Redesigning Nature: Ruthenium Flavonoid Complexes with Antitumour, Antimicrobial and Cardioprotective Activities
Abstract
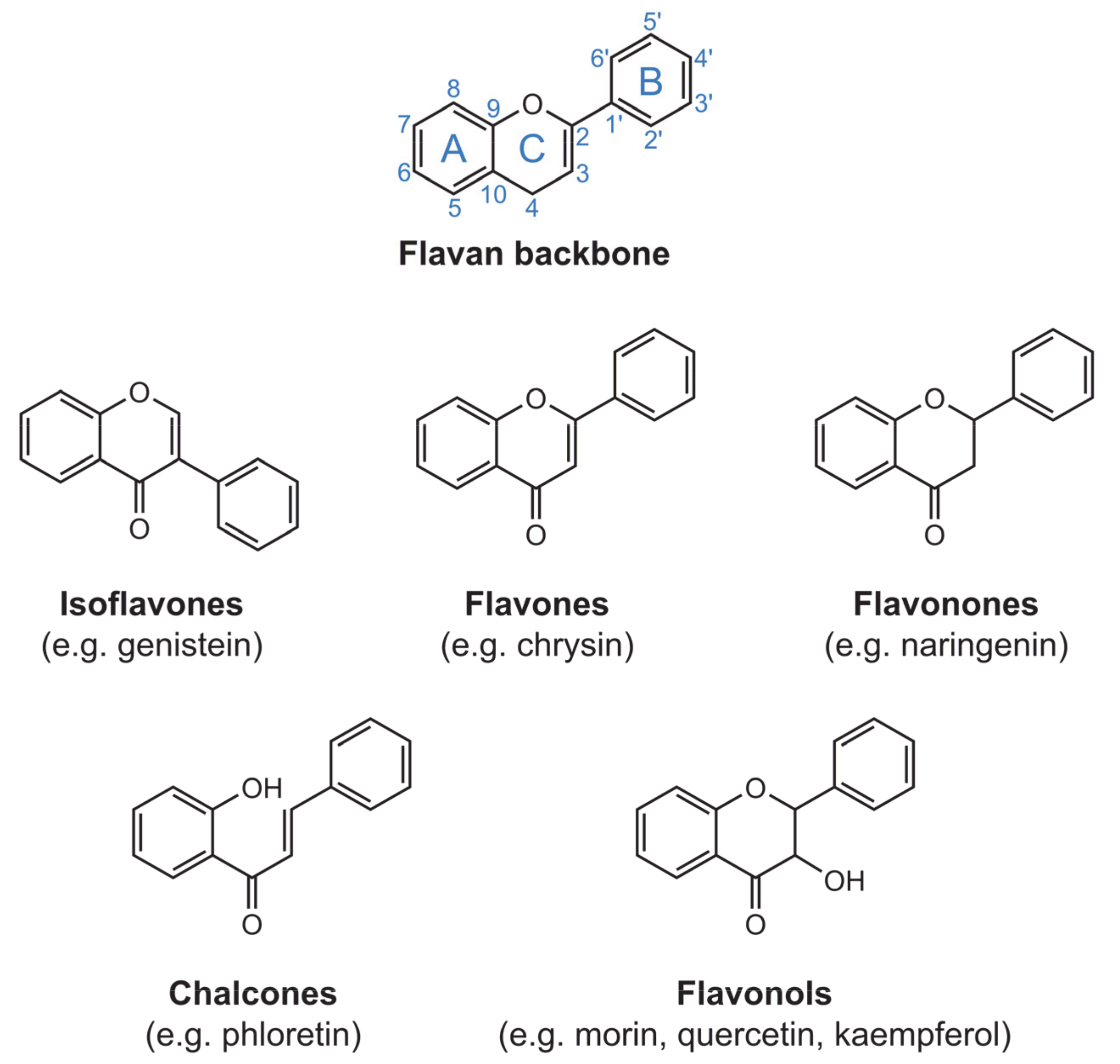
1. Introduction
Pharmacologic and Biophysical Properties of Model Ruthenium Complexes
2. Ruthenium Flavonoid Complexes with Cytotoxic Activity In Vitro
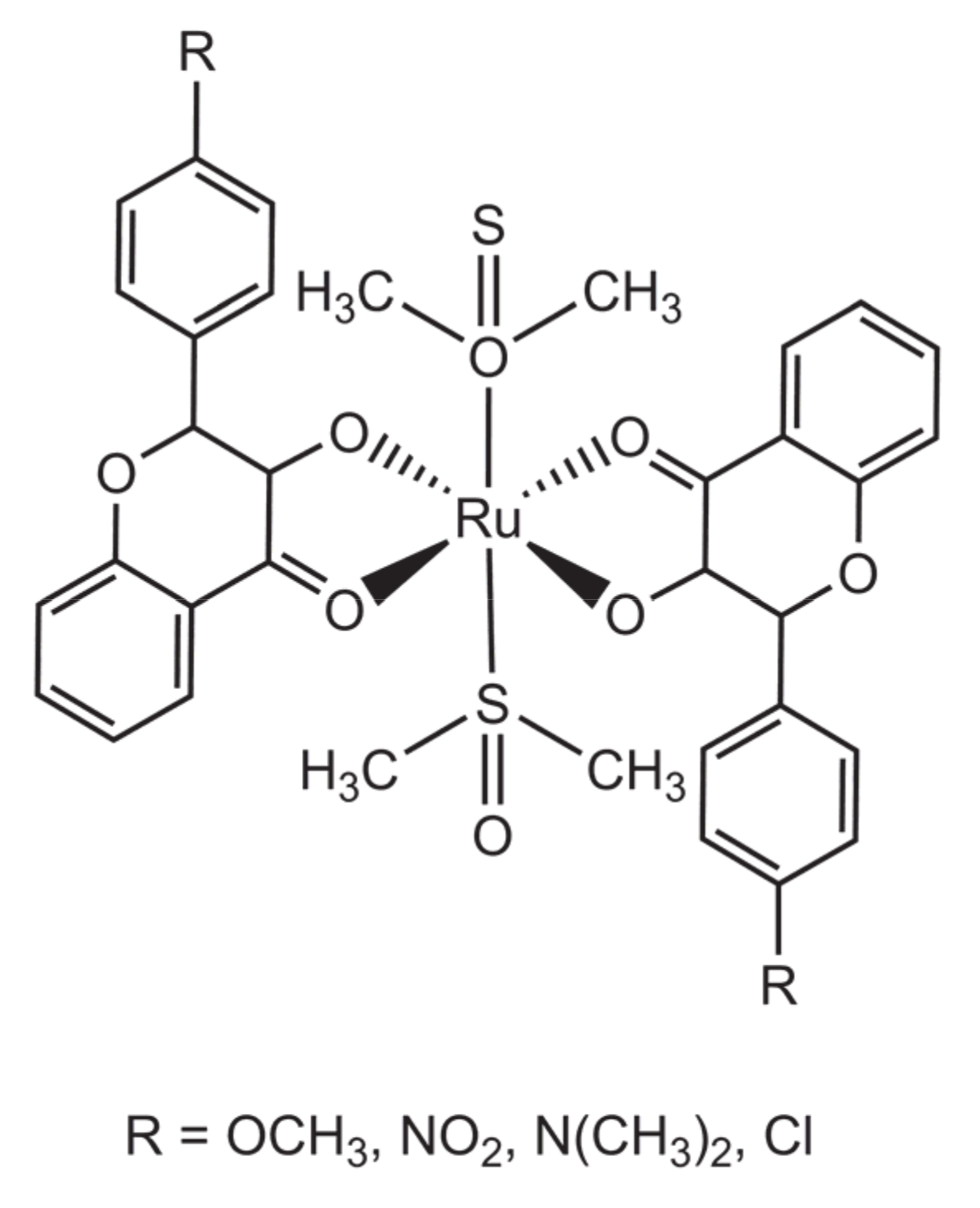
2.1. Complexes Obtained from [Ru(DMSO)4Cl2]
2.2. Ruthenium Acqua Complexes
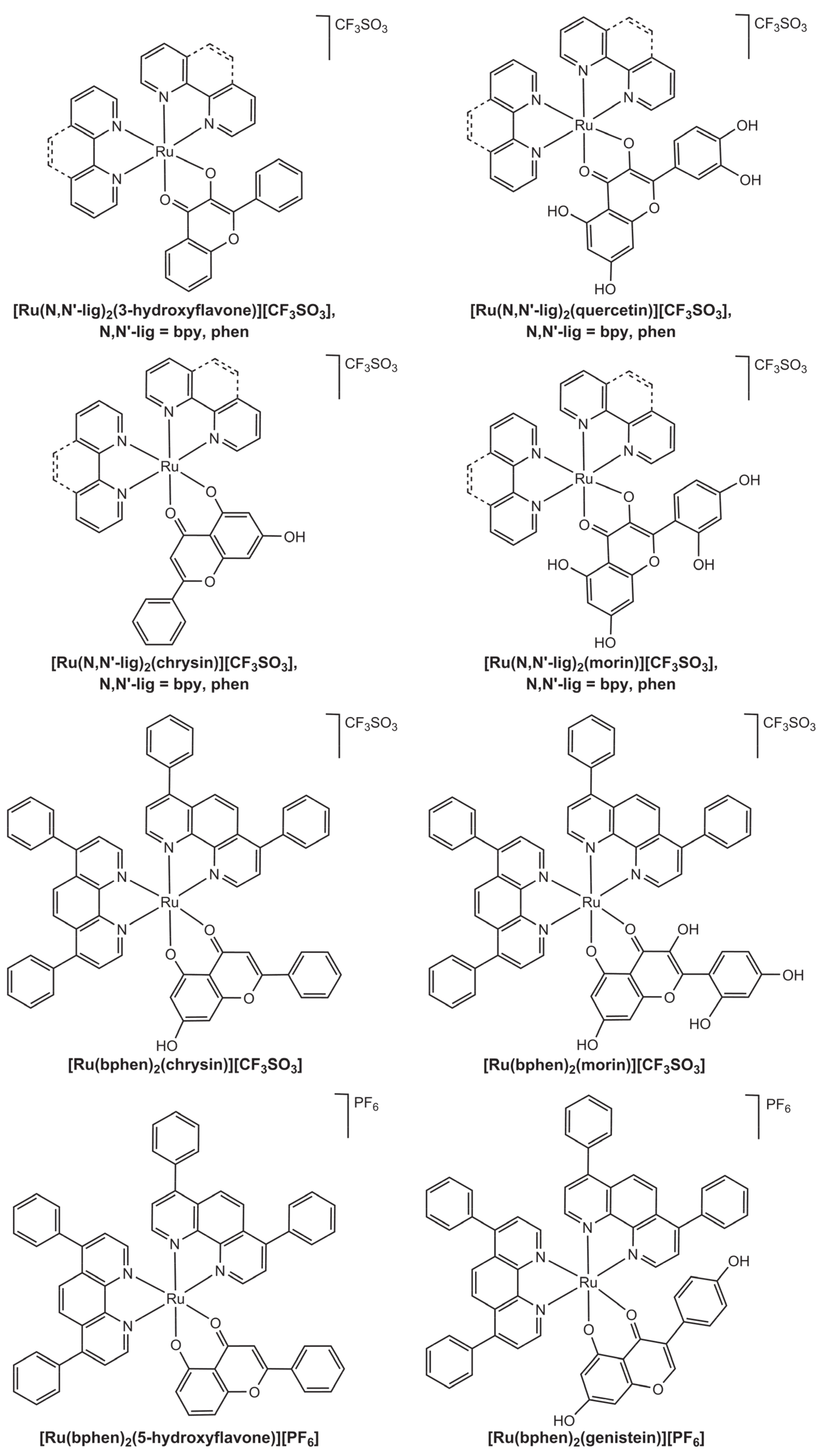
2.3. Ruthenium Polypyridyl Complexes
2.4. Ruthenium Trithiacyclononane Complexes
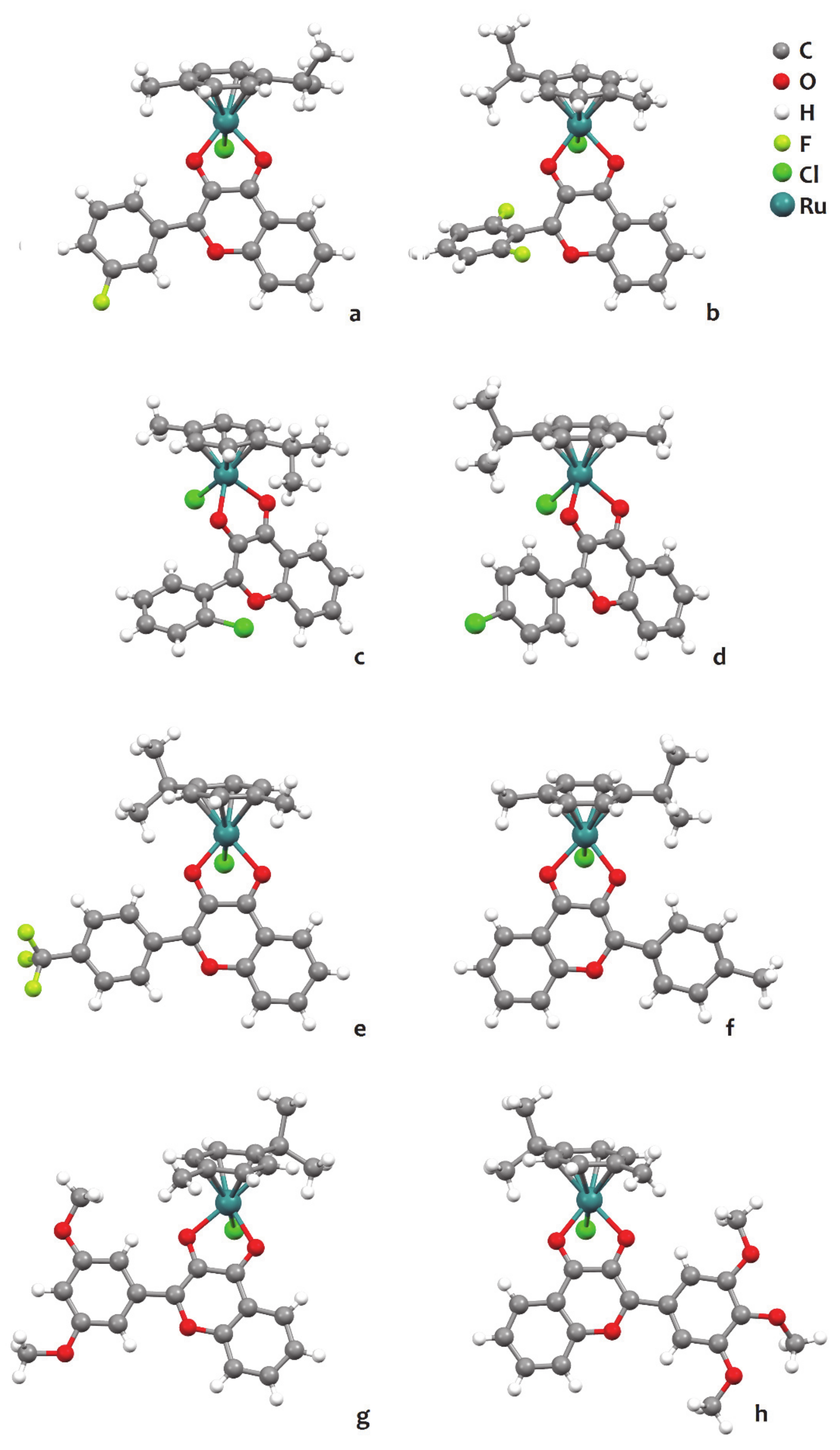
2.5. Ruthenium p-cymene Complexes
3. Antimicrobial Ruthenium Flavonoid Complexes
3.1. Ruthenium Polypyrydil Complexes for Antimicrobial Action in Human Medicine
3.2. Ruthenium Polypyrydil Complexes against Crop-Damaging Bacteria
4. Ruthenium Flavonoids Complexes in the Prevention and Treatment of Cardiovascular Diseases
4.1. A Ruthenium p-cymene Quercetin Complex for Cholesterol Regulation
4.2. Ruthenium p-cymene Complexes with Chrysin and Thiochrysin as Antithrombotic Agents
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gürler, S.B.; Kiraz, Y.; Baranala, Y. Flavonoids in cancer therapy: Current and future trends. In Biodiversity and Biomedicine, 1st ed.; Munir Ozturk, M., Egamberdieva, D., Pešić, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; Chapter 21; pp. 403–440. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R., Jr.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Barros, L.; Ferreira, I.C.F.R. Chemical characterization of Ginkgo biloba L. and antioxidant properties of its extracts and dietary supplements. Ind. Crops Prod. 2013, 51, 244–248. [Google Scholar] [CrossRef]
- Christie, S.; Walker, A.F.; Hicks, S.M.; Abeyasekera, S. Flavonoid supplement improves leg health and reduces fluid retention in pre-menopausal women in a double-blind, placebo-controlled study. Phytomedicine 2004, 11, 11–17. [Google Scholar] [CrossRef][Green Version]
- Maher, P. Preventing and treating neurological disorders with the flavonol fisetin. Brain Plast. 2020, 6, 155–166. [Google Scholar] [CrossRef]
- Kaempferol Powder Extract 50%. Available online: https://purebulk.com/products/kaempferol (accessed on 19 April 2021).
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D. Effects of the dietary flavonoid quercetin upon performance and health. Curr. Sports Med. Rep. 2009, 8, 206–213. [Google Scholar] [CrossRef]
- Vida, R.G.; Fittler, A.; Somogyi-Végh, A.; Poór, M. Dietary quercetin supplements: Assessment of online product informations and quantitation of quercetin in the products by high-performance liquid chromatography. Phytother. Res. 2019, 33, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- GRAS Notice Inventory-Agency Response Letter GRAS Notice No. GRN 000341. Available online: https://wayback.archive-it.org/7993/20171031012354/https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm235935.htm (accessed on 19 April 2021).
- Ueno, I.; Nakano, N.; Hirono, I. Metabolic fate of [14C] quercetin in the ACI rat. Jpn. J. Exp. Med. 1983, 53, 41–50. [Google Scholar]
- Kim, K.A.; Park, P.W.; Park, J.Y. Short-term effect of quercetin on the pharmacokinetics of fexofenadine, a substrate of P-glycoprotein, in healthy volunteers. Eur. J. Clin. Pharmacol. 2009, 65, 609–614. [Google Scholar] [CrossRef]
- Wu, L.-X.; Guo, C.-X.; Chen, W.-Q.; Yu, J.; Qu, Q.; Chen, Y.; Tan, Z.-R.; Wang, G.; Fan, L.; Li, Q.; et al. Inhibition of the organic anion-transporting polypeptide 1B1 by quercetin: An in vitro and in vivo assessment. Br. J. Clin. Pharmacol. 2012, 73, 750–757. [Google Scholar] [CrossRef]
- Dwyer, F.P.; Gyarfas, E.C.; Rogers, W.P.; Koch, J.H. Biological activity of complex ions. Nature 1952, 170, 190–191. [Google Scholar] [CrossRef]
- Shulman, A.; Dwyer, F.P. Metal Chelates in Biological Systems. In Chelating Agents and Metal Chelates, 1st ed.; Dwyer, F.P., Mellor, D.P., Eds.; Academic Press: New York, NY, USA; London, UK, 1963; Chapter 9; pp. 383–439. [Google Scholar]
- Dwyer, F.P.; Humpoletz, J.E.; Nyholm, R.S. The chemistry of ruthenium. Part I. The redox potential of the tris-orthophenanthroline ruthenous ion. J. Proc. R. Soc. NSW 1946, 80, 212–216. [Google Scholar]
- Dwyer, F.P.; Reid, I.K.; Shulman, A.; Laycock, G.M.; Dixson, S. The biological actions of 1,10-phenanthroline and 2,2′-bipyridine hydrochlorides, quaternary salts and metal chelates and related compounds. 1. Bacteriostatic action on selected Gram-positive, Gram-negative and acid-fast bacteria. Aust. J. Exp. Biol. Med. Sci. 1969, 47, 203–218. [Google Scholar] [CrossRef]
- Howe-Grant, M.; Lippard, S.J. Binding of platinum(II) intercalation reagents to deoxyribonucleic acid. Dependence on base-pair composition, nature of the intercalator, and ionic strength. Biochemistry 1979, 18, 5762–5769. [Google Scholar] [CrossRef]
- Li, F.; Feterl, M.; Warner, J.M.; Keene, F.R.; Collins, J.G. Dinuclear polypyridylruthenium(II) complexes: Flow cytometry studies of their accumulation in bacteria and the effect on the bacterial membrane. J. Antimic. Chemother. 2013, 68, 2825–2833. [Google Scholar] [CrossRef]
- Koch, J.H.; Rogers, W.P.; Dwyer, F.P.; Gyarfas, E.C. The metabolic fate of tris-1,10-phenanthroline 106ruthenium(II) perchlorate, a compound with anticholinesterase and curare-like activity. Aust. J. Biol. Sci. 1957, 10, 342–350. [Google Scholar]
- Murray, B.S.; Babak, M.V.; Hartinger, C.G.; Dyson, P.J. The development of RAPTA compounds for the treatment of tumors. Coord. Chem. Rev. 2016, 306, 86–114. [Google Scholar] [CrossRef]
- Pujante-Galián, M.A.; Pérez, S.A.; Montalbán, M.G.; Carissimi, G.; Fuster, M.G.; Víllora, G.; García, G. p-Cymene complexes of ruthenium(II) as antitumor agents. Molecules 2020, 25, 5063. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Carreon, T.; Iniguez, E.; Anzellotti, A.; Sánchez, A.; Tyan, M.; Sattler, A.; Herrera, L.; Maldonado, R.A.; Sánchez-Delgado, R.A. Searching for new chemotherapies for tropical diseases: Ruthenium–clotrimazole complexes display high in vitro activity against Leishmania major and Trypanosoma cruzi and low toxicity toward normal mammalian cells. J. Med. Chem. 2012, 55, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Glans, L.; Ehnbom, A.; de Kock, C.; Martínez, A.; Estrada, J.; Smith, P.J.; Haukka, M.; Sánchez-Delgado, R.A.; Nordlander, E. Ruthenium(II) arene complexes with chelating chloroquine analogue ligands: Synthesis, characterization and in vitro antimalarial activity. Dalton Trans. 2012, 41, 2764–2773. [Google Scholar] [CrossRef]
- Dyson, P.J. Systematic design of a targeted organometallic antitumour drug in pre-clinical development. CHIMIA 2007, 61, 698–703. [Google Scholar] [CrossRef]
- Scolaro, C.; Bergamo, A.; Brescacin, L.; Delfino, R.; Cocchietto, M.; Laurenczy, G.; Geldbach, T.J.; Sava, G.; Dyson, P.J. In vitro and in vivo evaluation of ruthenium(II)-arene PTA complexes. J. Med. Chem. 2005, 48, 4161–4171. [Google Scholar] [CrossRef]
- Thangavel, P.; Viswanath, B.; Kim, S. Synthesis and characterization of kaempferol-based ruthenium(II) complex: A facile approach for superior anticancer application. Mat. Sci. Eng. C 2018, 89, 87–94. [Google Scholar] [CrossRef]
- Prajapati, R.; Dubey, S.K.; Gaur, R.; Koiri, R.K.; Maurya, B.K.; Trigun, S.K.; Mishra, L. Structural characterization and cytotoxicity studies of ruthenium(II)–dmso–chloro complexes of chalcone and flavone derivatives. Polyhedron 2010, 29, 1055–1061. [Google Scholar] [CrossRef]
- Singh, A.K.; Saxena, G.; Sahabjada; Arshad, M. Synthesis, characterization and biological evaluation of ruthenium flavanol complexes against breast cancer. Spectrochim. Acta A 2017, 180, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Das, R.; Ghosh, B.; Chakraborty, T. Deciphering the biochemical and molecular mechanism underlying the in-vitro and in-vivo chemotherapeutic efficacy of ruthenium quercetin complex in colon cancer. Mol. Carcinog. 2018, 57, 700–721. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Zhao, Z.; Chakraborty, T.; Mandal, A.; Roy, A.; Roy, S.; Guo, Z. Decrypting the molecular mechanistic pathways delineating the chemotherapeutic potential of ruthenium-phloretin complex in colon carcinoma correlated with the oxidative status and increased apoptotic events. Oxid. Med. Cell. Longev. 2020, 7690845. [Google Scholar] [CrossRef]
- Zahirović, A.; Kahrović, E.; Cindrić, M.; Pavelić, S.K.; Hukić, M.; Harej, A.; Turkušić, E. Heteroleptic ruthenium bioflavonoid complexes: From synthesis to in vitro biological activity. J. Coord. Chem. 2017, 70, 4030–4053. [Google Scholar] [CrossRef]
- Munteanu, A.C.; Notaro, A.; Jakubaszek, M.; Cowell, J.; Tharaud, M.; Goud, B.; Uivarosi, V.; Gasser, G. Synthesis, characterization, cytotoxic activity, and metabolic studies of ruthenium(II) polypyridyl complexes containing flavonoid ligands. Inorg. Chem. 2020, 59, 4424–4434. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.; Silva, A.M.S.; Marques, M.P.M.; Braga, S.S. Ruthenium(II) trithiacyclononane complexes of 7,3′,4′-trihydroxyflavone, chrysin and tectochrysin: Synthesis, characterisation, and cytotoxic evaluation. Inorg. Chim. Acta 2019, 488, 71–79. [Google Scholar] [CrossRef]
- Kurzwernhart, A.; Kandioller, W.; Bartel, C.; Bächler, S.; Trondl, R.; Mühlgassner, G.; Jakupec, M.A.; Arion, V.B.; Marko, D.; Keppler, B.K.; et al. Targeting the DNA-topoisomerase complex in a double-strike approach with a topoisomerase inhibiting moiety and covalent DNA binder. Chem. Commun. 2012, 48, 4839–4841. [Google Scholar] [CrossRef]
- Kurzwernhart, A.; Kandioller, W.; Bächler, S.; Bartel, C.; Martic, S.; Buczkowska, M.; Mühlgassner, G.; Jakupec, M.A.; Kraatz, H.-B.; Bednarski, P.J.; et al. Structure−activity relationships of targeted RuII(η6-p-cymene) anticancer complexes with flavonol-derived ligands. J. Med. Chem. 2012, 55, 10512–10522. [Google Scholar] [CrossRef]
- Kubanik, M.; Tu, J.K.Y.; Hejl, M.; Jakupec, M.A.; Kandioller, W.; Keppler, B.K.; Hartinger, C.G. Expanding on the structural diversity of flavone-derived rutheniumII(ƞ6-arene) anticancer agents. Metallodrugs 2015, 1, 24–35. [Google Scholar] [CrossRef]
- Pastuszko, A.; Niewinna, K.; Czyz, M.; Jóźwiak, A.; Ma1ecka, M.; Budzisz, E. Synthesis, X-ray structure, electrochemical properties and cytotoxic effects of new arene ruthenium(II) complexes. J. Organomet. Chem. 2013, 745–746, 64–70. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.-N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial silver in medicinal and consumer applications: A patent review of the past decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.I.; Braga, T.M.; Braga, S.S. Ru(II)-based antimicrobials: Looking beyond organic drugs. Mini-Rev. Med. Chem. 2012, 12, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S.; Lysenko, K.; Santos, N.E. Ruthenophenanthroline complexes active against tripanosomids. In A Closer Look at Phenanthroline, 1st ed.; Amies, W., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2020; Chapter 3; pp. 101–123. ISBN 978-1-53617-953-8. [Google Scholar]
- Singh, A.K.; Saxena, G.; Kumar, A.; Sharma, R. Antimycobacterial activities of ruthenium(II) complexes of hydroxyl flavone hydrazones. Lett. Appl. NanoBioScience 2020, 10, 1760–1791. [Google Scholar] [CrossRef]
- da Silva, D.F.; Amaral, J.C.; Carlos, R.M.; Ferreira, A.G.; Forim, M.R.; Fernandes, J.B.; da Silva, M.F.G.F.; della Colleta Filho, H.; de Souza, A.A. Octahedral ruthenium and magnesium naringenin 5-alkoxide complexes: NMR analysis of diastereoisomers and in-vivo antibacterial activity against Xylella fastidiosa. Talanta 2021, 225, 122040. [Google Scholar] [CrossRef]
- Kahrović, E.; Zahirović, A.; Pavelić, S.K.; Turkušić, E.; Harej, A. In vitro anticancer activity of binuclear Ru(II) complexes with Schiff bases derived from 5-substituted salicylaldehyde and 2-aminopyridine with notably low IC50 values. J. Coord. Chem. 2017, 70, 1683–1697. [Google Scholar] [CrossRef]
- Kahrović, E.; Zahirović, A.; Turkušic, E.; Bektaš, S. A dinuclear ruthenium(II) schiff base complex with dissimilar coordination: Synthesis, characterization, and biological Activity. Z. Anorg. Allg. Chem. 2016, 642, 480–485. [Google Scholar] [CrossRef]
- Schneider, K.; van der Werf, W.; Cendoya, M.; Mourits, M.; Navas-Cortés, J.A.; Vicent, A.; Lansink, A.O. Impact of Xylella fastidiosa subspecies pauca in European olives. Proc. Natl. Acad. Sci. USA 2020, 117, 9250–9259. [Google Scholar] [CrossRef] [PubMed]
- Cuccioloni, M.; Bonfili, L.; Mozzicafreddo, M.; Cecarini, V.; Pettinari, R.; Condello, F.; Pettinari, C.; Marchetti, F.; Angeletti, M.; Eleuteri, A.M. A ruthenium derivative of quercetin with enhanced cholesterol-lowering activity. RSC Adv. 2016, 6, 39636. [Google Scholar] [CrossRef]
- Ravishankar, D.; Salamah, M.; Attina, A.; Pothi, R.; Vallance, T.M.; Javed, M.; Williams, H.F.; Alzahrani, E.M.S.; Kabova, E.; Vaiyapuri, R.; et al. Ruthenium-conjugated chrysin analogues modulate platelet activity, thrombus formation and haemostasis with enhanced efficacy. Sci. Rep. 2017, 7, 5738. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, C.Y.; Nam, T.G. Ruthenium complexes as anticancer agents: A brief history and perspectives. Drug Des. Devel. Ther. 2020, 14, 5375–5392. [Google Scholar] [CrossRef] [PubMed]
- Intravesical Photodynamic Therapy (PDT) in BCG Refractory High-Risk Non-Muscle Invasive Bladder Cancer (NMIBC) Patients. Phase 1. Clinical Trial Identifier NCT03053635. Available online: https://clinicaltrials.gov/ct2/show/NCT03053635?term=TLD1433&draw=2&rank=1 (accessed on 24 June 2021).






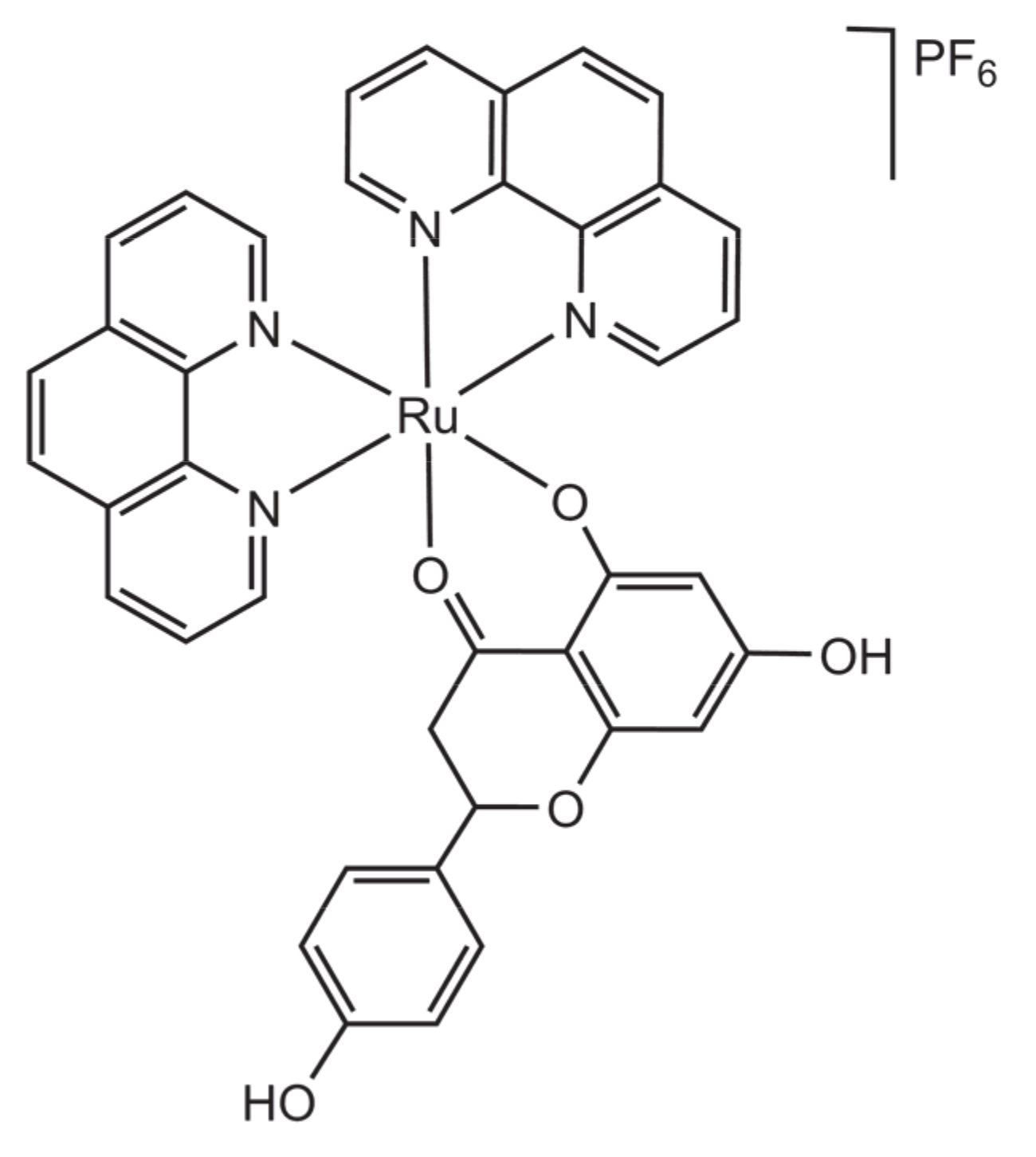

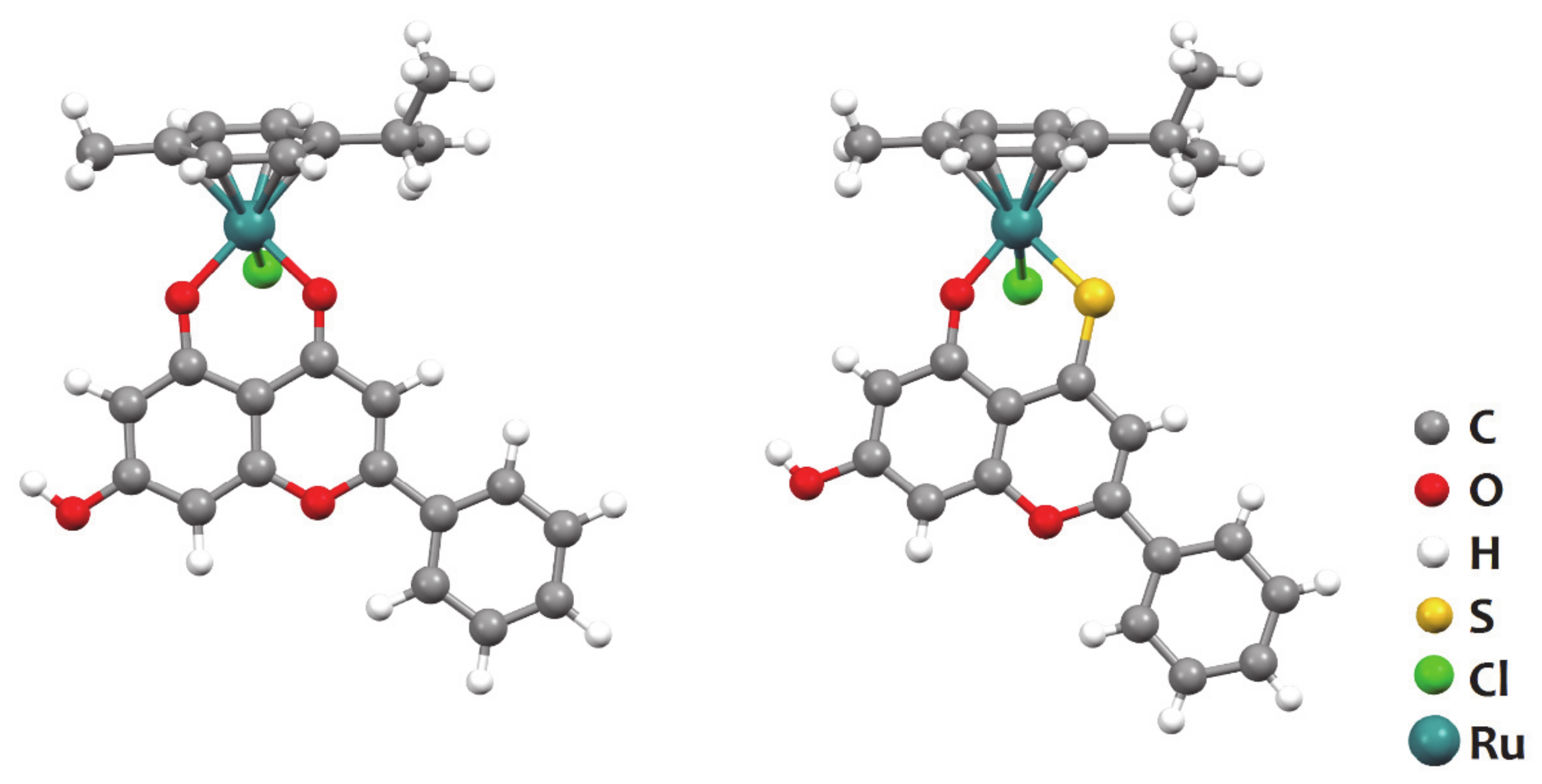
| Compound | IC50 Expressed as Mean ± s.d. (μM) | |||
|---|---|---|---|---|
| SW620 | HepG2 | MCF-7 | HeLa | |
| 3-hydroxyflavone | 50.73 ± 22.29 | 8.88 ± 17.68 | 42.06 ± 21.08 | 5.44 ± 31.22 |
| [Ru(bpy)2(3OHflav)][CF3SO3] | 0.75 ± 0.15 | 2.5 ± 0.67 | 0.52 ± 0.38 | 0.78 ± 0.20 |
| [Ru(phen)2(3OHflav)][CF3SO3] | 8.2 ± 46.4 | 11.4 ± 66.0 | 8.32 ± 0.86 | 19.3 ± 65.9 |
| [Ru(bpy)2Cl2] | - | >100 | - | >100 |
| [Ru(phen)2Cl2] | >100 | >100 | 92.38 ± 44.00 | >100 |
| Compound | IC50 Expressed as Mean ± s.d. (μM) | |||||
|---|---|---|---|---|---|---|
| MCF-7 | FaDU | MDA-MB-435S | U87 | RPE-1 | HEK293 | |
| 5-hydroxyflavone | >100 | >100 | >100 | >100 | >100 | >100 |
| [Ru(bphen)2(5OHFlv)][PF6] | >50 | 38.2 ± 5.2 | 24.5 ± 1.9 | 30.7 ± 1.5 | 19.7 ± 8.2 | 26.5 ± 3.2 |
| genistein | >100 | >100 | >100 | >100 | >100 | 75.9 ± 0.8 |
| [Ru(bphen)2(gen)][PF6] | 16.7 ± 3.9 | 5.2 ± 0.7 | 2.6 ± 0.4 | 5.2 ± 1.7 | 2.4 ± 0.8 | 0.7 ± 0.1 |
| chrysin | 62.6 ± 3.2 | 95.1 ± 11.6 | 79.4 ± 8.1 | 91.1 ± 13.8 | >100 | 26.8 ± 2.8 |
| [Ru(bphen)2(chr)][CF3SO3] | >50 | >50 | 27.73 ± 5.33 | 25.59 ± 0.29 | 23.21 ± 8.08 | 33.0 ± 3.3 |
| morin | >100 | >100 | >100 | >100 | >100 | >100 |
| [Ru(bphen)2(mor)][CF3SO3] | >50 | >50 | >50 | >50 | >50 | >50 |
| [Ru(bphen)2Cl2] | >50 | >50 | 27.7 ± 5.3 | 25.6 ± 0.3 | 3.1 ± 0.3 | 12.1 ± 1.30 |
| cisplatin | 19.7 ± 1.6 | 5.2 ± 0.2 | 17.6 ± 0.5 | 6.9 ± 0.5 | 39.9 ± 9.1 | 2.3 ± 0.7 |
| doxorubicin | 9.4 ± 1.4 | 1.6 ± 0.2 | 5.6 ± 1.4 | 0.6 ± 0.03 | 14.9 ± 1.3 | 0.2 ± 0.03 |
| Compound 1 | Topoisom | CH1 | SW480 | A549 | 5637 | LCLC-103H | DAN-G | Ref |
|---|---|---|---|---|---|---|---|---|
| flavonol | + | 1.9 ± 0.2 | 11 ± 3 | 25 ± 10 | n.d. | n.d. | n.d. | [34] |
| [Ru(p-cym)(flavonol)Cl] | ++ | 2.1 ± 0.2 | 9.6 ± 1.5 | 20 ± 2 | 11 ± 5 | 13 ± 6 | 12 ± 2 | [34,35] |
| 4′-methylflavonol | + | 1.1 ± 0.1 | 6.3 ± 1.1 | 81 ± 9 | n.d. | n.d. | n.d. | [34] |
| [Ru(p-cym)(4′MeFlv)Cl] | ++ | 1.8 ± 0.2 | 7.2 ± 0.5 | 17 ± 2 | 5.7 ± 3.2 | 5.2 ± 0.8 | 6.6 ± 2.5 | [34,35] |
| 3′,4′-dimethoxyflavonol | n.d. | 2.1 ± 0.2 | > 25 | > 25 | n.d. | n.d. | n.d. | [36] |
| [Ru(p-cym)(3′,4′dMFlv)Cl] | n.d. | 2.2 ± 0.5 | 8.7 ± 0.8 | 18 ± 2 | n.d. | n.d. | n.d. | [36] |
| 3′,5′-dimethoxyflavonol | n.d. | 1.4 ± 0.2 | > 25 | > 25 | n.d. | n.d. | n.d. | [36] |
| [Ru(p-cym)(3′,5′dMFlv)Cl] | n.d. | 1.5 ± 0.2 | 4.5 ± 0.2 | 9.0 ± 0.5 | n.d. | n.d. | n.d. | [36] |
| 3′,4′,5′-trimethoxyflavonol | n.d. | 2.0 ± 0.2 | 8.6 ± 1.5 | > 25 | n.d. | n.d. | n.d. | [36] |
| [Ru(p-cym)(3′,4′,5′tMFlv)Cl] | n.d. | 2.5 ± 0.3 | 9.7 ± 1.9 | 23 ± 5 | n.d. | n.d. | n.d. | [36] |
| 4′-fluoroflavonol | + | 1.56 ± 0.04 | 7.0 ± 0.9 | 37 ± 10 | n.d. | n.d. | n.d. | [34] |
| [Ru(p-cym)(4′FFlv)Cl] | ++ | 1.7 ± 0.4 | 7.9 ± 2.1 | 18 ± 1 | 33 ± 5 | 5.5 ± 5.2 | 12 ± 2 | [34,35] |
| [Ru(p-cym)(3′FFlv)Cl] | n.d. | 1.5 ± 0.1 | 7.0 ± 1.0 | 15 ± 1 | 4.3 ± 2.5 | 4.3 ± 1.1 | 5.3 ± 1.6 | [35] |
| [Ru(p-cym)(2′FFlv)Cl] | n.d. | 4.0 ± 0.8 | 24 ± 3 | 30 ± 1 | n.d. | n.d. | n.d. | [35] |
| 2′,6′-difluoroflavonol | n.d. | 18 ± 1 | > 25 | > 25 | n.d. | n.d. | n.d. | [36] |
| [Ru(p-cym)(2′,6′dFFlv)Cl] | n.d. | 5.1 ± 0.8 | 20 ± 4 | 55 ± 15 | n.d. | n.d. | n.d. | [36] |
| 4′-chloroflavonol | ++ | 0.60 ± 0.10 | 3.7 ± 0.4 | 7.9 ± 1.2 | n.d. | n.d. | n.d. | [34] |
| [Ru(p-cym)(4′ClFlv)Cl] | +++ | 0.86 ± 0.06 | 3.8 ± 0.5 | 9.5 ± 0.5 | 3.3 ± 1.1 | 13 ± 1 | 19 ± 7 | [34,35] |
| [Ru(p-cym)(3′ClFlv)Cl] | n.d. | 1.0 ± 0.1 | 7.0 ± 0.7 | 12 ± 2 | 30 ± 2 | 5.0 ± 3.5 | 19 ± 5 | [35] |
| [Ru(p-cym)(2′ClFlv)Cl] | n.d. | 7.9 ± 0.6 | 26 ± 1 | 51 ± 5 | n.d. | n.d. | n.d. | [35] |
| cisplatin | - | 0.14± 0.03 | 3.3 ± 0.4 | 1.3 ± 0.4 | n.d. | n.d. | n.d. | [34] |
| Compound | Staphylococcus aureus ATCC 25923 | Enterococcus faecalis ATCC 19433 | Streptococcus β-hemolytic group A | Methicillin-resistant Staphylococcus aureus | Klebsiella pneumoniae ATCC 1705 | Acinetobacter baumannii ATCC-BAA 747 | Pseudomonas aeruginosa | Escherichia coli | Candida albicans |
|---|---|---|---|---|---|---|---|---|---|
| Diameter of Inhibition Zone/mm | |||||||||
| quercetin | 17 | 15 | – | 21 | – | 18 | – | – | – |
| [Ru(bpy)2(quercetin)][CF3SO3] | – | – | – | – | – | – | – | – | 15 |
| [Ru(phen)2(quercetin)][CF3SO3] | – | – | – | – | – | 16 | – | – | – |
| morin | – | – | – | – | – | 16 | – | – | – |
| [Ru(bpy)2(morin)][CF3SO3] | – | – | – | – | – | 14 | – | – | – |
| [Ru(phen)2(morin)][CF3SO3] | – | – | – | – | – | 14 | – | – | 12.5 |
| chrysin | – | – | – | – | – | 14 | – | – | 14 |
| [Ru(bpy)2(chrysin)][CF3SO3] | 15 | – | 15 | 16 | – | 13 | – | – | 17 |
| [Ru(phen)2(chrysin)][CF3SO3] | – | – | – | – | – | 17 | – | – | – |
| flavonol | – | – | – | 12 | – | 16.5 | – | – | 18.5 |
| [Ru(bpy)2(flavonol)][CF3SO3] | 25 | 20 | 20 | 26 | – | 16 | 13 | – | 28 |
| [Ru(phen)2(flavonol)][CF3SO3] | – | – | – | 14 | – | 14 | – | – | – |
| cis-[Ru(bpy)2Cl2] | – | – | – | – | – | 15 | – | – | 14 |
| DMSO | – | – | – | – | – | – | – | – | – |
| vancomycin | 27 | 26 | 35 | – | – | – | – | – | – |
| gentamicin | – | – | – | 20 | 25 | 35 | 36 | 21 | – |
| nystatin | – | – | – | – | – | – | – | – | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, N.E.; Braga, S.S. Redesigning Nature: Ruthenium Flavonoid Complexes with Antitumour, Antimicrobial and Cardioprotective Activities. Molecules 2021, 26, 4544. https://doi.org/10.3390/molecules26154544
Santos NE, Braga SS. Redesigning Nature: Ruthenium Flavonoid Complexes with Antitumour, Antimicrobial and Cardioprotective Activities. Molecules. 2021; 26(15):4544. https://doi.org/10.3390/molecules26154544
Chicago/Turabian StyleSantos, Nádia E., and Susana Santos Braga. 2021. "Redesigning Nature: Ruthenium Flavonoid Complexes with Antitumour, Antimicrobial and Cardioprotective Activities" Molecules 26, no. 15: 4544. https://doi.org/10.3390/molecules26154544
APA StyleSantos, N. E., & Braga, S. S. (2021). Redesigning Nature: Ruthenium Flavonoid Complexes with Antitumour, Antimicrobial and Cardioprotective Activities. Molecules, 26(15), 4544. https://doi.org/10.3390/molecules26154544







