrolides. J. Org. Chem. 1972, 42, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.J.; Shen, P.Q.; Li, C.Y. Some thoughts on the Anti-cancer Research of Maytenus. Chin. Acad. Med. Org. 2003, 70, 70–72. [Google Scholar]
- American Cancer Research Institute. American Institute of Cancer Research on the study of maytansin. Drugs Clinic 1981, 2, 9–14. [Google Scholar]
- Hiroshi, M.; Yusuke, H.; Akihiro, M.; Tadashi, Y.; Setsuko, S.; Osamu, S. Antimitotic quinoid triterpenes from Maytenus chuchuhuasca. Bioorg. Med. Chem. Lett. 2008, 18, 1050–1052. [Google Scholar]
- Kuo, Y.H.; King, M.L.; Chen, C.F.; Chen, H.Y.; Chen, C.H.; Chen, K.; Lee, K.H. Two new macrolide sesquiterpene pyridine alkaloids from Maytenus emarginata: Emarginatine G and the cytotoxic emarginatine F. J. Nat. Prod. 1994, 57, 263–269. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Alvarenga, N.L.; Ravelo, A.G.; Jimenez, I.A.; Bazzocchi, I.L.; Canela, N.J.; Moujir, L.M. Antibiotic phenol nortriterpenes from Maytenus canariensis. Phtochemistry 1996, 43, 129–132. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Feng, J.T.; Zhang, X. Advances in studies on triterpenes constituents and their biological activities in species of Maytenus. Acta Bot. Boreali Occident. Sin. 2007, 27, 1484–1490. [Google Scholar]
- Pu, D.B. Study on the Chemical Consituents and Anti-Tumor Activity of M. austroyunnanensis. Master’s Thesis, Kun Ming University of Science and Technology, Yunnan, China, 2014. [Google Scholar]
- Pu, D.B.; Gao, Y.; Li, R.T.; Li, H.Z. Structures and 13C NMR Features Triterpenoid Compounds in Maytenus: A Review. Chin. J. Magn. Reson. 2014, 31, 437–445. [Google Scholar]
- Martinod, P. Separation eupirone and Prisminin from M. chuchuhuasca. Phytochemistry 1976, 15, 562. [Google Scholar] [CrossRef]
- Alvarenga, N.L.; Velázquez, C.A.; Gomez, R.; Canela, N.J.; Bazzocchi, I.L.; Ferro, E.A. A new antibiotic nortriterpene quinone methide from Maytenus catingarum. J. Nat. Prod. 1999, 62, 750–751. [Google Scholar] [CrossRef]
- Chávez, H.; Valdivi, E.; Estévez-Braun, A.; Ravelo, Á.G. Structure of new bioactive triterpenes related to 22-β-hydroxy-tingenone. Tetrahedron 1998, 54, 13579–13590. [Google Scholar] [CrossRef]
- Chávez, H.; Estévez-Braun, A.; Ravelo, A.G.; Gonzalez, A.G. New phenolic and quinone methide triterpenes from Maytenus amazonica. J. Nat. Prod. 1999, 62, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Chávez, H.; Estevez-Braun, A.; Ravelo, A.G.; Gonzalez, A.G. Friedelane triterpenoids from Maytenus macrocarpa. J. Nat. Prod. 1998, 61, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Chávez, H.; Rodriguez, G.; Estevez-Braun, A.; Ravelo, A.G.; Estévez-Reyes, R.; González, A.D.; Fdez-Puente, J.L.; García-Grávalos, D. Macrocarpins A-D, new cytotoxic nor-triterpenes from Maytenus macrocarpa. Bioorg. Med. Chem. Lett. 2000, 10, 759–762. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Ou, J.C.; Lee, K.H.; Chen, C.F. A new triterpene lactone, maytenfolone, and a new sesquiterpene pyridine alkaloid, emarginatine H, from the leaves of Maytenus diversifolia. J. Nat. Prod. 1995, 58, 1103–1108. [Google Scholar] [CrossRef]
- Rodrguez, F.M.; Lopez, M.R.; Jimenez, I.A.; Moujir, L.; Ravelo, A.G.; Bazzocchi, I.L. New phenolic triterpenes from Maytenus blepharodes, semisynthesis of deoxobleoharodol from pristimerin. Tetrahedron 2005, 61, 2513–2519. [Google Scholar] [CrossRef]
- de Almeida, M.T.R.; Rios-Luci, C.; Padron, J.M.; Palermo, J.A. Antiproliferative terpenoids and alkaloids from the roots of Maytenus vitis-idaea and Maytenus spinosa. Phtochemistry 2010, 71, 1741–1748. [Google Scholar] [CrossRef]
- Oramas-Royo, S.M.; Chavez, H.; Martin-Rodriguez, P.; Fernandez-Perez, L.; Ravelo, A.G.; Estevez-Braun, A. Cytotoxic triterpenoids from Maytenus retusa. J. Nat. Prod. 2010, 73, 2029–2034. [Google Scholar] [CrossRef]
- Kennedy, M.L.; Llanos, G.G.; Castanys, S.; Gamarro, F.; Bazzocchi, I.L.; Jiménez, I.A. Terpenoids from Maytenus species and assessment of their reversal activity against a multidrug-resistant leishmania tropica line. Chem. Biodivers. 2011, 8, 2191–2298. [Google Scholar] [CrossRef] [PubMed]
- Ardiles, A.E.; González-Rodríguez, Á.; Núñez, M.J.; Perestelo, N.R.; Pardo, V.; Jiménez, I.A.; Valverde, Á.M.; Bazzocchi, I.L. Studies of naturally occurring friedelane triterpenoids as insulin sensitizers in the treatment type 2 diabetes mellitus. Phtochemistry 2012, 84, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.F.; Duarte, L.P.; Alcântara, A.F.; Silva, G.D.; Vieira-Filho, S.A.; Silva, R.R.; Oliveira, D.M.; Takahashi, J.A. New triterpenes from Maytenus robusta: Structural elucidation based on NMR experimental data and theoretical calculations. Molecules 2012, 17, 13439–13456. [Google Scholar] [CrossRef]
- Magalhães, C.G.; de Fátima Silva, G.D.; Duartel, L.P.; Bazzocchi, I.L.; Diaz, A.J.; Moujir, L.; López, M.R.; Figueiredo, R.C.; Vieira Filho, S.A. Salicassin, an unprecedented chalconediterpene adduct and a quinone methide triterpenoid from Maytenus salicifolia. Helv. Chim. Acta 2013, 96, 1046–1054. [Google Scholar] [CrossRef]
- Touré, S.; Nirma, C.; Falkowski, M.; Dusfour, I.; Boulogne, I.; Jahn-Oyac, A.; Coke, M.; Azam, D.; Girod, R.; Moriou, C.; et al. Aedes aegypti larvicidal sesquiterpene alkaloids from Maytenus oblongata. J. Nat. Prod. 2017, 80, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.P.; Jiménez, I.A.; Bazzocchi, I.L. Pentacyclic Triterpenoids from Maytenus cuzcoina. Nat. Prod. Commun. 2017, 12, 675–678. [Google Scholar] [CrossRef]
- de Sousa, G.F.; de Aguilar, M.G.; Dias, D.F.; Takahashi, J.A.; Moreira, M.E.C.; Vieira Filho, S.A.; Silva, G.D.F.; Rodrigues, S.B.V.; Braga, M.M.C.T.; Duarte, L.P. Anti-inflammatory, antimicrobial and acetylcholinesterase inhibitory activities of friedelanes from Maytenus robusta branches and isolation of further triterpenoids. Phytochem. Lett. 2017, 21, 61–65. [Google Scholar] [CrossRef]
- Martin, J.D. The structure of dispermoquinone: A triterpenoid quinone methide from Maytenus dispermus. Tetrahedron 1973, 29, 2997–3000. [Google Scholar] [CrossRef]
- González, A.G.; Alvarenga, N.L.; Ravelo, A.G.; Bazzocchi, I.L.; Ferro, E.A.; Navarro, A.G.; Moujir, L.M. Scutione, a new bioactive norquinonemethide triterpene from Maytenus scutioides (Celastraceae). Bioorg. Med. Chem. 1996, 4, 815–820. [Google Scholar] [CrossRef]
- De León, L.; Moujir, L. Activity and mechanism of the action of zeylasterone against Bacillus subtilis. J. Appl. Microbiol. 2008, 104, 1266–1274. [Google Scholar] [CrossRef]
- Niero, R.; Mafra, A.P.; Lenzi, A.C.; Cechinel-Filho, V.; Tischer, C.; Malheiros, A.; De Souza, M.M.; Yunes, R.A.; Delle Monache, F. A new triterpene with antinociceptive activity from Maytenus robusta. Nat. Prod. Res. 2006, 20, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, H.; Suzuki, H.; Lee, K.H.; McPhail, A.T. Structure and stereochemistry of maytenfolic acid and maytenfoliol, two new antileukemic triterpenes from Maytenus diversifolia: X-ray crystal structures. J. Chem. Soc. 1982, 18, 1048–1051. [Google Scholar] [CrossRef]
- Itokawa, H.; Shirota, O.; Morita, H.; Takeya, K.; Tomioka, N.; Itai, A. New triterpene dimers from Maytenus ilicifolia. Tetrahedron Lett. 1990, 31, 6881–6882. [Google Scholar] [CrossRef]
- Gonzlez, A.G.; Jimenez, J.S.; Moujir, L.M.; Ravelo, A.G.; Luis, J.G.; Bazzocchi, I.L.; Gutierrez, A.M. Two new triterpene dimers from Celastraceae, their partial synthesis and antimicrobial activity. Tetrahedron 1992, 48, 769–774. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Alvarenga, N.L.; Bazzocchi, I.L.; Ravelo, A.G.; Laila, M. Triterpenet trimers from Maytenus scutioides: Cycioaddition compounds. J. Nat. Prod. 1999, 62, 1185–1187. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.G.; Rodriguez, F.M.; Bazzocchi, I.L.; Ravelo, A.G. New terpenoids from Maytenus blepharodes. J. Nat. Prod. 2000, 63, 48–51. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Kennedy, M.L.; Rodriguez, F.M.; Bazzocchi, I.L.; Jimenez, I.A.; Ravelo, A.G.; Moujir, L. Absolute configuration of triterpen dimers from Maytenus species(Celastraceae). Tetrahedron 2001, 57, 1283–1287. [Google Scholar] [CrossRef]
- González, A.G.; Jiménez, I.A.; Ravelo, A.G. Triterpenes from Maytenus canariensis and synthesis of a derivative from Betulin. Phtochemistry 1992, 31, 2069–2072. [Google Scholar] [CrossRef]
- Ohsaki, A.; Imai, Y.; Naruse, M.; Ayabe, S.; Komiyama, K.; Takashima, J. Four new triterpenoids from Maytenus ilicifolia. J. Nat. Prod. 2004, 67, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro de Souza e Silva, S.; de Fátima Silva, G.D.; de Almeida Barbosa, L.C.; Duarte, L.P.; Filho, S.A.V. Lupane pentacyclic triterpenes isolated from stems and branches of Maytenus imbricata (Celastraceae). Helv. Chim. Acta 2005, 88, 1102–1109. [Google Scholar] [CrossRef]
- Nunez, M.J.; Reyes, C.P.; Jiménez, I.A.; Moujir, L.; Bazzocchi, I.L. Lupane triterpenoids from Maytenus species. J. Nat. Prod. 2005, 68, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.P.; Nunez, M.J.; Jiménez, I.A.; Busserolles, J.; Alcaraz, M.J.; Bazzocchi, I.L. Activity of lupane truterpenoids from Maytenus species as inhibitors of nitric oxide and prostaglandin E2. Bioorg. Med. Chem. 2006, 14, 1573–1579. [Google Scholar] [CrossRef]
- Vazdeki, N.E.; Chávez, H.; Estevez-Braun, A.; Ravelo, A.G. Triterpenoids and a lignan from the aerial parts of Maytenus apurimacensis. J. Nat. Prod. 2009, 72, 1045–1048. [Google Scholar] [CrossRef]
- Delgado-Méndez, P.; Herrera, N.; Chávez, H.; Estévez-Braun, A.; Ravelo, Á.G.; Cortes, F.; Castanys, S.; Gamarro, F. New terpenoids from Maytenus apurimacensis as MDR reversal agents in the parasite Leishmania. Bioorg. Met. Chem. 2008, 16, 1425–1430. [Google Scholar] [CrossRef]
- Shirota, O.; Tamemura, T.; Morita, H.; Takeya, K.; Itokawa, H. Triterpenes from brazilian medicinal plant “chuchuhuasi” (Maytenus krukovii). J. Mat. Prod. 1996, 59, 1072–1075. [Google Scholar] [CrossRef]
- Muhammad, I.; El Sayed, K.A.; Mossa, J.S.; Al-Said, M.S.; El-Feraly, F.S.; Clark, A.M.; Hufford, C.D.; Oh, S.; Mayer, A.M.S. Bioactive 12-oleanene triterpene and secotriterpene acids from Maytenus undata. J. Nat. Prod. 2000, 63, 605–610. [Google Scholar] [CrossRef]
- Nakagawa, H.; Takaishi, Y.; Fujimoto, Y.; Duque, C.; Garzon, C.; Sato, M.; Okamoto, M.; Oshikawa, T.; Ahmed, S.U. Chemical constituents from the colombian medicinal plant Maytenus laevis. J. Nat. Prod. 2004, 67, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.M.; de Fátima Silva, G.D.; Duarte, L.P.; Vieira Filho, S.A. Chemical constituents isolated from roots of Maytenus acanthophylla reissk (Celastraceae). Biochem. Syst. Ecol. 2006, 34, 661–665. [Google Scholar] [CrossRef]
- Valladao, F.N.; De Miranda, R.R.; de Oliveira, G.S.; Silva, G.D.; Duarte, L.P.; Sidney Filho, A.V. Constituents of furuit pulp of Maytenus salicifolia and complete 1D/2D NMR date of 3β-hydroxy-D:B-friedo-olean-5-enen. Chem. Matural Compd. 2010, 46, 686–691. [Google Scholar] [CrossRef]
- Tan, W.; Pu, D.; Feng, Y.; Li, H. A new triterpenoid from Maytenus austroyunnanensis. Nat. Prod. Res. Dev. 2016, 28, 173–178. [Google Scholar]
- Miranda, R.R.S.; Silva, G.D.F.; Duarte, L.P.; Fortes1, I.C.P.; Filho, S.V. Structural determination of 3β-stearyloxy-urs-12-ene from Maytenus salicifolia by 1D and 2D NMR and quantitative 13C NMR spectroscopy. Magn. Reson. Chem. 2006, 44, 127–131. [Google Scholar] [CrossRef]
- Chavez, H.; Estevez-Braum, A.; Ravelo, A.G.; Gonzalea, A.G. First examples of dammarane triterpenes isolated form Celastraceae. Tetrahedro 1997, 53, 6465–6472. [Google Scholar] [CrossRef]
- Torpocco, V.; Chavez, H.; Estevez-Braum, A.; Ravelo, A.G.; Gonzalea, A.G. New damarane triterpenes from Maytenus macrocarpa. Chem. Pharm. Bull. 2007, 55, 812–814. [Google Scholar] [CrossRef]
- González, A.G.; Tincusi, B.M.; Bazzocchi, I.L.; Tokuda, H.; Nishino, H.; Konoshima, T.; Jiménez, I.A.; Ravelo, A.G. Anti-tumor promoting effects of sesquiterpenes from Maytenus cuzcoina (Celastraceae). Bioorg. Med. Chem. 2000, 8, 1773–1778. [Google Scholar] [CrossRef]
- González, A.C.; Jiménez, I.A.; Ravelo, A.G.; Luis, J.G.; Bazzocchi, I.L. β-Agarofurane sesquiterpene esters from Maytenus canariensis. Phtochemistry 1989, 28, 173–175. [Google Scholar] [CrossRef]
- Chavez, H.; Callo, N.; Estevez-Braun, A.; Ravelo, A.G.; Gonzalez, A.G. Sesquiterpene polyol esters from the leaves of Maytenus macrocarpa. J. Nat. Prod. 1999, 62, 1576–1577. [Google Scholar] [CrossRef]
- Kennedy, M.L.; Corts-Selva, F.; Prez-Victoria, J.M.; Jimnez, I.A.; Gonzlez, A.G.; Muoz, O.M.; Gamarro, F.; Castanys, S.; Ravelo, A.G. Chemosensitization of a multidrug-resistant leishmania tropica line by new sesquiterpenes from Maytenus magellanica and Maytenus chubutensis. J. Med. Chem. 2001, 44, 4668–4676. [Google Scholar] [CrossRef]
- Nunez, M.J.; Cortes-Selva, F.; Bazzocchi, I.L.; Jimenez, I.A.; Gonzalez, A.G.; Ravelo, A.G.; Gavin, J.A. Absolute configuration and complete assignment of 13C NMR data for new sesquiterpenes from Maytenus chiapensis. J. Nat. Prod. 2003, 66, 572–574. [Google Scholar] [CrossRef]
- Perestelo, N.R.; Sánchez-Cañete, M.P.; Gamarro, F.; Jiménez, I.A.; Castanys, S.; Bazzocchi, I.L. Overcoming human P-glycoprotein-dependent multidrug resistance with novel dihydro-β-agarofuran sesquiterpenes. Eur. J. Med. Chem. 2011, 46, 4915–4923. [Google Scholar] [CrossRef]
- Gutiérrez-Nicolás, F.; Oberti, J.C.; Ravelo, Á.G.; Estévez-Braun, A. β-Agarofurans and Sesquiterpene Pyridine Alkaloids from Maytenus spinosa. J. Nat. Prod. 2014, 77, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Núñez, M.J.; Jiménez, I.A.; Mendoza, C.R.; Chavez-Sifontes, M.; Martinez, M.L.; Ichiishi, E.; Tokuda, R.; Tokuda, H.; Bazzocchi, I.L. Dihydro-β-agarofuran sesquiterpenes from celastraceae species as anti-tumour-promoting agents: Structure-activity relationship(Article). Eur. J. Med. Chem. 2016, 111, 95–102. [Google Scholar] [CrossRef]
- Alarcon, J.; Becerra, J.; Silva, M.; Morgenstern, T.; Jakupovic, J. β–Agarofuran from seeds of Maytenus boaria. Phytochemistry 1995, 40, 1457–1460. [Google Scholar] [CrossRef]
- Wibowo, M.; Levrier, C.; Sadowski, M.C.; Nelson, C.C.; Wang, Q.; Holst, J.; Healy, P.C.; Hofmann, A.; Davis, R.A. Bioactive dihydro-β-agarofuran sesquiterpenoids from the Australian rainforest plant Maytenus bilocularis. J. Nat. Prod. 2016, 79, 1445–1453. [Google Scholar] [CrossRef]
- Wibowo, M.; Wang, Q.; Holst, J.; White, J.M.; Hofmann, A.; Davis, R.A. Dihydro-β-agarofurans from the roots of the Australian endemic rainforest tree Maytenus bilocularis act as leucine transport inhibitors. Phytochemistry 2018, 148, 71–77. [Google Scholar] [CrossRef]
- Paz, C.; Von Dossow, D.; Tiznado, V.; Suarez, S.; Cukiernik, F.D.; Baggio, R. A dihydro-β-agarofuran sesquiterpene from Maytenus boaria. Acta Crystallogr. Sect. C 2017, 73, 451–457. [Google Scholar] [CrossRef]
- Paz, C.; Heydenreich, M.; Schmidt, B.; Vadra, N.; Baggio, R. Three new dihydro-β-agarofuran sesquiterpenes from the seeds of Maytenus boaria. Ricardo Baggioe 2018, 74, 564–570. [Google Scholar] [CrossRef]
- Alarcon, J.; Cespedes, C.L. Triterpenes and β-agarofurane sesquiterpenes from tissue culture of Maytenus boaria. Boletín Latinoam. Y Del Caribe De Plantas Med. Y Aromáticas 2016, 15, 206–214. [Google Scholar]
- Orabi, K.Y.; Al-Qasoumi, S.I.; El-Olemy, M.M.; Mossa, J.S.; Muhammad, I. Dihydroagarofuran alkaloid and triterpenes from Maytenus heterophylla and Maytenus arbutifolia. Phytochemistry 2001, 58, 475–480. [Google Scholar] [CrossRef]
- Munoz, O.; Galeffi, C.; Federici, E.; Garbarino, J.A.; Piovano, M.; Nicoletti, M. Boarioside, a eudesmane glucoside from Maytenus boaria. Phytochemistry 1995, 40, 853–855. [Google Scholar] [CrossRef]
- Yuan, L.; Ma, J.; Wang, T.; Li, G.H.; Shen, Y.M.; Zhao, P.J. Chemical constituents from endophytic Phomopsis sp. Lz42 of Maytenus hookeri. Chem. J. Chin. Univ. 2009, 30, 78–81. [Google Scholar]
- Schaneberg, B.T.; Green, D.K.; Sneden, A.T. Dihydroagarofuran sesquiterpene alkaloids from Maytenus putterlickoides. J. Nat. Prod. 2001, 64, 624–626. [Google Scholar] [CrossRef]
- Monache, F.D.; Bettolo, G.M.; Bernays, E.A. Isolation of insect antifeedant alkaloids from Maytenus rigida (Celastraceae). Z. Fur Angew. Entomol. 1984, 97, 406–414. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Chen, C.H.; Kuo, L.M.Y.; King, M.L.; Wu, T.S.; Haruna, M.; Lee, K.H. Antitumor agents, 112. Emarginatine B, a novel potent cytotoxic sesquiterpene pyridine alkaloid from Maytenus emarginata. J. Nat. Prod. 1990, 53, 422–428. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Chen, C.H.; King, M.L.; Wu, T.S.; Lee, K.H. Sesquiterpene pyridine alkaloids from Maytenus emarginata: Emarginatine-C and -D and cytotoxic emarginatine-E and emarginatinine. Phytochemistry 1994, 35, 803–807. [Google Scholar]
- Itokawa, H.; Shirota, O.; Morita, H.; Takeya, K. Sesquiterpene alkaloids obtained from Maytenus ebenifolia. Heterocycles 1992, 34, 885–889. [Google Scholar] [CrossRef]
- Corsino, J.; da Silva Bolzani, V.; Pereira, A.M.S.; Franca, S.C.; Furlan, M. Bioactive sesquiterpene pyridine alkaloids from Maytenus aquifolium. Phytochemistry 1998, 48, 137–140. [Google Scholar] [CrossRef]
- Corsino, J.; Da Silva Bolzan, V.; Pereira, A.M.S.; Franca, S.C.; Furlan, M. Further sesquiterpene pyridine alkaloids from Maytenus aquifolium. Phytochemistry 1998, 49, 2181–2183. [Google Scholar] [CrossRef]
- Santos, V.A.; Regasini, L.O.; Nogueira, C.R.; Passerini, G.D.; Martinez, I.; da Silva, B.V.; Graminha, M.A.S.; Cicarelli, R.M.B.; Furlan, M. Antiprotozoal sesquiterpene pyridine alkaloids from Maytenus ilicifolia. J. Nat. Prod. 2012, 75, 991–995. [Google Scholar] [CrossRef]
- Piacente, S.; Tommasi, N.D.; Pizza, C. Laevisines A and B: Two new sesquiterpene-pyridine alkaloids from Maytenus laevis. J. Nat. Prod. 1999, 62, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Lhinhatrakool, T.; Prabpai, S.; Kongsaeree, P.; Sutthivaiyakit, S. Antiplasmodial Sesquiterpene Alkaloids from the Roots of Maytenus mekongensis. J. Nat. Prod. 2011, 74, 1386–1391. [Google Scholar] [CrossRef]
- Sekar, K.V.; Campagne, J.M.; Sneden, A.T. 5-benzoyl-5-deacetylwilforidine: A new sesquiterpene nicotinoyl alkaloid from Maytenus buchananii. Planta Med. 1996, 62, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Nunez, M.J.; Guadano, A.; Jimenez, I.A.; Ravelo, A.G.; Gonzalez-Coloma, A.; Bazzocchi, I.L. Insecticidal sesquiterpene pyridine alkaloids from Maytenus chiapensis. J. Nat. Prod. 2004, 67, 14–18. [Google Scholar] [CrossRef]
- Callies, O.; Núnez, M.J.; Perestelo, N.R.; Reyes, C.P.; Torres-Romero, D.; Jiménez, I.A.; Bazzocchi, I.L. Distinct sesquiterpene pyridine alkaloids from in Salvadoran and Peruvian Celastraceae species. Phytochemistry 2017, 142, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Sneden, A.T.; Beemsterboer, G.L. Maytansine, a new antileukemic ansa macrolide from Maytenus buchananii. J. Nat. Prod. 1980, 43, 637–640. [Google Scholar] [CrossRef]
- Larson, G.M.; Schaneberg, B.T.; Sneden, A.T. Two new maytansinoids from Maytenus buchananii. J. Nat. Prod. 1999, 62, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.I.; Shaw, C.C. Studies directed toward the total synthesis of maytansine. The preparation and properties of the carbinolamide moiety. Tetrahedron Lett. 1974, 15, 717–720. [Google Scholar] [CrossRef]
- Meyers, A.I.; Shaw, C.C.; Deane, H.; Trefonas, L.M.; Majeste, R.J. Progress toward the total synthesis of maytansine. A stereoselective synthesis of the C-1 to C-7 moiety (northern zone). Tetrahedron Lett. 1975, 16, 1745–1748. [Google Scholar] [CrossRef]
- Meyers, A.I.; Brinkmeyer, R.S. Progress toward the total synthesis of maytansine. A model system containing the C-7 to C-16 moiety (southern and eastern zone). Tetrahedron Lett. 1975, 16, 1749–1752. [Google Scholar] [CrossRef]
- Kane, J.M.; Meyers, A.I. Progress toward total synthesis of maytansinoids—facile route to aromatic moiety (western zone). Tetrahedron Lett. 1977, 18, 771–774. [Google Scholar] [CrossRef]
- Meyers, A.I.; Tomiok, K.; Roland, D.M.; Comins, D. Progress toward the total synthesis of maytansinoids. An efficient route to two major precursors (western-southern zone). Tetrahedron Lett. 1978, 19, 1375–1378. [Google Scholar] [CrossRef]
- Corey, E.J.; Bock, M.G. Stereocontrolled route to a key intermediate for the synthesis of maytansine. Tetrahedron Lett. 1975, 16, 2643–2646. [Google Scholar] [CrossRef]
- Corey, E.J.; Wetter, H.F.; Kozikowski, A.P.; Rama Rao, A.V. Preparation of a benzeynoid intermediate for use in the synthesis of maytansine. Tetrahedron Lett. 1977, 9, 777–778. [Google Scholar] [CrossRef]
- Corey, E.J.; Bock, M.G.; Kozikowski, A.P.; Rao, A.R.; Floyd, D.; Lipshutz, B. A key intermedlate for the synthesis of maytansine and related antitumor agents. Tetrahedron Lett. 1978, 12, 1051–1054. [Google Scholar] [CrossRef]
- Corey, E.J.; Weigel, L.O.; Chamberlin, A.R.; Cho, H.; Hua, D.H. Total synthesis of maytansine. J. Am. Chem. Soc. 1980, 102, 6613–6615. [Google Scholar] [CrossRef]
- Foy, J.E.; Ganem, B. Ansa preparation of the macrolide synthesis aromatic portion of maycansine. Tetrahedron Lett. 1977, 9, 775–776. [Google Scholar] [CrossRef]
- Edwards, O.E.; Ho, P.T. The cyclic carbamate unit of maytansine. Can. J. Chem. 1997, 55, 371–373. [Google Scholar] [CrossRef]
- Samson, M.; De Clercq, P.; De Wilde, H.; Vandewalle, M. A stereo controlled route to a key intermediate for the synthesis of maytansine. Tetrahedron Lett. 1977, 36, 3195–3198. [Google Scholar] [CrossRef]
- Gotschi, E.; Schneider, F.; Wagner, H.; Bernauer, K. Building Blocks for the Synthesis of Maytansinoids. Helv. Chim. Acta 1977, 60, 1416–1418. [Google Scholar] [CrossRef]
- Pan, B.C.; Zhang, H.; Pan, C.Y.; Shu, Y.; Wang, Z.F.; Gao, Y.S. Studies on the total syntheses of maytansinoidsⅠ. Stereoselective syntheses of C1-C8 fragnment of maytansine. Acta Chim. Sin. 1980, 38, 502–506. [Google Scholar]
- Zhou, Q.; Pai, D.L.; Sun, H.L.; Yang, Y.C.; Du, Y.H.; Xu, Y.B.; Chen, M.Q.; Gao, Y.S. Studies on the total syntheses of maytansinoids Ⅱ. Preparation of the C9-N molety. Acta Chim. Sin. 1980, 38, 507–510. [Google Scholar]
- Gu, X.Q.; Pan, B.C.; Zhou, Q.T.; Bai, D.L.; Gao, Y.S. Studies on the total syntheses of maytansinoids Ⅲ. Preparation of the C5-N molety. Sci. China Chem. 1987, 12, 1294–1300. [Google Scholar]
- Gu, X.; Pan, B.; Zhou, Q.; Bai, D.; Gao, Y. Studies on the total synthesis of maytansinoids (Ⅳ)—Synthesis of maytansine. Sci. China Chem. 1987, 12, 1301–1308. [Google Scholar]
- People’s Liberation Army 62nd Hospital. Preliminary clinical observation of 17 cases of malignant tumor treated with Maytenus hookeri Loes. J. New Med. 1978, 9, 116–118. [Google Scholar]
- Ning, A.; Pan, Q.Q.; Xue, X.H. Effect of Maytenus hookeri Loes on the fine structures in the cells of an esophageal carcinoma cell line. Acta Anat. Sin. 1980, 11, 199–204. [Google Scholar]
- Fan, Y.J.; He, S.X.; Wei, H.Y.; Xin, B.H.; Qu, Z.J.; Wang, Y.J.; Zhou, J.; Li, M.; Zhou, G.F.; Chen, L.L.; et al. Study on anti-tumor and pharmacological effects of Maytenus confertiflorus. Chin. Tradit. Herb. Drugs 1981, 12, 18–21. [Google Scholar]
- Gong, A.M.; Li, X.Y.; Yang, S.Z.; Feng, Z. The extract of Maytenus.hainanensis regulates the expression of apoptosis genes p53, Fas and bcl-2 in human hepatocarcinoma cells. Chin. J. Gerontol. 2015, 35, 2370–2372. [Google Scholar]
- Nabende, P.N.; Karanja, S.M.; Mwatha, J.K.; Wachira, S.W. Anti-proliferative Activity of Prunus africana, Warburgia stuhlmannii and Maytenus senegalensis Extracts in Breast and Colon Cancer Cell Lines. Eur. J. Med. Plants 2015, 5, 366–376. [Google Scholar] [CrossRef]
- Zeng, B.; Ge, C.; Zhao, W.; Fu, K.; Liu, L.; Lin, Z.; Fu, Q.; Li, Z.; Li, R.; Guo, H.; et al. Anticancer effect of the traditional Chinese medicine herb Maytenus compound via the EGFR/PI3K/AKT/GSK3β pathway(Article). Transl. Cancer Res. 2019, 8, 2130–2140. [Google Scholar] [CrossRef]
- Ni, Z.W.; Li, G.H.; Zhao, P.J.; Shen, Y.M. Antimicrobial components of the endophytic fungal strain Chaetomiumglobosum Ly50′ from Maytenus hookeri. Nat. Prod. Res. Dev. 2008, 20, 33–36. [Google Scholar]
- Wu, J.M.; Chen, P.J.; Xu, S.H.; Wu, Y.; Chen, Z.H. Study on antibacterial effect of mayteus. Microbiol. China 1982, 6, 277–279. [Google Scholar]
- Rubab, M.; Chellia, R.; Saravanakumar, K.; Mandava, S.; Khan, I.; Tango, C.N.; Hussain, M.S.; Daliri, E.B.; Kim, S.H.; Ramakrishnan, S.R.; et al. Preservative effect of Chinese cabbage (Brassica rapa subsp. pekinensis) extract on their molecular docking, antioxidant and antimicrobial properties. PLoS ONE 2018, 13, e0203306. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Hei, Y.R.; Tian, J. Study on antifungal activity from Gymnosporia varialilis Loes. J. Shanxi Agric. Sci. 2018, 46, 1626–1630. [Google Scholar]
- Zhang, X.Y. Bioactive Components of Phacellaria compressa Benth., Gymnosporia varialilis Loes. and Buddleja brachysta Diels. Ph.D. Thesis, Chegdu Institute of Miology, Chinese Academy of Sciences, Chengdu, China, 2005. [Google Scholar]
- Malebo, H.M.; Wiketye, V.; Katani, S.J.; Kitufe, N.A.; Nyigo, V.A.; Imeda, C.P.; Ogondiek, J.W.; Sunguruma, R.; Mhame, P.P.; Massaga, J.J.; et al. In vivo antiplasmodial and toxicological effect of Maytenus senegalensis traditionally used in the treatment of malaria in Tanzania. Malar. J. 2015, 14, 2–7. [Google Scholar] [CrossRef]
- Bishnoi, N.; Shrivastava, B.; Bairwa, R.; Sah, S.K. Evaluation of anti- hyperglycaemic acticity of maytenus emarginatus willd leaves extract on streptozotocin-induced diabetes in wistar rats. Int. J. Pharm. Sci. Res. 2016, 7, 14–19. [Google Scholar]
- Hussein, G.; Nakamura, N.; Meselhy, M.R.; Hattori, M. Phenolics from Maytenus senegalensis. Phytochemistry 1999, 50, 689–694. [Google Scholar] [CrossRef]
- Joshi, U.P.; Wagh, R.D. Estimation of Total Phenolic, Total Flavonoid Content and Assessment of in vitro Antioxidant Activity of Extracts of plant Maytenus emarginata stembark. Int. J. Pharm. Res. 2021, 13, 3332–3341. [Google Scholar]
- de Oliveira Meneguetti, D.U.; Lima, R.A.; da Silva, F.C.; Passarini, G.M.; Facundo, J.B.; de Cassia Pagotto, R.; Militão, J.S.L.T.; Facundo, V.A. Acute genotoxicity analysis in vivo of the aqueous extract of Maytenus guyanensis Amazonian chichuá. Rev. Bras. Farmacogn. 2015, 25, 164–169. [Google Scholar] [CrossRef][Green Version]
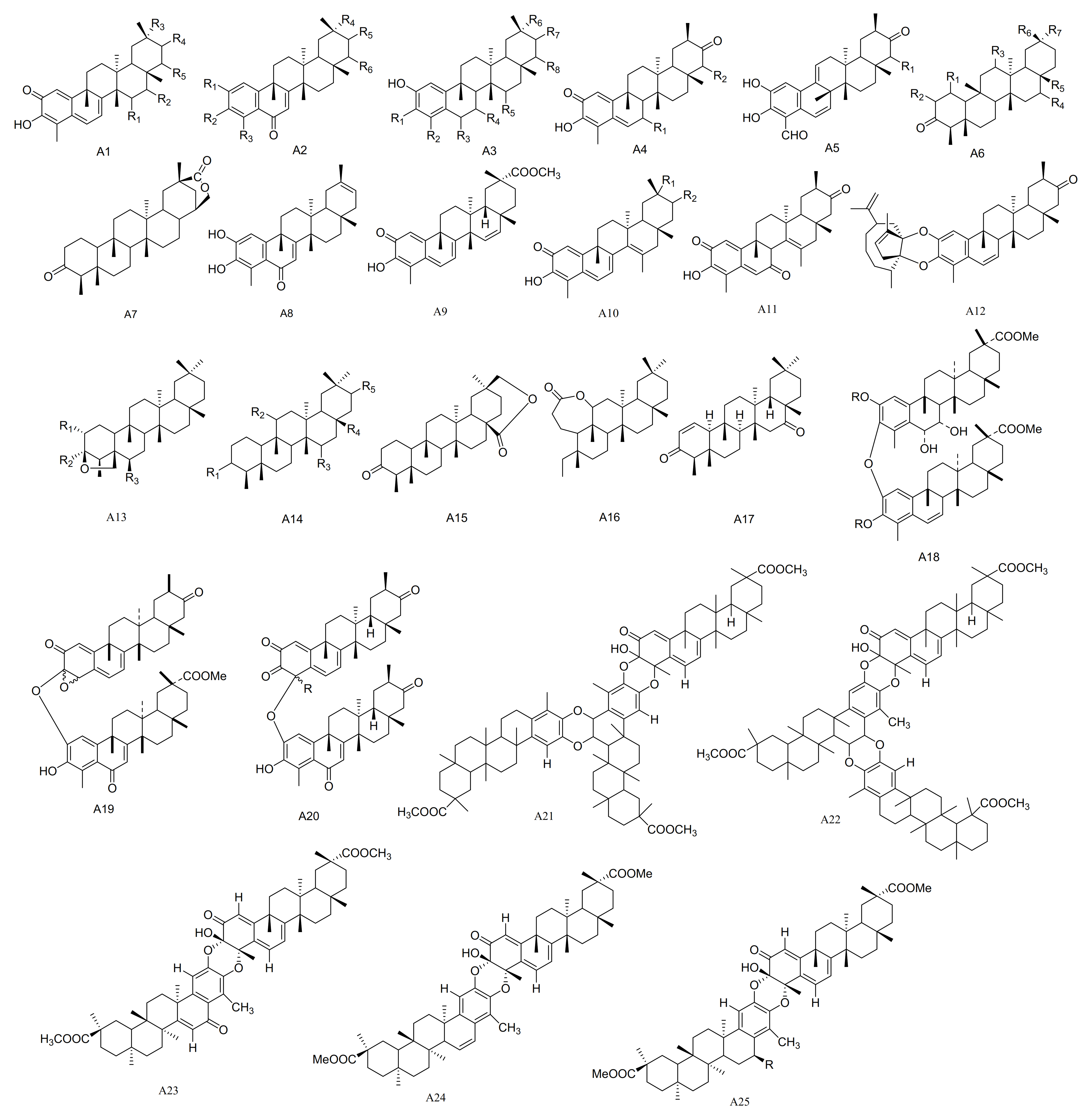
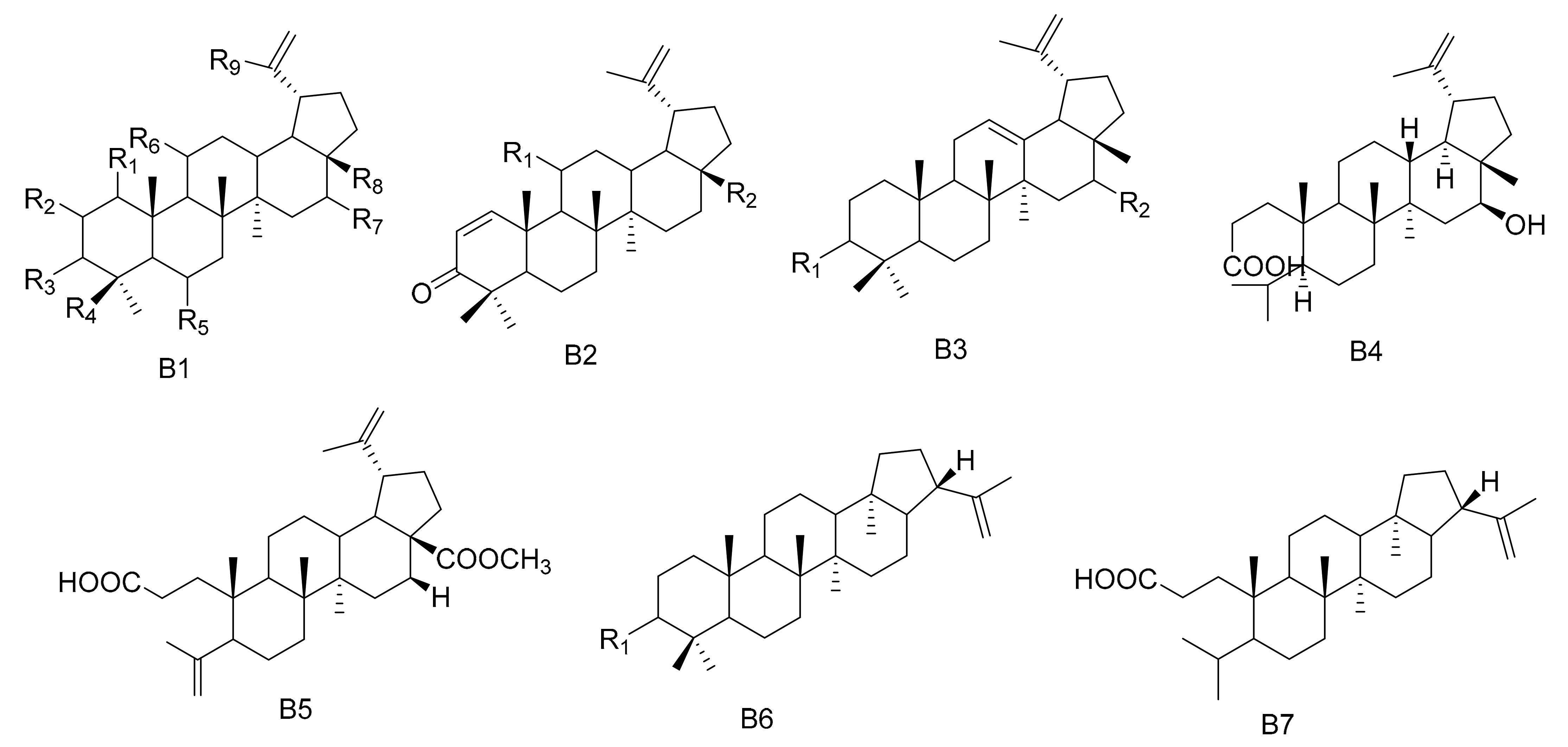
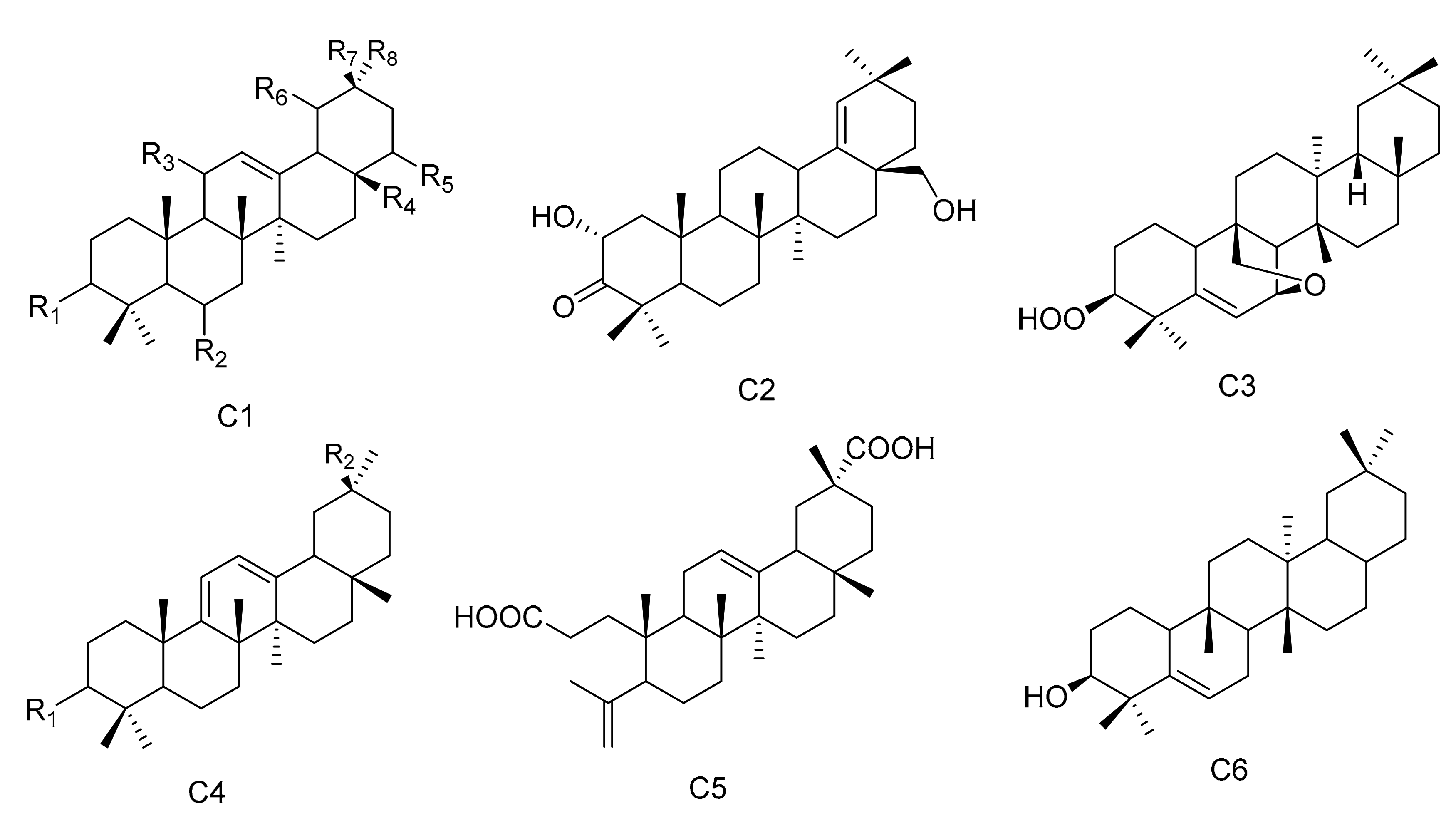
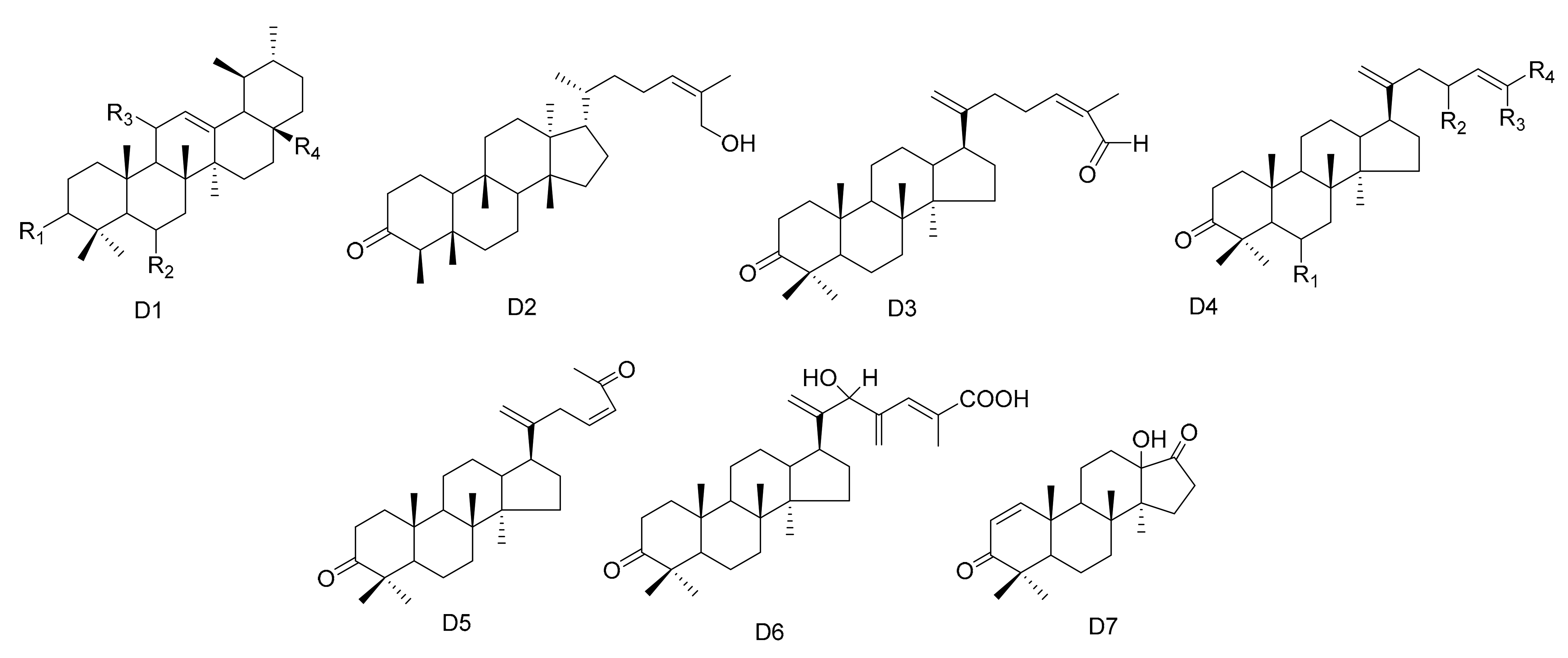
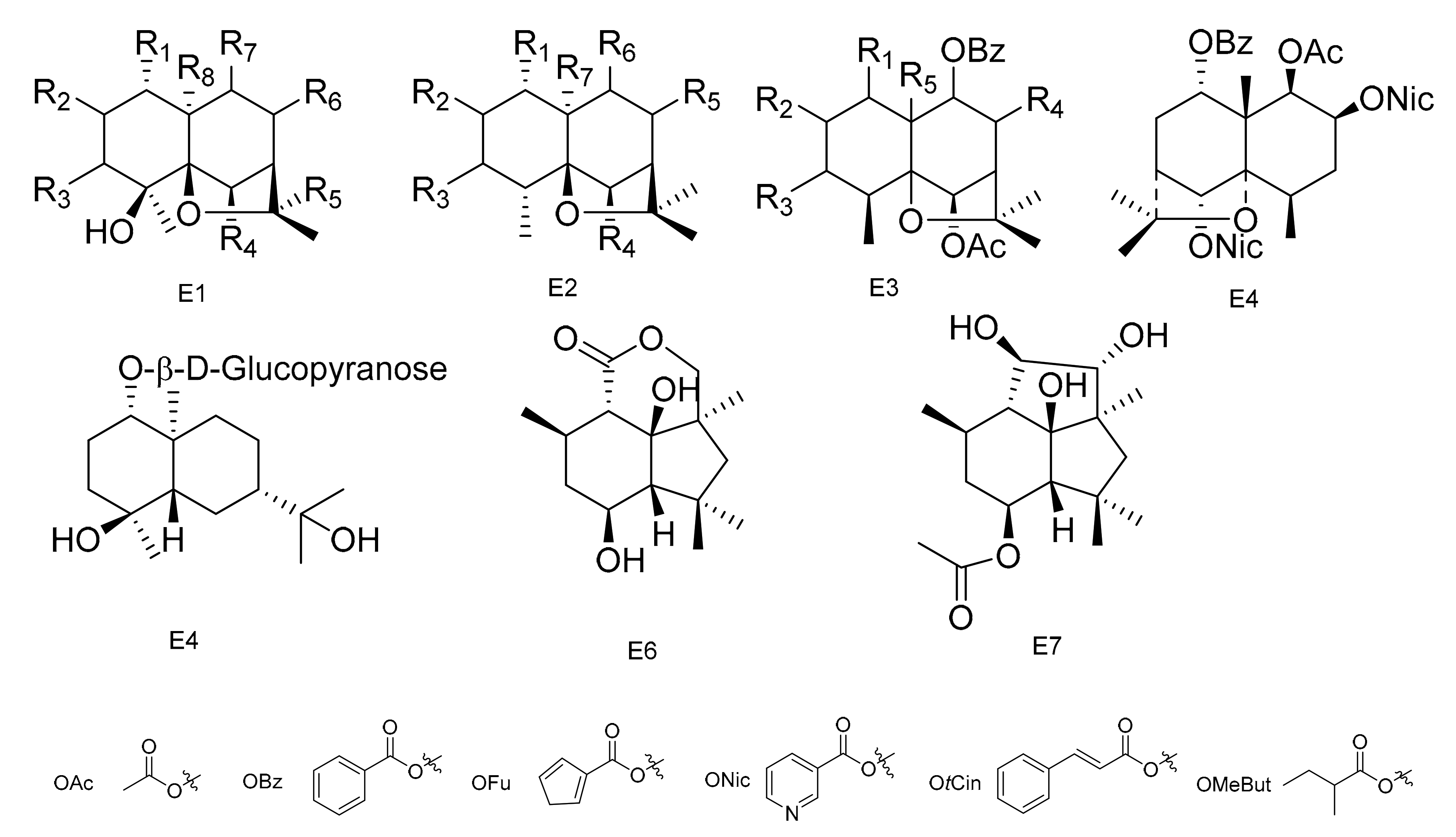
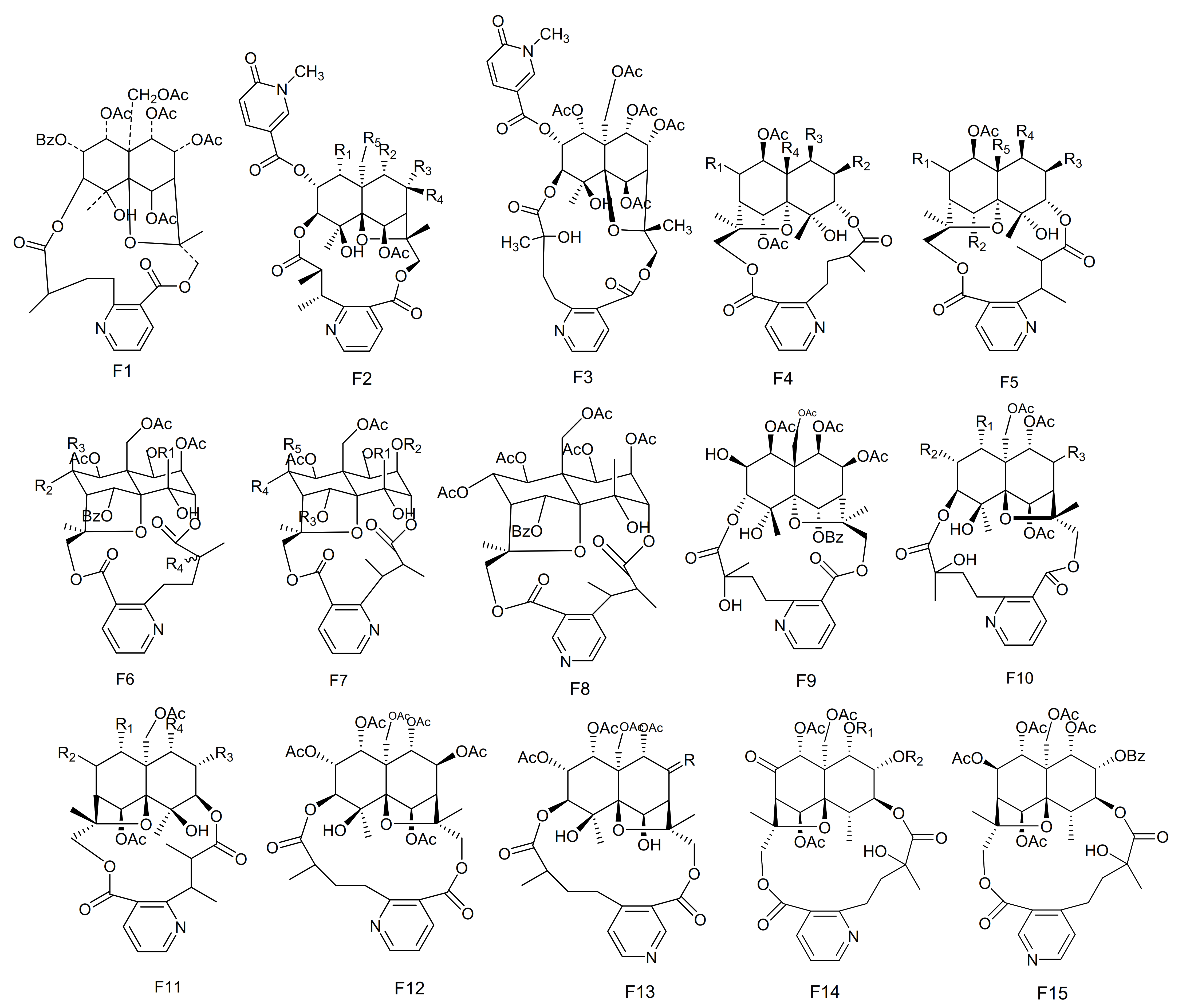
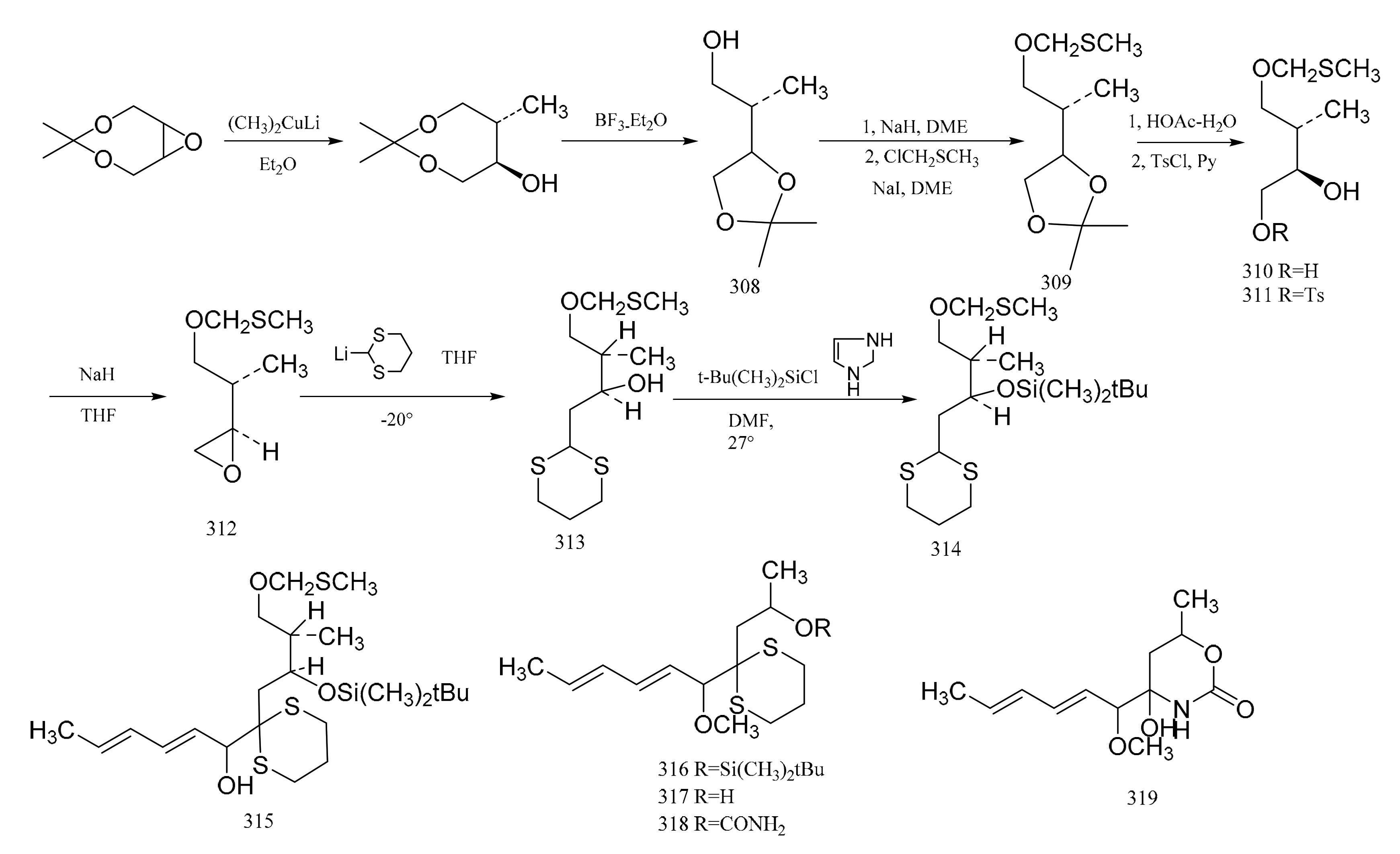
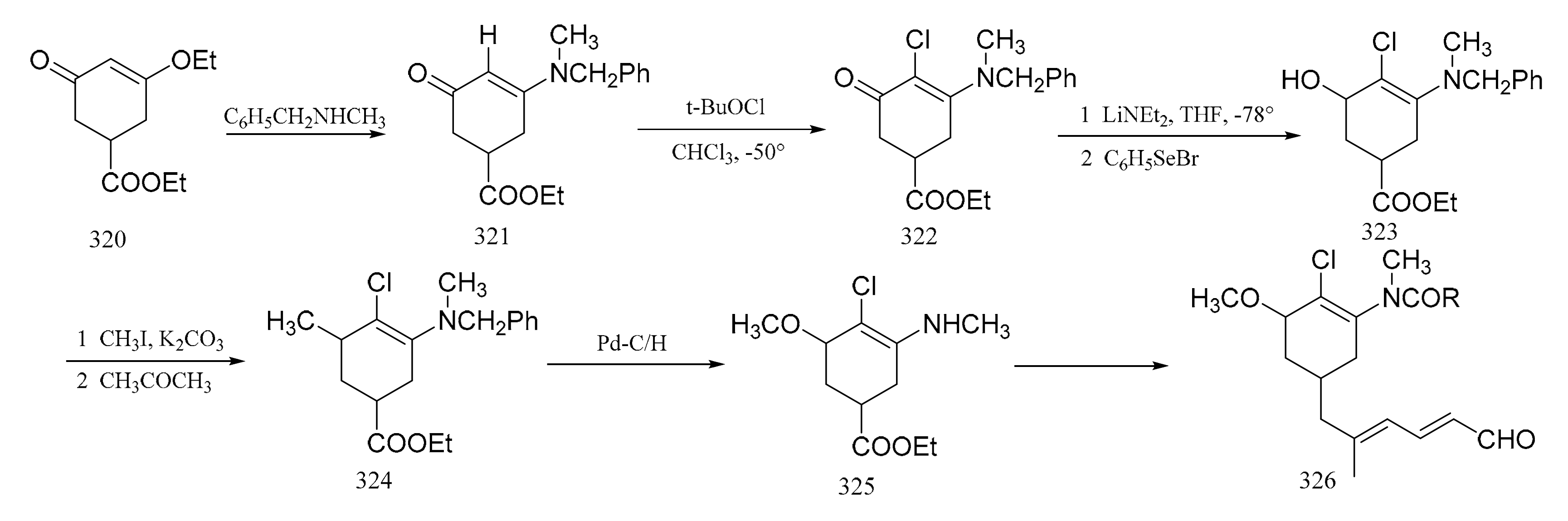
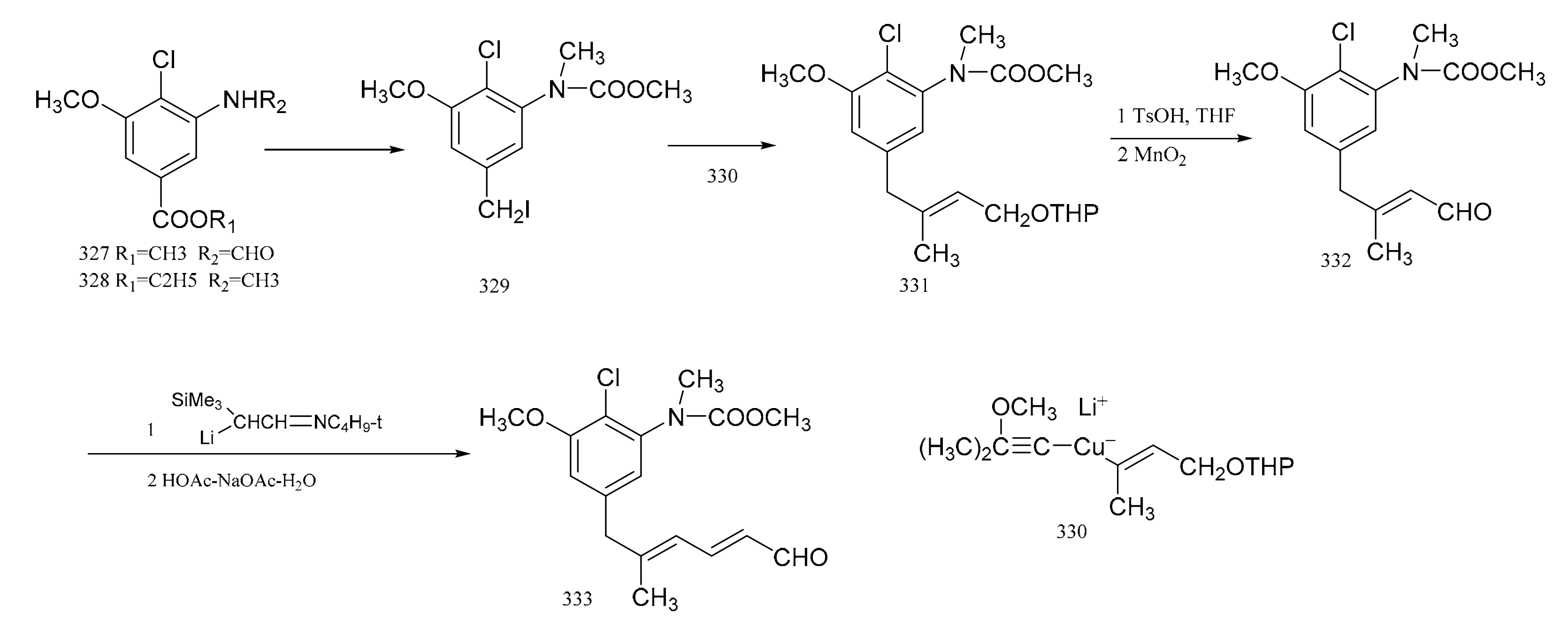
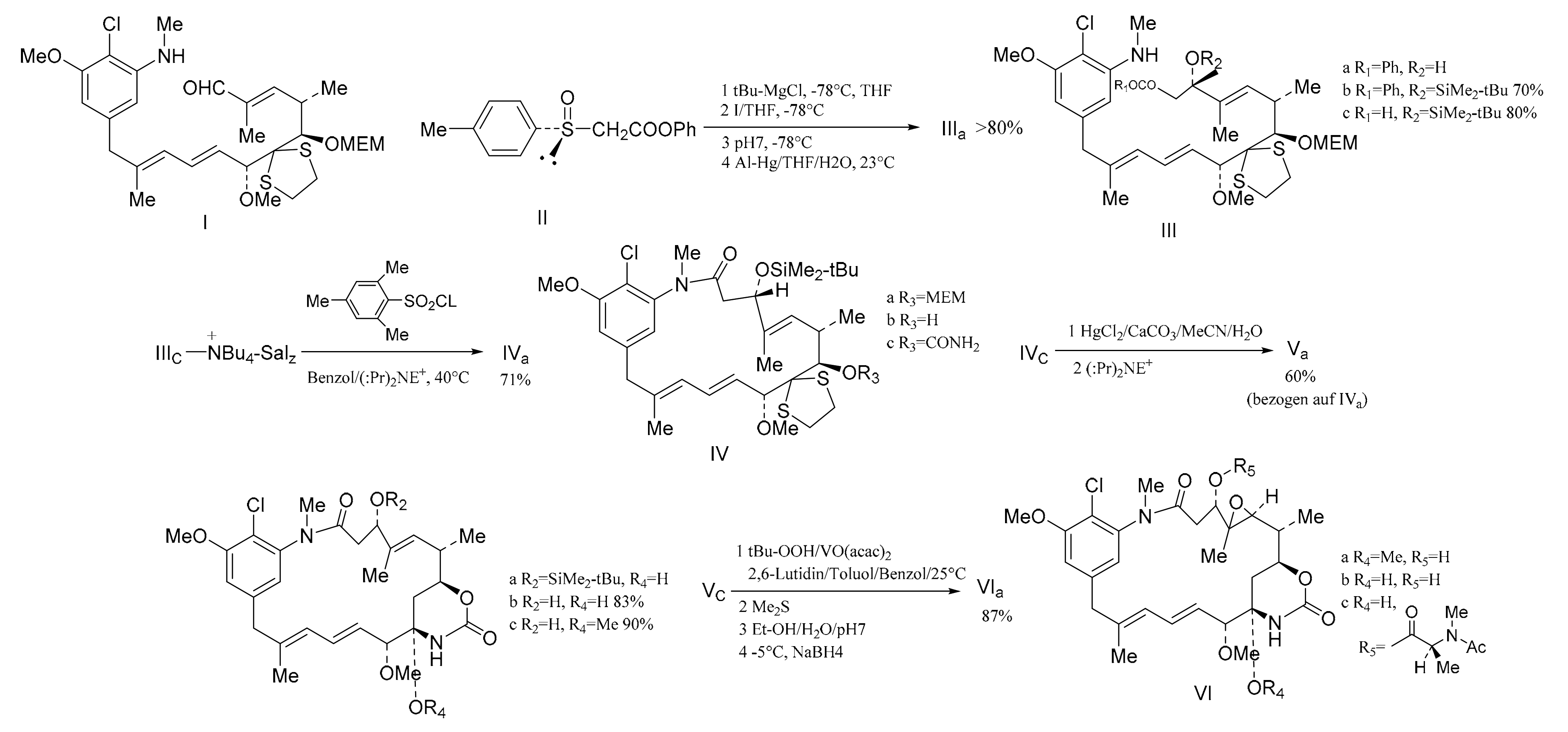
| No. | Name | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | Type | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pristimerin | H | H | COOMe | H | H | - | - | - | A1 | [16] |
| 2 | 15α-hydroxy-21-keto-pristimerine | α-OH | H | COOMe | =O | H | - | - | - | A1 | [17] |
| 3 | 2,3,22β-trihydroxy-24,29-dinor-1,3,5(10),7-friedelatetraene-6,21-dione-23-al | OH | OH | CHO | H | =O | β-OH | - | - | A2 | [18] |
| 4 | 2,22β-dihydroxyl-3-methoxy-24,29-dinor-1,3,5(10),7-friedelatetraene-6,21-dione | OH | OH | CH3 | H | =O | β-OH | - | - | A2 | [18] |
| 5 | 2,3,22β-triihydroxy-23,24,29-trinor-1,3,5(10),7-friedelatetr aene-6,21-dione | OH | OH | H | H | =O | β-OH | - | - | A2 | [18] |
| 6 | 2,22β-dihydroxyl-3-methoxy-24,29-dinor-1,3,5(10),7-friedelatetraene-6,21-dione | OH | OCH3 | CH3 | H | =O | β-OH | - | - | A2 | [18] |
| 7 | 2,3,22β-trihydroxy-24,29-dinor-1,3,5(10)–friedelatetraene -6,21-dione | OH | CH3 | =O | H | H | H | =O | β-OH | A3 | [18] |
| 8 | 2,15α,22β-trihydroxy-3-methoxy-24,29-dinor-1,3, 5(10)-friedelatriene-21-one | OCH3 | CH3 | H | H | α-OH | H | =O | β-OH | A3 | [18] |
| 9 | 3,22β-dihydroxy-24,29-dinor-l(10)-3,5-friedelatriene-2,7,21-trione | =O | β-OH | =O | H | - | - | - | - | A4 | [18] |
| 10 | 3,22β-dihydroxy-24,29-dinor-l(10),3,5–friedelatriene-21-one | H | β-OH | =O | H | - | - | - | - | A4 | [18] |
| 11 | 2,3,22β-trihydroxy-24,29-dinor-25(9→8)-1,3,5(10),7-friedelatetraene-21-one-23-al | β-OH | - | - | - | - | - | - | - | A5 | [18] |
| 12 | 23-oxo-iso-tingenone | H | - | - | - | - | - | - | - | A5 | [19] |
| 13 | (8S)-7,8-dihydro-7-oxo-tingenoe | =O | H | =O | H | - | - | - | - | A4 | [19] |
| 14 | (7S, 8S)-7-hydroxy-7,8-dihydro-tingenone | α-OH | H | =O | H | - | - | - | - | A4 | [19] |
| 15 | (8S)-7,8-dihydro-6-oxo-tingenol | OH | CH3 | =O | H | H | H | =O | H | A3 | [19] |
| 16 | 23-nor-6-oxo-tingenol | OH | OH | H | H | =O | H | - | - | A2 | [19] |
| 17 | 28-hydroxy-friedelane-1,3-dione | =O | H | H | H | CH2OH | CH3 | CH3 | - | A6 | [20] |
| 18 | Macrocarpin A | OH | CHO | =O | H | H | COOMe | H | H | A3 | [21] |
| 19 | Macrocarpin B | OH | OH | COOH | H | =O | β-OH | - | - | A2 | [21] |
| 20 | Macrocarpin C | OH | OCH3 | COOH | H | =O | β-OH | - | - | A2 | [21] |
| 21 | Macrocarpin D | α-OH | β-OH | =O | H | - | - | - | - | A4 | [21] |
| 22 | Maytenfolone | - | - | - | - | - | - | - | - | A7 | [22] |
| 23 | 6-oxo-iguesterol | - | - | - | - | - | - | - | - | A8 | [12] |
| 24 | 6-oxo-tingenol | OH | OH | CH3 | H | =O | H | - | - | A2 | [12] |
| 25 | 3-O-methoxy-6-oxo-tingenol | OH | OCH3 | CH3 | H | =O | H | - | - | A2 | [12] |
| 26 | Blepharotriol | OH | OH | OH | COOMe | H | H | - | - | A2 | [23] |
| 27 | 6-deoxoblepharodol | OH | CH3 | H | H | H | COOMe | H | H | A3 | [23] |
| 28 | Isoblepharodol | OH | CH3 | H | =O | H | COOMe | H | H | A3 | [23] |
| 29 | 7-oxo-blepharodol | OH | CH3 | =O | =O | H | COOMe | H | H | A3 | [23] |
| 30 | 15α-hydroxy-tingenone | α-OH | H | H | =O | H | - | - | - | A1 | [24] |
| 31 | 15-dihydro-pristimerin | - | - | - | - | - | - | - | - | A9 | [24] |
| 32 | Vitideasin | α-COOCH3 | H | - | - | - | - | - | - | A10 | [24] |
| 33 | 20β-hydroxy-scutione | α-OH | =O | - | - | - | - | - | - | A10 | [24] |
| 34 | 7-oxo-7,8-dihydro-scutione | - | - | - | - | - | - | - | - | A11 | [25] |
| 35 | 6,23-dioxo-7,8-dihydro-pristimerol-23-oic Acid | OH | COOH | =O | H | H | COOMe | H | H | A3 | [25] |
| 36 | 23-nor-blepharodol | OH | H | =O | H | H | COOMe | H | H | A3 | [25] |
| 37 | 3-methoxy-6-oxo-tingenol-23-oic Acid | OH | OCH3 | COOH | H | =O | H | - | - | A2 | [25] |
| 38 | Retusonine | - | - | - | - | - | - | - | - | A12 | [25] |
| 39 | 21-Oxopristimerine | H | H | COOMe | =O | H | - | - | - | A1 | [25] |
| 40 | 3-O-methyl-6-oxo-pristimerol | OH | OCH3 | CH3 | COOMe | H | H | - | - | A2 | [26] |
| 41 | 3β,24-epoxy-2α,3α-dihydroxy-D:A-friedooleanan-29-oic acid methyl ester | OH | OH | H | - | - | - | - | - | A13 | [27] |
| 42 | 2α-acetoxy-3β,24-epoxy-3α-hydroxy- D:A-friedooleanan-29-oic acid methyl ester | OAc | OH | H | - | - | - | - | - | A13 | [27] |
| 43 | 3α-hydroxy- D:A-friedooleanan-28-oic acid | α-OH | H | H | COOH | H | - | - | - | A14 | [27] |
| 44 | 3-oxo-D:A-friedooleanan-28,30-olide | - | - | - | - | - | - | - | - | A15 | [27] |
| 45 | 3β,11β-dihydroxyfriedelane | β-OH | β-OH | H | CH3 | H | - | - | - | A14 | [28] |
| 46 | 3,4-seco-friedelan-3,11β-olide | - | - | - | - | - | - | - | - | A16 | [28] |
| 47 | (16β)-16-hydroxy-pristimerin | H | β-OH | COOMe | H | H | - | - | - | A1 | [29] |
| 48 | 12,16-dihydroxyfriedelan-3-one | H | H | α-OH | β-OH | CH3 | CH3 | CH3 | - | A6 | [30] |
| 49 | 3β,24β-epoxy-29-methoxy-2α,3α,6α-trihydroxy-D:A-friedelane | OH | OH | β-OH | - | - | - | - | - | A13 | [31] |
| 49a | 3β,24β-epoxy-29-methoxy-2α,3α,6α-triacetoxy-D:A-friedelane | OAc | OAc | β-OAc | - | - | - | - | - | A13 | [31] |
| 50 | Friedel-1-en-3,16-dione | - | - | - | - | - | - | - | - | A17 | [32] |
| 51 | 1α,29-dihydroxyfriedelan-3-one | α-OH | H | H | H | CH3 | CH3 | CH2OH | - | A6 | [32] |
| 52 | 16β,28,29-trihydroxyfriedelan-3-one | H | H | H | β-OH | CH2OH | CH3 | CH2OH | - | A6 | [32] |
| 53 | Dispemroquinone | =O | H | H | COOMe | - | - | - | - | A5 | [33] |
| 54 | Scutione | H | =O | - | - | - | - | - | - | A10 | [34] |
| 55 | Zeylasterone | OH | OH | COOH | COOMe | H | H | - | - | A2 | [35] |
| 56 | Demethylzeylasterone | OH | OH | COOH | COOH | H | H | - | - | A2 | [35] |
| 57 | 3,15-dioxo-21-hydroxy friedelane | =O | H | =O | CH3 | α-OH | - | - | - | A14 | [36] |
| 58 | Maytenfoliol | H | H | H | H | CH2OH | CH2OH | CH3 | - | A6 | [37] |
| 59 | Cangorosin A | H | - | - | - | - | - | - | - | A18 | [38] |
| 60 | Atropcangorosin A | H(atropisomer of 7-bu) | - | - | - | - | - | - | - | A18 | [38] |
| 61 | Dihydroatropcangorosin A | 6′,7′-dihydro derivative of 8-BU | - | - | - | - | - | - | - | A18 | [38] |
| 62 | Cangorosin B | - | - | - | - | - | - | - | - | A19 | [38] |
| 63 | Umbellatin α | α-Me | - | - | - | - | - | - | - | A20 | [39] |
| 64 | Umbeilatin β | β-Me | - | - | - | - | - | - | - | A20 | [39] |
| 65 | Tiscutin A | - | - | - | - | - | - | - | - | A21 | [40] |
| 66 | Triscutin B | - | - | - | - | - | - | - | - | A22 | [40] |
| 67 | Xuxuarine Eα | - | - | - | - | - | - | - | - | A23 | [41] |
| 68 | Scutionin αB | - | - | - | - | - | - | - | - | A24 | [42] |
| 69 | 6′,7′-dihydro-scutionin αB | H | - | - | - | - | - | - | - | A25 | [42] |
| 70 | 6′β-methoxy-6′,7′dihydro-scutionin αB | OCH3 | - | - | - | - | - | - | - | A25 | [42] |
| No. | Name | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | Type | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 71 | 3β,28,30-Lup-20(29)-ene triol | H | H | OH | CH3 | H | H | H | CH2OH | CH2OH | B1 | [43] |
| 72 | 28,30-Dihyroxylup-20(29)-ene-3-one | H | H | =O | CH3 | H | H | H | CH2OH | CH2OH | B1 | [43] |
| 73 | Maytefolins A | H | α-OH | =O | CH3 | H | H | H | CH2OH | CH3 | B1 | [44] |
| 74 | 3-oxolup-20(29)-en-30-al | H | H | =O | CH3 | H | H | H | CH3 | CHO | B1 | [45] |
| 75 | 30-hydroxylup-20(29)-en-3-one | H | H | =O | CH3 | H | H | H | CH3 | CH2OH | B1 | [45] |
| 76 | (11α)-11-hydroxylup-20(29)-en-3-one | H | H | =O | CH3 | H | α-OH | H | CH3 | CH3 | B1 | [45] |
| 77 | (3β)-lup-20(30)-ene-3,29-diol | H | H | β-OH | CH3 | H | H | H | CH3 | CH2OH | B1 | [45] |
| 78 | 11α-hydroxy-epi-betuin | H | H | α-OH | CH3 | H | α-OH | H | CH2OH | CH3 | B1 | [46] |
| 79 | 6β-hydroxybetulin | H | H | β-OH | CH3 | β-OH | H | H | CH2OH | CH3 | B1 | [46] |
| 80 | 24-hydroxybetulin | H | H | =O | CH2OH | H | H | H | CH2OH | CH3 | B1 | [46] |
| 81 | Rigidenol-28-aldehyde | H | H | =O | CH3 | H | α-OH | H | CHO | CH3 | B1 | [46] |
| 82 | 28-hydroxyglochidone | H | CH2OH | - | - | - | - | - | - | - | B2 | [46] |
| 83 | 11α-hydroxy-glochidone | α-OH | CH3 | - | - | - | - | - | - | - | B2 | [47] |
| 84 | 3-epi-nepeticin | H | H | α-OH | CH3 | H | α-OH | H | CH3 | CH3 | B1 | [47] |
| 85 | 3-epi-calenduladiol | H | H | α-OH | CH3 | H | H | H | OH | CH3 | B1 | [47] |
| 86 | 3α,16β,28-Trihydroxylup-20(29)-ene | H | H | α-OH | CH3 | H | H | β-OH | CH2OH | CH3 | B1 | [48] |
| 87 | 3α,16β-dihydroxylup-12-ene | α-OH | β-OH | - | - | - | - | - | - | - | B3 | [48] |
| 88 | 3β,16β-dihydroxylup-12-ene | β-OH | β-OH | - | - | - | - | - | - | - | B3 | [48] |
| 89 | 16β-3,4-secolup-20(29)-en-3-oic acid | - | - | - | - | - | - | - | - | - | B4 | [48] |
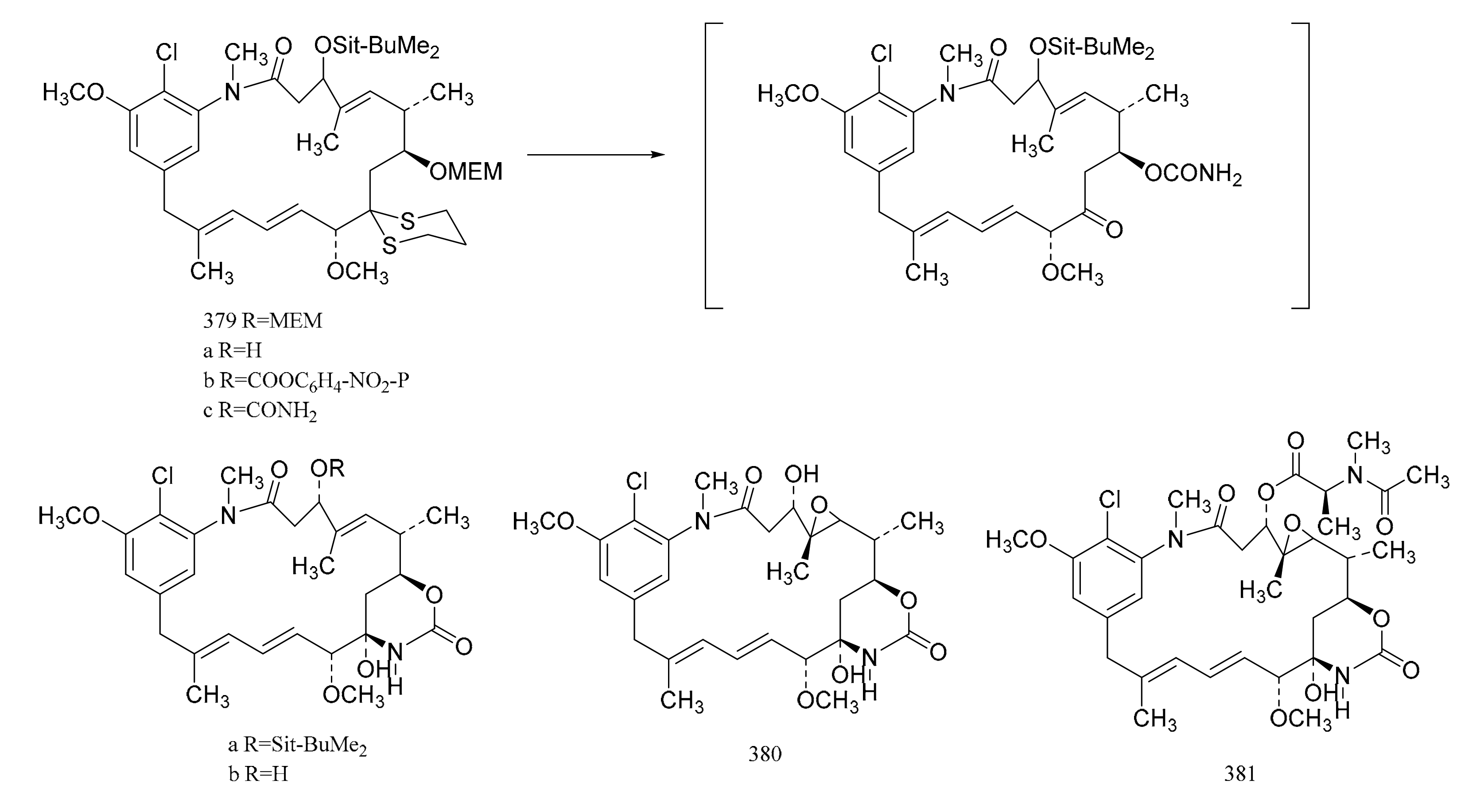
| 90 | 3-(E)-β-coumaroylnepeticin | H | H | 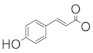 | CH3 | H | α-OH | H | CH3 | CH3 | B1 | [25] |
| 91 | 3,4-seco-lupa-4(23):20(29)-diene-3,28-dioicacid 28-methyl ester | - | - | - | - | - | - | - | - | - | B5 | [26] |
| 92 | 1β-Hydroxy-3β-caffeate lup-20(29)-ene | β-OH | H | OCaf | CH3 | H | H | H | CH3 | CH3 | B1 | [49] |
| 93 | 3-oxo-21β-H-hop-22(29)-ene | =O | - | - | - | - | - | - | - | - | B6 | [28] |
| 94 | 3β-hydroxy-21β-H-hop-22(29)-ene | β-OH | - | - | - | - | - | - | - | - | B6 | [28] |
| 95 | 3,4-seco-21β-H-hop-22(29)-en-3-oic acid | - | - | - | - | - | - | - | - | - | B7 | [28] |
| No. | Name | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | Type | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 96 | 3β,19α-dihydroxyolean-12-en-29-oic acid | β-OH | H | H | CH3 | H | α-OH | CH3 | COOH | C1 | [14] |
| 97 | 3α,19α-dihydroxyolean-12-en-29-oic acid | α-OH | H | H | CH3 | H | α-OH | CH3 | COOH | C1 | [14] |
| 98 | 3-oxo-11α-methoxyolean-12-ene | =O | H | α-OCH3 | CH3 | H | H | CH3 | CH3 | C1 | [24] |
| 99 | 22α-hydroxy-29-methoxy-3β-tetradecanoate-olean-12-ene | β-COOC13H27 | H | H | CH3 | α-OH | H | CH3 | COOMe | C1 | [31] |
| 100 | Maytefolin B | - | - | - | - | - | - | - | - | C2 | [44] |
| 101 | 3β-peroxy-7β,25-epoxy-D:B-friedoolean-5-ene | - | - | - | - | - | - | - | - | C3 | [48] |
| 102 | 28-hydroxyolean-12-ene-3,11-dione | =O | H | =O | CH2OH | H | H | CH3 | CH3 | C1 | [50] |
| 103 | 6β,28-dihydroxyolean-12-ene-3,11-dione | =O | β-OH | =O | CH2OH | H | H | CH3 | CH3 | C1 | [50] |
| 104 | 3-oxo-11α-methoxyolean-12-ene-30-oic acid | =O | H | α-OCH3 | CH3 | H | H | COOH | CH3 | C1 | [51] |
| 105 | 3-oxo-11α-hydroxyolean-12-ene-30-oic acid | =O | H | α-OH | CH3 | H | H | COOH | CH3 | C1 | [51] |
| 106 | 3-oxo-olean-9(11),12-diene-30-oic acid | =O | COOH | - | - | - | - | - | - | C4 | [51] |
| 107 | 3,4-seco-olean-4(23),12-diene-3,29-dioic acid | - | - | - | - | - | - | - | - | C5 | [51] |
| 108 | 3α-22β-dihydroxyolean-12-en-29-oicacid | α-OH | H | H | CH3 | β-OH | H | CH3 | COOH | C1 | [52] |
| 109 | Olean-9 (11):12-dien-3β-ol | β-OH | CH3 | - | - | - | - | - | - | C4 | [53] |
| 110 | 3β-Hydroxy-D:B-friedo-olean-5-ene | - | - | - | - | - | - | - | - | C6 | [54] |
| 111 | 19α-hydroxy-3-olean-12-en-29-oic acid | =O | H | H | CH3 | H | α-OH | CH3 | COOH | C1 | [55] |
| No. | Name | R1 | R2 | R3 | R4 | Type | Ref. |
|---|---|---|---|---|---|---|---|
| 112 | 3-Oxo-methoxyurs-12-ene | =O | H | α-OCH3 | CH3 | D1 | [24] |
| 113 | Krukovines B | =O | H | CH2OH | H | D1 | [50] |
| 114 | Krukovines D | =O | OH | CH2OH | H | D1 | [50] |
| 115 | Krukovines E | =O | H | OH | H | D1 | [50] |
| 116 | Maytefolins C | - | - | - | - | D2 | [44] |
| 117 | 28-hydroxy-12-ursene-3β-yl-caffeate | β-OCaf | H | CH2OH | H | D1 | [52] |
| 118 | 3β-stearyloxy-urs-12-ene | 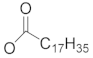 | H | CH3 | H | D1 | [56] |
| 119 | 24-(E)-3-oxo-dammara-20,24-dien-26-al | - | - | - | - | D3 | [57] |
| 120 | 24-(Z)-3-oxo-dammara-20,24-dien-26-al | - | - | - | - | D3 | [57] |
| 121 | 24-(E)-3-oxo-dammara-20,24-dien-26-ol | H | H | CH3 | CH2OH | D4 | [57] |
| 122 | 24-(E)-3-oxo-dammara-23-α-hydroxy-20,24-dien-26-al | H | α-OH | CH3 | CHO | D4 | [57] |
| 123 | 24-(E)-3-oxo-dammara-23-β-hydroxy-20,24-dien-26-al | H | β-OH | CH3 | CHO | D4 | [57] |
| 124 | 24-(E)-3-oxo-dammara-6-β-hydroxy-20,24-dien-26-al | β-OH | H | CH3 | CHO | D4 | [57] |
| 125 | 24-(E)-3-oxo-dammara-6-β-hydroxy-20,24-dien-26-ol | β-OH | H | CH3 | CH2OH | D4 | [57] |
| 126 | 23-(Z)-3,25-dioxo-25-nor-dammara-20,24-diene | - | - | - | - | D5 | [57] |
| 127 | 24-(E)-3-oxo-23-methylene-dammara-20,24-dien-26-oico | - | - | - | - | D6 | [58] |
| 128 | 24(Z)-3-oxodammara20(21),24-dien-27-oic acid | H | H | COOH | CH3 | D4 | [58] |
| 129 | Octa-nor-13-hydroxydammara-1-en-3,17-dione | - | - | - | - | D7 | [58] |
| No. | Name | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | Type | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 130 | 1α,9α-dibenzoyloxy-6β,8α,15-triacetoxy-4β-hydroxy-dihydro-β-agarofurane | OBz | H | H | OAc | CH3 | α-OAc | α-OBz | CH2OAc | E1 | [60] |
| 131 | 1α,9α-dibenzoyloxy-2α,6β,8α,15-tetracetoxy-4β-hydroxydihydro-β-agrofurane | OBz | α-OAc | H | OAc | CH3 | α-OAc | α-OBz | CH2OAc | E1 | [60] |
| 132 | 6β,8β-15-triacetoxy-1α,9α-dibenzoyloxy-4β-hydroxy-β-dihydroagarofuran | OBz | H | H | OAc | CH3 | β-OAc | α-OBz | CH2OAc | E1 | [61] |
| 133 | 1α,6β,8β-15-tetraacetoxy-9α-benzoyloxy-4β-hydroxy-β-dihydroagarofuran | OAc | H | H | OAc | CH3 | β-OAc | α-OBz | CH2OAc | E1 | [61] |
| 134 | (1S,4S,6R,7S,8S,9R)-1,6,15- triacetoxy-8,9-dibenzoyloxy-4β-hydroxy-β-dihydroagarofuran | OAc | H | H | OAc | CH3 | α-OBz | β-OBz | CH2OAc | E1 | [61] |
| 135 | (1R,2S,4S,5S,6R,7R,9S,10S)-6,15-diacetoxy-1,2,9-tribenzoyloxy-4-hrdroxy-8-oxo-dihydro-β-agarofuran | OBz | α-OBz | H | OAc | CH3 | O | α-OBz | CH2OAc | E1 | [41] |
| 136 | 9β-cinnamoyloxy-2β,3β-diacetoxy-6β-hydroxy-lα-nicotinoyloxydihidro-β-agarofuran | ONic | β-OAc | β-OAc | OH | CH3 | H | β-OCin | CH3 | E1 | [41] |
| 137 | 1α-acetoxy-2α,6β,9β-trtifuroyloxy-4β-hydroxy-dihydro-β- agarofuran | OAc | α-OFu | H | OFu | CH3 | H | β-OFu | CH3 | E1 | [59] |
| 138 | 1α,2α-diacetoxy-6β,9β-difuroyloxy-4β-hydroxy-dihydro-β- agarofuran | OAc | α-OAc | H | OFu | CH3 | H | β-OFu | CH3 | E1 | [59] |
| 139 | 1α-acetoxy-6β,9β-difuroyloxy-2α,4β-dihydroxy-dihydro-β-agarofuran | OAc | α-OH | H | OFu | CH3 | H | β-OFu | CH3 | E1 | [59] |
| 140 | 1α-acetoxy-2α-benzoyloxy-6β,9β-difuroyloxy-4β-dihydro-β-agarofuran | OAc | α-OBz | H | OFu | CH3 | H | β-OFu | CH3 | E1 | [59] |
| 141 | 1α-acetoxy-6β,9β-difuroyloxy-2α-propyonyloxy-4β-hydroxy-dihydro-β-agarofuran | OAc | α-OPr | H | OFu | CH3 | H | β-OFu | CH3 | E1 | [59] |
| 142 | 1α-acetoxy-6α,9β-difuroyloxy-2α-(2)-methylbutyroyloxy-4β-hydroxy-dihydro-β-agarofuran | OAc | α-OButMe | H | OFu | CH3 | H | β-OFu | CH3 | E1 | [59] |
| 143 | 1α, 2α,15-triacetoxy-6β,9β-difuroyloxy-4β- hydroxy-dihydro-β-agarofuran | OAc | α-OAc | H | OFu | CH3 | H | β-OFu | CH2OAc | E1 | [59] |
| 144 | 1α, 2α,15-triacetoxy-6β,9β-dibenzoyloxy-4β- hydroxy-dihydro-β-agarofuran | OAc | α-OAc | H | OBz | CH3 | H | β-OBz | CH2OAc | E1 | [59] |
| 145 | (1R,2R,4S,5R,7S,9S,10R)-2-acetoxy-1-benzoyloxy-9-cinnamoyloxy-4-hydroxy- dihydro-β-agarofuran | OBz | β-OAc | H | H | CH3 | H | β-OH | CH3 | E1 | [62] |
| 146 | (1R,2S,3S,5R,7R,9S,10R)-2-acetoxy-9-benzoyloxy-1-cinnamoyloxy-3-nicotinoyloxy-4-hydroxy-dihydro-β-agarofuran | OCin | β-OAc | β-ONic | H | CH3 | H | H | CH3 | E1 | [62] |
| 147 | (1R,2S,3S,4S,5S,6R,7R,9S,10R)-2,6-diacetoxy-1-benzoyloxy-9-cinnamoyloxy-3-nicotinoyloxy-4-hydroxy-dihydro-β-agarofuran | OBz | β-OAc | β-ONic | OAc | CH3 | H | β-OCin | CH3 | E1 | [62] |
| 148 | (1R,2S,3S,4S,5S,6R,7R,9S,10R)-2,6-diacetoxy-1,9-dibenzoyloxy-3-nicotinoyloxy-4-hydroxy-dihydro-β-agarofuran | OBz | β-OAc | β-ONic | H | CH3 | H | β-OBz | CH3 | E1 | [62] |
| 149 | (1R,2S,3S,4S,5R,7S,8S,9R,10R)-2,3-diacetoxy-8,9-dibenzoyloxy-1-nicotinoyloxy-4-hydroxy-dihydro-β-agarofuran | ONic | β-OAc | β-OAc | H | CH3 | β-OBz | β-OBz | CH3 | E1 | [62] |
| 150 | (1R,2S,4S,5S,6R,7R,8S,9R,10S)-6,8-diacetoxy-1,2,9-tribenzoyloxy-4-hydroxy-dihydro-β-agarofuran | OBz | α-OBz | H | OAc | CH3 | β-OAc | β-OBz | CH3 | E1 | [62] |
| 151 | (1R,2S,3S,4S,5R,7S,8S,9R,10R)-2,8-diacetoxy-3,9-dibenzoyloxy-1-nicotinoyloxy-4-hydroxy-dihydro-β-agarofuran | ONic | β-OAc | β-OBz | H | β-OAc | β-OBz | CH3 | - | E2 | [62] |
| 152 | (1R,2S,4R,5S,6R,7R,8S,9R,10S)-6,8-diacetoxy-1,9-dibenzoyloxy-2-nicotinoyloxy-dihydro-β-agarofuran | OBz | α-ONic | H | OAc | β-OAc | β-OBz | CH3 | - | E2 | [62] |
| 153 | 1α,15-diacetoxy-6β,9β-dibenzoyloxy-2α-nicotinoyloxy-dihydro-β-agarofuran | OAc | α-ONic | H | OBz | H | β-OBz | CH3 | - | E2 | [62] |
| 154 | 1α,15-diacetoxy-6β,9β-dibenzoyloxy-2α-nicotinoyloxy-4β-hydroxy-dihydro-β-agarofuran | OAc | α-ONic | H | OBz | CH3 | H | β-OBz | CH2OAc | E1 | [62] |
| 155 | (1R,2S,4S,5S,6R,7R,9S,10S)-1,2,6,9,15-pentaacetoxy-4-hydroxy-8-oxo-dihydro-β-agarofuran | OAc | α-OAc | H | OAc | CH3 | O | α-OAc | CH2OAc | E1 | [63] |
| 156 | (1R,2S,4S,5S,6R,7R,9S,10S)-1,2,9,15-taacetoxy-4,6-dihydroxy-8-oxo-dihydro-β-agarofuran | OAc | α-OAc | H | OH | CH3 | O | α-OAc | CH2OAc | E1 | [63] |
| 157 | (1R,2S,4S,5S,6R,7R,9S,10S)-1,9,15-triacetoxy-2,4,6-trihydroxy-8-oxo-dihydro-β-agarofuran | OAc | α-OH | H | OH | CH3 | O | α-OAc | CH2OAc | E1 | [63] |
| 158 | (1R,2S,3S,4S,5S,6R,7R,9S,10S)-1,2,3,6,9,12,15-heptaacetoxy-4-hydroxy-8-oxo-dihydro-β-agarofuran | OAc | α-OAc | β-OAc | OAc | CH2OAc | O | α-OAc | CH2OAc | E1 | [63] |
| 159 | 1α,2α,3β,6β,8α,9α,12,15-octaacetoxy-4β-hydroxy-dihydro-β-agarofuran | OAc | α-OAc | β-OAc | OAc | CH2OAc | α-OAc | α-OAc | CH2OAc | E1 | [63] |
| 160 | (1S,4S,5S,6R,7R,8S,9R,10R)-8-acetoxy-1,9-dibenzoyloxy-6-nicotynoyloxy-dihydro-β-agarofuran | OBz | H | H | ONic | α-OAc | α-OBz | CH3 | - | E2 | [49] |
| 161 | (1S,4R,5R,6R,7R,8S,9R,10R)-8-acetoxy-1,9-dibenzoyloxy-4-hydroxy-nicotynoyloxy-dihydro-β-agarofuran | OBz | H | H | ONic | CH3 | α-OAc | α-OBz | CH3 | E1 | [49] |
| 162 | (1R,2S,4S,5S,6R,7R,9S,10R)-1,15-diacetoxy-2,6-dibenzoyloxy-9-(3-furoyloxy)-4-hydroxy-dihydro-β-agarofuran | OAc | α-OBz | H | OBz | CH3 | H | α-OFu | CH2OAc | E1 | [64] |
| 163 | (1R,2S,4S,5S,6R,7R,9S,10R)-1,2,15-triacetoxy-6-benzoyloxy-9-(3-furoyloxy)-4-hydroxy-dihydro-β-agarofuran | OAc | α-OAc | H | OBz | CH3 | H | α-OFu | CH2OAc | E1 | [64] |
| 164 | (1R,2S,4S,5S,6R,7R,9S,10R)-1,15-diacetoxy-6-benzoyloxy-9-(3-furoyloxy)-2,4-dihydroxy-dihydro-β-agarofuran | OAc | α-OH | H | OBz | CH3 | H | α-OFu | CH2OAc | E1 | [64] |
| 165 | (1R,2S,4S,5S,6R,7R,9S,10R)-1,15-diacetoxy-6,9-dibenzoyloxy-2,4-hydroxy-dihydro-β-agarofuran | OAc | α-OH | H | OBz | CH3 | H | α-OBz | CH2OAc | E1 | [64] |
| 166 | (1R,2S,4S,5S,6R,7R,9S,10R)-1,2,6,15-tetracetoxy-9-(3-furoyloxy)-4-hydroxy-dihydro-β-agarofuran | OAc | α-OAc | H | OAc | CH3 | H | α-OFu | CH2OAc | E1 | [64] |
| 167 | (1R,2S,4S,5S,6R,7R,9S,10R)-1-Acetoxy-2,6-dibenzoyloxy-9-(3-furoyloxy)-4-hydroxy-dihydro-β-agarofuran | OAc | α-OBz | H | OBz | CH3 | H | α-OFu | CH3 | E1 | [64] |
| 168 | (1S,2S,3S,4S,5R,7R,9S,10R)-2,3-diacetoxy-9-benzoyloxy-1-(3-furoyloxy)-4-hydroxy-dihydro-β-agarofuran | OFu | β-OAc | β-OAc | H | CH3 | H | α-OBz | CH3 | E1 | [64] |
| 169 | (1S,2R,4S,5R,7R,9S,10R)-2-acetoxy-9-benzoyloxy-1-(3-furoyloxy)-4-hydroxy-dihydro-β-agarofuran | OFu | β-OAc | H | H | CH3 | H | α-OBz | CH3 | E1 | [64] |
| 170 | (1S,2R,4S,5R,7R,9S,10R)-2-Acetoxy-1,9-di-(3-furoyloxy)-4-hydroxy-dihydro-β-agarofuran | OFu | β-OAc | H | H | CH3 | H | α-OFu | CH3 | E1 | [64] |
| 171 | (1S,2R,4S,5R,7R,9S,10R)-2-Acetoxy-9-trans-cynamoiloxy-1-(3-furoyloxy)-4-hydroxy-dihydro-β-agarofuran | OFu | β-OAc | H | H | CH3 | H | α-OCin | CH3 | E1 | [64] |
| 172 | (1S,4S,5R,7R,9S,10S)-9-Benzoyloxy-1-(3-furoyloxy)-4-hydroxy-dihydro-β-agarofuran | OFu | H | H | H | CH3 | H | α-OBz | CH3 | E1 | [64] |
| 173 | (1S,2R,3R,4R,5S,7R,9S,10R)-2,3-diacetoxy-9-benzoyloxy-1-(3-furoyloxy)-dihydro-β-agarofuran | OFu | β-OAc | β-OAc | H | H | α-OBz | CH3 | - | E2 | [64] |
| 174 | (1S,2R,4R,5S,7R,9S,10R)-2-Acetoxy-9-benzoyloxy-1-(3-furoyloxy)-dihydro-β-agarofuran | OFu | β-OAc | H | H | H | α-OBz | CH3 | - | E2 | [64] |
| 175 | 1α,2α,9β,15-tetracetoxy-8β-benzoyloxy-β-dihydroagarofuran | OAc | α-OAc | H | H | β-OBz | α-OAc | CH2OAc | - | E2 | [65] |
| 176 | 1α-benzoyloxy-2α,6β,8α-triacetoxy-9α-methyllbutyroyloxy-β-dihydroagarofuran | OBz | α-OAc | H | OAc | α-OAc | α-OMeBut | CH3 | - | E2 | [65] |
| 177 | 1α,6β-diacetoxy-2α,8α,9α-tribenzoyloxy-β-dihydroagarofuran | OAc | α-OBz | H | OAc | α-OBz | α-OBz | CH3 | - | E2 | [65] |
| 178 | 1α-benzoyloxy-2α,6β,8α,9α-tetraacetoxy-β-dihydroagarofuran | OBz | α-OAc | H | OAc | α-OAc | α-OAc | CH3 | - | E2 | [65] |
| 179 | 1α,6β,8α-triacetoxy-9α-benzoyloxy-2α-hydroxy-β-dihydroagarofuran | OAc | α-OH | H | OAc | α-OAc | α-OBz | CH3 | - | E2 | [65] |
| 180 | (1R,2S,4R,5S,6R,7R,8R,9S,10S)-1,6-diacetoxy-8,9-dibenzoyloxy-2-h ydroxy-β-d ihydroagarofuran | OAc | α-OH | H | OAc | α-OBz | α-OBz | CH3 | - | E2 | [65] |
| 181 | 1α,6β,15-triacetoxy-8α-methylbutyroyloxy-9α-benzoyloxy-2α-hydroxy-β-dihydroagaro-furan | OAc | α-OH | H | OAc | α-OMeBut | α-OBz | CH2OAc | - | E2 | [65] |
| 182 | 1α,6β,15-triacetoxy-8α,9α-dibenzoyloxy-2α-hydroxy-β-dihydroagarofuran | OAc | α-OH | H | OAc | α-OBz | α-OBz | CH2OAc | - | E2 | [65] |
| 183 | 1α,6β,8β,15-tetracetoxy-2α-hydroxy-9α-benzoyloxy-β-dihydroagarofuran | OAc | α-OH | H | OAc | β-OAc | α-OBz | CH2OAc | - | E2 | [65] |
| 184 | Chiapens A | OH | α-OAc | H | OAc | α-OBz | α-OBz | CH2OAc | - | E2 | [66] |
| 185 | Chiapens B | OAc | α-OAc | H | OAc | α-OBz | α-OBz | CH2OAc | - | E2 | [66] |
| 186 | Chiapens C | OH | H | H | OAc | α-OBz | α-OBz | CH2OAc | - | E2 | [66] |
| 187 | Chiapens D | OAc | α-OAc | H | OBut | H | α-OBz | CH2OAc | - | E2 | [66] |
| 188 | Chiapens E | OAc | α-OAc | β-OAc | OAc | O | α-OBz | CH2OAc | - | E2 | [66] |
| 189 | 1α,6β-diacetoxy-8α-hydroxy-9β-furoyloxy-β-agarofuran | OAc | H | H | OAc | α-OH | β-OFu | CH3 | - | E2 | [67] |
| 190 | 1α-acetoxy-6β,8α-dihydroxy-9β-furoyloxy-β-agarofuran | OAc | H | H | OH | α-OH | β-OFu | CH3 | - | E2 | [67] |
| 191 | 1α-benzoyloxy-2α,3β,6β,9β,14-pentaacetoxy-8-oxo-β-agarofuan | OBz | α-OAc | β-OAc | OBz | O | OAc | CH2OAc | - | E2 | [67] |
| 192 | 1α-furoyloxy-2α,3β,6β,9β,14-pentaacetoxy-8-oxo-β-agarofuan | OFu | α-OAc | β-OAc | OFu | O | OAc | CH2OAc | - | E2 | [67] |
| 193 | Bilocularins A | OAc | H | H | OAc | α-OH | α-OBz | CH2OAc | - | E2 | [68] |
| 194 | Bilocularins B | OAc | H | H | OH | α-OAc | α-OBz | CH2OAc | - | E2 | [68] |
| 195 | Bilocularins C | OAc | H | H | OAc | O | α-OBz | CH2OAc | - | E2 | [68] |
| 196 | Bilocularins D | OHAc | α-OAc | H | OAc | CH3 | H | β-OtCin | CH2OBz | E1 | [69] |
| 197 | Bilocularins E | OHAc | α-OAc | H | OAc | CH3 | H | β-OtCin | CH2OtCin | E1 | [69] |
| 198 | Bilocularins F | OHAc | α-OAc | H | OAc | CH3 | H | β-OtCin | CH2OH | E1 | [69] |
| 199 | Bilocularins G | OAc | α-OAc | H | OAc | CH3 | H | β-OtCin | CH2OAc | E1 | [69] |
| 200 | Bilocularins H | OAc | α-OH | H | OAc | CH3 | H | β-OtCin | CH2OH | E1 | [69] |
| 201 | Bilocularins I | OAc | α-OH | H | ONic | CH3 | H | β-OtCin | CH3 | E1 | [69] |
| 202 | (1S,4S,5S,6R,7R,8R,9R,10S)-6-acetoxy-4,9,10-trihydroxy-2,2,5a,9-tetramethyloctahydro-2H-3,9a-methanobenzo[b]-oxepin-5-yl furan-3-carboxylate | OAc | H | H | OH | CH3 | α-OH | β-OFu | CH3 | E1 | [70] |
| 203 | (1S,4S,5S,6R,7R,8R,9R,10S)-6-acetoxy-4,9-dihydroxy-2,2,5a,9-tetramethyloctahydro-2H-3,9a-methanobenzo[b]oxepine-5,10-diylbis(furan-3-carboxylate) | OAc | H | H | OFu | CH3 | α-OH | β-OFu | CH3 | E1 | [71] |
| 204 | (1S,4S,5S,6R,7R,9S,10S)-6-acetoxy-9-hydroxy-2,2,5a,9-tetramethyloctahydro-2H-3,9a-methanobenzo[b]oxepine-5,10-diyl bis(furan-3-carboxylate) | OAc | H | H | OFu | CH3 | H | β-OFu | CH3 | E1 | [71] |
| 205 | (1S,4S,5S,6R,7R,9S,10S)-6-acetoxy-10-(benzoyloxy)-9-hydroxy-2,2,5a,9-tetramethyloctahydro-2H-3,9a-methanobenzo[b]-oxepin-5-yl furan-3-carboxylate | OAc | H | H | OBz | CH3 | H | β-OFu | CH3 | E1 | [71] |
| 206 | 2β,6β-diacetoxy-1α,9β-dibenzoyl-3β-hydroxy-dihydro-β-agarofuran | α-OBz | β-OAc | β-OH | H | β-CH3 | - | - | - | E3 | [72] |
| 207 | 1α,2α,6β,8α-tetraacetoxy-9β-benzoyl-15-hydroxy-dihydro-β-agarofuran | α-OAc | α-OAc | H | α-OAc | α-CH2OH | - | - | - | E3 | [72] |
| 208 | 1α,2α,6β,8α,15-pentaacetoxy-9β-benzoyl-dihydro-β-agarofuran | α-OAc | α-OAc | H | α-OAc | α-CH2OAc | - | - | - | E3 | [72] |
| 209 | 1β-acetoxy-9α-benzoyloxy-2β,6α-dinicotinoyioxy-β-dihydro-agarofuran(heterophylline) | - | - | - | - | - | - | - | - | E4 | [73] |
| 210 | Boarioside | - | - | - | - | - | - | - | - | E5 | [74] |
| 211 | 4-deacetyl-10-oxo-dihydrobotrydial | - | - | - | - | - | - | - | - | E6 | [75] |
| 212 | 4β-acetoxy-9β,10β,15α-trihydroxyp robotrydial | - | - | - | - | - | - | - | - | E7 | [75] |
| No. | Name | R1 | R2 | R3 | R4 | R5 | Type | Ref. |
|---|---|---|---|---|---|---|---|---|
| 213 | Wilforine | - | - | - | - | - | F1 | [77] |
| 214 | Emarginatine-A | OAc | OAc | OAc | H | OAc | F2 | [78] |
| 215 | Emarginatine-B | OAc | Benzoate | H | OAc | OAc | F2 | [78] |
| 216 | Emarginatine-C | OAc | OH | OAc | H | OAc | F2 | [79] |
| 217 | Emarginatine-D | OA | OAc | OAc | H | OAc | F2 | [79] |
| 218 | Emarginatine-E | OH | OH | H | OAc | OAc | F2 | [79] |
| 219 | Emarginatine-F |  | OAc | H | OH | OAc | F2 | [11] |
| 220 | Emarginatine-G | CH3CH=CCH3OO | OAc | OAc | H | OAc | F2 | [11] |
| 221 | Emarginatine-H | OAc | OAc | OAc | H | OH | F2 | [22] |
| 222 | Emarginatinine | - | - | - | - | - | F3 | [79] |
| 223 | Ebenifoline W-I | β-OAc | OBz | OBz | OAc | - | F4 | [80] |
| 224 | Ebenifoline E-I | β-OAc | OAc | OH | OBz | OAc | F5 | [80] |
| 225 | Ebenifoline E-II | β-OAc | OBz | OAc | OBz | OAc | F5 | [80] |
| 226 | Aquifoliunine E-I | α-OBz | OAc | OAc | OAc | CH2OAc | F5 | [81] |
| 227 | Aquifoliunine E-II | α-OH | OAc | OH | OAc | CH2OAc | F5 | [81] |
| 228 | Aquifoliunine E-III | α-OH | OAc | OAc | OAc | CH2OAc | F5 | [82] |
| 229 | Aquifoliunine E-IV | α-ONic | OAc | OAc | OAc | CH2OAc | F5 | [82] |
| 230 | Ilicifoliunines A | α-OBz | OH | OAc | OAc | CH2OAc | F5 | [83] |
| 231 | Ilicifoliunines B | α-OBz | OAc | OAc | CH2OAc | - | F4 | [83] |
| 232 | Mayteine | β-OAc | OAc | OAc | OBz | CH2OAc | F5 | [83] |
| 233 | Laevisines A | β-OAc | OAc | OAc | OCOC(CH3)=CHCH3 | CH2OAc | F5 | [84] |
| 234 | Laevisines B | β-OAc | OAc | ONic | CH2OAc | - | F4 | [84] |
| 235 | Mekongensine | Ac | H | OAc | OAc | - | F6 | [85] |
| 236 | 7-epi-mekongensine | Ac | OAc | H | OAc | - | F6 | [85] |
| 237 | 1-O-benzoyl-1-deacetylmekongensine | Bz | H | OAc | OAc | - | F6 | [85] |
| 238 | 9′-deacetoxymekongensine | Ac | H | OAc | H | - | F6 | [85] |
| 239 | 1-O-benzoyl-1-deacetyl-9′-deacetoxymekongensine | Bz | H | OAc | H | - | F6 | [85] |
| 240 | 7-epi-euojaponine | Bz | Ac | H | OAc | H | F7 | [85] |
| 241 | 2-O-benzoyl-2-deacetylmayteine | Bz | Bz | Ac | H | OAc | F7 | [85] |
| 242 | 7-epi-5-O-benzoyl-5-deacetylperitassine A | - | - | - | - | - | F8 | [85] |
| 243 | 5-benzoyl-5-deacetylwilforidine | - | - | - | - | - | F9 | [86] |
| 244 | Putterines A | 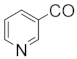 | OAc | COOCH3 | COOCH3 | CH2OAc | F5 | [76] |
| 245 | Putterines B | 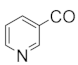 | OAc | COOCH(CH3)2 | COOCH3 | CH2OAc | F5 | [76] |
| 246 | 7-(acetyloxy)-O11-benzoyl-O2,11-deacetyl-7-deoxoevonine | β-OAc | OAc | OH | OAc | CH2OBz | F5 | [52] |
| 247 | Chiapenines ES-I | OBz | OBz | α-OAc | - | - | F10 | [87] |
| 248 | Chiapenines ES-II | OBz | OBz | =O | - | - | F10 | [87] |
| 249 | Chiapenines ES-III | OBz | OH | =O | - | - | F10 | [87] |
| 250 | Chiapenines ES-IV | OAc | OH | =O | - | - | F10 | [87] |
| 251 | Jelskiine | OiBut | α-OAc | OH | OAc | - | F11 | [88] |
| 252 | O9-benzoyl-O9-deacetylevonine | OBz | =O | OAc | OAc | - | F11 | [24] |
| 253 | 8β-acetoxy-O1-benzoyl- O1-deacetyl-8-deoxoevonine | OAc | β-OAc | OAc | OBz | - | F11 | [24] |
| 254 | 1α,2α,6β,8β,9α,15-hexacetoxy-4β-hydroxy-3β,13-[2′-(3-carboxybutyl)]nicotinicacid-dicarbo-lactone-β-dihydroagarofuran | - | - | - | - | - | F12 | [65] |
| 255 | 1α,2α,9α,15-tetracetoxy-4β,6β-dihydroxy-8-oxo,3β,13-[4′-(3-carboxybutyl)]nicotinicacid-dicarbolactone-β-dihydroagarofuran | =O | - | - | - | - | F13 | [65] |
| 256 | 1α,2α,9α,15-tetracetoxy-4β,6β,8β-trihydroxy-3β,13-[4′-(3-carboxybutyl)]nicotinicacid-dicarbolactone-β-dihydroagarofuran | β-OH | - | - | - | - | F13 | [65] |
| 257 | 1α,2α,8β,9α,15-pent acetoxy-4β,6β-dihydroxy-3β,13-[4′-(3-carboxybutyl)]nicotinicaciddicarbolactne-β-dihydroagarofuran | β-OAc | - | - | - | - | F13 | [65] |
| 258 | 4-deoxyalatamine | OAc | OAc | - | - | - | F14 | [31] |
| 259 | 1-O-benzoyl-1-deacetyl-4-deoxy-alatamine | OBz | OAc | - | - | - | F14 | [31] |
| 260 | 1,2-O-dibenzoyl-1,2-deacetyl-4-deoxyalatamine | OBz | OBz | - | - | - | F14 | [31] |
| 261 | 4-deoxyisowilfordine | - | - | - | - | - | F15 | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-Y.; Chen, L.; Ma, G.-X.; Xu, X.-D.; Jia, X.-G.; Deng, F.-S.; Li, X.-J.; Yuan, J.-Q. A Review on Phytochemicals of the Genus Maytenus and Their Bioactive Studies. Molecules 2021, 26, 4563. https://doi.org/10.3390/molecules26154563
Huang Y-Y, Chen L, Ma G-X, Xu X-D, Jia X-G, Deng F-S, Li X-J, Yuan J-Q. A Review on Phytochemicals of the Genus Maytenus and Their Bioactive Studies. Molecules. 2021; 26(15):4563. https://doi.org/10.3390/molecules26154563
Chicago/Turabian StyleHuang, Yuan-Yuan, Lu Chen, Guo-Xu Ma, Xu-Dong Xu, Xue-Gong Jia, Fu-Sheng Deng, Xue-Jian Li, and Jing-Quan Yuan. 2021. "A Review on Phytochemicals of the Genus Maytenus and Their Bioactive Studies" Molecules 26, no. 15: 4563. https://doi.org/10.3390/molecules26154563
APA StyleHuang, Y.-Y., Chen, L., Ma, G.-X., Xu, X.-D., Jia, X.-G., Deng, F.-S., Li, X.-J., & Yuan, J.-Q. (2021). A Review on Phytochemicals of the Genus Maytenus and Their Bioactive Studies. Molecules, 26(15), 4563. https://doi.org/10.3390/molecules26154563





