Neobavaisoflavone May Modulate the Activity of Topoisomerase Inhibitors towards U-87 MG Cells: An In Vitro Study
Abstract
:1. Introduction
2. Results
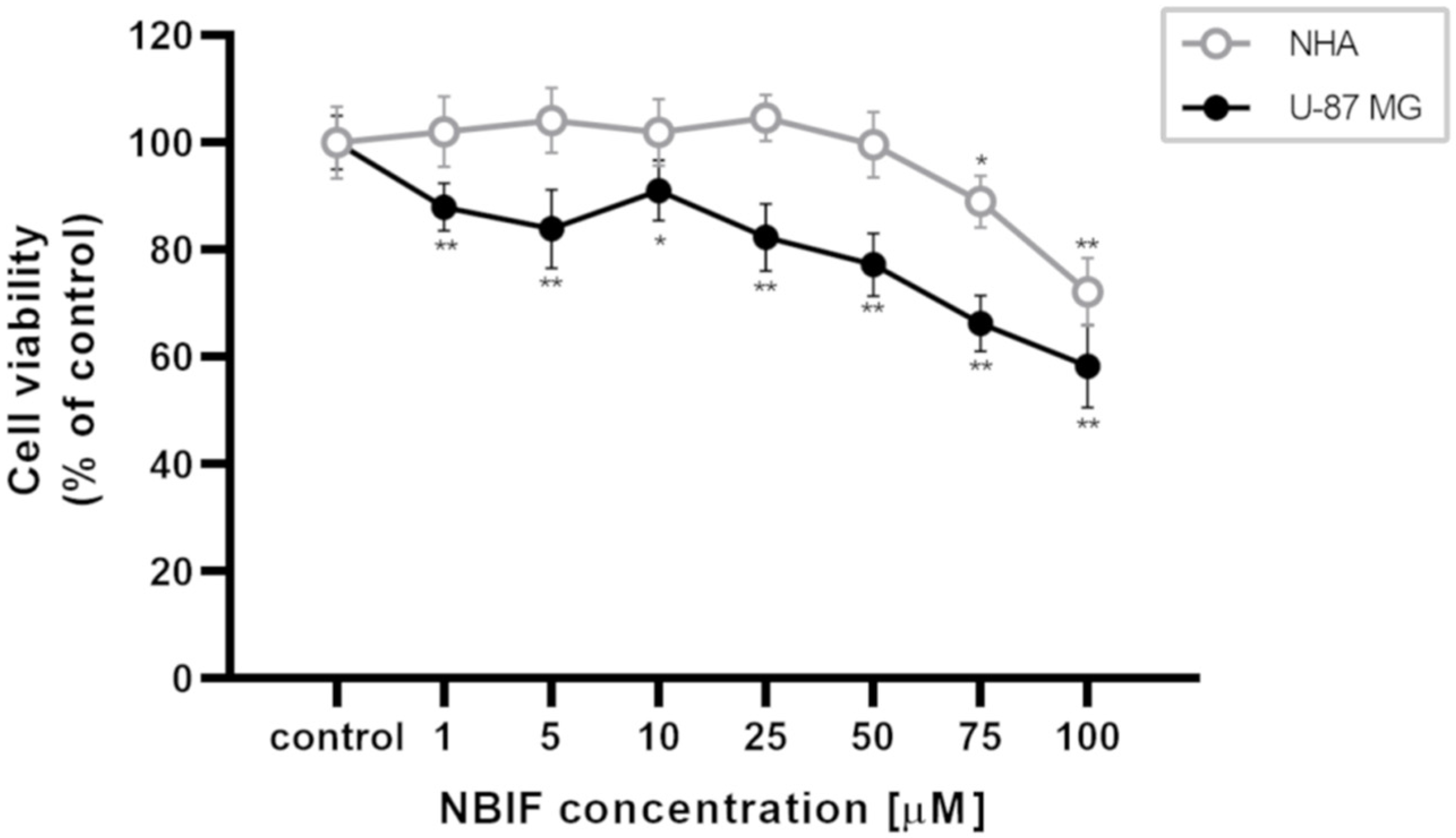
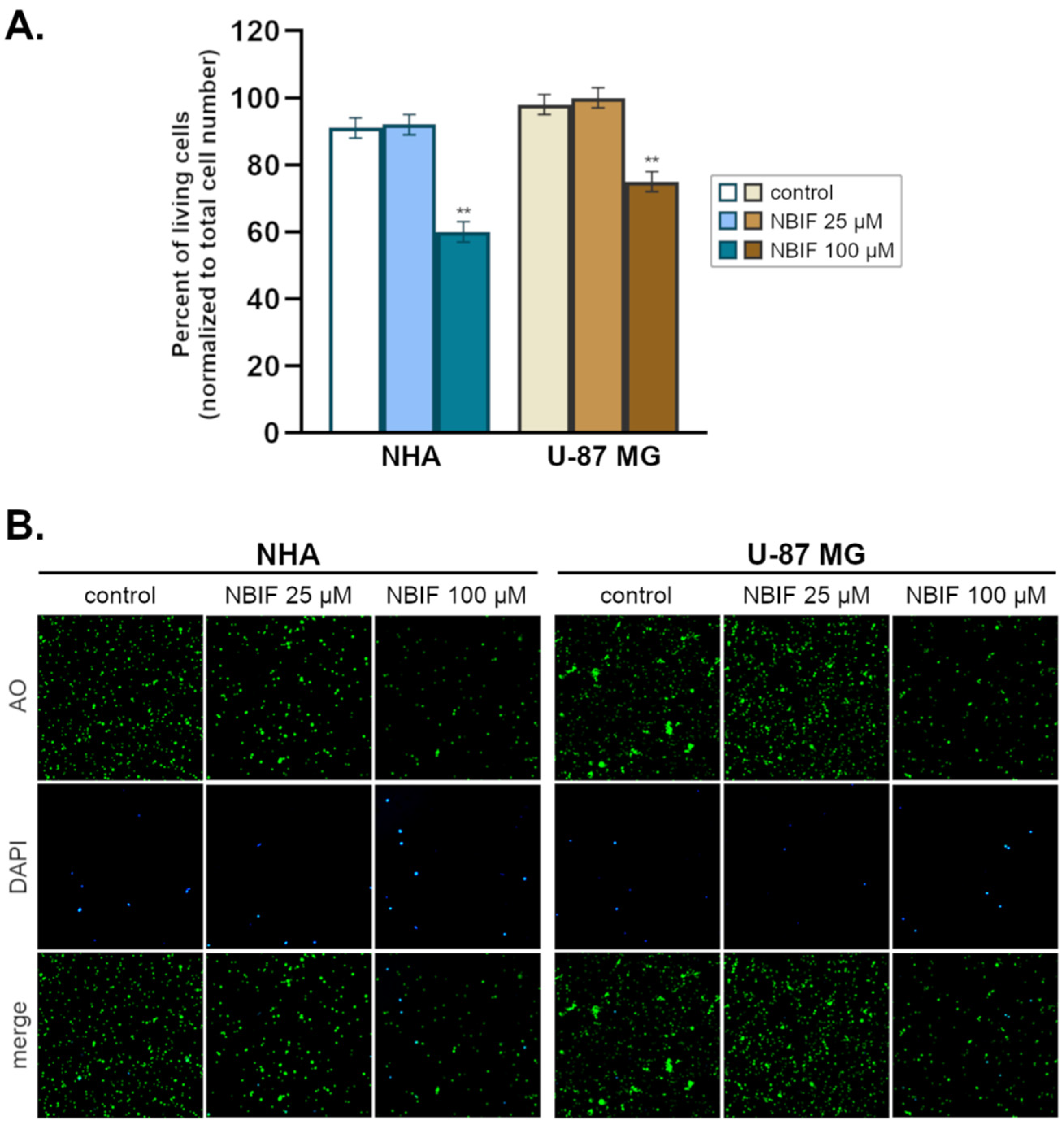
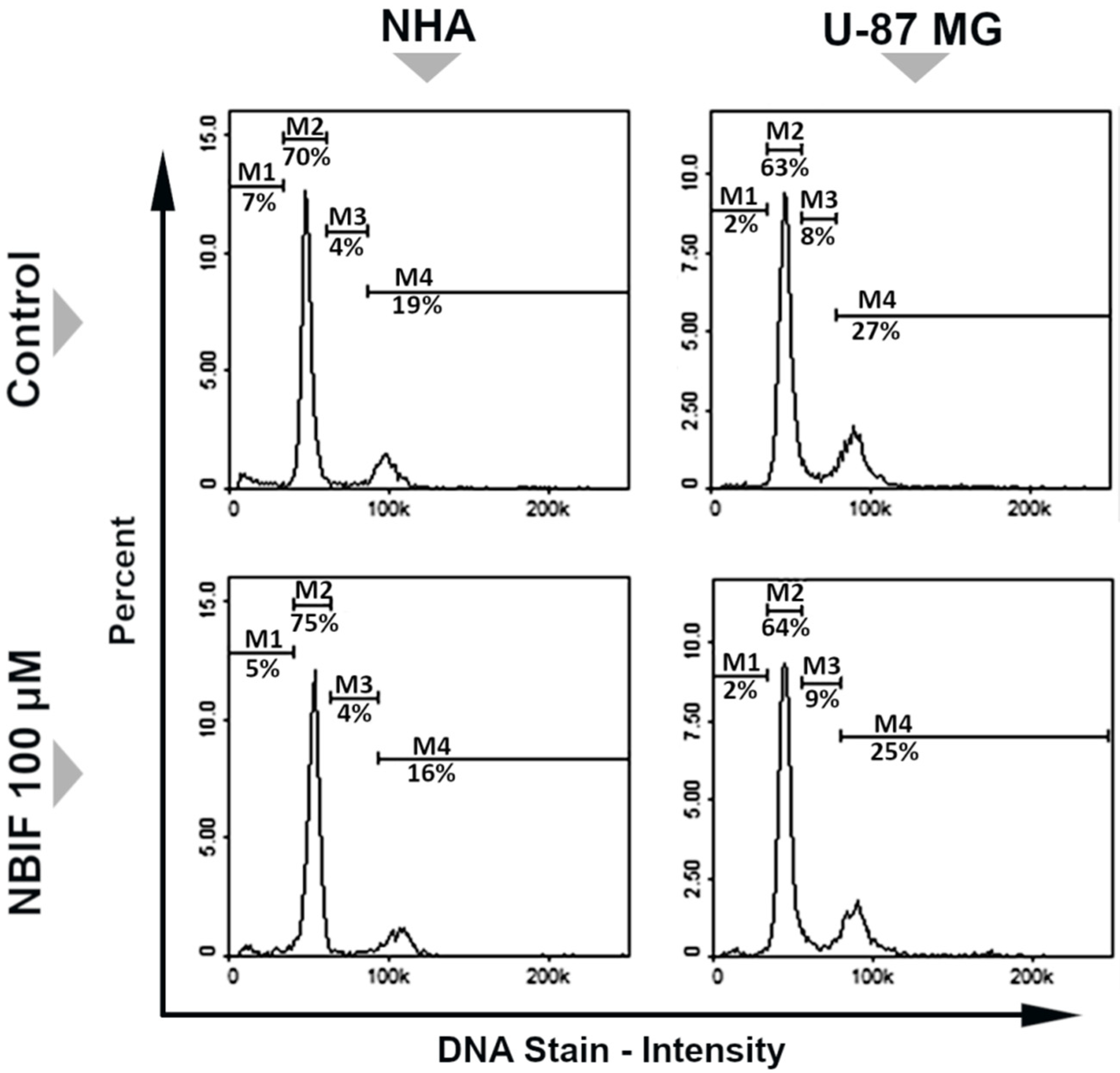
2.1. NBIF Decreases Viability and Amount of NHA and U-87 MG Cells without Affecting the Cell Cycle
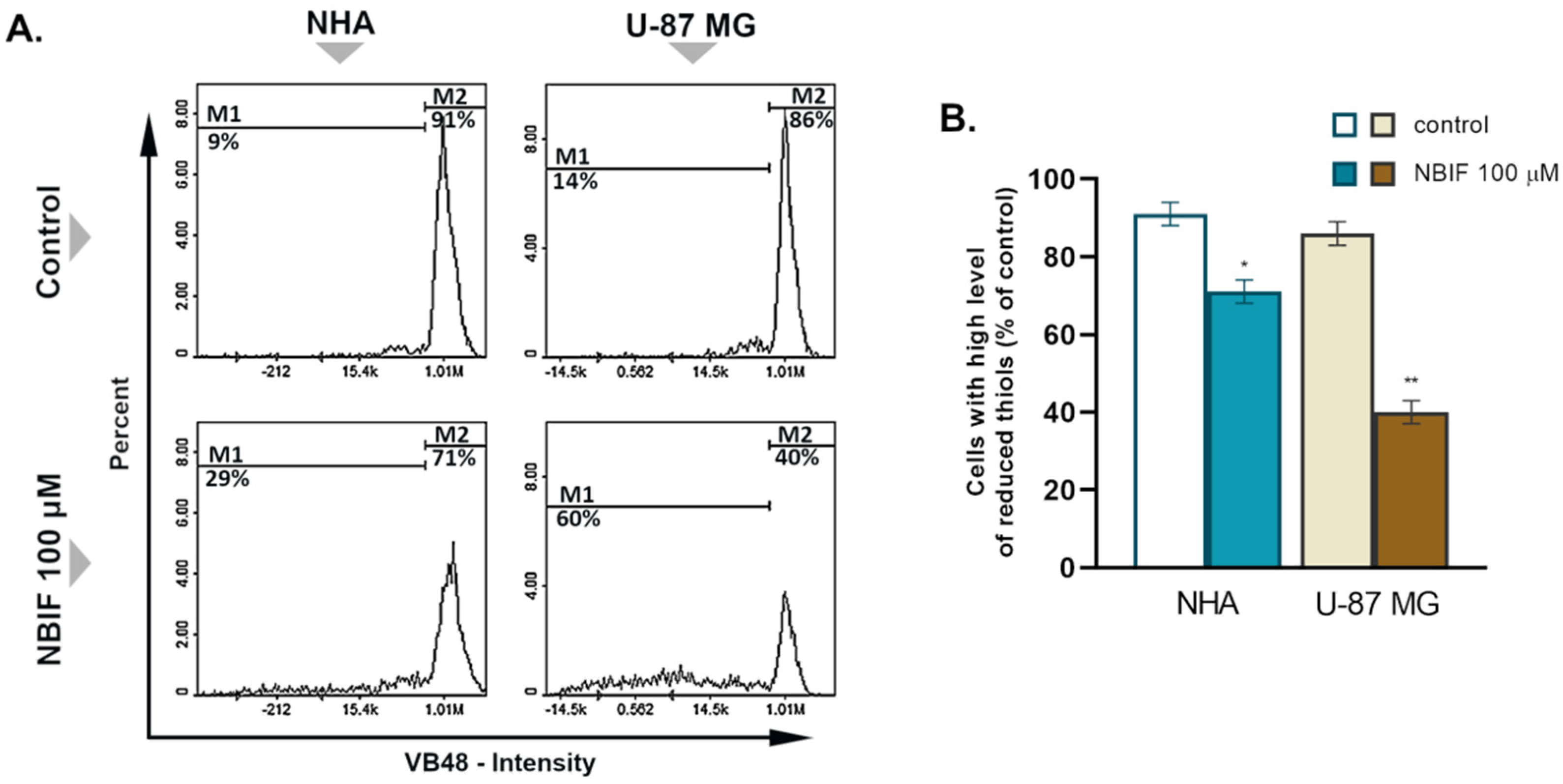
2.2. NBIF Causes Reduction in the Level of Cellular GSH in U-87 MG Cells
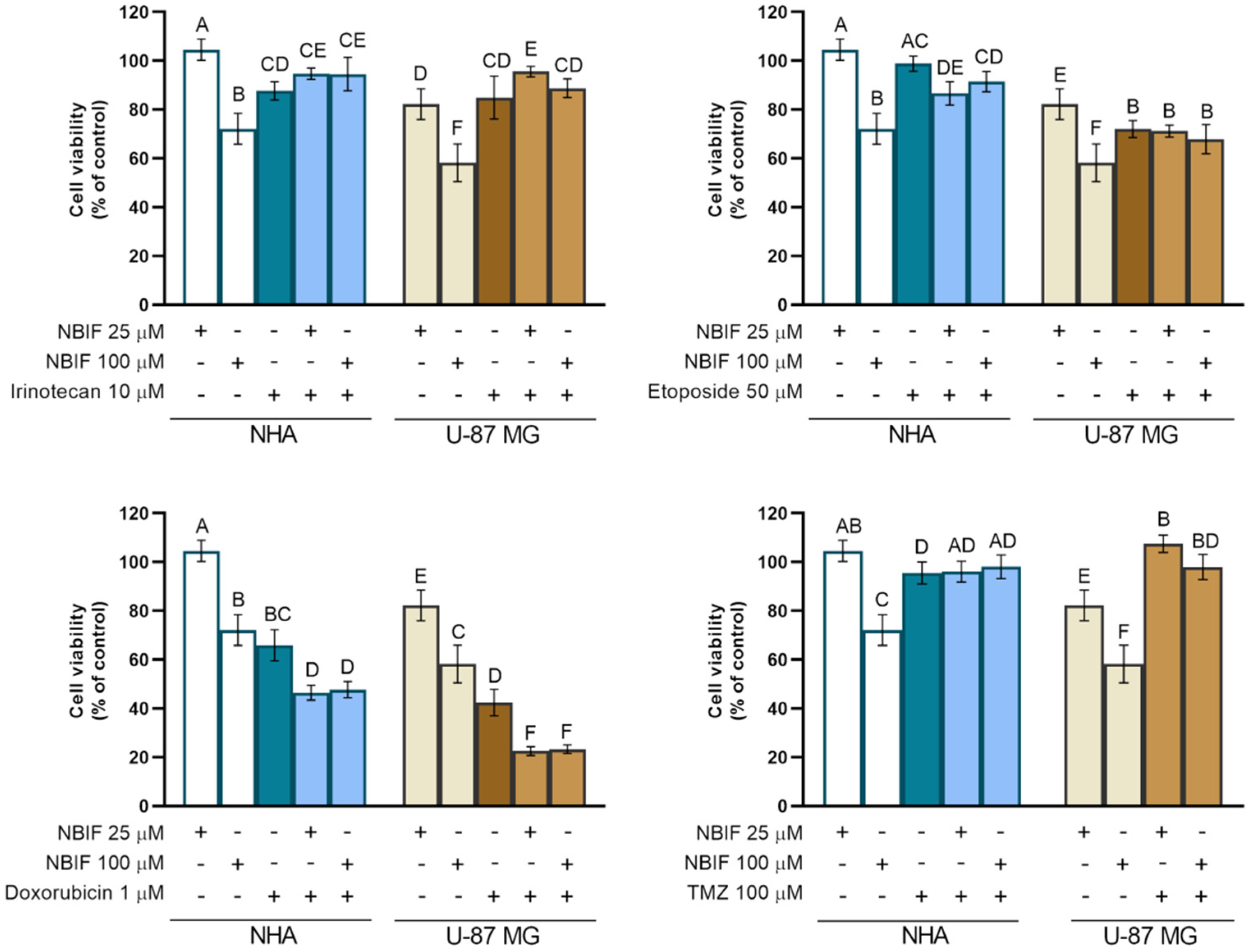
2.3. The NBIF Effect on the Activity of Irinotecan, Etoposide, Doxorubicin, and Temozolomide on the Cell Viability
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Cell Count Assay
4.5. Cell Vitality Assay—Assessment of the Level of Cellular Reduced Glutathione (GSH)
4.6. Fixed Cell Cycle-DAPI Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sehm, T.; Fan, Z.; Weiss, R.; Schwarz, M.; Engelhorn, T.; Hore, N.; Doerfler, A.; Buchfelder, M.; Eyüpoglu, I.Y.; Savaskan, N.E. The impact of dietary isoflavonoids on malignant brain tumors. Cancer Med. 2014, 3, 865–877. [Google Scholar] [CrossRef]
- Chen, R.; Smith-Cohn, M.; Cohen, A.L.; Colman, H. Glioma Subclassifications and Their Clinical Significance. Neurotherapeutics 2017, 14, 284–297. [Google Scholar] [CrossRef] [Green Version]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Eastman Langer, C.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro Oncol. 2014, 16, 896–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanders, E.D.; Svensson, F.; Bailey, D.S. Therapy for glioblastoma: Is it working? Drug. Discov. Today 2019, 24, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Osuka, S.; Van Meir, E.G. Overcoming therapeutic resistance in glioblastoma: The way forward. J. Clin. Investig. 2017, 127, 415–426. [Google Scholar] [CrossRef] [Green Version]
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma Multiforme: An Overview of Emerging Therapeutic Targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef] [Green Version]
- Respondek, M.; Beberok, A.; Rok, J.; Rzepka, Z.; Wrześniok, D.; Buszman, E. MIM1, the Mcl-1 specific BH3 mimetic induces apoptosis in human U87MG glioblastoma cells. Toxicol. In Vitro 2018, 53, 126–135. [Google Scholar] [CrossRef]
- Fan, C.H.; Liu, W.L.; Cao, H.; Wen, C.; Chen, L.; Jiang, G. O6-methylguanine DNA methyltransferase as a promising target for the treatment of temozolomide-resistant gliomas. Cell Death Dis. 2013, 4, e876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.W.; Schiff, D. Treatment considerations for MGMT-unmethylated glioblastoma. Curr. Neurol. Neurosci. Rep. 2015, 15, 507. [Google Scholar] [CrossRef]
- De Man, F.M.; Goey, A.K.L.; Van Schaik, R.H.N.; Mathijssen, R.H.J.; Bins, S. Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics. Clin. Pharmacokinet. 2018, 57, 1229–1254. [Google Scholar] [CrossRef] [Green Version]
- Horescu, C.; Cioc, C.E.; Tuta, C.; Sevastre, A.S.; Tache, D.E.; Alexandru, O.; Artene, S.A.; Danoiu, S.; Dricu, A.; Oana, P.S. The effect of temozolomide in combination with doxorubicin in glioblastoma cells in vitro. J. Immunoass. Immunochem. 2020, 31, 1033–1043. [Google Scholar] [CrossRef]
- Vredenburgh, J.J.; Desjardins, A.; Herndon, J.E., II; Marcello, J.; Reardon, D.A.; Quinn, J.A.; Rich, J.N.; Sathornsumetee, S.; Gururangan, S.; Sampson, J.; et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 2007, 25, 4722–4729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; Gururangan, S.; Sampson, J.H.; Sathornsumetee, S.; McLendon, R.E.; Herndon, J.E., II; Marcello, J.E.; Norfleet, J.; et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: A phase II study. Br. J. Cancer 2009, 101, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.A.; Hsu, F.P.K.; Delashaw, J.; Bota, D.A. Efficacy and safety of bevacizumab and etoposide combination in patients with recurrent malignant gliomas who have failed bevacizumab. RHC 2014, 5, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Mesti, T.; Moltara, M.E.; Boc, M.; Rebersek, M.; Ocvirk, J. Bevacizumab and irinotecan in recurrent malignant glioma, a single institution experience. Radiol. Oncol. 2015, 49, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Villodre, E.S.; Kipper, F.C.; Silva, A.O.; Lenz, G.; Da Costa Lopez, P.L. Low Dose of Doxorubicin Potentiates the Effect of Temozolomide in Glioblastoma Cells. Mol. Neurobiol. 2018, 55, 4185–4194. [Google Scholar] [CrossRef]
- Lesniak, M.S.; Upadhyay, U.; Goodwin, R.; Tyler, B.; Brem, H. Local delivery of doxorubicin for the treatment of malignant brain tumors in rats. Anticancer Res. 2005, 25, 3825–3831. [Google Scholar]
- Maksimenko, O.; Malinovskaya, J.; Shipulo, E.; Osipova, N.; Razzhivina, V.; Arantseva, D.; Yarovaya, O.; Mostovaya, U.; Khalansky, A.; Fedoseeva, V.; et al. Doxorubicin-loaded PLGA nanoparticles for the chemotherapy of glioblastoma: Towards the pharmaceutical development. Int. J. Pharm. 2019, 572, 118733. [Google Scholar] [CrossRef] [PubMed]
- Dréan, A.; Goldwirt, L.; Verreault, M.; Canney, M.; Schmitt, C.; Guehennec, J.; Delattre, J.Y.; Carpentier, A.; Idbaih, A. Blood-brain barrier, cytotoxic chemotherapies and glioblastoma. Expert Rev. Neurother. 2016, 16, 1285–1300. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatmentof Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Ullah, H.; Castilho, P.C.M.F.; Gomila, A.S.; D’Onoforio, G.; Filosa, R.; Wang, F.; Nabavi, S.M.; Dalgia, M.; Silva, A.S.; et al. Targeting NF-κB signaling pathway in cancer by dietary polyphenols. Crit. Rev. Food Sci. Nutr. 2019, 60, 2790–2800. [Google Scholar] [CrossRef]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef]
- Szliszka, E.; Skaba, D.; Czuba, Z.P.; Krol, W. Inhibition of inflammatory mediators by neobavaisoflavone in activated RAW264.7 macrophages. Molecules 2011, 16, 3701–3712. [Google Scholar] [CrossRef] [PubMed]
- Don, M.J.; Lin, L.C.; Chiou, W.F. Neobavaisoflavone stimulates osteogenesis via p38-mediated up-regulation of transcription factors and osteoid genes expression in MC3T3-E1 cells. Phytomedicine 2012, 19, 551–561. [Google Scholar] [CrossRef]
- Ye, H.; He, X.; Feng, X. Developing neobavaisoflavone nanoemulsion suppresses lung cancer progression by regulating tumor microenvironment. Biomed. Pharmacother. 2020, 129, 110369. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Sędek, Ł.; Paradysz, A.; Król, W. Enhanced TRAIL-mediated apoptosis in prostate cancer cells by the bioactive compounds neobavaisoflavone and psoralidin isolated from Psoralea corylifolia. Pharmacol. Rep. 2011, 63, 139–148. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, W.I.; Ko, H.; So, Y.; Kang, K.S.; Kim, I.K.; Kim, K.; Yoon, H.G.; Kim, T.J.; Choi, K.C. Neobavaisoflavone sensitizes apoptosis via the inhibition of metastasis in TRAIL-resistant human glioma U373MG cells. Life Sci. 2014, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lim, H.S.; Lee, J.; Jeong, S.J. Quantitative Analysis of Psoralea corylifolia Linne and its Neuroprotective and Anti-Neuroinflammatory Effects in HT22 Hippocampal Cells and BV-2 Microglia. Molecules 2016, 21, 1076. [Google Scholar] [CrossRef]
- Sahin, I.; Bilir, B.; Ali, S.; Sahin, K.; Kucuk, O. Soy isoflavones in integrative oncology: Increased efficacy and decreased toxicity of cancer therapy. Integr. Cancer Ther. 2019, 18. [Google Scholar] [CrossRef] [Green Version]
- Brandes, N.; Schmitt, S.; Jakob, U. Thiol-based redox switches in eukaryotic proteins. Antioxid. Redox Signal. 2009, 11, 997–1014. [Google Scholar] [CrossRef]
- Li, Y.; Ahmed, F.; Ali, S.; Philip, P.A.; Kucuk, O.; Sarkar, F.H. Inactivation of Nuclear Factor KB by Soy Isoflavone Genistein Contributes to Increased Apoptosis Induced by Chemotherapeutic Agents in Human Cancer Cells. Cancer Res. 2005, 65, 6934–6942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papazisis, K.T.; Kalemi, T.G.; Zambouli, D.; Geromichalos, G.D.; Lambropoulos, A.F.; Kotsis, A.; Boutis, L.L.; Kortsaris, A.H. Synergistic effects of protein tyrosine kinase inhibitor genistein with camptothecins against three cell lines in vitro. Cancer Lett. 2006, 233, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.P.; Wang, G.; Zhao, Z.B.; Wang, Q.; Shi, Y. Synergistic cytotoxic effect of genistein and doxorubicin on drug-resistant human breast cancer MCF 7/Adr cells. Oncol. Rep. 2014, 32, 1647–1653. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Sun, Y.; Zheng, J.M.; Yan, X.L.; Chen, H.M.; Chen, J.K.; Huang, H.Q. Formononetin sensitizes glioma cells to doxorubicin through preventing EMT via inhibition of histone deacetylase 5. Int. J. Clin. Exp. Pathol. 2015, 8, 6434–6441. [Google Scholar]
- Chen, C.C.; Chen, C.Y.; Ueng, S.H.; Hsueh, C.; Yeh, C.T.; Ho, J.Y.; Chou, L.F.; Wang, T.H. Corylin increases the sensitivity of hepatocellular carcinoma cells to chemotherapy through long noncoding RNA RAD51-AS1-mediated inhibition of DNA repair. Cell Death Dis. 2018, 9, 543. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.N.; Shyu, H.W.; Hu, T.W.; Yeh, J.P.; Lin, Y.W.; Lee, L.Y.; Yeh, Y.T.; Dai, H.Y.; Perng, D.S.; Su, S.H.; et al. Anti-proliferative activity of biochanin A in human osteosarcoma cells via mitochondrial-involved apoptosis. Food Chem. Toxicol. 2018, 112, 194–204. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Formulation and Optimization of Doxorubicin and Biochanin A Combinational Liposomes for Reversal of Chemoresistance. AAPS PharmSciTech 2016, 18, 1116–1124. [Google Scholar] [CrossRef]
- Sun, N.J.; Woo, S.H.; Cassady, J.M.; Snapka, R.M. DNA Polymerase and Topoisomerase II Inhibitors from Psoralea corylifolia. J. Nat. Prod. 1998, 61, 362–366. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of Glutathione in Cancer Progression and Chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef] [Green Version]
- Desideri, E.; Ciccarone, F.; Ciriolo, M.R. Targeting glutathione metabolism: Partner in crime in anticancer therapy. Nutrients 2019, 11, 1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kachadourian, R.; Day, B.J. Flavonoid-induced glutathione depletion: Potential implications for cancer treatment. Free Radic. Biol. Med. 2006, 41, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.G.; Kim, J.S.; Lee, J.H.; Cho, E.W. Genistein decreases cellular redox potential, partially suppresses cell growth in HL-60 leukemia cells and sensitizes cells to γ-radiation-induced cell death. Mol. Med. Rep. 2014, 10, 2786–2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sznarkowska, A.; Kostecka, A.; Meller, K.; Bielawski, K.P. Inhibition of cancer antioxidant defence by natural compounds. Oncotarget 2017, 8, 15996–16016. [Google Scholar] [CrossRef] [Green Version]
- Illesca, P.; Valenzuela, R.; Espinosa, A.; Echeverría, F.; Soto-Alarcon, S.; Ortiz, M.; Videla, L.A. Hydroxytyrosol supplementation ameliorates the metabolic disturbances in white adipose tissue from mice fed a high-fat diet through recovery of transcription factors Nrf2, SREBP-1c, PPAR-γ and NF-κB. Biomed. Pharmacother. 2019, 109, 2472–2481. [Google Scholar] [CrossRef]
- Tascioglu Aliyev, A.; Panieri, E.; Stepanić, V.; Gurer-Orhan, H.; Saso, L. Involvement of NRF2 in breast cancer and possible therapeutical role of polyphenols and melatonin. Molecules 2021, 26, 1853. [Google Scholar] [CrossRef] [PubMed]

| Irinotecan (μM) | Etoposide (μM) | Doxorubicin (μM) | ||||
|---|---|---|---|---|---|---|
| 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | |
| NHA | 85.9 | 30.3 | 168.9 | 117.7 | 45.9 | 1.6 |
| U-87 MG | 42.4 | 21.5 | 126.6 | 97.2 | 7.6 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maszczyk, M.; Rzepka, Z.; Rok, J.; Beberok, A.; Wrześniok, D. Neobavaisoflavone May Modulate the Activity of Topoisomerase Inhibitors towards U-87 MG Cells: An In Vitro Study. Molecules 2021, 26, 4516. https://doi.org/10.3390/molecules26154516
Maszczyk M, Rzepka Z, Rok J, Beberok A, Wrześniok D. Neobavaisoflavone May Modulate the Activity of Topoisomerase Inhibitors towards U-87 MG Cells: An In Vitro Study. Molecules. 2021; 26(15):4516. https://doi.org/10.3390/molecules26154516
Chicago/Turabian StyleMaszczyk, Mateusz, Zuzanna Rzepka, Jakub Rok, Artur Beberok, and Dorota Wrześniok. 2021. "Neobavaisoflavone May Modulate the Activity of Topoisomerase Inhibitors towards U-87 MG Cells: An In Vitro Study" Molecules 26, no. 15: 4516. https://doi.org/10.3390/molecules26154516
APA StyleMaszczyk, M., Rzepka, Z., Rok, J., Beberok, A., & Wrześniok, D. (2021). Neobavaisoflavone May Modulate the Activity of Topoisomerase Inhibitors towards U-87 MG Cells: An In Vitro Study. Molecules, 26(15), 4516. https://doi.org/10.3390/molecules26154516