Degradable Elastomers: Is There a Future in Tyre Compound Formulation?
Abstract
:1. Introduction
1.1. Thermoplastic Elastomers
1.1.1. The General Structure of Thermoplastic Elastomers (TPEs)
1.1.2. A Glance at Thermoplastic Elastomers Proposed by Michelin: Features and Mechanical Properties Required for Tyre Compounds
1.1.3. Review Structure
2. Copolymers Having Poly(lactide) as a Rigid Block
2.1. Copolymers Having Poly(isoprene) as Soft Block
2.2. Copolymers Having Aliphatic Polyesters as Soft Block
2.3. Copolymers Having Caprolactone-Lactide Copolymers as Soft Block
2.4. Copolymers Based on ε-Decalactone Monomer
3. Copolymers Having a Rigid Block Other Than Polylactide
3.1. Rigid Block Based on Tulipanin a Monomer
3.2. Semi-Aromatic Polyester as Rigid Block
3.3. Poly(hexamethylene 2,5-furanodicarboxylate) as Rigid Block
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lechtenboehmer, A.; Moneypenny, H.G.; Merschand, F. A Review of polymer interfaces in tyre technology. Br. Polym. J. 1990, 22, 265–301. [Google Scholar] [CrossRef]
- European Union Parliament Regulation (EC) No 1222/2009 of the European Parliament and of the Council of 25 November 2009 on the labelling of tyres with respect to fuel efficiency and other essential parameters. Off. J. Eur. Union 2009, L 342/46, 13.
- European Union. European Parliament Commission Regulation (EU) No 68/2014. Off. J. Eur. Union 2014, L 23/9.
- Zhang, P.; Morris, M.; Doshi, D. Materials development for lowering rolling resistance of tires. Rubber Chem. Technol. 2016, 89, 79–116. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Recycling waste tires into ground tire rubber (Gtr)/rubber compounds: A review. J. Compos. Sci. 2020, 4, 103. [Google Scholar] [CrossRef]
- Markl, E.; Lackner, M. Devulcanization technologies for recycling of tire-derived rubber: A review. Materials 2020, 13, 1246. [Google Scholar] [CrossRef] [Green Version]
- Grammelis, P.; Margaritis, N.; Dallas, P.; Rakopoulos, D.; Mavrias, G. A review on management of End of Life Tires (ELTs) and alternative uses of textile fibers. Energies 2021, 14, 571. [Google Scholar] [CrossRef]
- Kim, J.K.; Saha, P.; Thomas, S.; Haponiuk, J.T.; Aswathi, M.K. (Eds.) Rubber Recycling: Challenges and Developments, 1st ed.; The Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Jan Kole, P.A.; Löhr, J.; Van Belleghem, F.G.A.J.; Ragas, A.M.J. Wear and Tear of Tyres: A stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef]
- Baensch-Baltruschat, B.; Kocher, B.; Stock, F.; Reifferscheid, G. Tyre and road wear particles (TRWP)—A review of generation, properties, emissions, human health risk, ecotoxicity, and fate in the environment. Sci. Total Environ. 2020, 733, 137823. [Google Scholar] [CrossRef]
- Eisentraut, P.; Dümichen, E.; Ruhl, A.S.; Jekel, M.; Albrecht, M.; Gehde, M.; Braun, U. Two Birds with One Stone—Fast and simultaneous analysis of microplastics: Microparticles derived from thermoplastics and tire wear. Environ. Sci. Technol. Lett. 2018, 5, 608–613. [Google Scholar] [CrossRef]
- Klöckner, P.; Reemtsma, T.; Eisentraut, P.; Braun, U.; Ruhl, A.S.; Wagner, S. Tire and road wear particles in road environment—Quantification and assessment of particle dynamics by Zn determination after density separation. Chemosphere 2019, 222, 714–721. [Google Scholar] [CrossRef]
- Klöckner, P.; Seiwert, B.; Eisentraut, P.; Braun, U.; Reemtsma, T.; Wagner, S. Characterization of tire and road wear particles from road runoff indicates highly dynamic particle properties. Water Res. 2020, 185, 116262. [Google Scholar] [CrossRef]
- Wypych, G. (Ed.) Handbook of UV Degradation and Stabilization, 3rd ed.; ChemTec Publishing: Scarborough, ON, Canada, 2020. [Google Scholar]
- Linos, A.; Steinbüchel, A. Biodegradation of Natural and Synthetic Rubbers. Biopolym. Online 2005. [Google Scholar] [CrossRef]
- Bockstal, L.; Berchem, T.; Schmetz, Q.; Richel, A. Devulcanisation and reclaiming of tires and rubber by physical and chemical processes: A review. J. Clean. Prod. 2019, 236, 117574. [Google Scholar] [CrossRef]
- Pehlken, A.; Müller, D.H. Using information of the separation process of recycling scrap tires for process modelling. Resour. Conserv. Recycl. 2009, 54, 140–148. [Google Scholar] [CrossRef]
- Bowie, M.D. Tire Industry Association OTR Tire and Rim Weight Chart. In Proceedings of the Off-the-Road Tire Conference, Saddlebrook Resort, Tampa, FL, USA, 27 February 2019. [Google Scholar]
- Stevenson, K.; Stallwood, B.; Hart, A.G. Tire Rubber Recycling and Bioremediation: A Review. Bioremediat. J. 2008, 12, 1–11. [Google Scholar] [CrossRef]
- Cao, R.; Deng, L.; Feng, Z.; Zhao, X.; Li, X.; Zhang, L. Preparation of natural bio-based Eucommia ulmoides gum/styrene-butadiene rubber composites and the evaluation of their damping and sound absorption properties. Polymer 2021, 213, 123292. [Google Scholar] [CrossRef]
- Zhai, X.; Chen, Y.; Han, D.; Zheng, J.; Wu, X.; Wang, Z.; Li, X.; Ye, X.; Zhang, L. New designed coupling agents for silica used in green tires with low VOCs and low rolling resistance. Appl. Surf. Sci. 2021, 558, 149819. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, R.; Lei, W.; Qiao, H.; Ji, H.; Zhang, L.; Hua, K.C.; Kulig, J. Design and synthesis by redox polymerization of a bio-based carboxylic elastomer for green tire. Sci. China Chem. 2015, 58, 1561–1569. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Wang, Y. Microbial Desulfurization of Ground Tire Rubber by Sphingomonas sp.: A novel technology for crumb rubber composites. J. Polym. Environ. 2012, 20, 372–380. [Google Scholar] [CrossRef]
- Bredberg, K.; Persson, J.; Christiansson, M.; Stenberg, B.; Holst, O. Anaerobic desulfurization of ground rubber with the thermophilic archaeon Pyrococcus furiosus—A new method for rubber recycling. Appl. Microbiol. Biotechnol. 2001, 55, 43–48. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Wang, Y. Improvement of the properties of natural rubber/ground tire rubber composites through biological desulfurization of GTR. J. Polym. Res. 2012, 19, 9864. [Google Scholar] [CrossRef]
- Valdés, C.; Hernández, C.; Morales-vera, R.; Andler, R. Desulfurization of Vulcanized Rubber Particles Using Biological and Couple Microwave-Chemical Methods. Res. Sq. 2020, 9, 1–19. [Google Scholar]
- Chicu, N.; Prioteasa, A.-L.; Deaconu, A. Current trends and perspectives in tyre industry. Stud. Univ. “Vasile Goldis” Arad–Econ. Ser. 2020, 30, 36–56. [Google Scholar] [CrossRef]
- Barlow, F.W. Rubber Compounding, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Rodgers, B.; Waddell, W. The Science and Technology of Rubber, 4th ed.; Mark, J., Erman, B., Roland, M., Eds.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Kruželák, J.; Sýkora, R.; Hudec, I. Sulphur and peroxide vulcanisation of rubber compounds—Overview. Chem. Pap. 2016, 70, 1533–1555. [Google Scholar] [CrossRef]
- British Standards Institution. BS EN ISO 18064: 2014 Thermoplastic Elastomers—Nomenclature and Abbreviated Terms; British Standards Institution: London, UK, 2014; ISBN 978 0 580 85117 9. [Google Scholar]
- Drobny, J.G. Handbook of Thermoplastic Elastomers, 2nd ed.; William, A., Ed.; Elsevier Inc.: Oxford, UK, 2014. [Google Scholar]
- Shanks, R.; Kong, I. Thermoplastic Elastomers; El-Sonbati, A., Ed.; In Tech: Rijeka, Croatia, 2012. [Google Scholar]
- Wang, W.; Lu, W.; Goodwin, A.; Wang, H.; Yin, P.; Kang, N.-G.; Hong, K.; Mays, J.W. Recent advances in thermoplastic elastomers from living polymerizations: Macromolecular architectures and supramolecular chemistry. Prog. Polym. Sci. 2019, 95, 1–31. [Google Scholar] [CrossRef]
- Whelan, D. Thermoplastic Elastomers. Brydson’s Plast. Mater. Eighth Ed. 2017, 653–703. [Google Scholar] [CrossRef]
- Abad, V.; Custodero, E.; Chouvel, C.; Greiveldinger, M. Tire Provided with a Tread Comprising a Thermoplastic Elastomer. Compagnie generale des etablissements Michelin and Michelin Recherche et Technique. S.A. Patent WO/2015/113966, 6 August 2015. [Google Scholar]
- Chouvel, C.; Da Silva, J.C.A. Tire Tread Comprising a Thermoplastic Elastomer-Patent. US10081723B2, 26 December 2017. [Google Scholar]
- Chouvel, C. Tire Provided With a Tread Comprising a Diene Elastomer and Thermoplastic Elastomer System. Compagnie Generale Des Etablissements. Michelin. Patent US10780740B2, 22 September 2020. [Google Scholar]
- Da Silva, J.C.A.; Gornard, B. Tire Comprising a Tread Comprising a Thermoplastic Elastomer and a Crosslinking System Based on Sulfur Michelin. U.S. Patent 20190322136A1, 24 October 2019. [Google Scholar]
- Frick, E.M.; Zalusky, A.S.; Hillmyer, M.A. Characterization of Polylactide-b-polyisoprene-b-polylactide Thermoplastic Elastomers. Biomac 2003, 4, 216–223. [Google Scholar] [CrossRef]
- Wanamaker, C.L.; Bluemle, M.J.; Pitet, L.M.; O’Leary, L.E.; Tolman, W.B.; Hillmyer, M.A. Consequences of Polylactide Stereochemistry on the Properties of Polylactide-Polymenthide-Polylactide Thermoplastic Elastomers. Biomac 2009, 10, 2904–2911. [Google Scholar] [CrossRef]
- Martello, M.T.; Hillmyer, M.A. Polylactide–Poly(6-methyl-ε-caprolactone)–Polylactide Thermoplastic Elastomers. Macromolecules 2011, 44, 8537–8545. [Google Scholar] [CrossRef]
- Xiong, M.D.; Schneiderman, K.; Bates, F.S.; Hillmyer, M.A.; Zhang, K. Scalable production of mechanically tunable block polymers from sugar. Proc. Natl. Acad. Sci. USA 2014, 111, 8357–8362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, A.; Kurokawa, N.; Hillmyer, M.A. Strong, Resilient, and Sustainable Aliphatic Polyester Thermoplastic Elastomers. Biomac 2017, 18, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Aihara, K.; Yamanishi, H.; Fukuoka, H.; Tanaka, R.; Cai, Z.; Shiono, T. Synthesis of biodegradable thermoplastic elastomers from ε-caprolactone and lactide. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 489–495. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.; Kim, Y.W.; Shin, J. Preparation and characterization of a renewable pressure-sensitive adhesive system derived from ε-decalactone, l-lactide, epoxidized soybean oil, and rosin ester. ACS Sustain. Chem. Eng. 2015, 3, 2309–2320. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.; Jang, J.; Choung, J.S.; Choi, W.J.; Kim, G.J.; Kim, Y.W.; Shin, J. Sustainable poly(ε-decalactone)−poly(l-lactide) multiarm star copolymer architectures for thermoplastic elastomers with fixed molar mass and block ratio. Polymer 2017, 112, 306–317. [Google Scholar] [CrossRef]
- Shin, J.; Lee, Y.; Tolman, W.B.; Hillmyer, M.A. Thermoplastic Elastomers Derived from Menthide and Tulipalin A. Biomac 2012, 13, 3833–3840. [Google Scholar] [CrossRef]
- Lee, I.; Bates, F.S. Synthesis, Structure, and Properties of Alternating and Random Poly(styrene-b-butadiene) Multiblock Copolymers. Macromolecules 2013, 46, 4529–4539. [Google Scholar] [CrossRef]
- Akkapeddi, M.K. The free radical copolymerization characteristics of α-methylene γ-butyrolactone. Polymer 1979, 20, 1215–1216. [Google Scholar] [CrossRef]
- Gregory, G.L.; Sulley, G.S.; Carrodeguas, L.P.; Chen, T.T.D.; Santmarti, A.; Terrill, N.J.; Lee, K.Y.; Williams, C.K. Triblock polyester thermoplastic elastomers with semi-aromatic polymer end blocks by ring-opening copolymerization. Chem. Sci. 2020, 11, 6567–6581. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Poland, R.R.; Escobedo, C. Kinetic Studies of the Alternating Copolymerization of Cyclic Acid Anhydrides and Epoxides, and the Terpolymerization of Cyclic Acid Anhydrides, Epoxides, and CO2 Catalyzed by (salen)CrIIICl. Macromolecules 2012, 45, 2242–2248. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Irska, I.; Zubkiewicz, A.; Szymczyk, A. Biobased Thermoplastic Elastomers: Structure-Property Relationship of Poly(hexamethylene 2,5-furanodicarboxylate)-Block-Poly(tetrahydrofuran) Copolymers Prepared by Melt Polycondensation. Polymers 2021, 13, 397. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, A.C.; Hakkarainen, M. Designed to degrade. Science 2017, 358, 872–873. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, D.; Mathisen, T.; Finne-Wistrand, A. Biocompatibility of Resorbable Polymers: A Historical Perspective and Framework for the Future. Biomac 2019, 20, 1465–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakkarainen, M. Aliphatic Polyesters: Abiotic and Biotic Degradation and Degradation Products. Adv. Polym. Sci. 2002, 157, 113–138. [Google Scholar] [CrossRef]
- Mecking, S. Nature or Petrochemistry?—Biologically Degradable Materials. Angew. Chem. Int. Ed. 2004, 43, 1078–1085. [Google Scholar] [CrossRef] [Green Version]
- Laycock, B.; Nikolic, M.; Colwell, J.M.; Gauthier, É.; Halley, P.; Bottle, S.; George, G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017, 71, 144–189. [Google Scholar] [CrossRef] [Green Version]
- Schneiderman, D.K.; Hill, E.M.; Martello, M.T.; Hillmyer, M.A. Poly(lactide)-block-poly(ε-caprolactone-co-ε-decalactone)-block-poly(lactide) copolymer elastomers. Polym. Chem. 2015, 6, 3641–3651. [Google Scholar] [CrossRef]
- Fuoco, T.; Pappalardo, D. Aluminum Alkyl Complexes Bearing Salicylaldiminato Ligands: Versatile Initiators in the Ring-Opening Polymerization of Cyclic Esters. Catalysts 2017, 7, 64. [Google Scholar] [CrossRef]
- Brannigan, R.P.; Dove, A.P. Synthesis, properties and biomedical applications of hydrolytically degradable materials based on aliphatic polyesters and polycarbonates. Biomater. Sci. 2017, 5, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Longo, J.M.; Sanford, M.J.; Coates, G.W. Ring-Opening Copolymerization of Epoxides and Cyclic Anhydrides with Discrete Metal Complexes: Structure–Property Relationships. Chem. Rev. 2016, 116, 15167. [Google Scholar] [CrossRef]
- Romain, C.; Zhu, Y.; Dingwall, P.; Paul, S.; Rzepa, H.S.; Buchard, A.; Williams, C.K. Chemoselective Polymerizations from Mixtures of Epoxide, Lactone, Anhydride, and Carbon Dioxide. J. Am. Chem. Soc. 2016, 138, 4120–4131. [Google Scholar] [CrossRef] [Green Version]
- Romain, C.; Williams, C.K. Chemoselective Polymerization Control: From Mixed-Monomer Feedstock to Copolymers. Angew. Chem. Int. Ed. 2014, 53, 1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Auria, I.; Santulli, F.; Ciccone, F.; Giannattasio, A.; Mazzeo, M.; Pappalardo, D. Synthesis of Semi-Aromatic Di-Block Polyesters by Terpolymerization of Macrolactones, Epoxides, and Anhydrides. ChemCatChem. 2021, 13, 1–10. [Google Scholar]
- Roy, K.; Debnath, S.C.; Potiyaraj, P. Application of cellulose as green filler for the development of sustainable rubber technology. J. Polym. Environ. 2020, 28, 367–387. [Google Scholar] [CrossRef]
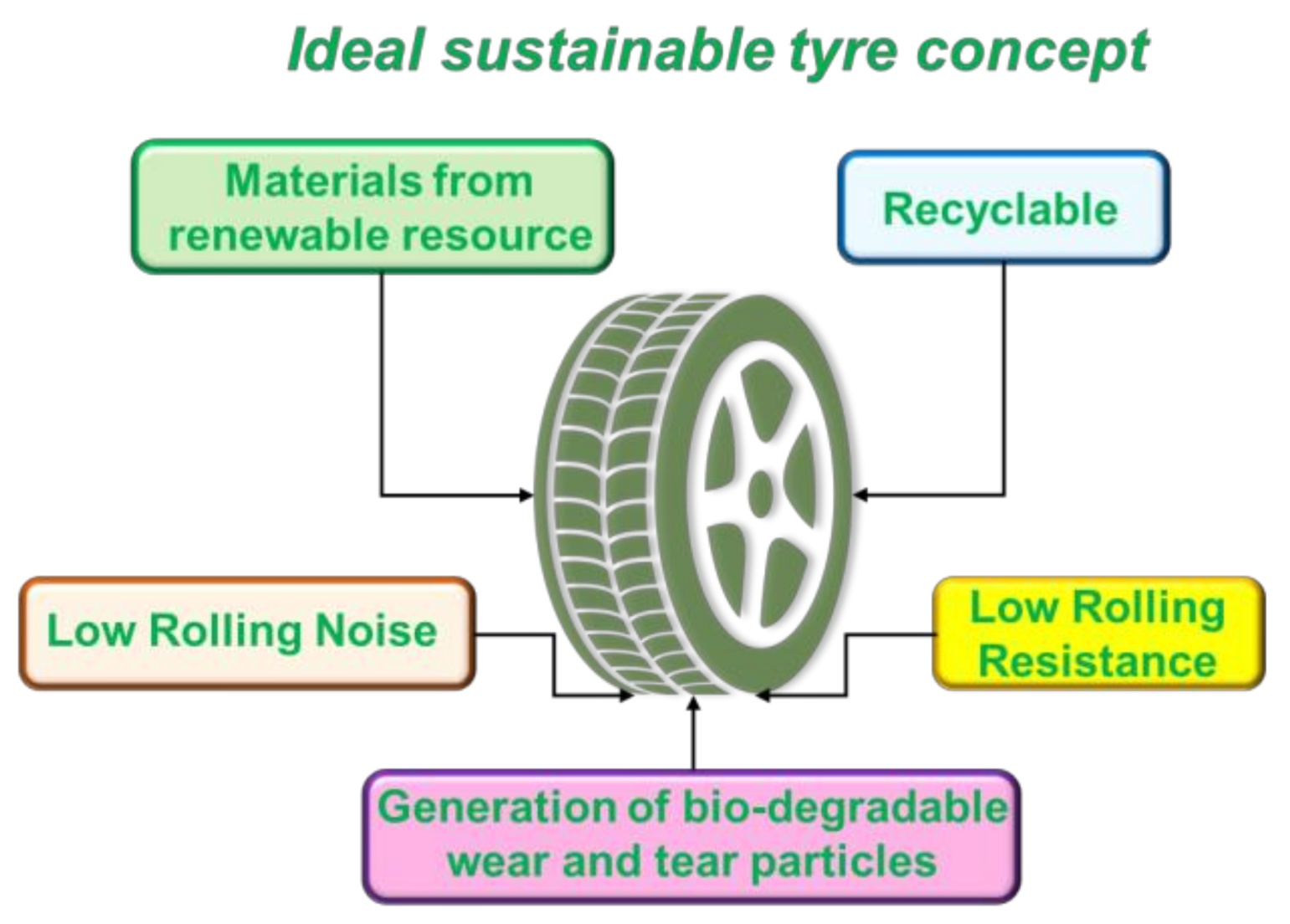

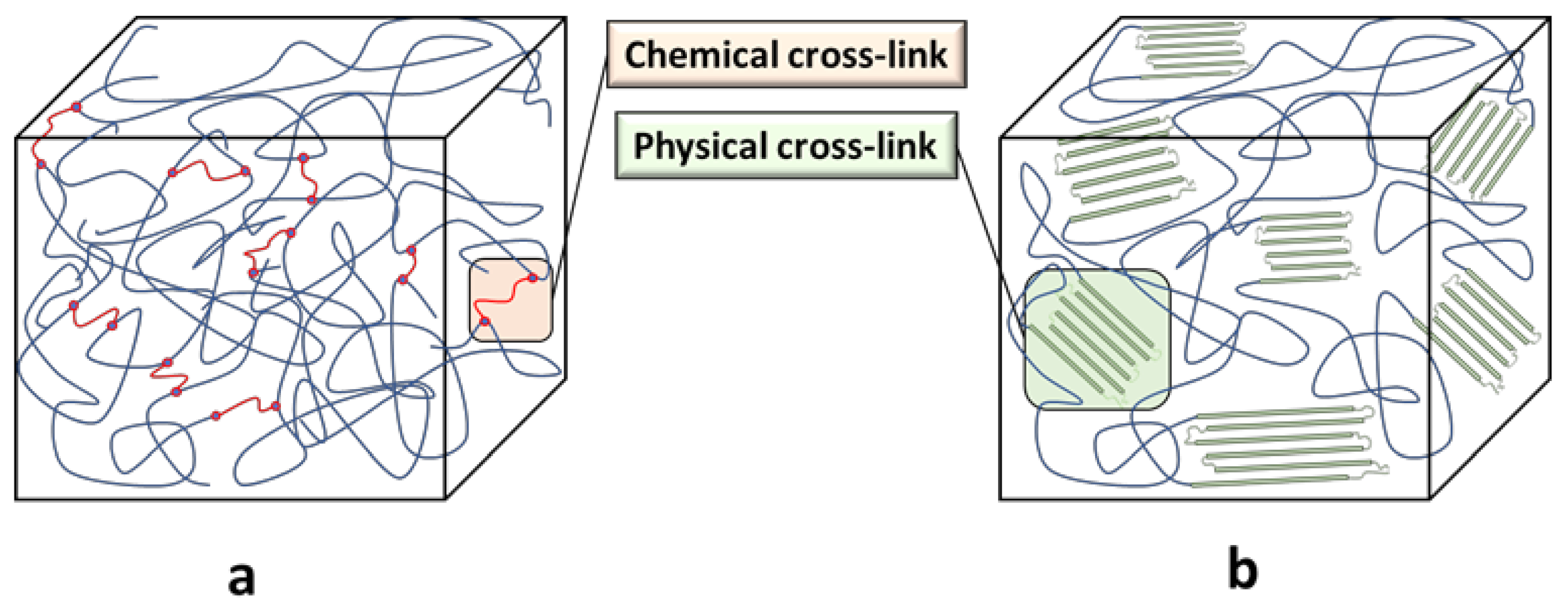


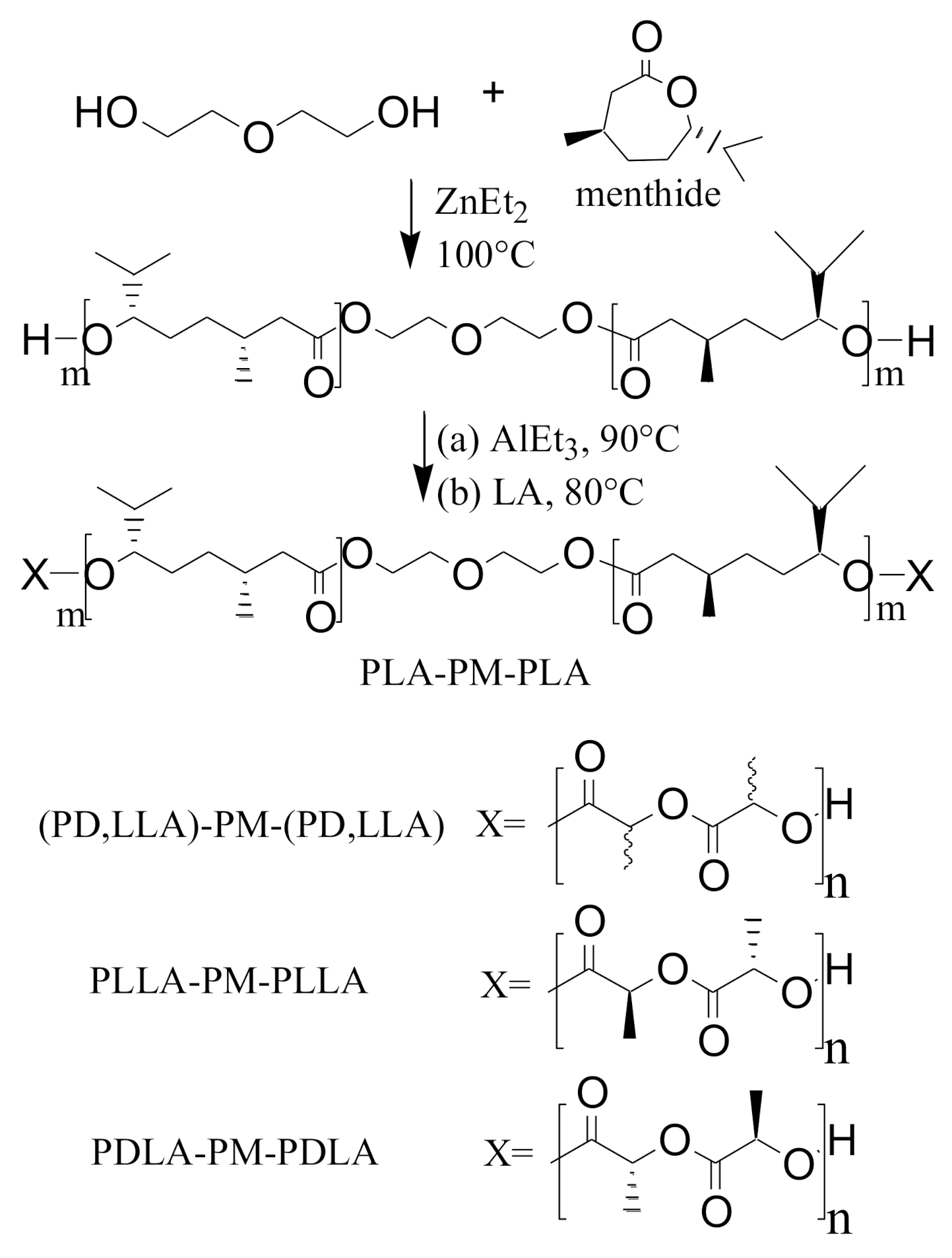
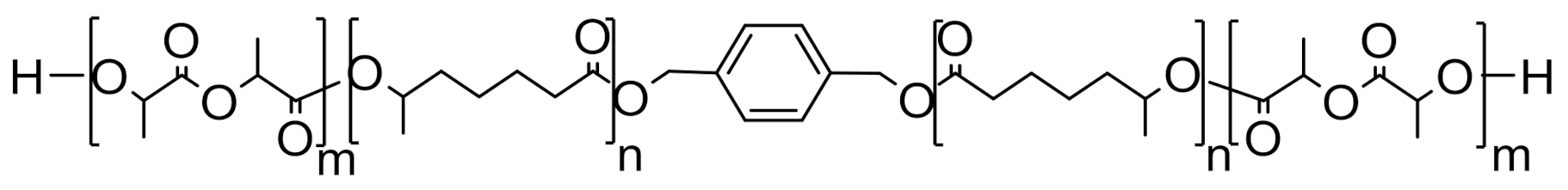

| Material | Passenger Car | Truck and Bus | Off the Road (OTR) |
|---|---|---|---|
| Elastomers | 47 | 45 | 47 |
| Carbon black and silica | 22.5 | 21 | 22 |
| Metal fabrics | 14 | 23.5 | 12 |
| Textiles | 5.5 | 1 | 10 |
| Vulcanization agents | 2.5 | 3 | 3 |
| Additives | 8.5 | 6.5 | 6 |
| Estimated weight of new tire (kg) | 8.5 | 65 | >100 |
| Elastomer | Main Feature |
|---|---|
| Natural Rubber (NR) | Tear resistance/Low rolling resistance |
| Polyisoprene (IR) | Tear resistance |
| Butadiene Rubber (BR) | Abrasion resistance |
| Styrene-Butadiene Rubber (SBR) | Skid resistance |
| Isobutylene Isoprene Rubber (IIR) | Low gas permeability |
| Halogenated Isobutylene Isoprene Rubber (HIIR) | Low gas permeability |
| TPE | Commercial Name | Supplier | Rigid Block | Soft Block | M300% | Tensile Strength | Elongation at Break |
|---|---|---|---|---|---|---|---|
| Polystyrene Content | EB/I/B | ||||||
| (%) | (MPa) | (MPa) | (%) | ||||
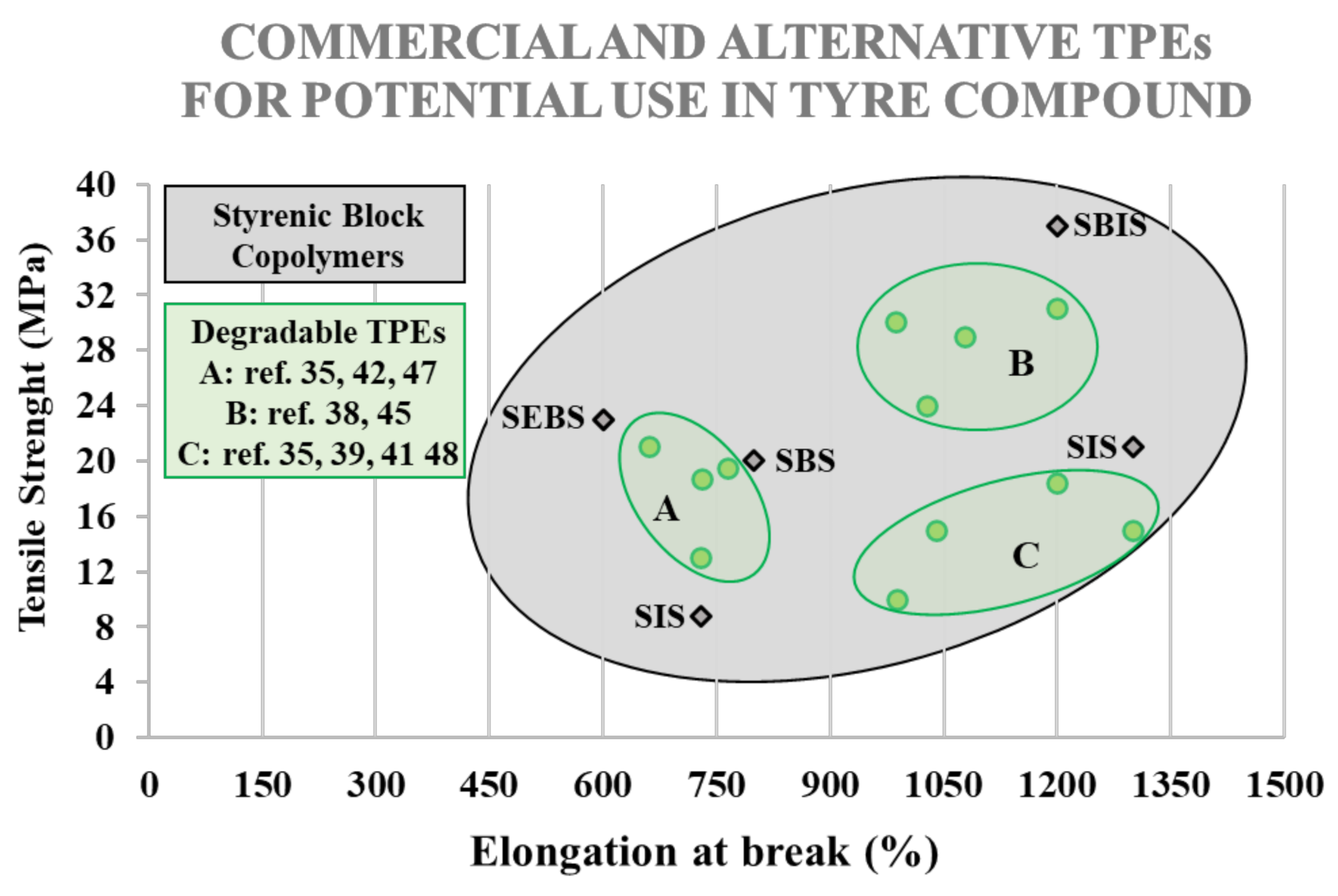
| Styrene and isoprene Vinyl-Rich triblock copolymer (SIS) | Hybrar 5125TM | Kuraray | 20 | 80 | 2.8 | 8.8 | 730 |
| Styrene and isoprene triblock copolymer (SIS) | KRATONTMD1161 | Kraton | 15 | 85 | 0.9 | 21 | 1300 |
| Styrene and butadiene triblock copolymer (SBS) | EUROPRENE®SOL T 166 | Versalis | 30 | 70 | 2.5 | 20 | 800 |
| Styrene, butadiene and isoprene triblock copolymer (SBIS) | KRATONTMD1170 | Kraton | 19 | 81 | 1.4 | 37 | 1200 |
| Hydrogenated Styrenic Thermoplastic Elastomer (SEBS) | S.O.E.TM S1611 | Asahi Kasei | 62 | 38 | 4.0 | 23 | 600 |
| Triblock (Morphology a) | Mn(PLA)-Mn(PI)-Mn(PLA) b (g/mol) | fPLAc | PI PDI d | Block PDI d | De (μm) | Tg(PI)f (K) | Tg(PLA)f (K) |
|---|---|---|---|---|---|---|---|
| Lamellae (L) | 14000-33000-14000 | 0.40 | 108 | 1.10 | 0.0045 | 210.15 | 333.15 |
| Cylinders (C) of PLA | 9000-33000-9000 | 0.28 | 1.08 | 1.12 | 0.0038 | 212.15 | 334.15 |
| Spheres (S) of PLA | 5100-35000-5100 | 0.18 | 1.07 | 1.12 | 0.0029 | 211.15 | 325.15 |
| Hard Block | Soft Block | Tensile Strength (MPa) | Elongation at Break (%) | Reference |
|---|---|---|---|---|
| P(L,L-LA) | Polymenthide | 19.5 | 765 | [41] |
| P(D,L-LA) | Polymenthide | 18.7 | 731 | |
| P(L,L-LA)/P(D,L-LA) | Polymenthide | 10.0 | 990 | |
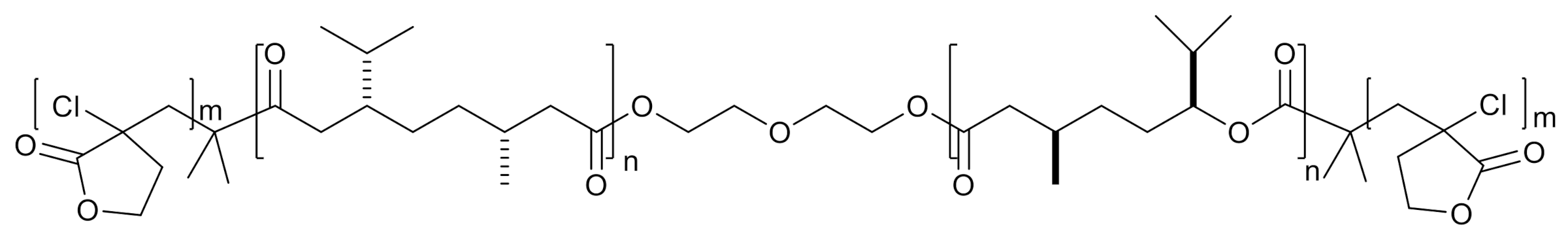
| P(D,L-LA) | Poly(ε-caprolactone-co-ε-decalactone) | 18.4 | 1200 | [59] |
| P(L,L-LA) | Poly(γ-methyl-ε-caprolactone) | 31.0 | 1200 | [44] |
| P(L,L-LA) | Poly(γ-methyl-ε-caprolactone) | 24.0 | 1029 | |
| P(L,L-LA) | Poly(γ-methyl-ε-caprolactone) | 30.0 | 998 | |
| P(L,L-LA)/P(D,L-LA) | Poly(ε-caprolactone -ran-L,L-LA)/Poly(ε- caprolactone -ran-D,L-lactide) | 15.0 | 1300 | [45] |
| [P(L,L-LA)]6 | [Poly(ε-decalactone)]6 | 15.2 | 1041 | [47] |
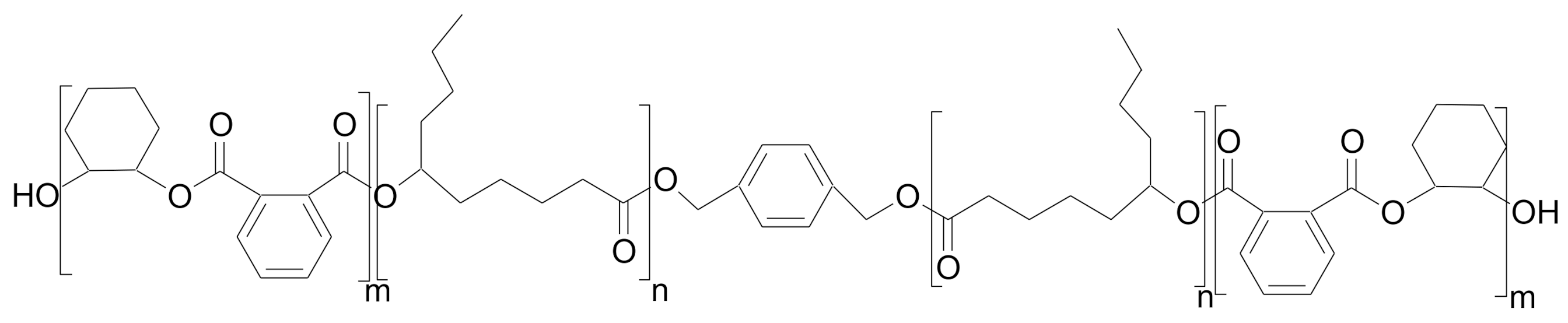
| Poly(cyclohexene-alt-phthalate) | Poly(ε-decalactone) | 29.1 | 1079 | [51] |
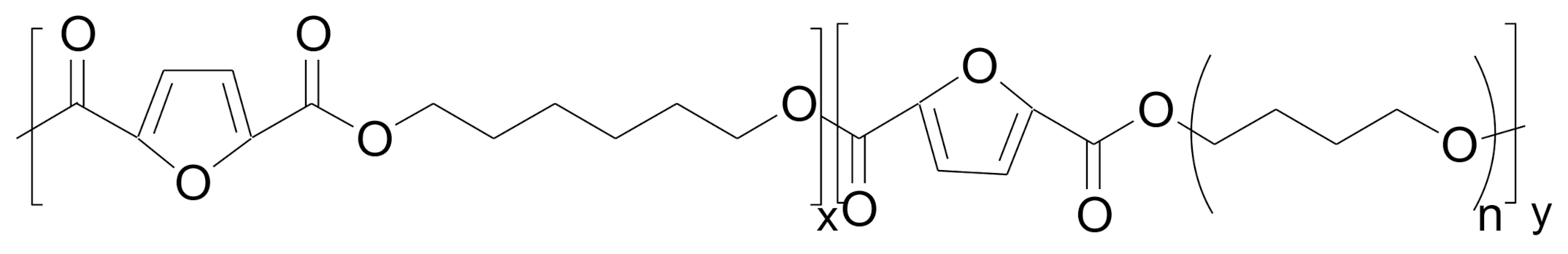
| Poly(hexamethylene 2,5-furanodicarboxylate) | poly(tetrahydrofuran) | 21.8 | 662 | [53] |
| Tulipanin A | Polymenthide | 13.0 | 730 | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naddeo, M.; Viscusi, G.; Gorrasi, G.; Pappalardo, D. Degradable Elastomers: Is There a Future in Tyre Compound Formulation? Molecules 2021, 26, 4454. https://doi.org/10.3390/molecules26154454
Naddeo M, Viscusi G, Gorrasi G, Pappalardo D. Degradable Elastomers: Is There a Future in Tyre Compound Formulation? Molecules. 2021; 26(15):4454. https://doi.org/10.3390/molecules26154454
Chicago/Turabian StyleNaddeo, Marco, Gianluca Viscusi, Giuliana Gorrasi, and Daniela Pappalardo. 2021. "Degradable Elastomers: Is There a Future in Tyre Compound Formulation?" Molecules 26, no. 15: 4454. https://doi.org/10.3390/molecules26154454
APA StyleNaddeo, M., Viscusi, G., Gorrasi, G., & Pappalardo, D. (2021). Degradable Elastomers: Is There a Future in Tyre Compound Formulation? Molecules, 26(15), 4454. https://doi.org/10.3390/molecules26154454









