Application of Compound-Specific Isotope Analysis in Environmental Forensic and Strategic Management Avenue for Pesticide Residues
Abstract
:1. Introduction
2. Pesticides and Current Affairs
2.1. Several Problems of Pesticides in History and Chemical Control
2.2. Emerging Problems of Pesticide: Unintended Pollution to Environments, Crops, and Humans
3. Highlighted Approach: Stable Isotope Analysis
3.1. Practical Use of Stable Isotope for Tracing Source
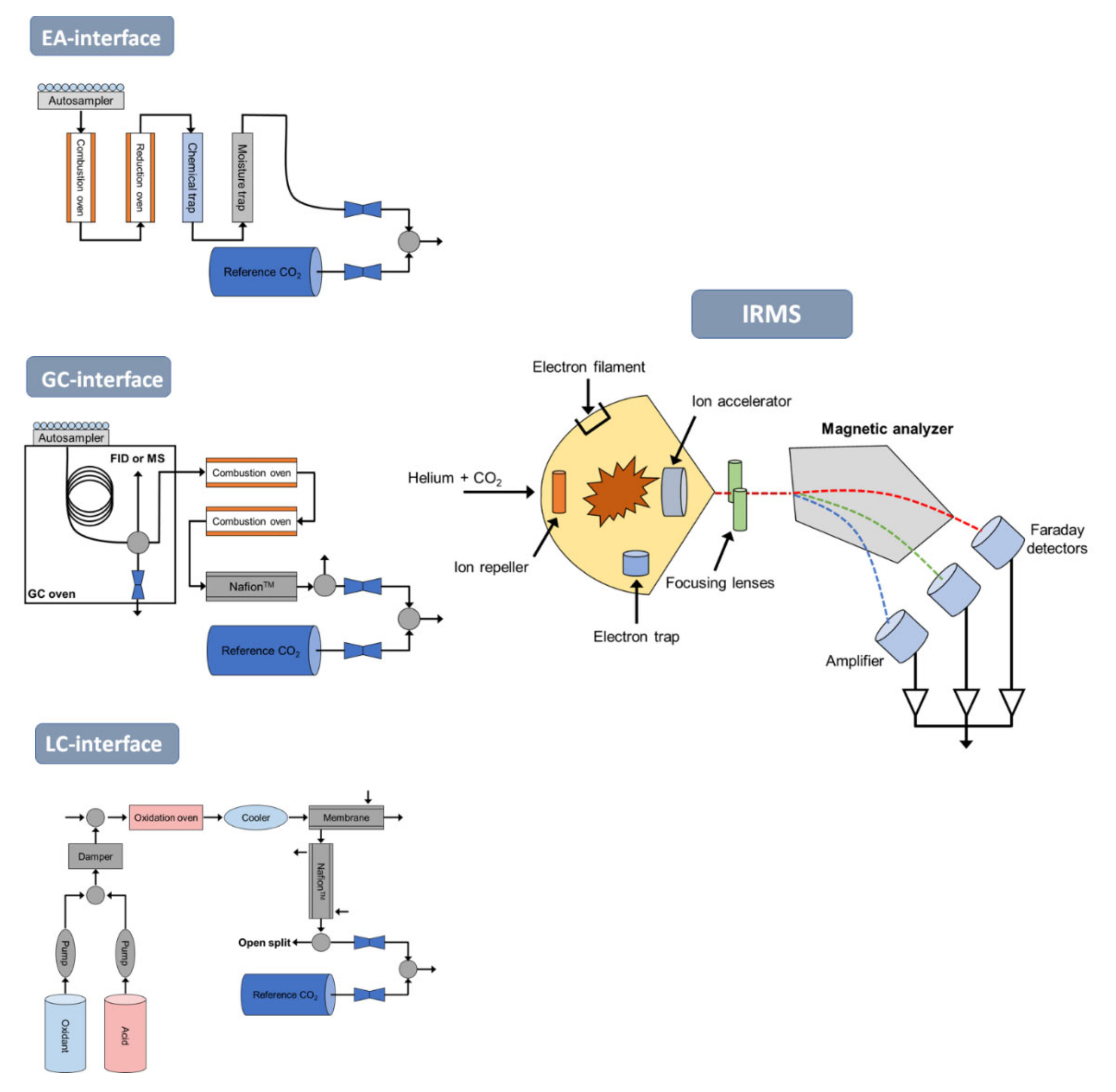
3.2. Applications of Compound-Specific Isotope Analysis (CSIA) to Pollutants in Environmental Sciences
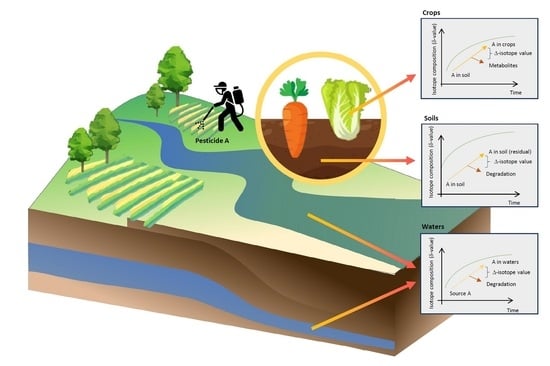
4. Applications of CSIA for Monitor Pesticide Pollution in Environments and Agricultural Products
4.1. Pesticide Tracing Using Isotope of Parental Compounds
4.2. Isotopic Fractionations in Degradations of Pesticides
| Investigated Pesticide | Elements (CSIA) | Study Objectives or Target Mechanisms (for Isotopic Fractionation) | Sample Types (or Field Site) | Special Features | References |
|---|---|---|---|---|---|
| Hexachlorocyclohexane (HCH, Lindane) (OC, insecticide) | C (α-, γ-HCH) | - Reductive dechlorination (Sulfate reducing bacteria) | - Lab experiment (pesticide chemicals itself) | - HCH dechlorination can be monitored in anoxic environments | [113,114] |
| C (α-HCH) | - Biotransformation (Dehalococcoides species) | - Enrichment culture | - The first ESIA study for understanding the biodegradation process of α-HCH | [115] | |
| C (α-HCH) | - Direct and indirect photolysis, alkaline hydrolysis, electro-reduction and reduction by Fe (0) | - Lab experiment (pesticide chemicals itself) | - The study showed discriminate results in chemical and biological transformations. | [106] | |
| C (α-, β- HCH) | - Fate of HCH in environments | - Field sample (sediment, waters, cow milk, plants, etc.) | - The first study using the combination of isotope fractionation and EF to trace the reactive transport processes in the environment, including food web | [110] | |
| Cl, C | - Isotopic values of each compound | - Pesticide product | - The first study applying isotopes, and has strength to provide a baseline for future work employing isotope ratios to study the environmental fate of SVOCs (semi-volatile organochlorine compounds) | [22] | |
| Chlordene (OC, insecticide) | Cl, C | - Isotopic values of each compound | - Pesticide product | ||
| Heptachlor (OC, insecticide) | |||||
| Dieldrin (OC, insecticide) | |||||
| Aldrin (OC, insecticide) | |||||
| Mirex (OC, insecticide) | |||||
| Hexachlorocyclopentadiene | |||||
| Cypermethrin (class II pyrethroid, insecticide) | C | - Isotopic values of different production | - 16 products | - The study shows the possibility as a forensic tool in criminology | [116] |
| Isoproturon (Phenylurea, herbicide) | C, N, H | - Hydrolysis - Biodegradation - Fungal/bacterial—hydroxylation | - Lab experiment (pesticide chemicals itself) - Pure culture experiments | - Studies showed quantifying contaminant degradation using isotope and also showed biotransformation and photolytic reactions of the phenylurea herbicide. | [117,118] |
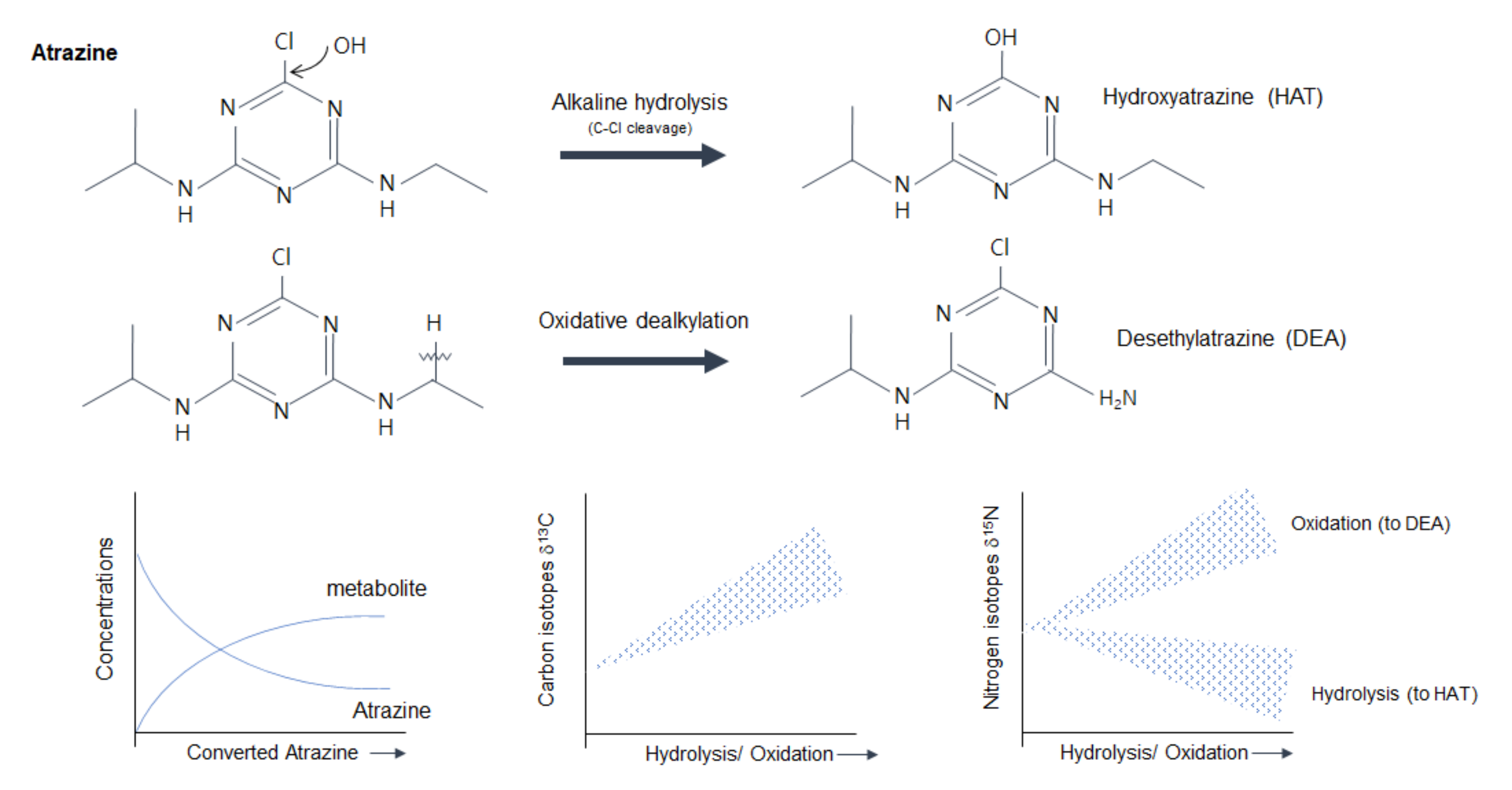
| Atrazine (triazine, herbicide) | C, N, H | - Photolysis (254 nm) | - Lab model system (Surface waters) | - The study provided isotope fractionations that can be used in environments for determining phototransformation pathways. | [105] |
| C, N, H | - Experimental condition - Alkaline hydrolysis | - Lab experiment (pesticide chemicals itself) | - The study found conditions for accurate and precise isotope values in its procedure and provided fractionation of C and N at alkaline hydrolysis. | [23] | |
| C, N, H | - Biodegradation | - Lab experiment (pesticide chemicals itself) | - Different transformation occurred in biotic and abiotic experiments. The mechanistic insights on enzymatic reactions were discussed. | [109] | |
| Cl | - Biodegradation | - Pure culture experiments | - The first study on the fractionation in Cl | [119] | |
| C, N | - Abiotic hydrolysis | - Pesticide standards | - Significant isotopic fraction was measured at alkalic condition (pH 9). | [45] | |
| C, N | - Biodegradation | - Bacteria with atrazine | - Found fractionation for bioavailability | [120] | |
| C, N | - Extraction method | - Drainage waters | - Study suggests solid-phase extraction with consecutive clean-up by HPLC | [46] | |
| C | - Extraction method | - Ground waters | - This study showed the fractionation in an extraction procedure and mentioned the careful method evaluation of sample preparation and sample pretreatment prior to reliable CSIA. | [121] | |
| 2,6-dichlorobenzamide (benzamides, herbicide) | C | - Extraction method | - Ground waters | [121] | |
| C, N | - Fractionations in mesoscale aquifer | - Aqueous samples | - Application study to monitor the reactivity of micropollutants in aquifers and guide future efforts to accomplish CSIA at even lower concentrations | [122] | |
| Diazinon (OP, Insecticide) | C | - Source tracking | - Eight diazinon products and paddy soils | - Carbon isotopes values of diazinon narrow down the candidate products | [43] |
| Alachlor, Acetochlor and Metolachlor (Chloroacetanilide, herbicide) | C, N | - Biodegradation | - Pure culture experiments | - Provide assessing method on the fate of the metabolite (BAM) of pesticide in environments | [108] |
| C, N | - Degradation | - Lab-scale wet land samples | - The first study on isotopic enrichment revealed by biodegradation of chloroacetanilides | [24] | |
| C, N | - Abiotic hydrolysis | - Drainage water | - The study showed different fractionations of pesticides in pH conditions. | [45] | |
| C, N | - Extraction procedure in method | - Tap water/drainage water | - This study validated the SPE approach for large samples to analyze low concentrations of aquatic pesticides. | [46] | |
| S-metolachlor/Acetochlor (Chloroacetanilide, herbicide) | C | - Degradation (field) | - Catchment | - The first study applying isotopes to assess pesticide transformation at catchment scale using a conceptual model | [44] |
| Glyphosate (OP, herbicide) | O (water) | - UV degradation | - Lab experiment (pesticide chemicals itself) | - Confirmation of cleavage through oxygen isotope measurement using EA-gas bench-CF-IRMS | [123] |
| C, N | - Analytical procedure (derivatization) | - Lab experiment (pesticide chemicals itself) | - Showed high precisions of isotope values in LC-IRMS approach | [124] | |
| Dimethoate (OP, insecticide) | C, H | - Photolysis/hydrolysis | - Aqueous sample | - The study for developing a method for the analysis of carbon isotope signatures of three OP pesticides | [111], [125] |
| dichlorobenzene isomers (insecticide) | C | - Anaerobic degradation | - Lab experiment (pesticide chemicals itself) | - Result showed small isotopic fractionation in anaerobic biodegradation and the limitation of CSIA to quantify in situ biodegradation. | [126] |
| Parathion (OP, insecticide) | C | - Hydrolysis - Photolysis | - Lab experiment (pesticide chemicals itself) | - The study showed different fractionations in hydrolysis of parathion according to pH conditions. | [107] |
| Metalaxyl (Acylalanine, fungicide) | C, N | - Abiotic hydrolysis | - Pesticide standard | - Significant isotopic fraction was measured at alkalic condition (pH 9). | [45] |
4.3. Analytical Method and Trials for Residual Pesticides
| Target Compounds | Conventional Platform | Objectives (Purpose of the Method Development) | Samples | Additional Procedures | Platform | Strength Points of New Method | References |
|---|---|---|---|---|---|---|---|
| Amino acids (Methionine) | GC- IRMS | For using source amino acids combined with other source AA (Phe) | 23 terrestrial and coastal organisms | Purification of methionine isolation (HPLC-CAD) | GC-IRMS | Measures low concentrations of Methionine | [134] |
| Amino acids | GC-IRMS GC-IRMS | For high precision | Standard and squid | Purification (Multidimensional HPLC) | EA-IRMS | No derivatization | [127] |
| Ethanol and glucose | EA-IRMS (IC/LC extraction and purification) | To develop the criteria to check brewers’ alcohol and sugar | Sake (Alcohol) | – | LC-IRMS | Less sample volume, Fast analysis | [129] |
| Pesticide—Atrazine | – | To find conditions for accurate and precise isotope values, the types of combustion reactor tube fillings and the effects of Ni/NiO furnace on N isotopes were tested. | Pesticide itself | – | GC-IRMS | Combustion fillings | [23] |
| Pesticide—Atrazine and desethylatrazine | EA-IRMS | To measure the isotope values from a large volume of samples having low concentrations | Groundwaters | SPE and HPLC cleanup | GC- IRMS | HPLC reduces matrix effects, and increases sensitivities by on-column injection | [130] |
| Pesticide—Alachlor, Acetochlor | EA-IRMS | To compare the results of each method and additional extraction procedure efficiency | Waters (Lab-scale wetland water) | SPE extraction procedure | GC- IRMS | High accuracy | [24] |
| Organophosphate Pesticide—Dichlorvos, Omethoate, Dimethoate | EA-IRMS | To develop the method for pesticide from aqueous samples | Aqueous sample | SPE | GC- IRMS | – | [111] |
| Pesticide metabolites—Desphenylchloridazon (metabolite of herbicide chloridazon) | GC-IRMS | For the development of methods applicable to small amounts of environmental samples | Agricultural drainage water | Solid-phase extraction (SPE) approaches | LC-IRMS | Isotope ratios can be accurately measured at analyte concentrations in the sub-μg L−1 range | [46] |
| Pesticide—Atrazine, Desethylatrazine, 2,6-dichlorobenzamide (ng/L)) | GC-IRMS | To get a high precision for low-concentration water samples | Ground waters | SPE with consecutive clean-up by HPLC | GC-IRMS | High accuracy of isotope values from a large volume of water samples by SPE | [121] |
| Pesticide (and Its Metabolite) | Residual Quantification Platform | Platform for CSIA (Target Elements) | Reference | ||
|---|---|---|---|---|---|
| EA-IRMS | GC-IRMS | LC-IRMS | |||
| Atrazine | LC-MS/MS a,b, LC/MS | O | O | O | [23,35,45,46,105,109,120,121,130] |
| Desethylatrazine | SPE-LC-MS/MS a | O | [122] | ||
| Desphenylchloridazon † | GC-MS/MS a | O | O | [46] | |
| DDT | GC-MS/MS b,c | O | [81] | ||
| Heptachlor | GC-MS/MS a,b,c | O | [22] | ||
| Aldrin | GC-MS/MS a,c | O | [22] | ||
| 2,6-dichlorobenzamide | GC-MS/MS a | O | [122] | ||
| Diazinon | GC-MS/MS a,c, LC-MS/MS b | O | O | O | [43,45,46] |
| Dimethoate | LC-MS/MS a,c | O | O | [111] | |
| Parathion | GC-MS/MS a,c, LC-MS/MS b | O | [107] | ||
| Chlordene | GC-platform | O | [22] | ||
| Dieldrin | GC-MS/MS b | O | [22] | ||
| Mirex (Dechlorane) | GC-MS/MS b | O | [22] | ||
| Hexachlorocyclopentadiene (HCCPD) § | GC-platform | O | [22] | ||
| Cypermethrin | GC-MC/MS b,c | O | [116] | ||
| Isoproturon | LC-MS/MS c | O | [117,118] | ||
| Alachlor, Acetochlor, Butachlor, and Metolachlor, S-metolachlor | GC-MS/MS a,c, LC-MS/MS c | O | [24,29,44,45,108] | ||
| Chloroacetanilide | LC-MS/MS d | O | [24] | ||
| Glyphosate | LC- and GC-MS/MS | O | O | O | [123] e [124] f |
| Hexachlorocyclohexane (HCH, Lindane) | GC-FID, -MS/MS | O | [22] | ||
| O | [106,110,113,114,115,132] | ||||
| Dichlobenil (2,6-Dichlorobenzonitrile) | GC-platform | O | [122] | ||
| Acylalanine (Metalaxyl) | LC-MS/MS c | O | O | [45] | |
5. Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- León, V.M.; García-Agüera, I.; Moltó, V.; Fernández-González, V.; Llorca-Pérez, L.; Andrade, J.M.; Muniategui-Lorenzo, S.; Campillo, J.A. PAHs, pesticides, personal care products and plastic additives in plastic debris from Spanish Mediterranean beaches. Sci. Total Environ. 2019, 670, 672–684. [Google Scholar] [CrossRef]
- Diamanti, K.S.; Alygizakis, N.A.; Nika, M.-C.; Oswaldova, M.; Oswald, P.; Thomaidis, N.S.; Slobodnik, J. Assessment of the chemical pollution status of the Dniester River Basin by wide-scope target and suspect screening using mass spectrometric techniques. Anal. Bioanal. Chem. 2020, 412, 4893–4907. [Google Scholar] [CrossRef]
- UNDESA; Stockholm Convention; UNEP. Practices in the Sound Management of Chemicals; United Nations: New York, NY, USA, 2010. [Google Scholar]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassini, P.; Eskridge, K.M.; Cassman, K.G. Distinguishing between yield advances and yield plateaus in historical crop production trends. Nature Comm. 2013, 4, 2918. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cornejo, J.; Nehring, R.; Osteen, C.; Wechsler, S.; Martin, A.; Vialou, A. Pesticide Use in U.S. Agriculture: 21 Selected Crops, 1960-2008, EIB-124, U.S.; Department of Agriculture, Economic Research Service: Washington, DC, USA, 2014.
- UNEP. Stockholm Convention on Persistent Organic Pollutants, Secretariat of the Stockholm Convention Report No.; UNEP: Nairobi, Kenya, 2001. [Google Scholar]
- CDPR, California Department of Pesticide Regulation. Toward Safer and More Sustainable Alternatives to Chlorpyrifos: An Action Plan for California; The Alternatives to Chlorpyrifos Work Group, Ed.; California Department of Pesticide Regulation: Sacramento, CA, USA, 2020.
- DeLorenzo, M.E.; Scott, G.I.; Ross, P.E. Toxicity of pesticides to aquatic microorganisms: A review. Environ. Toxicol. Chem. 2001, 20, 84–98. [Google Scholar] [CrossRef]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Eugenio, N.; McLaughlin, M.; Pennock, D. Soil Pollution: A Hidden Reality; FAO: Roma, Italy, 2018. [Google Scholar]
- Özkara, A.; Akyil, D.; Konuk, M. Pesticides, environmental pollution and health. In Environmental Health Risk—Hazardous Factors to Living Species; Larramendy, M.L., Soloneski, S., Eds.; Intech: Rijeka, Croatia, 2016; pp. 1–27. [Google Scholar]
- Atreya, K.; Sitaula, B.K.; Johnsen, F.H. Continuing issues in the Limitations of Pesticide Use in Developing Countries. J. Agric. Environ. Eth. 2011, 24, 49–62. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.G.J.; Tienstra, M.; Ritsema, G.J.; Geissen, V. Pesticide residues in European agricultural soils- a hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Vander Zanden, M.J.; Casselman, J.M.; Rasmussen, J.B. Stable isotope evidence for the food web consequences of species invasions in lakes. Nature 1999, 401, 464–467. [Google Scholar] [CrossRef]
- Gilbert, P.M.; Middelburg, J.J.; McClelland, J.W.; Vander Zanden, M.J. Stable isotope tracers: Enriching our perspectives and questions on sources, fate, rates, and pathways of major elements in aquatic systems. Limnol. Oceanogr. 2018, 64, 950–981. [Google Scholar] [CrossRef] [Green Version]
- Horii, Y.; Kannan, K.; Petrick, G.; Gamo, T.; Falandysz, J.; Yamashita, N. Congener-specific carbon isotope analysis of technical PCB and PCN mixtures using two-dimensional gas chromatography-isotope ratio mass spectrometry. Environ. Sci. Technol. 2005, 39, 4206–4212. [Google Scholar] [CrossRef] [PubMed]
- Bosch, C.; Andersson, A.; Kruså, M.; Bandh, C.; Hovorková, I.; Klánová, T.D.J.; Pancost, R.D. Source apportionment of polycyclic aromatic hydrocarbons in central European soils with compound-specific triple isotopes (δ13C, δ14C, and δ2H). Environ. Sci. Technol. 2015, 49, 7657–7665. [Google Scholar] [CrossRef] [Green Version]
- Wiederhold, J.G. Metal stable isotope signatures as tracers in environmental geochemistry. Environ. Sci. Technol. 2015, 49, 2606–2624. [Google Scholar] [CrossRef]
- Dreznek, N.J.; Tarr, C.H.; Eglinton, T.I.; Heraty, L.J.; Sturchio, N.C.; Shiner, V.J.; Reddy, C.M. Stable chlorine and carbon isotopic compositions of selected semi-volitle organochlorine compounds. Org. Geochem. 2002, 33, 437–444. [Google Scholar]
- Meyer, A.H.; Penning, H.; Lowag, H.; Elsner, M. Precise and accurate compound specific carbon and nitrogen isotope analysis of atrazine: Critical role of combustion oven condition. Environ. Sci. Technol. 2008, 42, 7757–7763. [Google Scholar] [CrossRef]
- Elsayed, O.F.; Maillard, E.; Vuilleumier, S.; Nijenhuis, I.; Richnow, H.H.; Imfeld, G. Using compound-specific isotope analysis to assess the degradation of chloroacetanilide herbicides in lab-scale wetlands. Chemosphere 2014, 99, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Elsner, M.; Imfeld, G. Compound-specific isotope analysis (CSIA) of micropollutants in the environment—Current developments and future challenges. Curr. Opin. Biotechnol. 2016, 41, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattani, D.; Cavalli, V.L.; De Liz, O.; Rieg, C.E.H.; Domingues, J.T.; Dal-Cim, T.; Tasca, C.I.; Silva, F.R.M.B.; Zamoner, A. Mechanisms underlying the neurotoxicity induced by glyphosate-based herbicide in immature rat hippocampus: Involvement of glutamate excitotoxicity. Toxicology 2014, 320, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Lushchak, V.I.; Marviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. Exp. Clinic. Sci. 2018, 17, 1101–1136. [Google Scholar]
- Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J.; Mejuto, J.-C.; García-Río, L. The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric. Ecosyst. Environ. 2008, 123, 247–260. [Google Scholar] [CrossRef]
- Alvarez-Zaldívar, P.; Payraudeau, S.; Meite, F.; Masbou, J. Pesticide degradation and export losses at the catchment scale: Insights from compound-specific isotope analysis (CSIA). Water Res. 2018, 139, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Al-Saleh, I.A. Pesticides: A review article. J. Environ. Pathol. Toxicol. Oncol. 1994, 13, 151–161. [Google Scholar]
- Mishra, S.; Zhang, W.; Lin, Z.; Pang, S.; Huang, Y.; Bhatt, P.; Chen, S. Carbofuran toxicity and its microbial degradation in contaminated environments. Chemosphere 2020, 259, 127419. [Google Scholar] [CrossRef] [PubMed]
- Drogué, S.; DeMaria, F. Pesticide residues and trade, the apple of discord? Food Policy 2012, 37, 641–649. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. 2006. Available online: https://www.mhlw.go.jp/ (accessed on 29 June 2021).
- Ministry of Food and Drug Safety. White Paper 2016. Available online: https://www.mfds.go.kr/ (accessed on 29 June 2021).
- Annable, W.K.; Frape, S.K.; Shouakar-Stach, O.; Shanoff, T.; Drimmie, R.J.; Harvey, F.E. 37Cl, 15N, 13C isotope analysis of common agro-chemicals for identifying non-point source agricultural contaminants. Appl. Geochem. 2007, 22, 1530–1536. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Safety. Result on Collection and Inspection for Extended Application of Inspection Substances for Eggs Distributed on the Market, Osong; Press Release: Cheongju, Korea, 2017.
- Chen, L.; Yin, L.; Song, F.; Liu, Z.; Zheng, Z.; Xing, J.; Liu, S. Determination of pesticide residues in ginseng by dispersive liquid-liquid microextraction and ultra high performance liquid chromatography-tandem mass spectrometry. J. Chromatogra. B Analyt. Technol. Biomed. Life Sci. 2013, 917, 71–77. [Google Scholar] [CrossRef]
- Willis, G.H.; McDowell, L.L. Pesticide persistence on foliage. Rev. Environ. Contam. Toxicol. 1987, 100, 23–73. [Google Scholar]
- Fantke, P.; Gillespie, B.W.; Juraske, R.; Jolliet, O. Estimating half-lives for pesticide dissipation from plants. Environ. Sci. Technol. 2014, 48, 8588–8602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.Y.; Yang, D.B.; Hong, G.H.; Shin, K.-H. Distribution and bioaccumulation of polychlorinated biphenyls and organochlorine pesticides residues in sediments and Manila clams (Ruditapes philippinarum) from along the Mid-Western coast of Korea. Mar. Pollut. Bull. 2014, 85, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Mitra, J.; Raghu, K. Pesticides-non target plants interactions: An overview. Arch. Agron. Soil Sci. 1988, 43, 445–500. [Google Scholar] [CrossRef]
- Kawashima, H.; Katayama, Y. Source evaluation of diazinon using stable carbon isotope ratio. Environ. Forensic 2010, 11, 363–371. [Google Scholar] [CrossRef]
- Lutz, S.R.; Van Der Velde, Y.; Elsayed, O.F.; Imfeld, G.; Lefrrancq, M.; Payraudeau, S.; Van Breukelen, B.M. Pesticide fate at catchment scale: Conceptual modelling of stream CSIA data. Hydrol. Earth Syst. Sci. 2017, 21, 5243–5261. [Google Scholar] [CrossRef] [Green Version]
- Masbou, J.; Drouin, G.; Payraudeau, S.; Infeld, G. Carbon and nitrogen stable isotope fractionation during abiotic hydrolysis of pesticide. Chemosphere 2018, 213, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Torrentó, C.; Bakkour, R.; Glauser, G.; Melsbach, A.; Ponsin, V.; Hofstetter, T.B.; Elsner, M.; Hunkeler, D. Solid-phase extraction method for stable isotope analysis of pesticides from large volume environmental water samples. Analyst 2019, 144, 2898–2908. [Google Scholar] [CrossRef] [Green Version]
- OECD. Guidance Document on Residues in Rotational Crops, Series on Pesticides and Biocides, No. 97; OECD Publishing: Paris, France, 2018. [Google Scholar] [CrossRef]
- FAO; WHO. Pesticide residues in food 2019—Report 2019. In Proceedings of the Joint FAO/WHO Meeting on Pesticide Residues, Rome, Italy, 13 October 2020. [Google Scholar]
- KREI (Korea Rural Economic Institute). The Introduction of Positive List System and Countermeasures in Agricultural Sector; KREI: Naju-si, Korea, 2019. [Google Scholar]
- Carvalho, F.P.; Fowler, S.W.; Readman, J.W.; Mee, L.D. Pesticide residues in tropical coastal lagoon. Use of 14C labelled compounds to study the cycling and fate of agrochemicals. In Proceedings Series, Proceedings of the International Symposium on Conservation of the Environment, Karlsruhe, Germany, 9–13 March 1992; International Atomic Energy Agency: Vienna, Austria, 1992; pp. 637–653. [Google Scholar]
- Carvalho, F.P.; Gonzalez-Farias, F.; Villeneuve, J.-P.; Cattini, C.; Hernandez-Garza, M.; Mee, L.D. Distribution, fate and effects of pesticide residues in tropical coastal lagoons of the northwest of Mexico. Environ. Technol. 2002, 23, 1257–1270. [Google Scholar] [CrossRef]
- Carvalho, F.P.; Villeneuve, J.P.; Cattini, C.; Rendón, J.; Oliveira. J.M. Pesticide and PCB residues in the aquatic ecosystems of Laguna de Terminos, a protected area of the coast of Campeche, Mexico. Chemosphere 2009, 74, 988–995. [Google Scholar] [CrossRef]
- Müller, K.; Bach, M.; Hartmann, H.; Spiteller, M.; Grede, H.-G. Point and nonpoint source pesticide contamination in the Zwester Ohm catchment, Germany. J. Environ. Qual. 2002, 31, 309–318. [Google Scholar] [CrossRef]
- Munaron, D.; Tapie, N.; Budzinski, H.; Andral, B.; Gonzalez, J.-L. Pharmaceuticals, alkylphenols and pesticides in Mediterranean coastal waters: Results from a pilot survey using passive samplers. Estuar. Coast. Shelf Sci. 2012, 114, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Radajewski, S.; Ineson, P.; Parekh, N.R.; Murrell, J.C. Stable-isotope probing as a tool in microbial ecology. Nature 2000, 403, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Chen, L.; Rabinowitz, J.D. Metabolomics and isotope tracing. Cell 2018, 173, 822–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Evaluation of pesticide residues for estimation of maximum residue levels and calculation of dietary intake. In Training Manual; Livestock and Crop Metabolism Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; Chapter 3. [Google Scholar]
- Cupples, A.M.; Sims, G.K. Identification of in situ 2,4-dichlorophenoxyacetic acid-degrading soil microorganisms using DNA-stable isotope probing. Soil Biol. Biochem. 2007, 39, 232–238. [Google Scholar] [CrossRef]
- Fry, B. Stable isotope diagrams of freshwater food webs. Ecology 1991, 72, 2293–2297. [Google Scholar] [CrossRef]
- Elsner, M. Stable isotope fractionation to investigate natural transformation mechanisms of organic contaminants: Principles, prospects and limitations. J. Environ. Monit. 2010, 12, 2005–2031. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.H. Carbon isotope in photosynthesis. Bioscience 1988, 38, 328–336. [Google Scholar] [CrossRef]
- Hobson, K.A. Tracing origins and migration of wildlife using stable isotopes: A review. Oecologia 1999, 120, 314–326. [Google Scholar] [CrossRef]
- IAEA (International Atomic Energy Agency). Manual for the Use of Stable Isotopes in Entomology; IAEA: Vienna, Austria, 2019. [Google Scholar]
- Hinz, H.; Moranta, J.; Balestrini, S.; Sciberras, M.; Pantin, J.R.; Monnington, J.; Zalewski, A.; Kaiser, M.J.; Sköld, M.; Jonsson, P.; et al. Stable isotopes reveal the effect of trawl fisheries on the diet of commercially exploited species. Sci. Rep. 2017, 7, 6334. [Google Scholar] [CrossRef] [Green Version]
- Trueman, C.N.; Mackenzie, K.M.; Palmer, M.R. Identifying migrations in marine fishes through stable-isotope analysis. J. Fish Biol. 2012, 81, 826–847. [Google Scholar] [CrossRef]
- Suh, Y.J.; Shin, K.-H. Size-related and seasonal diet of the manila clam (Ruditapes philippinarum), as determined using dual stable isotopes. Estuar. Coast. Shelf Sci. 2013, 135, 94–105. [Google Scholar] [CrossRef]
- Chesson, L.A.; Barnette, J.E.; Bowen, G.J.; Brooks, J.R.; Casale, J.F.; Cerling, T.E.; Cook, C.S.; Douthitt, C.B.; Howa, J.D.; Herley, J.M.; et al. Applying the principles of isotope analysis in plant and animal ecology to forensic science in the Americas. Oecologia 2019, 187, 1077–1094. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, L.A.; Moreira, M.Z.; Ommetto, J.P.H.B.; Alcarde, A.R.; Rizzon, L.A.; Stange, E.; Ehleringer, J.R. Stable carbon isotopic composition of the wine and CO2 bubbles of sparkling wines: Detecting C4 sugar additions. J. Agric. Food Chem. 2003, 51, 2625–2631. [Google Scholar] [CrossRef] [PubMed]
- Dindyal, D.; Brizmohun, R.; Fanny, J.O.Y.; Sacchi, E. Assessment of groundwater quality in the Western aquifers of Mauritius using isotope techniques. In Proceedings of the Isotopes in Hydrology, Marine Ecosystems and Climate Change Studies Proceedings of an International Symposium, Monaco, 27 March–1 April 2011; pp. 311–318. [Google Scholar]
- Horacek, M.; Hansel-Hohl, K.; Burg, K.; Soja, G.; Okello-Anyanga, W.; Fluch, S. Control of origin of sesame oil from various countries by stable isotope analysis and DNA based markers—A pilot study. PLoS ONE 2015, 10, e0123020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Taylor, M.P.; Salouros, H.; Prasad, S. Authenticity and geographic origin of global honeys determined using carbon isotope ratios and trace elements. Sci. Rep. 2018, 8, 14639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Hong, S.; Kim, M.-S.; Kim, D.; Choi, B.-J.; Hur, J.; Khim, J.S.; Shin, K.-H. Identification of sources and seasonal variability of organic matter in Lake Shihwa and surrounding inland creeks, South Korea. Chemosphere 2017, 177, 109–119. [Google Scholar] [CrossRef]
- Naito, Y.I.; Chikaraishi, Y.; Ohkouchi, N.; Drucker, D.G.; Bocherens, H. Nitrogen isotopic composition of collagen amino acids as an indicator of aquatic resource consumption: Insights from Mesolithic and Epipalaeolithic archaeological sites in France. World Archaeol. 2013, 45, 338–359. [Google Scholar] [CrossRef]
- Cristiani, E.; Radini, A.; Borić, D.; Robson, H.K.; Caricola, I.; Carra, M.; Mutri, G.; Oxilia, G.; Zupancich, A.; Šlaus, M.; et al. Dental calculus and isotopes provide direct evidence of fish and plant consumption in Mesolithic Mediterranean. Sci. Rep. 2018, 8, 8147. [Google Scholar] [CrossRef]
- Cooper, C.G.; Lupo, K.D.; Zena, A.G.; Schmitt, D.N.; Richards, M.P. Stable isotope ratio analysis (C, N, S) of hair from modern humans in Ethiopia shows clear differences related to subsistence regimes. Archaeol. Anthropol. Sci. 2018, 11, 3213–3223. [Google Scholar] [CrossRef]
- Kim, M.; Kennicutt, M.C., II; Qian, Y. Source characterization using compound composition and stable carbon isotope ratio of PAHs in sediments from lakes, harbor, and shipping waterway. Sci. Total Environ. 2008, 389, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Hirner, A.V.; Lyon, G.L. Stable isotope geochemistry of crude oils and of possible source rocks from New Zealand—1: Carbon. Appl. Geochem. 1989, 4, 109–120. [Google Scholar] [CrossRef]
- Park, Y.-M.; Park, K.-S.; Kim, H.; Yu, S.-M.; Noh, S.; Kim, M.-S.; Kim, J.-Y.; Ahn, J.-Y.; Lee, M.-D.; Seok, K.-W.; et al. Characterizing isotopic compositions of TC-C, NO3—N, and NH4+-N in PM2.5 in South Korea: Impact of China’s winter heating. Environ. Pollut. 2018, 233, 735–744. [Google Scholar] [CrossRef]
- Han, X.; Guo, Q.; Liu, C.; Fu, P.; Strauss, H.; Yang, J.; Hu, J.; Wei, L.; Ren, H.; Peters, M.; et al. Using stable isotopes to trace sources and formation processes of sulfate aerosols from Beijing, China. Sci. Rep. 2016, 6, 29958. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-H.; Luo, X.-J.; Yu, L.-H.; Chen, H.-S.; Wu, J.-P.; Chen, S.-J.; Mai, B.-X. Using compound-specific stable carbon isotope analysis to trace metabolism and trophic transfer of PCBs and PBDEs in fish from and e-waste site, South China. Environ. Sci. Technol. 2013, 47, 4062–4068. [Google Scholar] [CrossRef] [Green Version]
- Niu, L.; Xu, C.; Zhu, S. Enantiomer signature and carbon isotope evidence for the migration and transformation of DDTs in arable soils across China. Sci. Rep. 2016, 6, 38475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier-Augenstein, W.; Fraser, I. Forensic isotope analysis leads to identification of a multilated murder victim. Sci. Justice 2008, 48, 153–159. [Google Scholar] [CrossRef] [PubMed]
- West, J.B.; Hurley, J.M.; Ehleringer, J.R. Stable isotope ratios of marijuana. I. Carbon and nitrogen stable isotopes describe growth conditions. J. Forensic Sci. 2009, 54, 84–89. [Google Scholar] [CrossRef]
- Song, B.-Y.; Gwak, S.; Jung, M.; Nam, G.; Kim, N.Y. Tracing the source of methomyl using stable isotope analysis. Rapid Commun. Mass Specrom. 2018, 32, 235–240. [Google Scholar] [CrossRef]
- Rojas, J.M.M.; Serra, F.; Giani, I.; Moretti, V.M.; Reniero, F.; Guillou, C. The use of stable isotope ratio analyses to discriminate wild and farmed gilthead sea bream (Sparus aurata). Rapid Commun. Mass Spectrom. 2007, 21, 207–211. [Google Scholar] [CrossRef]
- Won, E.-J.; Kim, S.H.; Go, Y.-S.; Kumar, K.S.; Kim, M.-S.; Yoon, S.-H.; Bayon, G.; Kim, J.-H.; Shin, K.-H. A multi-elements isotope approach to assess the geographic provenance of manila clams (Ruditapes philippinarum) via recombining appropriate elements. Foods 2021, 10, 646. [Google Scholar] [CrossRef]
- Ballentine, D.C.; Macko, S.A.; Turekian, V.C.; Gilhooly, W.P.; Martincigh, B. Compound specific isotope analysis of fatty acids and polycyclic aromatic hydrocarbons in aerosols: Implications for biomass burning. Org. Geochem. 1996, 25, 97–104. [Google Scholar] [CrossRef]
- Dempster, H.S.; Lollar, B.S.; Feenstra, S. Tracing organic contaminants in groundwater: A new methodology using compound-specific isotope analysis. Environ. Sci. Technol. 1997, 31, 3193–3197. [Google Scholar] [CrossRef]
- Meckenstock, R.U.; Morasch, B.; Griebler, C.; Richnow, H.-H. Stable isotope fractionation analysis as a tool to monitor biodegradation in contaminated aquifers. J Contam. Hydrol. 2004, 75, 215–255. [Google Scholar] [CrossRef]
- Philp, R.P. The emergence of stable isotopes in environmental and forensic geochemistry studies: A review. Environ. Chem. Lett. 2007, 5, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, T.C.; Zwank, L.; Elsner, M.; Berg, M.; Meckenstock, R.U.; Haderlein, S.B. Compound-specific stable isotope analysis of organic contaminants in natural environments: A critical review of the state of the art, prospects, and future challenges. Anal. Bioanal. Chem. 2004, 378, 283–300. [Google Scholar]
- Muccio, Z.; Jackson, G.P. Isotope ratio mass spectrometry. Analyst 2009, 134, 213–222. [Google Scholar] [CrossRef]
- Kuntze, K.; Eisenmann, H.; Richnow, H.H.; Fischer, A. Compound-specific stable isotope analysis (CSIA) for evaluating degradation of organic pollutants: An overview of field case studies. In Anaerobic Utilization of Hydrocarbons, Oils, and Lipids. Handbook of Hydrocarbon and Lipid Microbiology; Boll, M., Ed.; Springer: Cham, Switzerland, 2020; pp. 323–360. [Google Scholar]
- Yang, J.; Sun, P.; Zhang, X.; Wei, X.-Y.; Huang, Y.-P.; Du, W.-N.; Qadeer, A.; Liu, M.; Huang, Y. Source apportionment of PAHs in roadside agricultural soils of a megacity using positive matrix factorization receptor model and compound-specific carbon isotope analysis. J. Hazard. Mater. 2021, 403, 123592. [Google Scholar] [CrossRef]
- Zwank, L.; Berg, M.; Schmidt, T.C.; Haderlein, S.B. Compound-specific carbon isotope analysis of volatile organic compounds in the low-microgram per liter range. Anal. Chem. 2003, 75, 5575–5583. [Google Scholar] [CrossRef]
- Sigman, D.M.; Casciotti, K.L.; Andreani, M.; Barford, C.; Galanter, M.; Böhlke, J.K. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal. Chem. 2001, 73, 4145–4153. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Dudley, S.; Sun, C.; Schlenk, D.; Gan, J. Stable isotope labeling-assisted metabolite probing for emerging contaminants in plants. Anal. Chem. 2018, 90, 11040–11047. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Rhee, J.S.; Jung, J.M.; Lee, H.S. Pesticide poisoning deaths detected at the national forensic service headquarters in Seoul of Korea: A five-year survey (2005–2009). Environ. Health Toxicol. 2010, 25, 263–271. [Google Scholar]
- Bonvoisin, T.; Utyasheva, L.; Knipe, D.; Gunnell, D.; Eddleston, M. Suicide by pesticide poisoning in India: A review of pesticide regulations and their impact on suicide trends. BMC Public Health 2020, 20, 251. [Google Scholar] [CrossRef] [Green Version]
- Burrows, H.D.; Canle, L.M.; Santaballa, J.A.; Steenken, S. Reaction pathways and mechanisms of photodegradation of pesticides. J. Photochem. Photobiol. B 2002, 67, 71–108. [Google Scholar] [CrossRef] [Green Version]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Env. Sci. Tec. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Sherwood-Lollar, B.; Slater, G.F.; Sleep, B.; Witt, M.; Klecka, G.M.; Harkness, M.; Spivack, J. Stable carbon isotope evidence for intrinsic bioremediation of tetrachloroethene and trichloroethene at area 6, Dover Air Force Base. Environ. Sci. Technol. 2001, 35, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Hunkeler, D.; Meckenstock, R.U.; Lollar, B.S.; Schmidt, T.C.; Wilson, J.T. A Guide for Assessing Biodegradation and Source Identification Of Organic Ground Water Contaminants Using Compound Specific Isotope Analysis (CSIA); US EPA: Washington, DC, USA, 2008.
- Thullner, M.; Richnow, H.-H.; Fischer, A. Characterization and quantification of in situ biodegradation of groundwater contaminants using stable isotope fractionation analysis: Advantages and limitations. In Environmental and Regional Air Pollution: Air, Water and Soil Pollution Science and Technology; Gallo, D., Mancini, R., Eds.; Nova Science Publishers: New York, NY, USA, 2009; pp. 41–81. [Google Scholar]
- Hartenbach, A.E.; Hofstetter, T.B.; Tentscher, P.R.; Canonica, S.; Berg, M.; Schwarzenbach, R.P. Carbon, hydrogen, and nitrogen isotope fractionation during light-induced transformations of atrazine. Environ. Sci. Technol. 2008, 42, 7751–7756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Bashir, S.; Qin, J.; Schindelka, J.; Fischer, A.; Nijenhuis, I.; Herrmann, H.; Wick, L.Y.; Richnow, H.H. Compound specific stable isotope analysis (CSIA) to characterize transformation mechanisms of hexachlorocyclohexane. J. Hazard. Mater. 2014, 280, 750–757. [Google Scholar] [CrossRef]
- Wu, L.; Chládková, B.; Lechtenfeld, O.; Lian, S.; Schindelka, S.; Herrmann, H.; Richnow, H.H. Characterizing chemical transformation of organophosphorus compounds by 13C and 2H stable isotope analysis. Sci. Total Environ. 2018, 615, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Reinnicke, S.; Simonsen, A.; Sørensen, S.R.; Aamand, J.; Elsner, M. C and N isotope fractionation during biodegradation of the pesticide metabolite 2,6-Dichlorobenzamide (BAM): Potential for environmental assessments. Environ. Sci. Technol. 2012, 46, 1447–1454. [Google Scholar] [CrossRef]
- Meyer, A.H.; Penning, H.; Elsner, M. C and N isotope fractionation suggests similar mechanisms of microbial atrazine transformation despite involvement of different enzymes (AtzA and TrZN). Environ. Sci. Technol. 2009, 43, 8079–8085. [Google Scholar] [CrossRef]
- Wu, L.; Lui, Y.; Bajaj, A.; Sharma, M.; Lal, R.; Richnow, H.H. Isotope fractionation approach to characterize the reactive transport processes governing the fate of hexachlorocyclohexanes at a contaminated site in India. Environ. Int. 2019, 132, 105036. [Google Scholar] [CrossRef]
- Wu, L.; Yao, J.; Trabse, P.; Zhang, N.; Richnow, H.H. Compound specific isotope analysis of organophosphorus pesticides. Chemosphere 2014, 111, 458–463. [Google Scholar] [CrossRef]
- Lutz, S.R.; Van Breukelen, B.M. Combined source apportionment and degradation quantification of organic pollutants with CSIA: 1. Model derivation. Environ. Sci. Technol. 2014, 48, 6220–6228. [Google Scholar] [CrossRef]
- Badea, S.-L.; Vogt, C.; Weber, S.; Danet, A.-F.; Richnow, H.-H. Stable isotope fractionation of gamma-hexachloroctclohexane (lindane) during reductive dechlorination by two strains of sulfate-reducing bacterial. Environ. Sci. Technol. 2009, 43, 3155–3161. [Google Scholar] [CrossRef]
- Badea, S.-L.; Vogt, C.; Gehre, M.; Fischer, A.; Danet, A.-F.; Richnow, H.-H. Development of an enantiomer-specific stable carbon isotope analysis (ESIA) method for assessing the fate of α-hexachlorocyclo-hexane in the environment. Rapid Commun. Mass Specrom. 2011, 25, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Fischer, A.; Nijenhuis, I.; Richnow, H.-H. Enantioselective carbon stable isotope fractionation of hexachlorocyclohexane during aerobic biodegradation by Sphingobium spp. Environ. Sci. Technol. 2013, 47, 11432–11439. [Google Scholar] [CrossRef]
- Kawashima, H.; Kariya, T. Use of stable carbon isotope ratios to determine the source of cypermethrin in so-called natural plant extract formulations used for organic farming. Isotopes Environ. Health Stud. 2017, 53, 70–79. [Google Scholar] [CrossRef]
- Penning, H.; Cramer, C.J.; Elsner, M. Rate-dependent carbon and nitrogen kinetic isotope fractionation in hydrolysis of isoproturon. Environ. Sci. Technol. 2008, 42, 7764–7771. [Google Scholar] [CrossRef] [PubMed]
- Penning, H.; Sorensen, S.R.; Meyer, A.H.; Aamand, J.; Elsner, M. C, N, and H isotope fractionation of the herbicide isoproturon reflects different microbial transformation pathways. Environ. Sci. Technol. 2008, 44, 2372–2378. [Google Scholar] [CrossRef] [PubMed]
- Lihl, C.; Heckel, B.; Grybkowska, A.; Defratyka, A.D.; Ponsin, V.; Torrentó Hunkeler, D.; Elsner, M. Compound-specific chlorine isotope fractionation in biodegradation of atrazine. Environ. Sci. Process Impact 2020, 22, 792–801. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, K.; Jha, R.K.; Chen, C.; Yu, H.; Liu, Y.; Ma, L. Isotope fractionations in atrazine degradation reveals rate-limiting, energy-dependent transport across the cell membrane of gram-negative rhizobium sp. CX-Z. Environ. Pollut. 2019, 248, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Melsbach, A.; Pittois, D.; Bayerle, M.; Daubmeier, M.; Meyer, A.H.; Hölzer, K.; Gallé, T.; Elsner, M. Isotope fractionation of micropollutants during large-volume extraction: Heads-up from a critical method evaluation for atrazine, desethylatrazine and 2,6-dichlorobenzamide at low ng/L concentrations in groundwater. Isotopes Environ. Health Stud. 2021, 51, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Schürner, H.K.V.; Maier, M.P.; Eckert, D.; Brejcha, R.; Neumann, C.-C.; Stumpp, C.; Cirpka, O.A.; Elsner, M. Compound-specific stable isotope fractionation of pesticides and pharmaceuticals in a Mesoscale Aquifer. Environ. Sci. Technol. 2016, 50, 5729–5739. [Google Scholar] [CrossRef] [PubMed]
- Sandy, E.H.; Blake, R.E.; Chang, S.J.; Jun, Y.; Yu, C. Oxygen isotope signature of UV degradation of glyphosate and phosphonoacetate: Tracing sources and cycling of phosphonates. J. Hazard. Mater. 2013, 260, 947–954. [Google Scholar] [CrossRef]
- Mogusu, E.O.; Wolbert, J.B.; Kujawinski, D.M.; Jochmann, M.A.; Elsner, M. Dual element (15N/14N, 13C/12C) isotope analysis of glyphosate and AMPA by derivatization—gas chromatography isotope ratio mass spectrometry (GC/IRMS) combined with LC/IRMS. Anal. Bioanal. Chem. 2015, 407, 5249–5260. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Kummel, S.; Richnow, H.H. Validation of GC-IRMS techniques for delta 13C and delta 2H CSIA of organophosphorus compounds and their potential for studying the mode of hydrolysis in the environment. Anal. Bioanal. Chem. 2017, 409, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Mundle, S.O.C.; Nelson, J.L.; Passeport, E.; Chan, C.C.H.; Lacrampe-Couloume, G.; Zinder, S.H.; Lollar, B.S. Distinct carbon isotope fractionation during anaerobic degradation of dichlorobenzene isomer. Environ. Sci. Technol. 2014, 48, 4844–4851. [Google Scholar] [CrossRef]
- Sun, Y.; Ishikawa, N.F.; Ogawa, N.O.; Kawahata, H.; Takano, Y.; Ohkouchi, N. A method for stable carbon isotope measurement of underivatized individual amino acids by multi-dimensional high-performance liquid chromatography and elemental analyzer/isotope ratio mass spectrometry. Rapid Commun. Mass Specrom. 2020, 34, e8885. [Google Scholar] [CrossRef]
- Melsbach, A.; Ponsin, V.; Torrentó, C.; Lihl, C.; Hofstetter, T.B.; Hunkeler, D.; Elsner, M. 13 C- and 15 N-Isotope Analysis of Desphenylchloridazon by Liquid Chromatography-Isotope-Ratio Mass Spectrometry and Derivatization Gas Chromatography-Isotope-Ratio Mass Spectrometry. Anal. Chem. 2019, 91, 3412–3420. [Google Scholar] [CrossRef] [Green Version]
- Suto, M.; Kawashima, H. Compound specific carbon isotope analysis in sake by LC/IRMS and brewers’ alcohol proportion. Sci. Rep. 2019, 9, 17635. [Google Scholar] [CrossRef]
- Schreglmann, K.; Hoeche, M.; Stainbeiss, S.; Reinnicke, S.; Elsner, M. Carbon and nitrogen isotope analysis of atrazine and desethylatrazine at sub-microgram per liter concentrations in groundwater. Anal. Bioannal. Chem. 2013, 405, 2857–2867. [Google Scholar] [CrossRef]
- Wang, X.; Qi, P.; Wang, W.; Zhang, Q.; Wang, Z.; Xu, X.; Xu, H.; Zhang, H.; Wang, Q. An efficient cleanup method coupled with gas chromatography and mass spectrometry for multi pesticides reidue analysis in complex plant matrices. J. Sep. Sci. 2019, 40, 2438–2450. [Google Scholar] [CrossRef]
- Alder, L.; Greulich, K.; Kempe, G.; Vieth, B. Residue analysis of 500 high priority pesticides: Better by GC-MS or LC-MS/Ms? Mass Spectrom. Rev. 2006, 25, 838–865. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.; Richnow, H.H.; Lal, R. Compound-specific stable isotope analysis: Implications in hexachlorocyclohexane in-vitro and field assessment. Indian J. Microbiol. 2017, 57, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, N.F.; Chikaraishi, Y.; Takano, Y.; Sasaki, Y.; Takizawa, Y.; Tsuchiya, M.; Tayasu, I.; Nagata, T.; Ohkouchi, N. A new analytical method for determination of the nitrogen isotopic composition of methioneine: Its application to aquatic ecosystems with mixed resources. Limnol. Oceanogr. Methods 2018, 16, 607–620. [Google Scholar] [CrossRef] [Green Version]
- Asanuma, L.; Miller, D.; Jordan, R.; Churley, M.; Macherone, A. A Novel Comprehensive Strategy for Residual Pesticide Analysis in Cannabis Flower; Agilent Technologies: Santa Clara, CA, USA, 2018. [Google Scholar]
- Almeida, M.O.; Oloris, S.C.S.; Faria, V.H.F.; Ribeiro, M.C.M.; Cantini, D.M.; Soto-Blanco, B. Optimization of method for pesticide detection in honey by using liquid and gas chromatography coupled with mass spectrometric detection. Foods 2020, 9, 1368. [Google Scholar] [CrossRef]
- Rathod, H.N.; Mallappa, B.; Sidramappa, P.M.; Vennapusa, C.S.R.; Kamin, P.; Nidoni, U.R.; Desai, B.R.K.R.; Rao, S.N.; Mariappan, P. Determination of 77 multicalss pesticides and their metabolites in Capsicum and Tomato using GC-MS/MS and LC-MS/MS. Molecules 2021, 26, 1837. [Google Scholar] [CrossRef]
- EPA. Measurement of Chloroacetanilide and Other Acetamide Herbicide Degradates in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS), Method 535; U.S. Environmental Protection Agency: Washington, DC, USA, 2005.
| Study Field | Objectives of Analysis | Target Compound | Elements | Highlights | Reference |
|---|---|---|---|---|---|
| Environmental | - To define sources of pollutants | - Organic matter | C, N | - Stable isotope values of carbon and nitrogen were clearly distinguished between anthropogenic sources in an artificial lake | [72] |
| - PAH | C (PAHs) | - A study found the source of PAH in urban lake sediments | [76] | ||
| - Oil | C | - The results of the aromatic fraction and the isotopic type curves showed the source of oils | [77] | ||
| - PM2.5 (air samples) | C, N (NOx), S | - Result showed that coal combustion-related isotopic patterns increased during China’s winter heating period. | [78] | ||
| - Aerosol (Sulfate) | S, O | - The mean δ34S value of aerosol sulfate is similar to that of coal from North China, indicating that coal combustion is a significant contributor to atmospheric sulfate. | [79] | ||
| - Pesticide | C | - Carbon isotope values of 8 different products were compared with the 13C values of diazinon in paddy soil | [43] | ||
| - PCB | C | - Found correlations between PCB and PBDE in sediment and fish and showed the origins and its transfers in aquatic environments using δ13C values of each compound | [80] | ||
| - DDT (in soil) | C (o,p’-DDT) | - Interpret the results of DDT inflow through air transport | [81] | ||
| Criminology | - To distinguish diet and habitat characteristics of the corpse | - Tissue/bone/hair | C, H | - Relations between diet/product and geographic location made it possible to identify the murder victim or crime | [82] |
| - To find distribution route of marijuana | - Product | C, N | - The source of the sample is classified through the results showing differences in the cultivation environment, such as indoor growth | [83] | |
| - To get the information of criminal evidence (poison, crime tool, etc.) | - Poisons (methomyl) | C (methomyl) | - Identifying the matching isotope values among the chemicals held by the accused. | [84] | |
| Archeology | - To reconstruct dietary information from paleo-people (bone) and understand social and socioeconomic status and life history | - Bone | N (collagen-amino acid) | - The trials to evaluate freshwater resource consumption using amino acid nitrogen isotope values | [73] |
| - Micro-debris in dental calculus | C, N | - The study showing direct evidence of fish and plant consumption in Mesolithic Mediterranean | [74] | ||
| - Hair | C, N, S | - Shows the dietary form and economic situation that is distinct in this region of Ethiopia | [75] | ||
| Ecology | - To reconstruct food source, trophic guild, and energy chain in target ecosystem | - Tissue | C, N | - Effect of species invasion on food web structure and trophic level, understood as a stable isotope ratio | [17] |
| - To find origin (habitat) and migration of wildlife | - Tissue | C, N, S, H, Sr | - Integrated research on the mobility of wild animals using spatially reflected isotope signatures based on various biogeochemical processes | [62] | |
| Food Science | - To prevent food fraud by distinguishing geographical and source origin (fish/ shellfish/livestock product/honey/tea) | - Tissue/Shell | C, N, H, O, S (Sr, Nd) | - Studies have shown results that differentiate: | |
| - between wild and farmed fish | [85] | ||||
| - between natural honeys and sugar-fed honeys | [71] | ||||
| - values of Clams from three different countries | [86] | ||||
| Fishery | - To find suitable or economically favorable natural aquaculture conditions | - Tissue | C, N | - Found changes in preferred diet according to the growth of manila clams observed in culture and field samples | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, E.-J.; Yun, H.-Y.; Lee, D.-H.; Shin, K.-H. Application of Compound-Specific Isotope Analysis in Environmental Forensic and Strategic Management Avenue for Pesticide Residues. Molecules 2021, 26, 4412. https://doi.org/10.3390/molecules26154412
Won E-J, Yun H-Y, Lee D-H, Shin K-H. Application of Compound-Specific Isotope Analysis in Environmental Forensic and Strategic Management Avenue for Pesticide Residues. Molecules. 2021; 26(15):4412. https://doi.org/10.3390/molecules26154412
Chicago/Turabian StyleWon, Eun-Ji, Hee-Young Yun, Dong-Hun Lee, and Kyung-Hoon Shin. 2021. "Application of Compound-Specific Isotope Analysis in Environmental Forensic and Strategic Management Avenue for Pesticide Residues" Molecules 26, no. 15: 4412. https://doi.org/10.3390/molecules26154412
APA StyleWon, E.-J., Yun, H.-Y., Lee, D.-H., & Shin, K.-H. (2021). Application of Compound-Specific Isotope Analysis in Environmental Forensic and Strategic Management Avenue for Pesticide Residues. Molecules, 26(15), 4412. https://doi.org/10.3390/molecules26154412