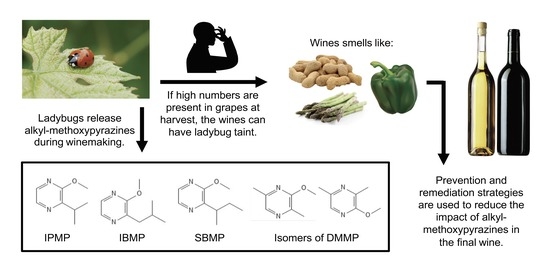
A Review of Ladybug Taint in Wine: Origins, Prevention, and Remediation
Abstract
1. What Is Ladybug Taint?
1.1. Alkyl-Methoxypyrazines Are the Molecules Responsible
1.2. How Much Is Too Much?
1.3. Coccinellidae Species Responsible
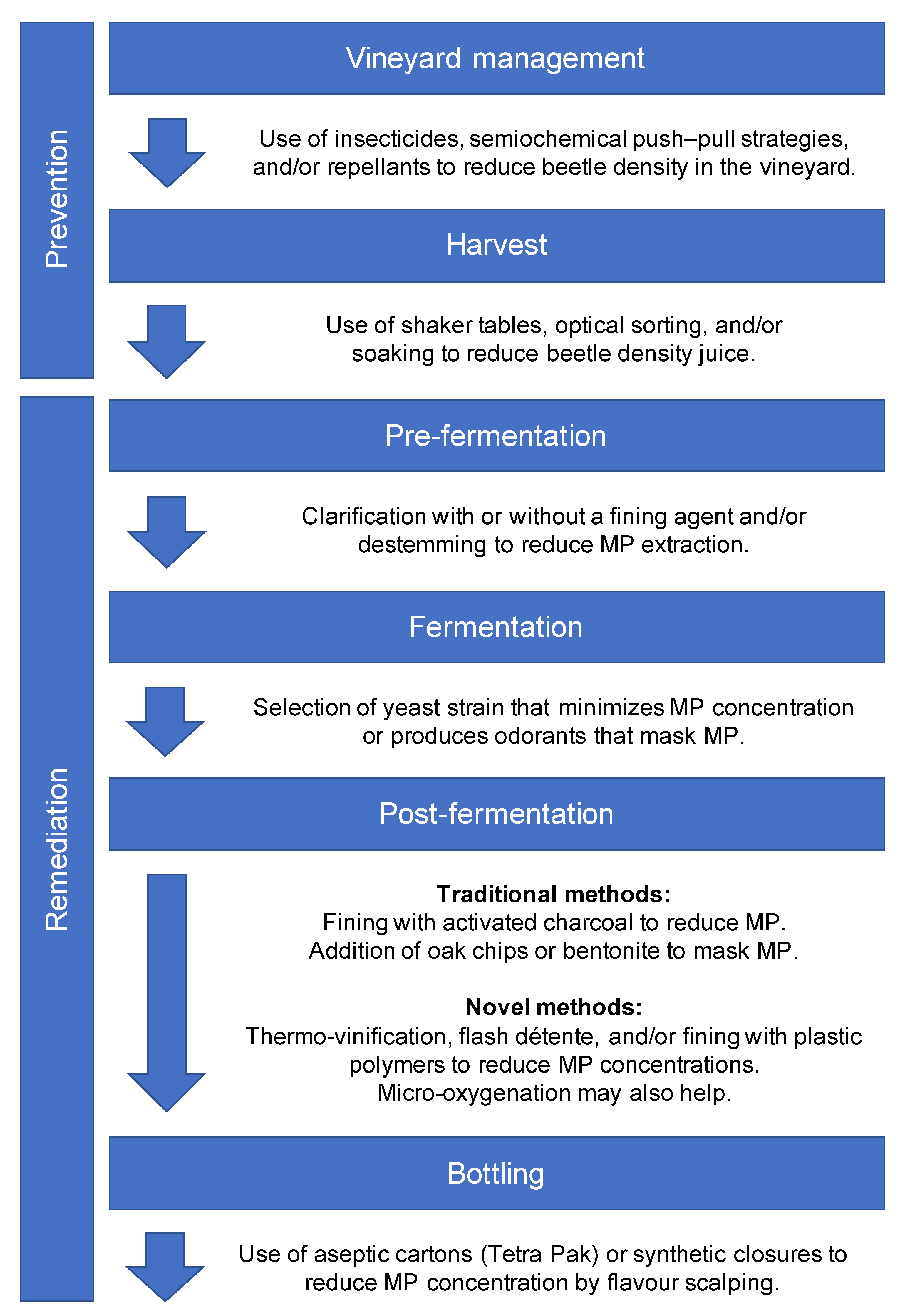
2. Preharvest Prevention in the Vineyard
2.1. Insecticides
2.2. Semiochemical-Based Push–Pull Strategies
2.3. Repellent Sprays
3. Postharvest Prevention and Remediation
3.1. Removing Beetles
3.2. Traditional Approaches to Treating Faulted Wine
3.3. Novel Interventions
3.3.1. Heat
3.3.2. Oxygen
3.3.3. The Panacea of Plastics?
3.3.4. Just Nuke It
3.4. The Challenge of Specificity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, R.D. The Coccinellidae (Coleoptera) of America north of Mexico. J. N. Y. Entomol. Soc. 1985, 93, 33–35. [Google Scholar]
- Koch, R.L.; Burkness, E.C.; Burkness, S.J.W.; Hutchison, W.D. Phytophagous preferences of the Multicolored Asian Lady Beetle (Coleoptera: Coccinellidae) for autumn-ripening fruit. J. Econ. Entomol. 2004, 97, 539–544. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vincent, C.; Pickering, G. Multicolored Asian ladybeetle, Harmonia axyridis (Coleoptera: Coccinellidae). In Biological Control Programmes in Canada 2001–2012; Mason, P.G., Gillespie, D.R., Eds.; CABI: Wallingford, UK, 2013; pp. 192–198. ISBN 9781780642574. [Google Scholar]
- Coderre, D.; Lucas, É.; Gagné, I. The occurrence of Harmonia axyridis (Pallas)(Coleoptera: Coccinellidae) in Canada. Can. Entomol. 1995, 127, 609–611. [Google Scholar] [CrossRef]
- Galvan, T.L.; Burkness, E.C.; Hutchison, W.D. Influence of berry Injury on Infestations of the Multicolored Asian Lady Beetle in wine grapes. Plant Health Prog. 2006, 7. [Google Scholar] [CrossRef]
- Pickering, G.; Lin, J.; Riesen, R.; Reynolds, A.; Brindle, I.; Soleas, G. Influence of Harmonia axyridis on the sensory properties of white and red wine. Am. J. Enol. Vitic. 2004, 55, 153–159. [Google Scholar]
- Pickering, G.J.; Spink, M.; Kotseridis, Y.; Brindle, I.D.; Sears, M.; Inglis, D. Morbidity of Harmonia axyridis mediates ladybug taint in red wine. J. Food Agric. Environ. 2008, 6, 133–138. [Google Scholar] [CrossRef]
- Pickering, G.J.; Ker, K.; Soleas, G. Determination of the critical stages of processing and tolerance limits for Harmonia axyridis for “ladybug taint” in wine. Vitis 2007, 46, 85–90. [Google Scholar]
- Pickering, G.J.; Lin, Y.; Reynolds, A.; Soleas, G.; Riesen, R.; Brindle, I. The influence of Harmonia axyridis on wine composition and aging. J. Food Sci. 2005, 70, S128–S135. [Google Scholar] [CrossRef]
- Ross, C.F.; Weller, K. Sensory evaluation of suspected Harmonia axyridis -tainted red wine using untrained panelists. J. Wine Res. 2007, 18, 187–193. [Google Scholar] [CrossRef]
- Cai, L.; Koziel, J.A.; O’Neal, M.E. Determination of characteristic odorants from Harmonia axyridis beetles using in vivo solid-phase microextraction and multidimensional gas chromatography–mass spectrometry–olfactometry. J. Chromatogr. A 2007, 1147, 66–78. [Google Scholar] [CrossRef]
- Slabizki, P.; Legrum, C.; Meusinger, R.; Schmarr, H.-G. Characterization and analysis of structural isomers of dimethyl methoxypyrazines in cork stoppers and ladybugs (Harmonia axyridis and Coccinella septempunctata). Anal. Bioanal. Chem. 2014, 406, 6429–6439. [Google Scholar] [CrossRef] [PubMed]
- Cudjoe, E.; Wiederkehr, T.B.; Brindle, I.D. Headspace gas chromatography-mass spectrometry: A fast approach to the identification and determination of 2-alkyl-3- methoxypyrazine pheromones in ladybugs. Analyst 2005, 130, 152. [Google Scholar] [CrossRef] [PubMed]
- Kögel, S.; Gross, J.; Hoffmann, C.; Ulrich, D. Diversity and frequencies of methoxypyrazines in hemolymph of Harmonia axyridis and Coccinella septempunctata and their influence on the taste of wine. Eur. Food Res. Technol. 2012, 234, 399–404. [Google Scholar] [CrossRef]
- Pickering, G.J.; Spink, M.; Kotseridis, Y.; Brindle, I.D.; Sears, M.; Inglis, D. The influence of Harmonia axyridis morbidity on 2-Isopropyl-3-methoxypyrazine in “Cabernet Sauvignon” wine. Vitis 2008, 47, 227–230. [Google Scholar] [CrossRef]
- Kögel, S.; Botezatu, A.; Hoffmann, C.; Pickering, G. Methoxypyrazine composition of Coccinellidae-tainted Riesling and Pinot noir wine from Germany. J. Sci. Food Agric. 2015, 95, 509–514. [Google Scholar] [CrossRef]
- Pickering, G.J.; Lin, Y.; Ker, K. Origin and remediation of Asian Lady Beetle (Harmonia axyridis) taint in wine. In Crops: Growth, Quality and Biotechnology. III. Quality Management of Food Crops for Processing Technology; Dris, R., Ed.; WFL Publisher: Helsinki, Findland, 2006; pp. 785–794. ISBN 952-91-8601-0. [Google Scholar]
- Galvan, T.L.; Kells, S.; Hutchison, W.D. Determination of 3-Alkyl-2-methoxypyrazines in Lady Beetle-infested wine by solid-phase microextraction headspace sampling. J. Agric. Food Chem. 2008, 56, 1065–1071. [Google Scholar] [CrossRef]
- Ross, C.F.; Rosales, M.U.; Fernandez-Plotka, V.C. Aroma profile of Niagara grape juice contaminated with multicoloured Asian lady beetle taint using gas chromatography/mass spectrometry/olfactometry. Int. J. Food Sci. Technol. 2010, 45, 789–793. [Google Scholar] [CrossRef]
- Botezatu, A.I.; Kotseridis, Y.; Inglis, D.; Pickering, G.J. Occurrence and contribution of alkyl methoxypyrazines in wine tainted by Harmonia axyridis and Coccinella septempunctata. J. Sci. Food Agric. 2013, 93, 803–810. [Google Scholar] [CrossRef]
- Allen, M.S.; Lacey, M.J.; Harris, R.L.N.; Brown, W.V. Contribution of methoxypyrazines to Sauvignon blanc wine aroma. Am. J. Enol. Vitic. 1991, 42, 109–112. [Google Scholar]
- Lacey, M.J.; Allen, M.S.; Harris, R.L.N.; Brown, W.V. Methoxypyrazines in Sauvignon blanc grapes and wines. Am. J. Enol. Vitic. 1991, 42, 103–108. [Google Scholar]
- Roujou de Boubée, D.; Van Leeuwen, C.; Dubourdieu, D. Organoleptic impact of 2-Methoxy-3-isobutylpyrazine on red Bordeaux and Loire wines. Effect of environmental conditions on concentrations in grapes during ripening. J. Agric. Food Chem. 2000, 48, 4830–4834. [Google Scholar] [CrossRef] [PubMed]
- Roujou de Boubée, D.; Cumsille, A.M.; Pons, M.; Dubourdieu, D. Location of 2-methoxy-3-isobutylpyrazine in Cabernet Sauvignon grape bunches and its extractability during vinifica-tion. Am. J. Enol. Vitic. 2002, 53, 1–5. [Google Scholar]
- Sala, C.; Busto, O.; Guasch, J.; Zamora, F. Contents of 3-alkyl-2-methoxypyrazines in musts and wines fromVitis vinifera variety Cabernet Sauvignon: Influence of irrigation and plantation density. J. Sci. Food Agric. 2005, 85, 1131–1136. [Google Scholar] [CrossRef]
- Belancic, A.; Agosin, E. Methoxypyrazines in grapes and wines of Vitis vinifera cv. Carmenere. Am. J. Enol. Vitic. 2007, 58, 462–469. [Google Scholar]
- Godelmann, R.; Limmert, S.; Kuballa, T. Implementation of headspace solid-phase-microextraction–GC–MS/MS methodology for determination of 3-alkyl-2-methoxypyrazines in wine. Eur. Food Res. Technol. 2007, 227, 449. [Google Scholar] [CrossRef]
- Botezatu, A.; Kotseridis, Y.; Inglis, D.; Pickering, G.J. A survey of methoxypyrazines in wine. J. Food Agric. Environ. 2016, 14, 24–29. [Google Scholar] [CrossRef]
- Ortega, C.; López, R.; Cacho, J.; Ferreira, V. Fast analysis of important wine volatile compounds: Development and validation of a new method based on gas chromatographic–flame ionisation detection analysis of dichloromethane microextracts. J. Chromatogr. A 2001, 923, 205–214. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Romero, R.; Chacón, J.L.; García, E.; Martínez, J. Pyrazine contents in four red grape varietes cultivated in warm climate. J. Int. Sci. Vigne Vin 2006, 40, 203–207. [Google Scholar] [CrossRef]
- Maga, J. Sensory and stability properties of added methoxypyrazines to model and authentic wines. In Flavors and Off-Flavors ’89, Proceedings of the 6th International Flavor Conference, Rethymnon, Crete, Greece, 5–7 July 1989; Charalambous, G., Ed.; Elsevier Science Limited: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Kotseridis, Y.; Beloqui, A.A.; Bertrand, A.; Doazan, J.P. An analytical method for studying the volatile compounds of Merlot noir clone wines. Am. J. Enol. Vitic. 1998, 49, 44–48. [Google Scholar]
- Pickering, G.J.; Karthik, A.; Inglis, D.; Sears, M.; Ker, K. Determination of ortho- and retronasal detection thresholds for 2-Isopropyl-3-Methoxypyrazine in wine. J. Food Sci. 2007, 72, S468–S472. [Google Scholar] [CrossRef] [PubMed]
- Pickering, G.J.; Karthik, A.; Inglis, D.; Sears, M.; Ker, K. Detection thresholds for 2-Isopropyl-3-methoxypyrazine in Concord and Niagara grape juice. J. Food Sci. 2008, 73, S262–S266. [Google Scholar] [CrossRef]
- Galvan, T.L.; Burkness, E.C.; Vickers, Z.; Stenberg, P.; Mansfield, A.K.; Hutchison, W.D. Sensory-based action threshold for Multicolored Asian Lady Beetle-related taint in winegrapes. Am. J. Enol. Vitic. 2007, 58, 518–522. [Google Scholar]
- Ross, C.; Ferguson, H.; Keller, M.; Walsh, D.; Weller, K.; Spayd, S. Determination of ortho-nasal aroma threshold for Multicolored Asian Lady Beetle in Concord grape juice. J. Food Qual. 2007, 30, 855–863. [Google Scholar] [CrossRef]
- Glemser, E.; McFadden-Smith, W.; Parent, J.-P. Evaluation of compounds for repellency of the multicoloured Asian lady beetle (Coleoptera: Coccinellidae) in vineyards. Can. Entomol. 2021, 1–12. [Google Scholar] [CrossRef]
- Koch, R.L. The multicolored Asian lady beetle, Harmonia axyridis: A review of its biology, uses in biological control, and non-target impacts. J. Insect Sci. 2003, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Glemser, E.J.; Dowling, L.; Inglis, D.; Pickering, G.J.; Mcfadden-Smith, W.; Sears, M.K.; Hallett, R.H. A novel method for controlling Multicolored Asian Lady Beetle (Coleoptera: Coccinellidae) in vineyards. Environ. Entomol. 2012, 41, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Seko, T.; Yamashita, K.; Miura, K. Residence period of a flightless strain of the ladybird beetle Harmonia axyridis Pallas (Coleoptera: Coccinellidae) in open fields. Biol. Control 2008, 47, 194–198. [Google Scholar] [CrossRef]
- Pickering, G.J.; Glemser, E.J.; Hallett, R.; Inglis, D.; McFadden-Smith, W.; Ker, K. Good bugs gone bad: Coccinellidae, sustainability and wine. WIT Trans. Ecol. Environ. 2011, 167, 239–251. [Google Scholar] [CrossRef]
- Leroy, P.D.; Schillings, T.; Farmakidis, J.; Heuskin, S.; Lognay, G.; Verheggen, F.J.; Brostaux, Y.; Haubruge, E.; Francis, F. Testing semiochemicals from aphid, plant and conspecific: Attraction of Harmonia axyridis. Insect Sci. 2012, 19, 372–382. [Google Scholar] [CrossRef]
- Pettersson, J.; Birkett, M.A.; Pickett, A. Pyrazines as Attractants for Insects of Order Coleoptera. WO1999037152 A1, 29 July 1999. Available online: https://www.google.com/patents/WO1999037152A1?cl=pt (accessed on 5 May 2021).
- Riddick, E.W.; Brown, A.E.; Chauhan, K.R. Harmonia axyridis adults avoid catnip and grapefruit-derived terpenoids in laboratory bioassays. Bull. Insectol. 2008, 61, 81–90. [Google Scholar]
- Riddick, E.W.; Aldrich, J.R.; De Milo, A.; Davis, J.C. Potential for modifying the behavior of the multicolored Asian lady beetle (Coleoptera: Coccinellidae) with plant-derived natural products. Ann. Entomol. Soc. Am. 2000, 93, 1314–1321. [Google Scholar] [CrossRef]
- Riddick, E.W.; Aldrich, J.R.; Davis, J.C. DEET repels Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) adults in laboratory bioassays. J. Entomol. Sci. 2004, 39, 373–386. [Google Scholar] [CrossRef]
- Botezatu, A.; Pickering, G. Ladybug (Coccinellidae) taint in wine. In Managing Wine Quality. Vol. 2, Oenology and Wine Quality; Reynolds, A.G., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2010; pp. 418–429. ISBN 9781845694845. [Google Scholar]
- Hashizume, K.; Umeda, N. Methoxypyrazine content of japanese red wines. Biosci. Biotechnol. Biochem. 1996, 60, 802–805. [Google Scholar] [CrossRef]
- Sala, C.; Busto, O.; Guasch, J.; Zamora, F. Influence of vine training and sunlight exposure on the 3-Alkyl-2-methoxypyrazines content in musts and wines from the Vitis vinifera variety Cabernet Sauvignon. J. Agric. Food Chem. 2004, 52, 3492–3497. [Google Scholar] [CrossRef]
- Sidhu, D.; Lund, J.; Kotseridis, Y.; Saucier, C. Methoxypyrazine analysis and influence of viticultural and enological procedures on their levels in grapes, musts, and wines. Crit. Rev. Food Sci. Nutr. 2015, 55, 485–502. [Google Scholar] [CrossRef]
- Kotseridis, Y.S.; Spink, M.; Brindle, I.D.; Blake, A.J.; Sears, M.; Chen, X.; Soleas, G.; Inglis, D.; Pickering, G.J. Quantitative analysis of 3-alkyl-2-methoxypyrazines in juice and wine using stable isotope labelled internal standard assay. J. Chromatogr. A 2008, 1190, 294–301. [Google Scholar] [CrossRef]
- Treloar, J.; Howell, G.S. Influence of yeast and malolactic bacteria strain choice on 3-isobutyl-2-methoxypyrazine concentration in Cabernet Sauvignon and Franc wines. In Wine Making Workshop, Vegetable & Farm Market EXPO, Great Lakes Fruit, Grand Rapids, MI, USA; 2006; Available online: www.glexpo.com/summaries/2006summaries/WineMaking2006.pdf (accessed on 5 May 2021).
- Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Sorption of volatile phenols by yeast cell walls. Int. J. Wine Res. 2009, 1, 11–18. [Google Scholar] [CrossRef][Green Version]
- Pickering, G.J.; Spink, M.; Kotseridis, Y.; Inglis, D.; Brindle, I.D.; Sears, M.; Beh, A.-L. Yeast strain affects 3-isopropyl-2-methoxypyrazine concentration and sensory profile in Cabernet Sauvignon wine. Aust. J. Grape Wine Res. 2008, 14, 230–237. [Google Scholar] [CrossRef]
- Pickering, G.; Lin, J.; Reynolds, A.; Soleas, G.; Riesen, R. The evaluation of remedial treatments for wine affected by Harmonia axyridis. Int. J. Food Sci. Technol. 2006, 41, 77–86. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Savitha, K.; Singhal, R.S.; Kanetkar, V.R. Scalping of flavors in packaged foods. Comp. Rev. Food Sci. Food Saf. 2007, 6, 17–35. [Google Scholar] [CrossRef]
- Blake, A.; Kotseridis, Y.; Brindle, I.D.; Inglis, D.; Sears, M.; Pickering, G.J. Effect of closure and packaging type on 3-Alkyl-2-methoxypyrazines and other impact odorants of Riesling and Cabernet Franc wines. J. Agric. Food Chem. 2009, 57, 4680–4690. [Google Scholar] [CrossRef]
- Pickering, G.J.; Blake, A.J.; Soleas, G.J.; Inglis, D.L. Remediation of wine with elevated concentrations of 3-alkyl-2-methoxypyrazines using cork and synthetic closures. J. Food Agric. Environ. 2010, 8, 97–101. [Google Scholar]
- Bakker, J.; Clarke, R.J. Wine Flavour Chemistry, 2nd ed.; Blackwell Publishing Ltd.: Chichester, UK, 2012; ISBN 9781444330427. [Google Scholar]
- Roujou de Boubée, D. Research on the vegetal green pepper character in grapes and wines. Rev. Oenol. 2004, 31, 6–10. [Google Scholar]
- Jackson, R.S. Wine Science: Principles and Applications, 3rd ed.; Academic Press: Burlington, MA, USA, 2008; ISBN 9780123736468. [Google Scholar]
- Maza, M.; Álvarez, I.; Raso, J. Thermal and non-thermal physical methods for improving polyphenol extraction in red winemaking. Beverages 2019, 5, 47. [Google Scholar] [CrossRef]
- Baggio, P. Flash extraction—what can it do for you? In Proceedings of the ASVO Proceedings: Managing the Best out of Difficult Vintages; 2017; pp. 29–31. Available online: https://www.dtpacific.com/dev/wp-content/uploads/2017/03/Art-ASVO-Flash-Bio-Thermo-Extraction-What-can-it-do-for-you.pdf (accessed on 1 April 2021).
- Vinsonneau, E.; Escaffre, P.; Crachereau, J.; Praud, S. Evaluation du Procédé de Vinification par “Flash Détente” Dans le Bordelais; ITV France Bordeaux: Blanquefort, France, 2006. [Google Scholar]
- Gómez-Plaza, E.; Cano-López, M. A review on micro-oxygenation of red wines: Claims, benefits and the underlying chemistry. Food Chem. 2011, 125, 1131–1140. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.-P.; Henschen, C.; Cantu, A.; Watrelot, A.A.; Waterhouse, A.L. Understanding microoxygenation: Effect of viable yeasts and sulfur dioxide levels on the sensory properties of a Merlot red wine. Food Res. Int. 2018, 108, 505–515. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Pérez-Coello, M.S.; Hermosín-Gutiérrez, I. Effect of wine micro-oxygenation treatment and storage period on colour-related phenolics, volatile composition and sensory characteristics. LWT 2011, 44, 866–874. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Hermosín-Gutiérrez, I.; Pérez-Coello, M.S. Improvement of Cencibel red wines by oxygen addition after malolactic fermentation: Study on color-related phenolics, volatile composition, and sensory characteristics. J. Agric. Food Chem. 2012, 60, 5962–5973. [Google Scholar] [CrossRef]
- Oberholster, A.; Elmendorf, B.L.; Lerno, L.A.; King, E.S.; Heymann, H.; Brenneman, C.E.; Boulton, R.B. Barrel maturation, oak alternatives and micro-oxygenation: Influence on red wine aging and quality. Food Chem. 2015, 173, 1250–1258. [Google Scholar] [CrossRef]
- Ryona, I.; Reinhardt, J.; Sacks, G.L. Treatment of grape juice or must with silicone reduces 3-alkyl-2-methoxypyrazine concentrations in resulting wines without altering fermentation volatiles. Food Res. Int. 2012, 47, 70–79. [Google Scholar] [CrossRef]
- Botezatu, A.; Pickering, G.J. Application of plastic polymers in remediating wine with elevated alkyl-methoxypyrazine levels. Food Addit. Contam. A 2015, 32, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Botezatu, A.; Kemp, B.; Pickering, G. Chemical and sensory evaluation of silicone and polylactic acid-based remedial treatments for elevated methoxypyrazine levels in wine. Molecules 2016, 21, 1238. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K. Applications of Radiation within the Wine Industry. Can. Bachelor’s Thesis, McMaster University, Hamilton, ON, Canada, 2003. [Google Scholar]
- Wilson, K.J.; Moran, G.; Boreham, D. Application of radiation within the wine industry. In Proceedings of the 12th Quadrennial Congress of the International Association for Radiation Research Incorporating the 50th Annual Meeting of Radiation Research Society, RANZCR Radiation Oncology Annual Scientific Meeting and AINSE Radiation Science Conference, Brisbane, Australia, 17–22 August 2003; 414p. [Google Scholar]
- Cavaggioni, A.; Findlay, J.B.C.; Tirindelli, R. Ligand binding characteristics of homologous rat and mouse urinary proteins and pyrazine-binding protein of calf. Comp. Biochem. Physiol. B 1990, 96, 513–520. [Google Scholar] [CrossRef]
- Pevsner, J.; Hou, V.; Snowman, A.M.; Snyder, S.H. Odorant-binding protein. Characterization of ligand binding. J. Biol. Chem. 1990, 265, 6118–6125. [Google Scholar] [CrossRef]
- Inglis, D.; Beh, A.L.; Brindle, I.D.; Pickering, G.; Humes, E.F. Method for Reducing Methoxypyrazines in Grapes and Grape Products. U.S. Patent No. 8859026B2, 14 October 2014. [Google Scholar]
- Pickering, G.; Inglis, D.; Botezatu, A.; Beh, A.; Humes, E.; Brindle, I. New approaches to removing alkyl-methoxypyrazines from grape juice and wine. In Scientific Bulletin. Series F. Biotechnologies, Vol. XVIII; The University of Agronomic Sciences and Veterinary Medicine: Bucharest, Romania, 2014; pp. 130–134. [Google Scholar]
- Graham, S.P.; El-Sharif, H.F.; Hussain, S.; Fruengel, R.; McLean, R.K.; Hawes, P.C.; Sullivan, M.V.; Reddy, S.M. Evaluation of molecularly imprinted polymers as synthetic virus neutralizing antibody mimics. Front. Bioeng. Biotechnol. 2019, 7, 115. [Google Scholar] [CrossRef]
- Liang, C.; Jeffery, D.W.; Taylor, D.K. Preparation of magnetic polymers for the elimination of 3-Isobutyl-2-methoxypyrazine from wine. Molecules 2018, 23, 1140. [Google Scholar] [CrossRef]
- Liang, C.; Ristic, R.; Stevenson, R.; Jiranek, V.; Jeffrey, D. Green characters: Using magnetic polymers to remove overpowering green capsicum flavour from Cabernet Sauvignon wine. Wine Vitic. J. 2019, 34, 24–26. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pickering, G.J.; Botezatu, A. A Review of Ladybug Taint in Wine: Origins, Prevention, and Remediation. Molecules 2021, 26, 4341. https://doi.org/10.3390/molecules26144341
Pickering GJ, Botezatu A. A Review of Ladybug Taint in Wine: Origins, Prevention, and Remediation. Molecules. 2021; 26(14):4341. https://doi.org/10.3390/molecules26144341
Chicago/Turabian StylePickering, Gary J., and Andreea Botezatu. 2021. "A Review of Ladybug Taint in Wine: Origins, Prevention, and Remediation" Molecules 26, no. 14: 4341. https://doi.org/10.3390/molecules26144341
APA StylePickering, G. J., & Botezatu, A. (2021). A Review of Ladybug Taint in Wine: Origins, Prevention, and Remediation. Molecules, 26(14), 4341. https://doi.org/10.3390/molecules26144341