A Novel Reagent for Radioiodine Labeling of New Chemical Entities (NCEs) and Biomolecules
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of Monochloramine
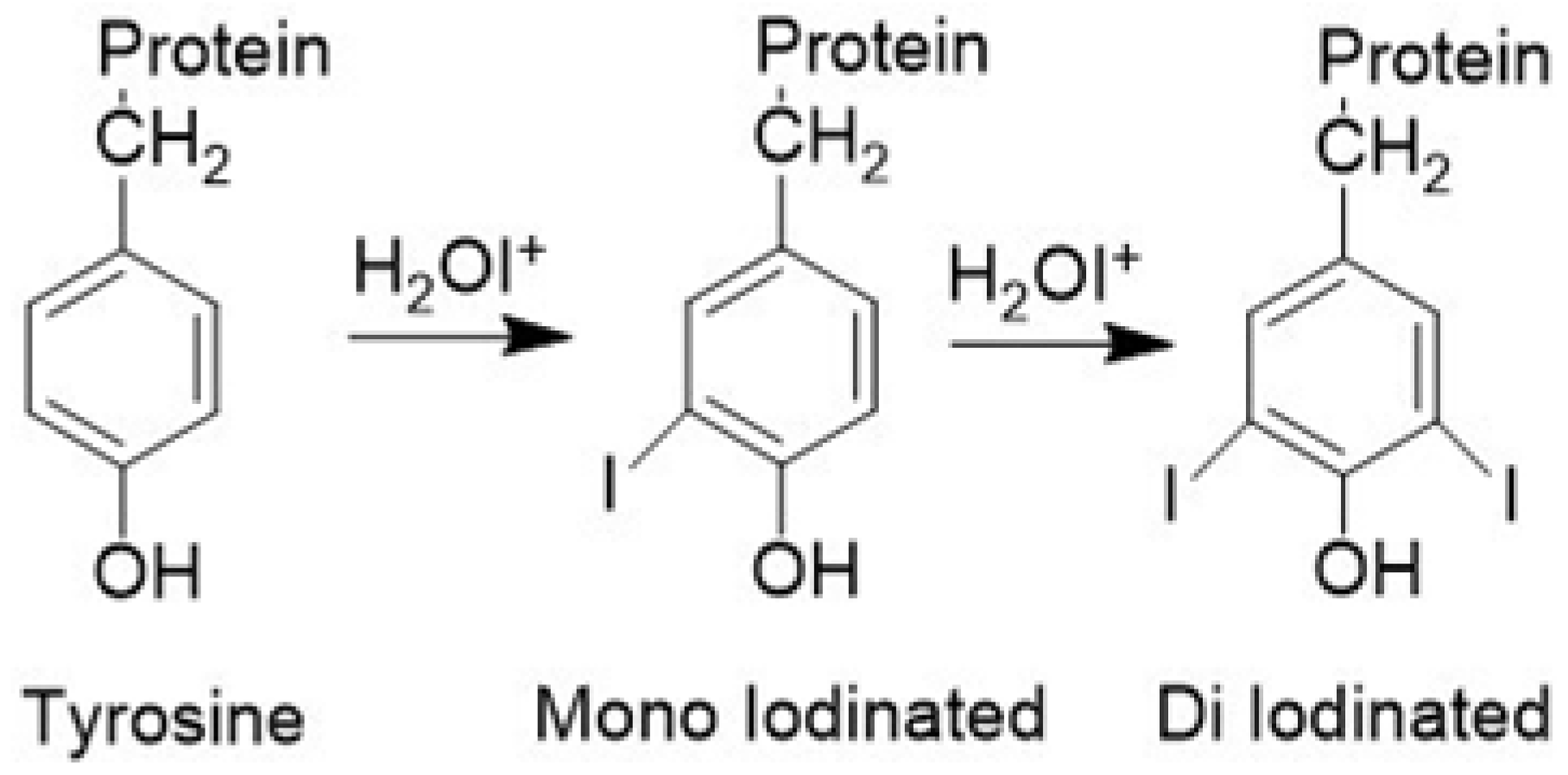
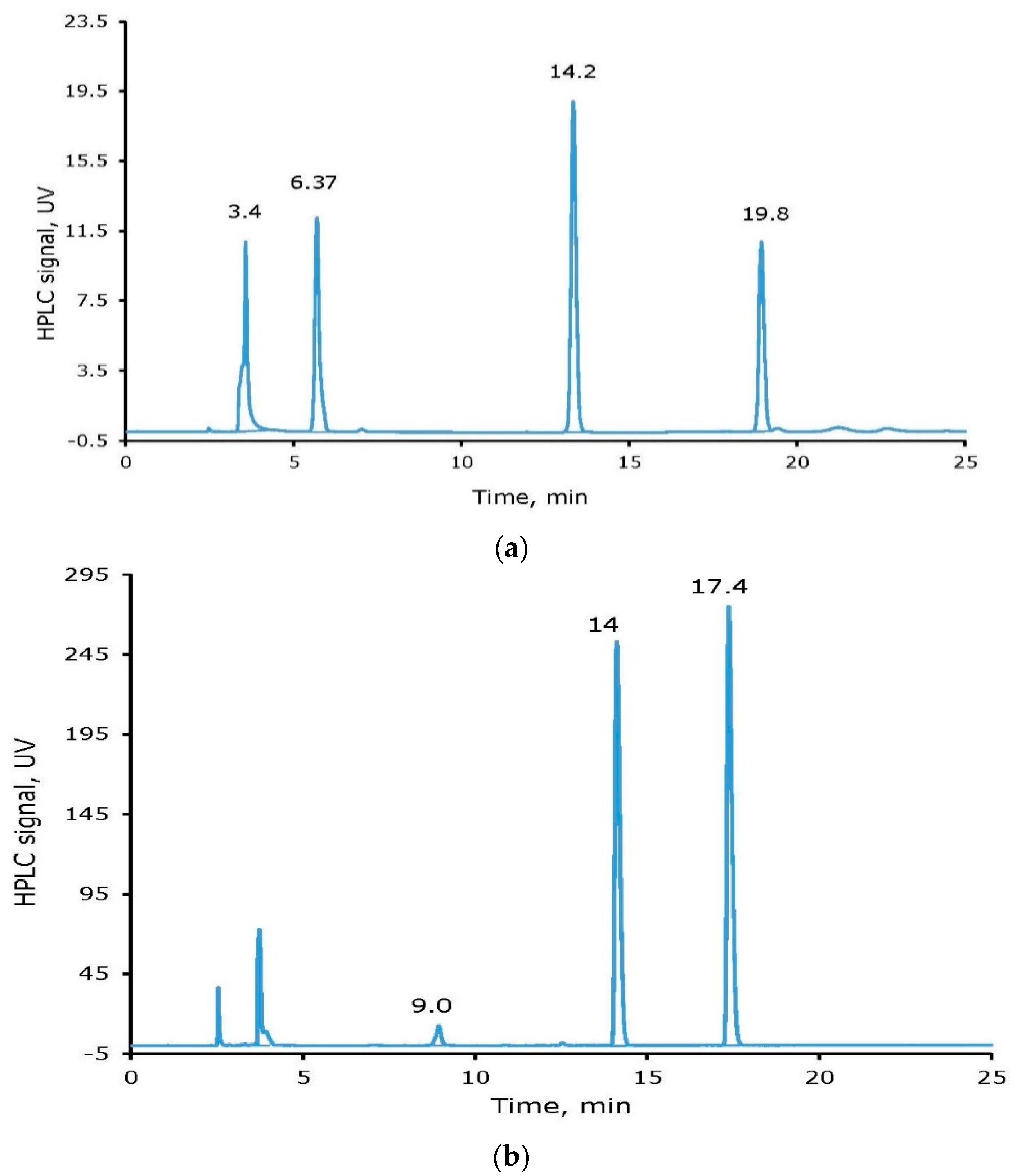
2.2. Non-Radioactive Iodine Labeling of Tyrosine and cRGDyK
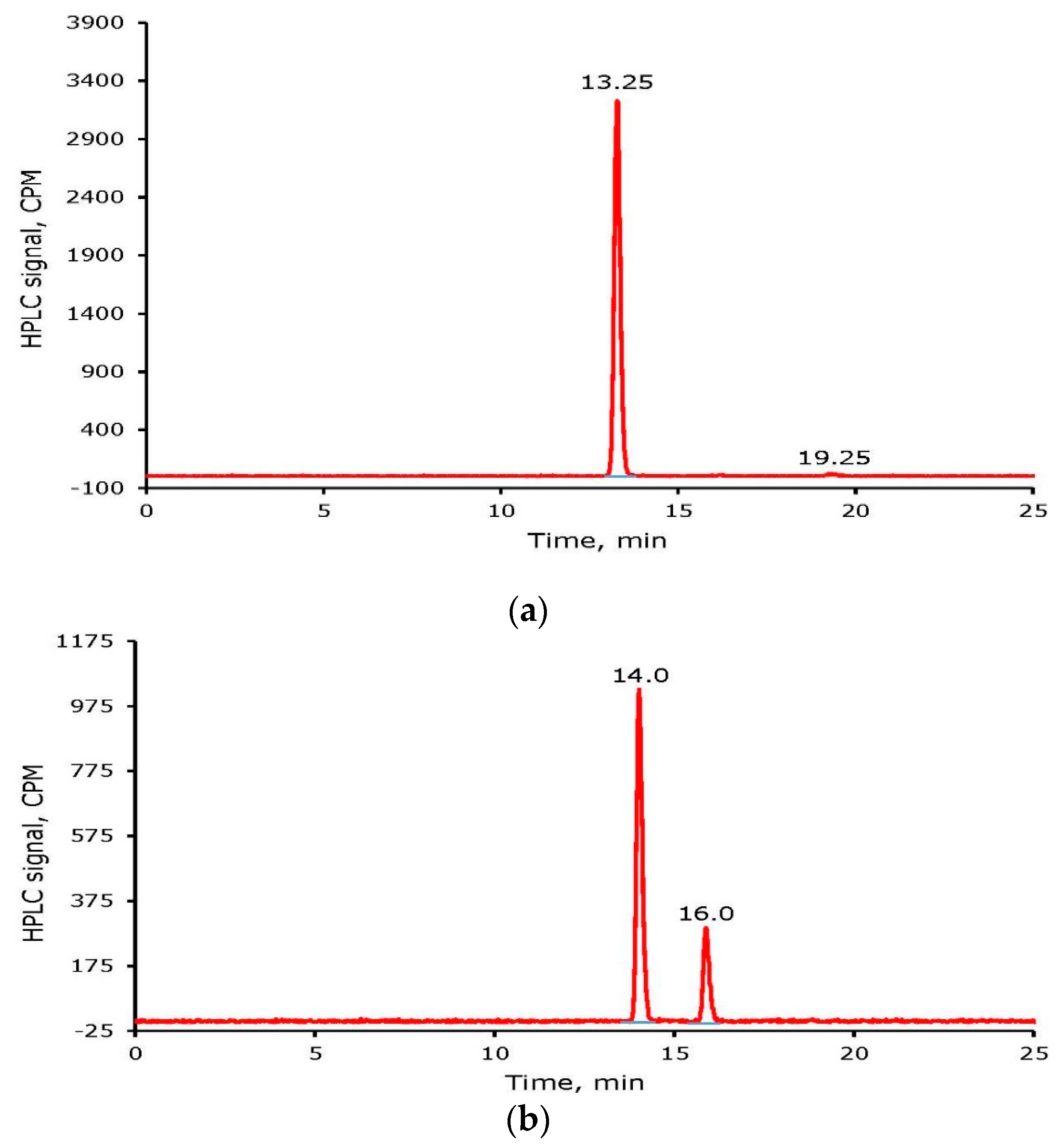
2.3. Radioiodine Labeling of Tyrosine and Cyclo Arg-Gly-Asp-d-Tyr-Lys (cRGDyK)
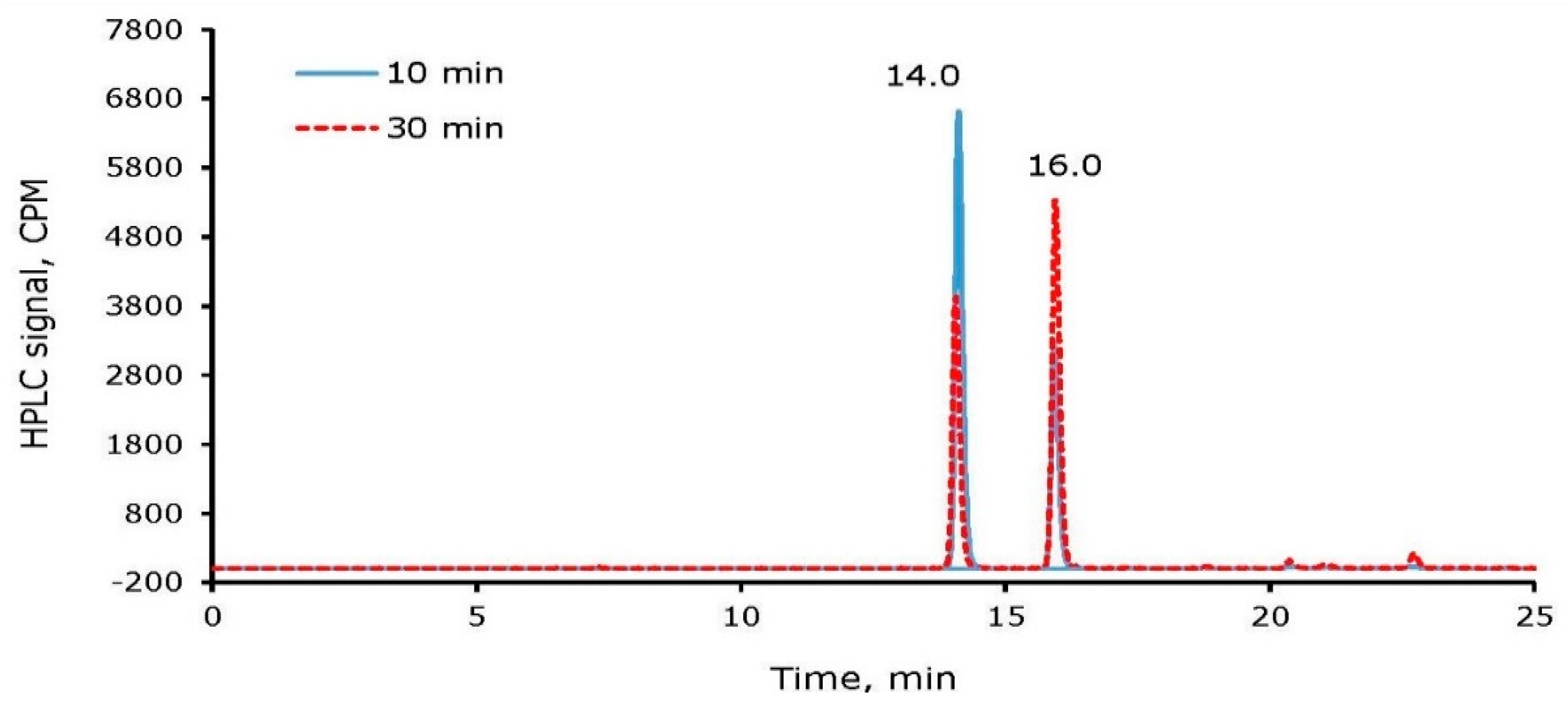
2.4. Radioiodine Labeling of Bovine Serum Albumin (BSA)
counts from top and the bottom halves) × 100
top and the bottom halves) × 100
2.5. Comparison of Monochloramine with Other Oxidizing Agents
3. Materials and Methods
3.1. General
3.2. Chemistry
3.3. Analytics
3.4. Radiochemistry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pressman, D.; Keighley, G. The zone of activity of antibodies as determined by the use of radioactive tracers; the zone of activity of nephritoxic antikidney serum. J. Immunol. 1948, 59, 141–146. [Google Scholar]
- Seevers, R.H.; Counsell, R.E. Radio-iodine labeling Techniques for Small Organic Molecules. Chem. Rev. 1982, 82, 575–590. [Google Scholar] [CrossRef]
- Kumar, K.; Ghosh, A. Radiochemistry, Production Processes, Labeling Methods, and ImmunoPET Imaging Pharmaceuticals of Iodine-124. Molecules 2021, 26, 414. [Google Scholar] [CrossRef]
- Redshaw, M.R.; Lynch, S.S. An improved method for the preparation of iodinated antigens for radioimmunoassay. J. Endocrinol. 1974, 60, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Eisen, H.N.; Keston, A.S. The Immunologic Reactivity of Bovine Serum Albumin Labelled with Trace-Amounts of Radioactive Iodine (I131). J. Immunol. 1949, 63, 71–80. [Google Scholar] [PubMed]
- Yalow, R.S.; Berson, S.A. Immunoassay of Endogenous Plasma Insulin in Man. J. Clin. Investig. 1960, 39, 1157–1175. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, R.C., Jr.; Robbins, M.C.; Reid, A.F. Labeling bovine and human albumin with I131. Nucleonics 1954, 12, 65–68. [Google Scholar]
- McFarlane, A.S. Labeling of plasma proteins with radioactive iodine. Biochem. J. 1956, 62, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Stadie, W.C.; Haugaard, N.; Vaughn, M. Studies of Insulin Binding with Isotopically Labeled Insulin. J. Biol. Chem. 1952, 199, 729–739. [Google Scholar] [CrossRef]
- Francis, G.E.; Mulligan, W.; Wormall, A. Labeling of proteins with iodine-131, Sulphur-35 and phosphorus-32. Nature 1951, 167, 748–751. [Google Scholar] [CrossRef]
- McFarlane, A.S. Efficient Trace-labeling of Proteins with Iodine. Nature 1958, 182, 53. [Google Scholar] [CrossRef]
- Hung, L.T.; Fermandjian, S.; Morgat, J.L.; Fromageot, P. Peptide and protein labeling with iodine, iodine monochloride reaction with aqueous solution of L-tyrosine, L-histidine, L-histidine-peptides, and his effect on some simple disulfide bridges. J. Label. Compd. 1974, 10, 3–21. [Google Scholar] [CrossRef]
- Doll, S.; Woolum, K.; Kumar, K. Radiolabeling of a Cyclic RGD (cyclo Arg-Gly-Asp-d-Tyr-Lys) Peptide Using Sodium Hypochlorite as an Oxidizing Agent. J. Label. Comp. Radiopharm. 2016, 59, 462–466. [Google Scholar] [CrossRef]
- Barluenga, J.; Garcia-Martin, M.A.; Gonzalez, J.M.; Clapes, P.; Valencia, G. Iodination of aromatic residues in peptides by reaction with IPy2BF4. Chem. Commun. 1996, 13, 1505–1506. [Google Scholar] [CrossRef]
- Tashtoush, B.M.; Traboulsi, A.A.; Dittert, L.; Hussain, A.A. Chloramine-T in radiolabeling techniques: IV. Pento-O-acetyl-N-chloro-N-methylglucamine as an oxidizing agent in radiolabeling techniques. Anal. Biochem. 2001, 288, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.A.; Bassam, T.; Dittert, L.W. Derivatives of N-chloro-N-Methyl Glucamine and N-Chloro-N-Methyl Glucamine Esters. U.S. Patent 5,985,239, 16 November 1999. [Google Scholar]
- Kaminski, J.J.; Bodor, N.; Higuchi, T. N-Halo Derivatives IV: Synthesis of Low Chlorine Potential Soft N-Chloramine Systems. J. Pharm. Sci. 1976, 65, 1733–1737. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.A.; Dittert, L.W. Non-Destructive Method for Radiolabeling Biomolecules by Halogenation. U.S. Patent 5,424,402, 13 June 1995. [Google Scholar]
- Hunter, W.M.; Greenwood, F.C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature 1962, 194, 495–496. [Google Scholar] [CrossRef]
- Greenwood, F.C.; Hunter, W.M.; Glover, J.S. The preparation of 131I-labelled human growth hormone of high specific radioactivity. Biochem. J. 1963, 89, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Markwell, M.A. A new solid-state reagent to iodinate proteins: Conditions for the efficient labeling of antiserum. Anal. Biochem. 1982, 125, 427–432. [Google Scholar] [CrossRef]
- Fracker, P.J.; Speck, J.C., Jr. Protein and cell membrane iodinations with a sparingly soluble chloramide 1,3,4,6-tetrachloro-3a,6a-diphenylglycoluril. Biochem. Biophys. Res. Commun. 1978, 80, 849–857. [Google Scholar] [CrossRef]
- Salacinski, P.; Hope, J.; McLean, C.; Clement-Jones, V.; Sykes, J.; Price, J.; Lowry, P.J. A new simple method which allows theoretical incorporation of radio-iodine into proteins and peptides without damage. J. Endocrinol. 1979, 81, 131. [Google Scholar]
- Markwell, M.A.K.; Fox, C.F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3a,6a-diphenylglycouril. Biochemistry 1978, 17, 4807–4817. [Google Scholar] [CrossRef]
- Boonkitticharoen, V.; Laohathai, K. Assessing performances of Iodogen-coated surfaces used for radio-iodine labeling of proteins. Nucl. Med. Commun. 1990, 11, 295–304. [Google Scholar] [CrossRef]
- Holohan, K.N.; Murphy, R.F.; Flanagan, R.W.J.; Buchanan, K.D.; Elmore, D.T. Enzymic iodination of the histidyl residue of secretin: A radioimmunoassay of the hormone. Biochim. Biophys. Acta 1973, 322, 178–180. [Google Scholar] [CrossRef]
- Holohan, K.N.; Murphy, R.F.; Elmore, D.T. The Site of Substitution in the Imidazole Nucleus after the Lactoperoxidase-Catalysed Iodination of Histidine Residues in Polypetides. Biochem. Soc. Trans. 1974, 2, 739–740. [Google Scholar] [CrossRef]
- Navarro, L.; Berdal, M.; Cherel, M.; Pecorari, F.; Gestin, J.-F.; Guerard, F. Prosthetic groups for radio-iodine labeling and astatination of peptides and proteins: A comparative study of five potential bioorthogonal labeling strategies. Bioorg. Med. Chem. 2019, 27, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Muramoto, E.; Colturato, M.T.; Siva, C.P.; Araujo, E.B. Radio-iodine labeling of proteins using prosthetic group: A convenient way to produce labelled proteins with in vivo stability. Cell. Mol. Biol. 2002, 47, 735–739. [Google Scholar]
- Kumar, K. Radio-iodine Labeling Reagents and Methods for New Chemical Entities (NCEs) and Biomolecules. Cancer Biother. Radiopharm. 2021. submitted. [Google Scholar]
- Victorin, K.; Hellstrom, K.-G.; Rylander, R. Redox potential measurements for determining the disinfecting power of chlorinated water. Epidemiol. Infect. 1972, 70, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.; Day, R.A.; Margerum, D.W. Atom-Transfer redox kinetics: General-acid-assisted oxidation of iodide by chloramines and hypochlorite. Inorg. Chem. 1986, 25, 4344–4350. [Google Scholar] [CrossRef]
- Haubner, R.; Wester, H.-J.; Reuning, U.; Senekowitsch-Schmidtke, R.; Diefenbach, B.; Kessler, H.; Stocklin, G.; Schwaiger, M. Radiolabeled αvβ3 Integrin Antagonists: A New Class of Tracers for Tumor Targeting. J. Nucl. Med. 1999, 40, 1061–1071. [Google Scholar]
- Haubner, R. αvβ3-integrin imaging: A new approach to characterise angiogenesis? Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.P.; Margerum, D.W. Kinetics of chlorine transfer from chloramine to amines, amino acids, and peptides. Inorg. Chem. 1982, 21, 2545–2550. [Google Scholar] [CrossRef]
- Cahn, J.W.; Powell, R.F. The Raschig Synthesis of Hydrazine. J. Am. Chem. Soc. 1954, 76, 2565–2567. [Google Scholar] [CrossRef]
- Jacangelo, J.G.; Olivieri, V.P.; Kawata, K. Oxidation of Sulfydryl Group by Monochloramine. Water Res. 1987, 21, 1339–1344. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, K.; Woolum, K. A Novel Reagent for Radioiodine Labeling of New Chemical Entities (NCEs) and Biomolecules. Molecules 2021, 26, 4344. https://doi.org/10.3390/molecules26144344
Kumar K, Woolum K. A Novel Reagent for Radioiodine Labeling of New Chemical Entities (NCEs) and Biomolecules. Molecules. 2021; 26(14):4344. https://doi.org/10.3390/molecules26144344
Chicago/Turabian StyleKumar, Krishan, and Karen Woolum. 2021. "A Novel Reagent for Radioiodine Labeling of New Chemical Entities (NCEs) and Biomolecules" Molecules 26, no. 14: 4344. https://doi.org/10.3390/molecules26144344
APA StyleKumar, K., & Woolum, K. (2021). A Novel Reagent for Radioiodine Labeling of New Chemical Entities (NCEs) and Biomolecules. Molecules, 26(14), 4344. https://doi.org/10.3390/molecules26144344






