Unravelling the Phytochemical Composition and the Pharmacological Properties of an Optimized Extract from the Fruit from Prunus mahaleb L.: From Traditional Liqueur Market to the Pharmacy Shelf
Abstract
:1. Introduction
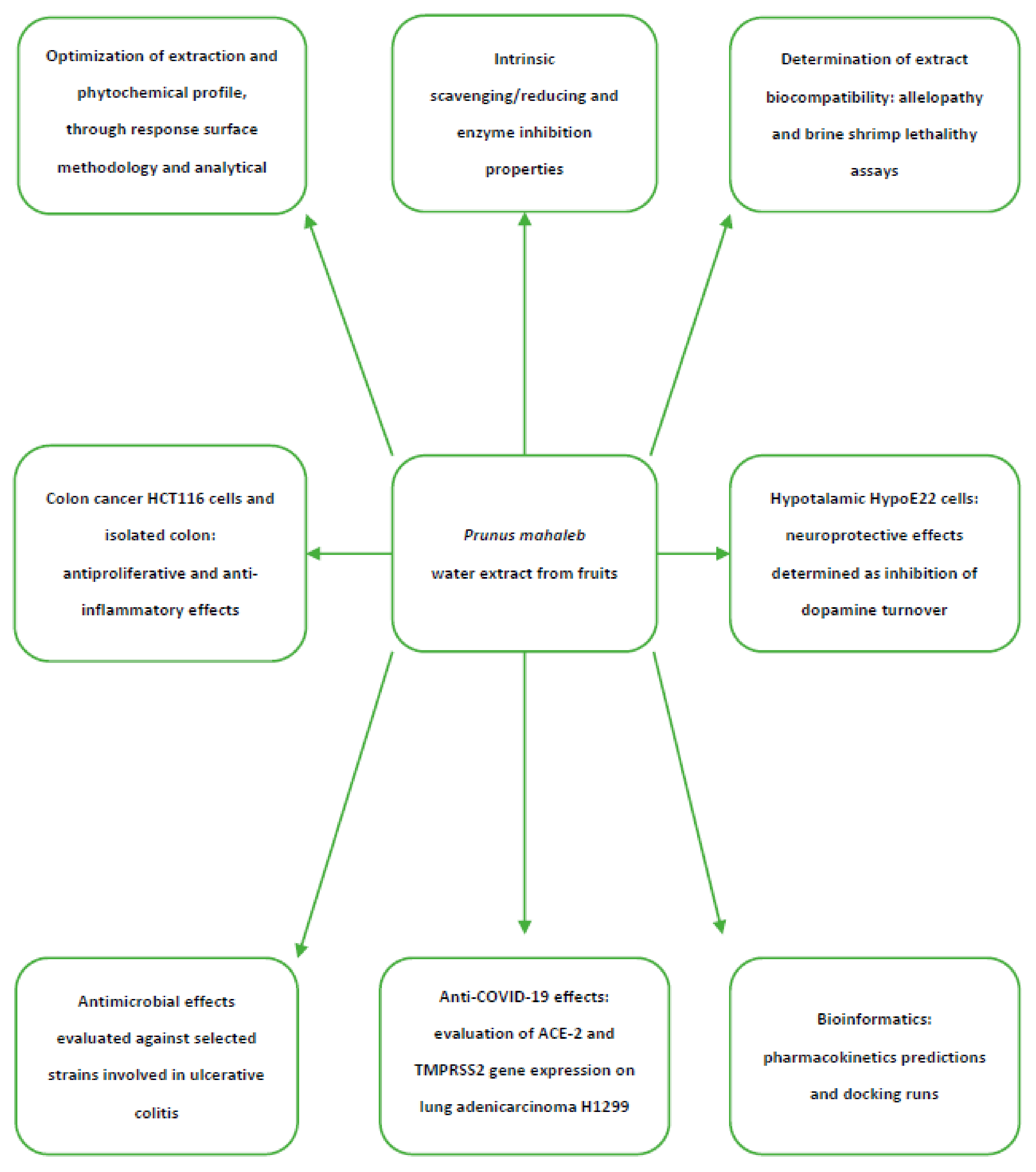
2. Results and Discussion
2.1. Phytochemical Analysis
2.2. Toxicological and Pharmacological Studies
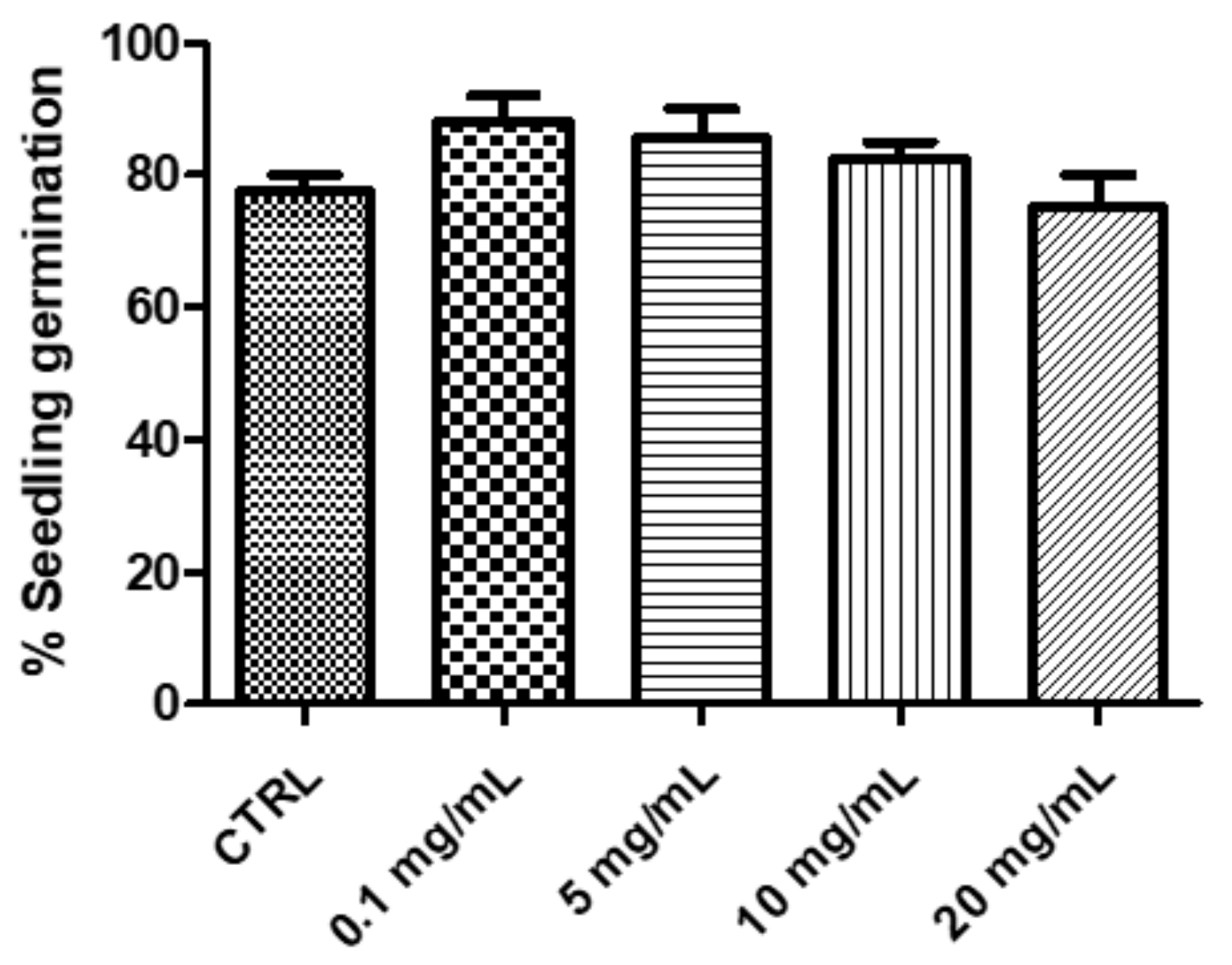
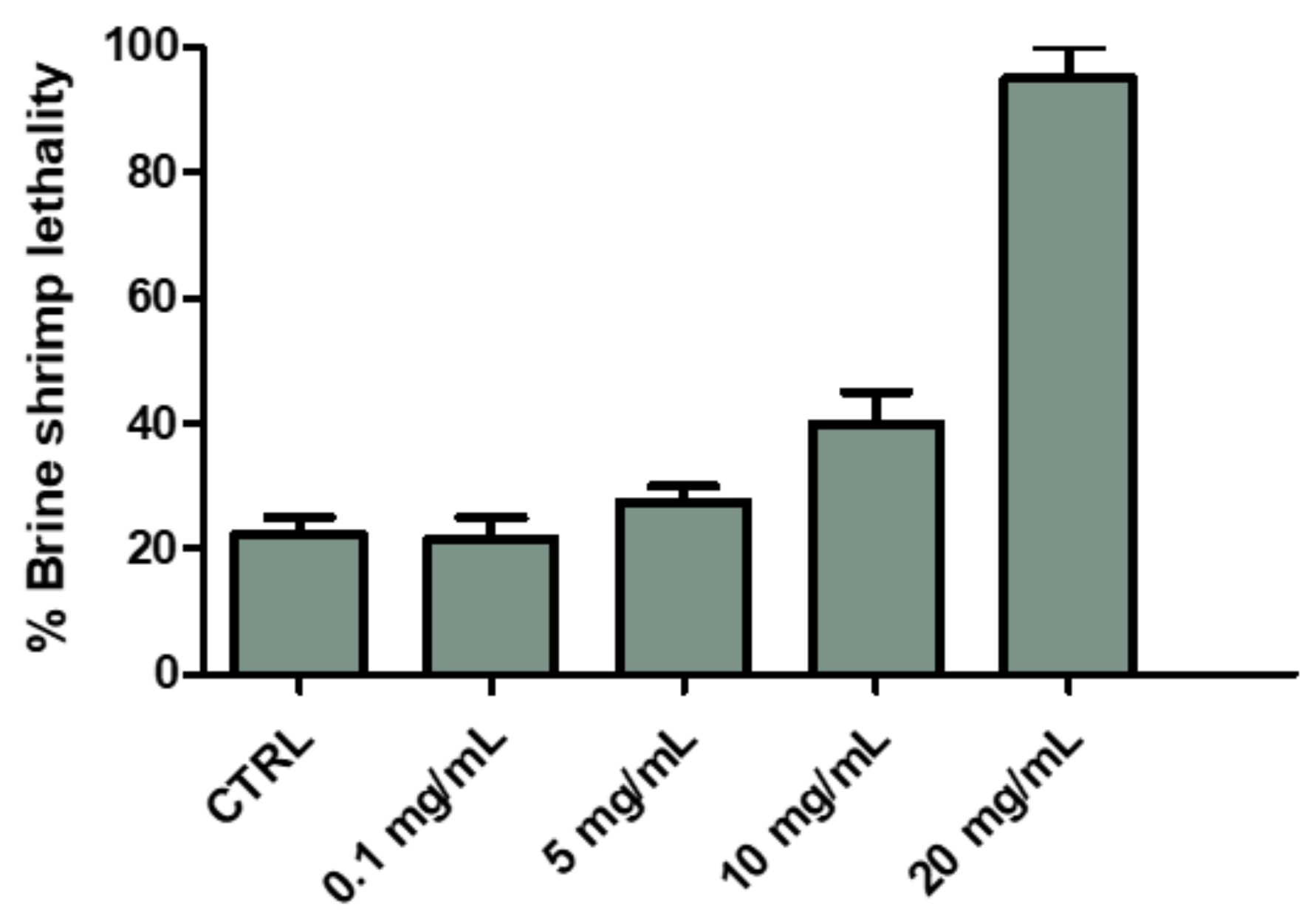
2.2.1. Eco-Toxicological Assays
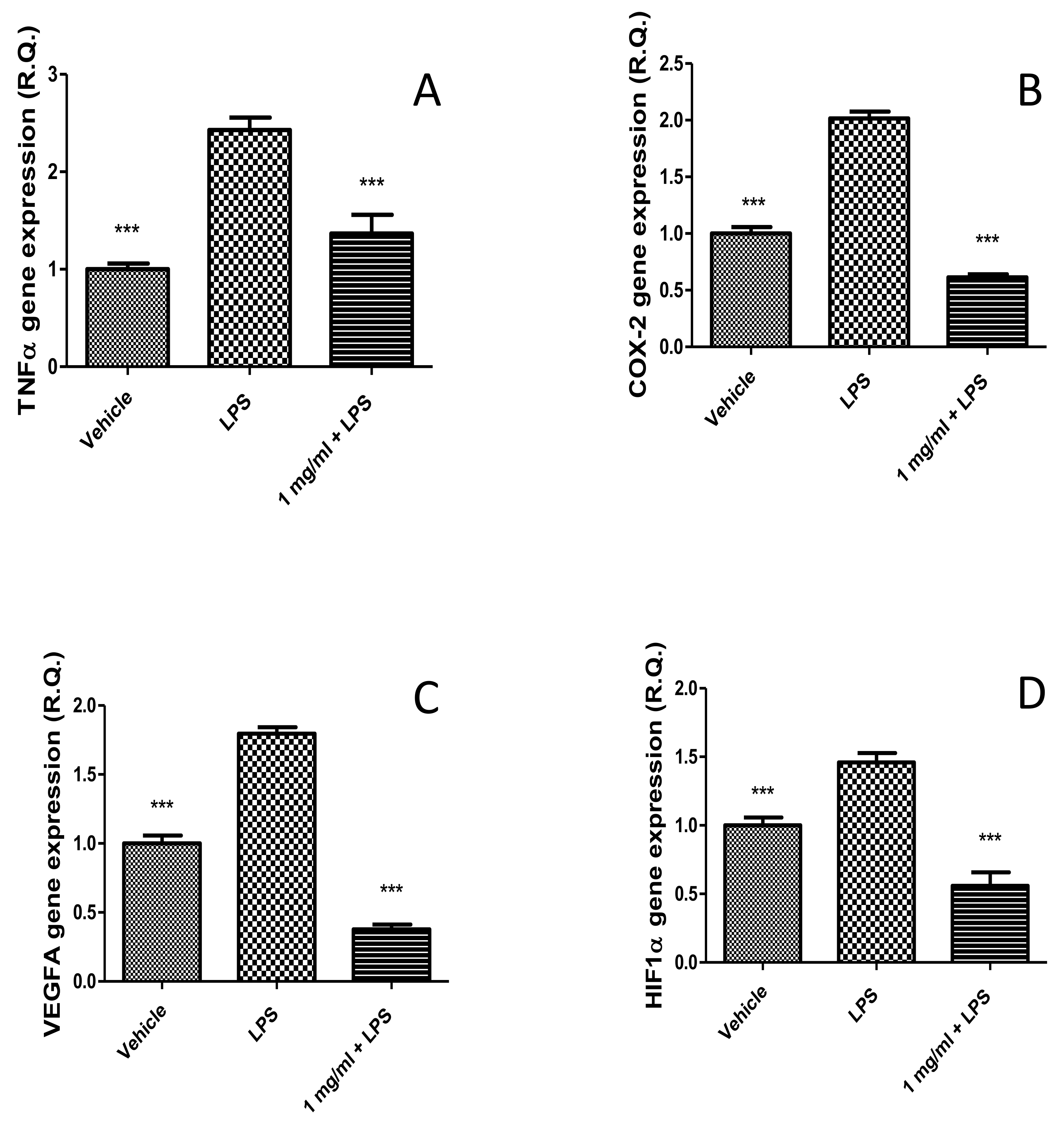
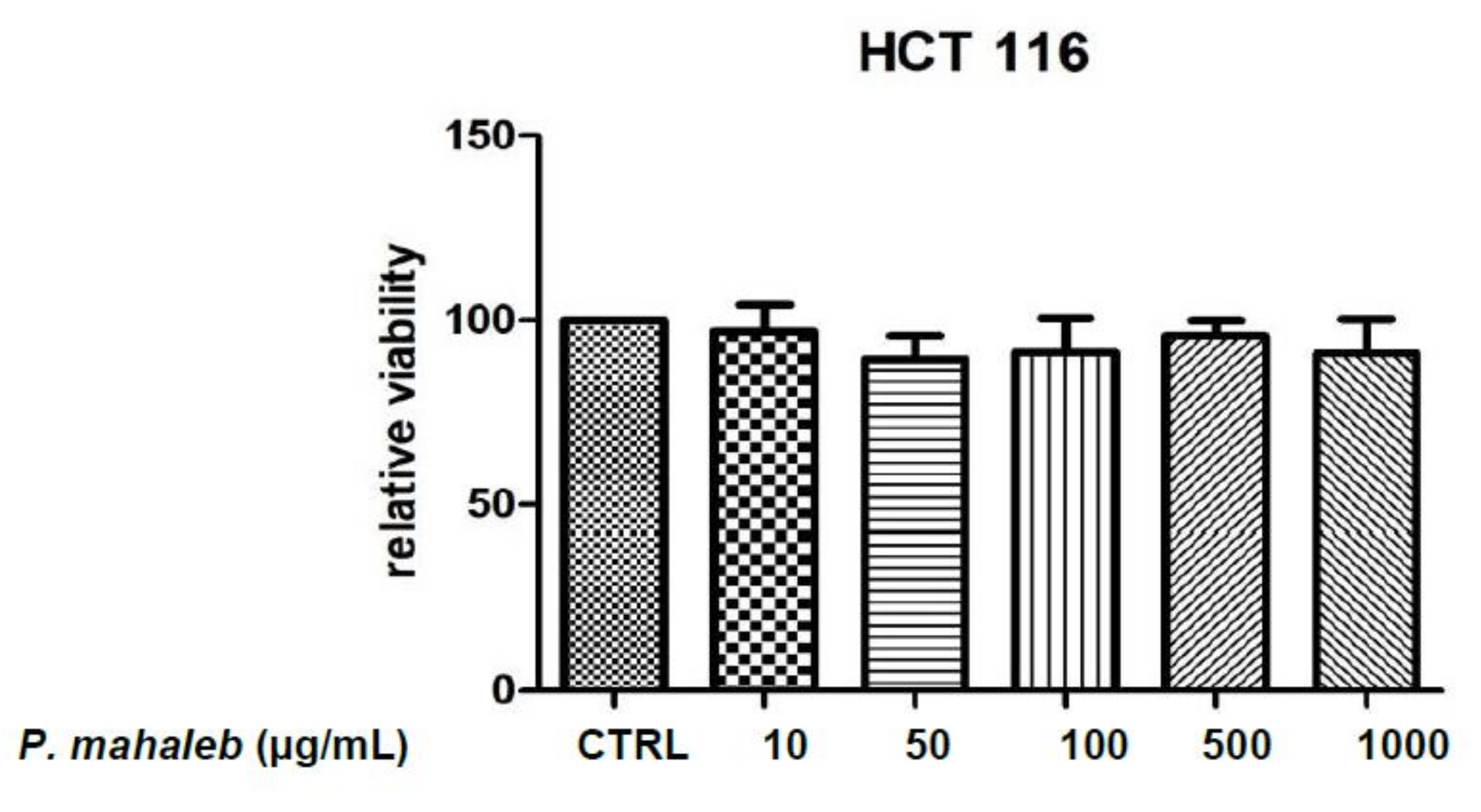
2.2.2. Anti-Inflammatory Effects in the Colon and Antimicrobial Properties
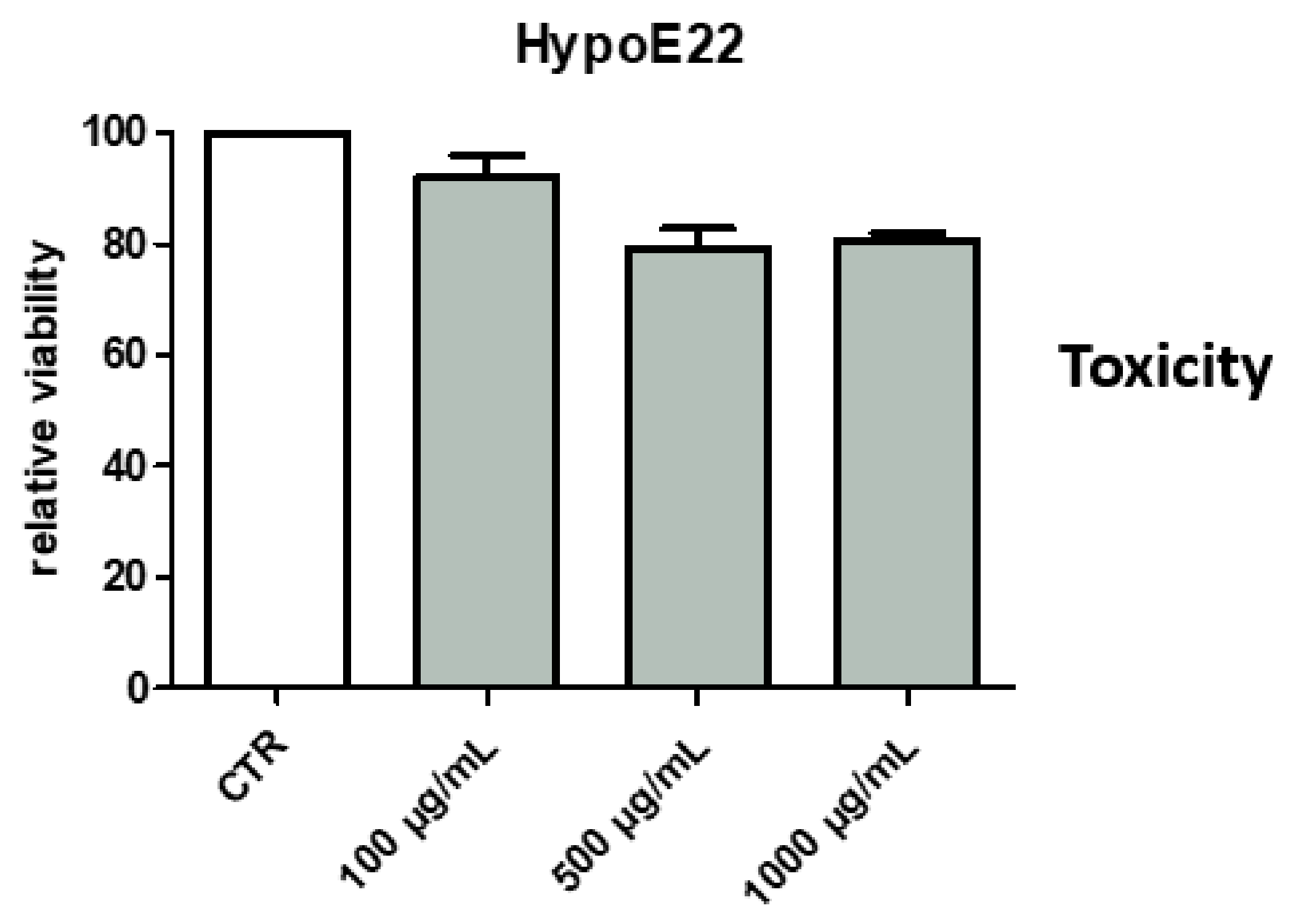
2.2.3. Neuroprotective Effects
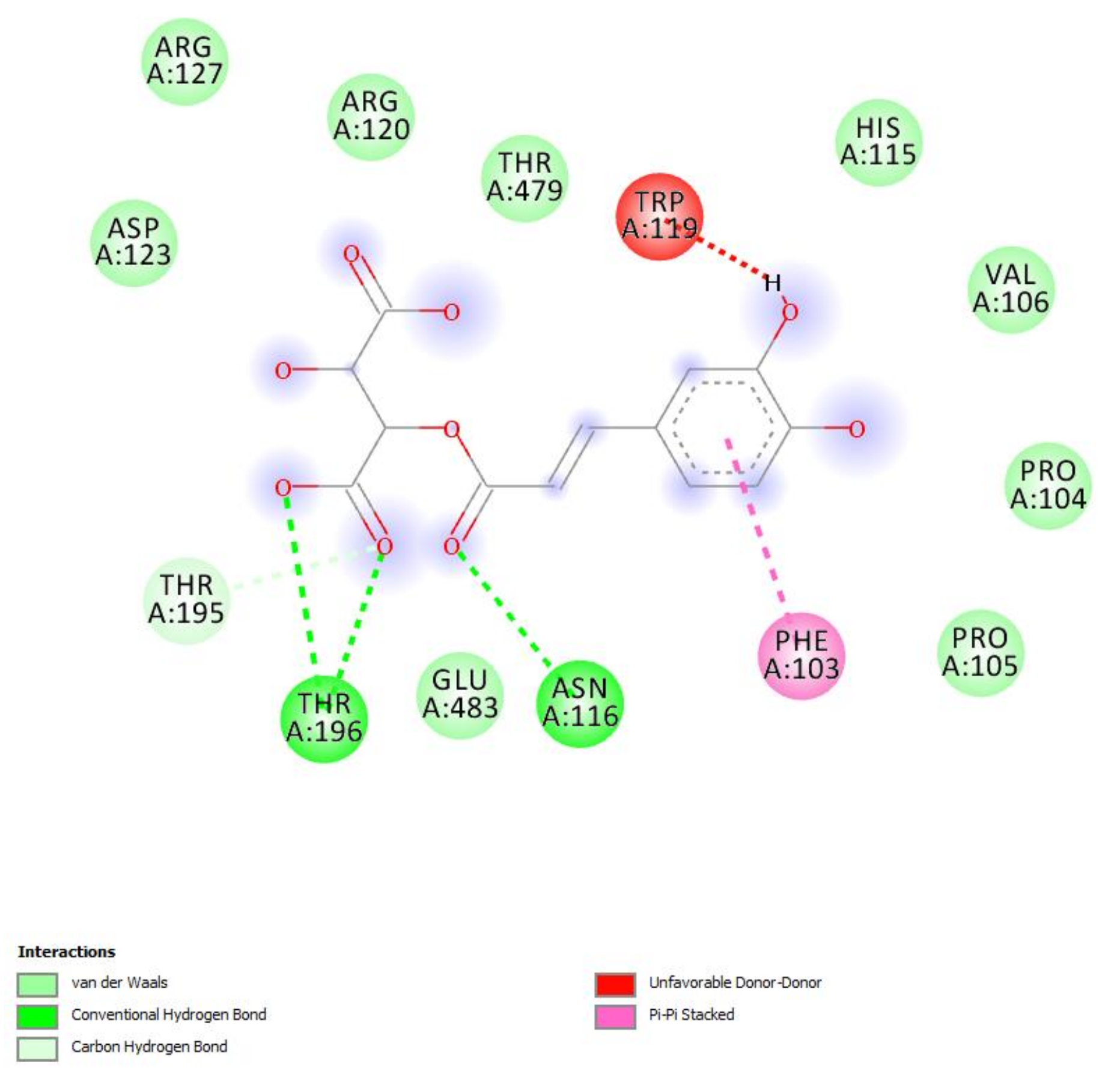
2.2.4. Protective Effects against COVID-19 Infection
4. Materials and Methods
4.1. Plant Material and Reagents
4.2. Response Surface Methodology (RSM)
4.3. Scavenging and Reducing and Enzyme Inhibition Properties
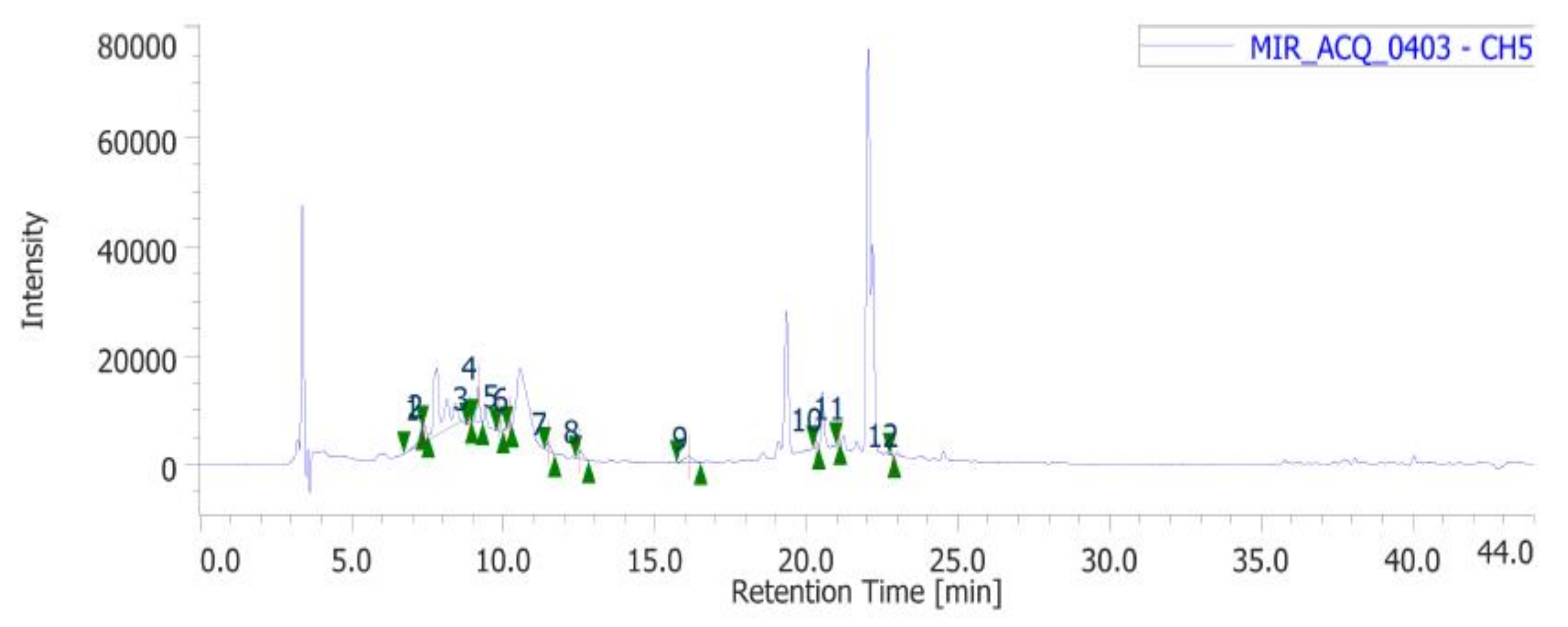
4.4. Phenolic and Flavonoid Determination: Colorimetric and HPLC-DAD-MS Analyses
4.5. Eco-Toxicological Profile: Allelopathy and Artemia salina (brine shrimp) Lethality Assays
4.6. Human Colon Cancer HCT116 Cells: Evaluation of Antiproliferative Effects
4.7. Isolated Mouse Colon Specimens: Evaluation of Anti-Inflammatory Effects
4.8. Hypothalamic HypoE22 Cells: Evaluation of Neuroprotective Effects
4.9. Human H1299 Lung Adenocarcinoma Cell Line: Anti-COVID-19 Effects
4.10. Gene Expression Analysis
4.11. Quantitative Determination of Dopamine (DA), Dihydroxyphenilacetic Acid (DOPAC)
4.12. Antibacterial Effects
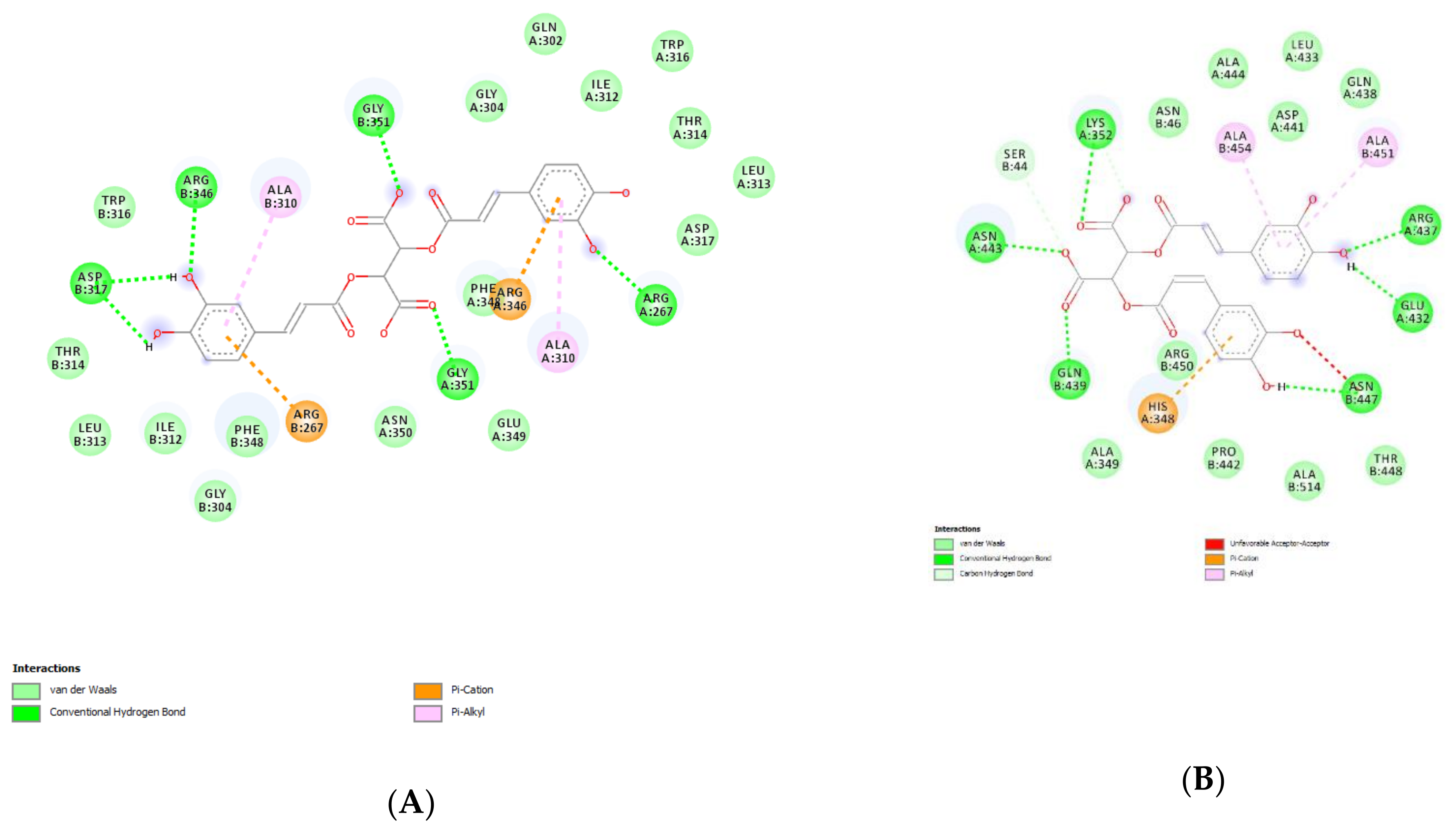
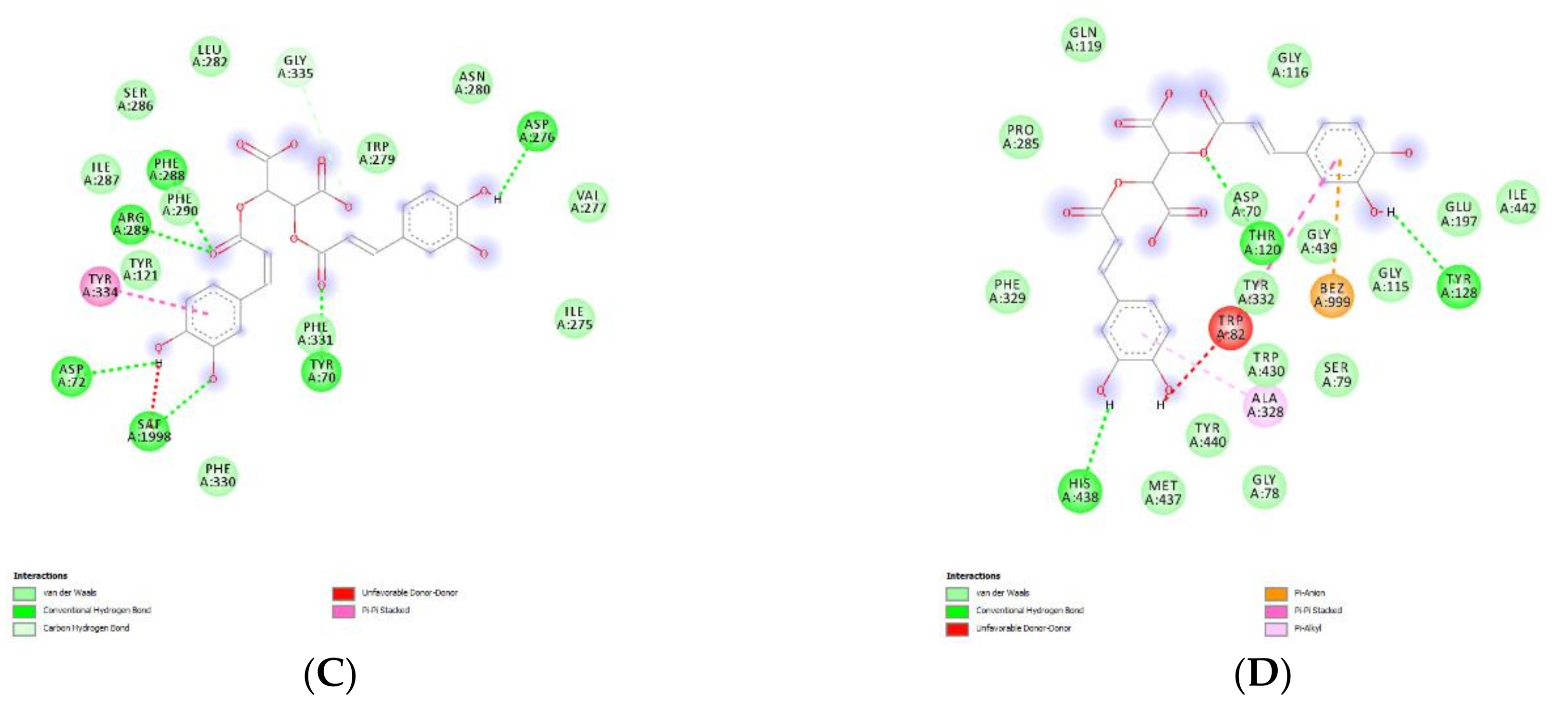
4.13. Bioinformatics
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, X.; Shen, X.; Jiang, D. Complete chloroplast genome sequence of Prunus mahaleb. Mitochondrial DNA Part B 2019, 4, 2204–2205. [Google Scholar] [CrossRef]
- Palasciano, M.; Ferrara, G.; Camposeo, S.; Laghezza, L. Studies on Prunus mahaleb in Apulia. Ital. J. Agron. 2009, 4, 705–708. [Google Scholar]
- Blando, F.; Albano, C.; Liu, Y.; Nicoletti, I.; Corradini, D.; Tommasi, N.; Gerardi, C.; Mita, G.; Kitts, D.D. Polyphenolic composition and antioxidant activity of the under-utilised Prunus mahaleb L. fruit. J. Sci. Food Agric. 2016, 96, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Mariod, A.A.; Ibrahim, R.M.; Ismail, M.; Ismail, N. Antioxidant activities of phenolic rich fractions (PRFs) obtained from black mahlab (Monechma ciliatum) and white mahlab (Prunus mahaleb) seedcakes. Food Chem. 2010, 118, 120–127. [Google Scholar] [CrossRef]
- Al-Said, M.S.; Hifnawy, M.S. Dihydrocoumarin and certain other coumarins from Prunus mahaleb seeds. J. Nat. Prod. 1986, 49, 721. [Google Scholar] [CrossRef]
- Ieri, F.; Pinelli, P.; Romani, A. Simultaneous determination of anthocyanins, coumarins and phenolic acids in fruits, kernels and liqueur of Prunus mahaleb L. Food Chem. 2012, 135, 2157–2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, F.; Dashti, A.; Vahedi, L.; Davoodi, A. Effect of Prunus mahaleb L. Seed Extract on Ethylene glycol-and Ammonium Chloride-Induced Urolithiasis in BALB/c Mice. Iran. J. Med. Sci. 2020, 45, 134. [Google Scholar] [PubMed]
- Abudayyak, M.; Nath, E.Ö.; Özhan, G. Toxic potentials of ten herbs commonly used for aphrodisiac effect in Turkey. Turk. J. Med. Sci. 2015, 45, 496–506. [Google Scholar] [CrossRef]
- di Giacomo, V.; Ferrante, C.; Ronci, M.; Cataldi, A.; Di Valerio, V.; Rapino, M.; Recinella, L.; Chiavaroli, A.; Leone, S.; Vladimir-Knežević, S.; et al. Multiple pharmacological and toxicological investigations on Tanacetum parthenium and Salix alba extracts: Focus on potential application as anti-migraine agents. Food Chem. Toxicol. 2019, 133, 110783. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef]
- Ferramosca, A.; Treppiccione, L.; Di Giacomo, M.; Aufiero, V.R.; Mazzarella, G.; Maurano, F.; Gerardi, C.; Rossi, M.; Zara, V.; Mita, G.; et al. Prunus Mahaleb Fruit Extract Prevents Chemically Induced Colitis and Enhances Mitochondrial Oxidative Metabolism via the Activation of the Nrf2 Pathway. Mol. Nutr. Food Res. 2019, 63, 1900350. [Google Scholar] [CrossRef]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Ben Lagha, A.; LeBel, G.; Grenier, D. Tart cherry (Prunus cerasus L.) fractions inhibit biofilm formation and adherence properties of oral pathogens and enhance oral epithelial barrier function. Phytother. Res. 2020, 34, 886–895. [Google Scholar] [CrossRef]
- Reddy, M.K.; Alexander-Lindo, R.L.; Nair, M.G. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J. Agric. Food Chem. 2005, 53, 9268–9273. [Google Scholar] [CrossRef]
- Šarić, A.; Sobočanec, S.; Balog, T.; Kušić, B.; Šverko, V.; Dragović-Uzelac, V.; Levaj, B.; Čosić, Z.; Šafranko, Ž.M.; Marotti, T. Improved antioxidant and anti-inflammatory potential in mice consuming sour cherry juice (Prunus Cerasus cv. Maraska). Plant Foods Hum. Nutr. 2009, 64, 231–237. [Google Scholar] [CrossRef]
- Bonaventura, M.V.M.D.; Martinelli, I.; Moruzzi, M.; Bonaventura, E.M.D.; Giusepponi, M.E.; Polidori, C.; Lupidi, G.; Tayebati, S.K.; Amenta, F.; Cifani, C.; et al. Brain alterations in high fat diet induced obesity: Effects of tart cherry seeds and juice. Nutrients 2020, 12, 623. [Google Scholar] [CrossRef] [Green Version]
- Ibars, M.; Aragonès, G.; Ardid-Ruiz, A.; Gibert-Ramos, A.; Arola-Arnal, A.; Suárez, M.; Bladé, C. Seasonal consumption of polyphenol-rich fruits affects the hypothalamic leptin signaling system in a photoperiod-dependent mode. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrante, C.; Chiavaroli, A.; Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Brunetti, L.; Petrucci, M.; Politi, M.; Menghini, L.; Leone, S.; et al. Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts. Antibiotics 2020, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Sinan, K.I.; Chiavaroli, A.; Orlando, G.; Bene, K.; Zengin, G.; Cziáky, Z.; Jekő, J.; Mahomoodally, M.F.; Picot-Allain, M.C.N.; Menghini, L.; et al. Evaluation of Pharmacological and Phytochemical Profiles Piptadeniastrum africanum (Hook. f.) Brenan Stem Bark Extracts. Biomolecules 2020, 10, 516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An hydroalcoholic chamomile extract modulates inflammatory and immune response in HT29 cells and isolated rat colon. Phytother. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Bouguen, G.; Chevaux, J.-B.; Peyrin-Biroulet, L. Recent advances in cytokines: Therapeutic implications for inflammatory bowel diseases. World J. Gastroenterol. 2011, 17, 547. [Google Scholar] [CrossRef]
- Chen, X.; Xu, C.; Hong, S.; Xia, X.; Cao, Y.; McDermott, J.; Mu, Y.; Han, J.-D.J. Immune cell types and secreted factors contributing to inflammation-to-cancer transition and immune therapy response. Cell Rep. 2019, 26, 1965–1977. [Google Scholar] [CrossRef] [Green Version]
- Gavrilaş, L.I.; Ionescu, C.; Bălăcescu, O.; Muresan, D.; Revnic, C.; Filip, L.; Miere, D. Intake of plant based foods and colorectal cancer. A case-control study in Romania. Bull. UASVM Food Sci. Technol. 2018, 75, 2. [Google Scholar] [CrossRef] [Green Version]
- Iguidbashian, J.P.; Parekh, J.D.; Kukrety, S.; Andukuri, V.G. Campylobacter jejuni and Pseudomonas coinfection in the setting of ulcerative colitis. Case Rep. 2018, 2018, bcr2018224941. [Google Scholar]
- Sünderhauf, A.; Pagel, R.; Künstner, A.; Wagner, A.E.; Rupp, J.; Ibrahim, S.M.; Derer, S.; Sina, C. Saccharin supplementation inhibits bacterial growth and reduces experimental colitis in mice. Nutrients 2020, 12, 1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, M.K.; Pratap, C.B.; Dixit, V.K.; Singh, T.B.; Shukla, S.K.; Jain, A.K.; Nath, G. Ulcerative colitis and its association with salmonella species. Interdiscip. Persp. Infect. Dis. 2016, 2016, 5854285. [Google Scholar]
- Anil, S.M.; Shalev, N.; Vinayaka, A.C.; Nadarajan, S.; Namdar, D.; Belausov, E.; Shoval, I.; Mani, K.A.; Mechrez, G.; Koltai, H. Cannabis compounds exhibit anti-inflammatory activity in vitro in COVID-19-related inflammation in lung epithelial cells and pro-inflammatory activity in macrophages. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mahrosh, H.S.; Mustafa, G. An in silico approach to target RNA-dependent RNA polymerase of COVID-19 with naturally occurring phytochemicals. Environ. Dev. Sustain. 2021, 1–14. [Google Scholar]
- Orlando, G.; Adorisio, S.; Delfino, D.; Chiavaroli, A.; Brunetti, L.; Recinella, L.; Leone, S.; D’Antonio, M.; Zengin, G.; Acquaviva, A.; et al. Comparative investigation of composition, antifungal, and anti-inflammatory effects of the essential oil from three industrial hemp varieties from Italian cultivation. Antibiotics 2021, 10, 334. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.J.; Hiscox, J.A.; Hooper, N.M. ACE2: From vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004, 25, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Bazrafkan, F.; Zarringhalami, S.; Ganjloo, A. Response surface optimization of conditions for debittering of white mahlab (Prunus mahaleb L.) juice using polystyrene resins. Food Sci. Biotechnol. 2017, 26, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Chatatikun, M.; Supjaroen, P.; Promlat, P.; Chantarangkul, C.; Waranuntakul, S.; Nawarat, J.; Tangpong, J. Antioxidant and Tyrosinase Inhibitory Properties of an Aqueous Extract of Garcinia atroviridis Griff. ex. T. Anderson Fruit Pericarps. Pharmacogn. J. 2020, 12, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Dorababu, A. Critical evaluation of current Alzheimer’s drug discovery (2018–19) & futuristic Alzheimer drug model approach. Bioorg. Chem. 2019, 93, 103299. [Google Scholar]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef]
- Wu, L.; Wu, W.; Cai, Y.; Li, C.; Wang, L. HPLC fingerprinting-based multivariate analysis of phenolic compounds in mango leaves varieties: Correlation to their antioxidant activity and in silico α-glucoidase inhibitory ability. J. Pharm. Biomed. Anal. 2020, 191, 113616. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; Rosa, L.A.; Vargas-Requena, C.L.; Bustos-Jaimes, I.; Alvarez-Parrilla, A.E. Polyphenolic compounds and digestive enzymes: In vitro non-covalent interactions. Molecules 2017, 22, 669. [Google Scholar] [CrossRef] [Green Version]
- Menghini, L.; Leporini, L.; Vecchiotti, G.; Locatelli, M.; Carradori, S.; Ferrante, C.; Zengin, G.; Recinella, L.; Chiavaroli, A.; Leone, S.; et al. Crocus sativus L. stigmas and byproducts: Qualitative fingerprint, antioxidant potentials and enzyme inhibitory activities. Food Res. Int. 2018, 109, 91–98. [Google Scholar] [CrossRef]
- Kondrashev, S.; Nesterova, N.; Luzin, A.; Kochanov, V.; Luzina, A.; Matyushin, A. Qualitative and Quantitative Assay of Hydroxycinnamates of Prunus spinosa L. Pharmacogn. J. 2020, 12, 157–161. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Scagel, C.F. Chicoric acid: Chemistry, distribution, and production. Front. Chem. 2013, 1, 40. [Google Scholar] [CrossRef] [Green Version]
- Jabłońska-Trypuć, A.; Wydro, U.; Wołejko, E.; Świderski, G.; Lewandowski, W. Biological Activity of New Cichoric Acid–Metal Complexes in Bacterial Strains, Yeast-Like Fungi, and Human Cell Cultures In Vitro. Qualitative and Quantitative Assay of Hydroxycinnamates of Prunus Spinosa, L. Nutrients 2020, 12, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Chen, Y.; Shen, C.; Xiao, Y.; Wang, Y.; Liu, Z.; Liu, X. Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-κB. FASEB J. 2017, 31, 1494–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.-L.; Chiu, C.-C.; Chen, J.Y.-F.; Chan, K.-C.; Lin, S.-D. Cytotoxic effects of Echinacea purpurea flower extracts and cichoric acid on human colon cancer cells through induction of apoptosis. J. Ethnopharmacol. 2012, 143, 914–919. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, G.; Liu, Q.; Duan, X.; Liu, Z.; Liu, X. Pharmacokinetics, tissue distribution, and plasma protein binding study of chicoric acid by HPLC-MS/MS. J. Chromatogr. B 2016, 1031, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Adem, Ş.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Zahoor, A.F.; Ali, M.; Abdalla, M.; Ibrahim, I.M.; Elfiky, A.A. Caffeic acid derivatives (CAFDs) as inhibitors of SARS-CoV-2: CAFDs-based functional foods as a potential alternative approach to combat COVID-19. Phytomedicine 2021, 85, 153310. [Google Scholar] [CrossRef]
- Bahar, B.; O’Doherty, J.; Hayes, M.; Sweeney, T. Extracts of brown seaweeds can attenuate the bacterial lipopolysaccharide-induced pro-inflammatory response in the porcine colon ex vivo. J. Anim. Sci. 2012, 90 (Suppl. 4), 46–48. [Google Scholar] [CrossRef]
- Aoki, T.; Narumiya, S. Prostaglandin E 2-EP2 signaling as a node of chronic inflammation in the colon tumor microenvironment. Inflamm. Regener. 2017, 37, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Hummel, D.M.; Fetahu, I.S.; Gröschel, C.; Manhardt, T.; Kállay, E. Role of proinflammatory cytokines on expression of vitamin D metabolism and target genes in colon cancer cells. J. Steroid Biochem. Mol. Biol. 2014, 144, 91–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdocca, M.; De Masi, C.; Pucci, S.; Mango, R.; Novelli, G.; Di Natale, C.; Sangiuolo, F. LOX-1 and cancer: An indissoluble liaison. Cancer Gene Ther. 2021, 1–11. [Google Scholar]
- Owczarek, K.; Lewandowska, U. The impact of dietary polyphenols on COX-2 expression in colorectal cancer. Nutr. Cancer 2017, 69, 1105–1118. [Google Scholar] [CrossRef]
- Lee, J.; Chang, C.; Liu, I.; Chi, T.; Yu, H.; Cheng, J. Changes in endogenous monoamines in aged rats. Clin. Exp. Pharmacol. Physiol. 2001, 28, 285–289. [Google Scholar] [CrossRef]
- Orlando, G.; Chiavaroli, A.; Leone, S.; Brunetti, L.; Politi, M.; Menghini, L.; Recinella, L.; Ferrante, C. Inhibitory effects induced by Vicia faba, Uncaria rhyncophylla, and Glycyrrhiza glabra water extracts on oxidative stress biomarkers and dopamine turnover in HypoE22 cells and isolated rat striatum challenged with 6-hydroxydopamine. Antioxidants 2019, 8, 602. [Google Scholar] [CrossRef] [Green Version]
- Bao, X.-X.; Ma, H.-H.; Ding, H.; Li, W.-W.; Zhu, M. Preliminary optimization of a Chinese herbal medicine formula based on the neuroprotective effects in a rat model of rotenone-induced Parkinson’s disease. J. Integr. Med. 2018, 16, 290–296. [Google Scholar] [CrossRef]
- Ye, S.; Koon, H.K.; Fan, W.; Xu, Y.; Wei, W.; Xu, C.; Cai, J. Effect of a traditional chinese herbal medicine formulation on cell survival and apoptosis of MPP+-treated MES 23.5 dopaminergic cells. Parkinson’s Dis. 2017, 2017, 4764212. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Fang, J.; Chen, P.; Die, Y.; Wang, J.; Liu, Z.; Liu, X. Chicoric acid improves neuron survival against inflammation by promoting mitochondrial function and energy metabolism. Food Funct. 2019, 10, 6157–6169. [Google Scholar] [CrossRef]
- Hussain, M.; Jabeen, N.; Amanullah, A.; Baig, A.A.; Aziz, B.; Shabbir, S.; Raza, F.; Uddin, N. Molecular docking between human TMPRSS2 and SARS-CoV-2 spike protein: Conformation and intermolecular interactions. AIMS Microbiol. 2020, 6, 350. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Crespo, Y.; Radice, M.; Bravo-Sanchez, L.R.; García-Quintana, Y.; Scalvenzi, L. Optimisation of ultrasound-assisted extraction of phenolic antioxidants from Ilex guayusa Loes. leaves using response surface methodology. Heliyon 2020, 6, e03043. [Google Scholar] [CrossRef] [Green Version]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Ceylan, R.; Sinan, K.I.; Ak, G.; Uysal, S.; Mahomoodally, M.F.; Lobine, D.; Aktumsek, A.; Cziáky, Z.; Jeko, J.; et al. Network analysis, chemical characterization, antioxidant and enzyme inhibitory effects of foxglove (Digitalis cariensis Boiss. ex Jaub. & Spach): A novel raw material for pharmaceutical applications. J. Pharm. Biomed. Anal. 2020, 191, 113614. [Google Scholar] [PubMed]
- Recinella, L.; Chiavaroli, A.; di Giacomo, V.; Antolini, M.D.; Acquaviva, A.; Leone, S.; Brunetti, L.; Menghini, L.; Ak, G.; Zengin, G.; et al. Anti-Inflammatory and Neuromodulatory Effects Induced by Tanacetum parthenium Water Extract: Results from In Silico, In Vitro and Ex Vivo Studies. Molecules 2021, 26, 22. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Orlando, G.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; Vacca, M. Central apelin-13 administration modulates hypothalamic control of feeding. J. Biol. Regul. Homeost. Agents 2016, 30, 883–888. [Google Scholar] [PubMed]
- Angelini, P.; Matei, F.; Flores, G.A.; Pellegrino, R.M.; Vuguziga, L.; Venanzoni, R.; Tirillini, B.; Emiliani, C.; Orlando, G.; Menghini, L.; et al. Metabolomic Profiling, Antioxidant and Antimicrobial Activity of Bidens pilosa. Processes 2021, 9, 903. [Google Scholar] [CrossRef]
- Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Tirillini, B.; Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Evaluation of Antioxidant, Antimicrobial and Tyrosinase Inhibitory Activities of Extracts from Tricholosporum goniospermum, an Edible Wild Mushroom. Antibiotics 2020, 9, 513. [Google Scholar] [CrossRef] [PubMed]



| Treatments | DPPH | ABTS | CUPRAC | FRAP | Chelating Ability | PBD |
|---|---|---|---|---|---|---|
| P. mahaleb water extract | 1.16 ± 0.01 | 1.11 ± 0.03 | 1.68 ± 0.09 | 0.97 ± 0.05 | 1.33 ± 0.04 | 2.76 ± 0.09 |
| TROLOX | 0.05 ± 0.01 | 0.08 ± 0.01 | 0.11 ± 0.01 | 0.04 ± 0.01 | nt | 0.60 ± 0.02 |
| EDTA | nt | nt | nt | nt | 0.03±0.01 | nt |
| Treatments | AchE | BChE | Tyrosinase | α-Amylase | α-Glucosidase |
| P. mahaleb water extract | 1.53 ± 0.10 | 1.34 ± 0.05 | 1.28 ± 0.04 | 3.44 ± 0.14 | 1.35 ± 0.04 |
| Galantamine | 0.003 ± 0.0001 | 0.004 ± 0.0001 | nt | nt | nt |
| Kojic acid | nt | nt | 0.08 ± 0.01 | nt | nt |
| Treatments | Bacterial Strains | MIC (µg/mL) |
|---|---|---|
| P. mahaleb water extract | E. coli (ATCC 10 536) | 31.49 (25–50) |
| P. mahaleb water extract | P. aeruginosa (ATCC 15442) | >200 |
| P. mahaleb water extract | B. cereus (ATCC 12826) | >200 |
| Ciprofloxacin | E. coli (ATCC 10536) | <0.12 |
| Ciprofloxacin | P. aeruginosa (ATCC 15442) | 1.23 (1.95–0.98) |
| Ciprofloxacin | B. cereus (ATCC 12826) | 0.62 (0.98–0.49) |
| Independent Variables | Levels | |
|---|---|---|
| −1 | 1 | |
| Time (min) | 5 | 60 |
| Temperature (°C) | 25 | 80 |
| Ethanol percentage | 0 | 100 |
| Solid/liquid (g/mL) | 0.010 | 0.10 |
| Variables | Experimental Results | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conditions | Time (min) | Temp (°C) | Ethanol % | Solid/Liquid (g/mL) | TPC | SD | TFC | SD | TTC | SD |
| 1 | 5 | 52.5 | 50 | 0.01 | 0.198 | 0.035 | 0.044 | 0.001 | 0.184 | 0.005 |
| 2 | 5 | 52.5 | 50 | 0.1 | 0.429 | 0.018 | 0.148 | 0.003 | 0.469 | 0.053 |
| 3 | 60 | 52.5 | 50 | 0.01 | 0.130 | 0.003 | 0.003 | 0.000 | 0.127 | 0.007 |
| 4 | 60 | 52.5 | 50 | 0.1 | 0.658 | 0.023 | 0.197 | 0.006 | 0.591 | 0.032 |
| 5 | 32.5 | 25 | 0 | 0.055 | 0.612 | 0.005 | 0.104 | 0.001 | 0.599 | 0.053 |
| 6 | 32.5 | 25 | 100 | 0.055 | 0.236 | 0.011 | 0.086 | 0.002 | 0.206 | 0.013 |
| 7 | 32.5 | 80 | 0 | 0.055 | 0.648 | 0.082 | 0.096 | 0.002 | 0.645 | 0.048 |
| 8 | 32.5 | 80 | 100 | 0.055 | 0.263 | 0.007 | 0.099 | 0.003 | 0.250 | 0.046 |
| 9 | 32.5 | 25 | 50 | 0.01 | 0.176 | 0.003 | 0.034 | 0.002 | 0.175 | 0.009 |
| 10 | 32.5 | 25 | 50 | 0.1 | 0.447 | 0.013 | 0.119 | 0.001 | 0.437 | 0.024 |
| 11 | 32.5 | 80 | 50 | 0.01 | 0.122 | 0.003 | 0.024 | 0.002 | 0.105 | 0.008 |
| 12 | 32.5 | 80 | 50 | 0.1 | 0.749 | 0.082 | 0.252 | 0.002 | 0.589 | 0.033 |
| 13 | 5 | 52.5 | 0 | 0.055 | 0.568 | 0.011 | 0.099 | 0.001 | 0.587 | 0.060 |
| 14 | 60 | 52.5 | 0 | 0.055 | 0.597 | 0.015 | 0.104 | 0.003 | 0.616 | 0.068 |
| 15 | 5 | 52.5 | 100 | 0.055 | 0.235 | 0.012 | 0.084 | 0.002 | 0.208 | 0.007 |
| 16 | 60 | 52.5 | 100 | 0.055 | 0.243 | 0.002 | 0.084 | 0.002 | 0.182 | 0.006 |
| 17 | 32.5 | 52.5 | 0 | 0.01 | 0.164 | 0.002 | 0.028 | 0.001 | 0.147 | 0.016 |
| 18 | 32.5 | 52.5 | 0 | 0.1 | 0.769 | 0.008 | 0.143 | 0.002 | 0.812 | 0.038 |
| 19 | 32.5 | 52.5 | 100 | 0.01 | 0.078 | 0.005 | 0.019 | 0.001 | 0.105 | 0.034 |
| 20 | 32.5 | 52.5 | 100 | 0.1 | 0.311 | 0.017 | 0.104 | 0.002 | 0.248 | 0.005 |
| 21 | 5 | 25 | 50 | 0.055 | 0.466 | 0.008 | 0.141 | 0.002 | 0.354 | 0.027 |
| 22 | 60 | 25 | 50 | 0.055 | 0.455 | 0.012 | 0.133 | 0.002 | 0.291 | 0.009 |
| 23 | 5 | 80 | 50 | 0.055 | 0.367 | 0.041 | 0.100 | 0.003 | 0.368 | 0.017 |
| 24 | 60 | 80 | 50 | 0.055 | 0.421 | 0.019 | 0.122 | 0.005 | 0.394 | 0.008 |
| 25 | 32.5 | 52.5 | 50 | 0.055 | 0.374 | 0.012 | 0.123 | 0.005 | 0.386 | 0.026 |
| 26 | 32.5 | 52.5 | 50 | 0.055 | 0.531 | 0.016 | 0.161 | 0.001 | 0.332 | 0.012 |
| 27 | 32.5 | 52.5 | 50 | 0.055 | 0.527 | 0.030 | 0.149 | 0.002 | 0.313 | 0.026 |
| Standard | m/z | Wavelengths (nm) | Retention Time (min) |
|---|---|---|---|
| Gallic acid | 169.1 | 254 | 7.303 |
| Catechin | 289.3 | 254 | 9.867 |
| Chlorogenic acid | 353.31 | 254 | 10.203 |
| Epicatechin | 289.3 | 254 | 11.473 |
| Caffeic acid | 179.16 | 254 | 12.533 |
| Chicoric acid | 473.37 | 254 | 16.117 |
| Coumaric acid | 163.04 | 254 | 20.293 |
| Ferulic acid | 193.1 | 254 | 21.033 |
| Rutin | 611.5 | 254 | 22.813 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlando, G.; Chiavaroli, A.; Adorisio, S.; Delfino, D.V.; Brunetti, L.; Recinella, L.; Leone, S.; Zengin, G.; Acquaviva, A.; Angelini, P.; et al. Unravelling the Phytochemical Composition and the Pharmacological Properties of an Optimized Extract from the Fruit from Prunus mahaleb L.: From Traditional Liqueur Market to the Pharmacy Shelf. Molecules 2021, 26, 4422. https://doi.org/10.3390/molecules26154422
Orlando G, Chiavaroli A, Adorisio S, Delfino DV, Brunetti L, Recinella L, Leone S, Zengin G, Acquaviva A, Angelini P, et al. Unravelling the Phytochemical Composition and the Pharmacological Properties of an Optimized Extract from the Fruit from Prunus mahaleb L.: From Traditional Liqueur Market to the Pharmacy Shelf. Molecules. 2021; 26(15):4422. https://doi.org/10.3390/molecules26154422
Chicago/Turabian StyleOrlando, Giustino, Annalisa Chiavaroli, Sabrina Adorisio, Domenico V. Delfino, Luigi Brunetti, Lucia Recinella, Sheila Leone, Gokhan Zengin, Alessandra Acquaviva, Paola Angelini, and et al. 2021. "Unravelling the Phytochemical Composition and the Pharmacological Properties of an Optimized Extract from the Fruit from Prunus mahaleb L.: From Traditional Liqueur Market to the Pharmacy Shelf" Molecules 26, no. 15: 4422. https://doi.org/10.3390/molecules26154422
APA StyleOrlando, G., Chiavaroli, A., Adorisio, S., Delfino, D. V., Brunetti, L., Recinella, L., Leone, S., Zengin, G., Acquaviva, A., Angelini, P., Flores, G. A., Venanzoni, R., Di Simone, S. C., Di Corpo, F., Mocan, A., Menghini, L., & Ferrante, C. (2021). Unravelling the Phytochemical Composition and the Pharmacological Properties of an Optimized Extract from the Fruit from Prunus mahaleb L.: From Traditional Liqueur Market to the Pharmacy Shelf. Molecules, 26(15), 4422. https://doi.org/10.3390/molecules26154422

















