Study on the Joint Toxicity of BPZ, BPS, BPC and BPF to Zebrafish
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single Acute Toxicity of BPZ, BPC, BPF and BPS to Zebrafish
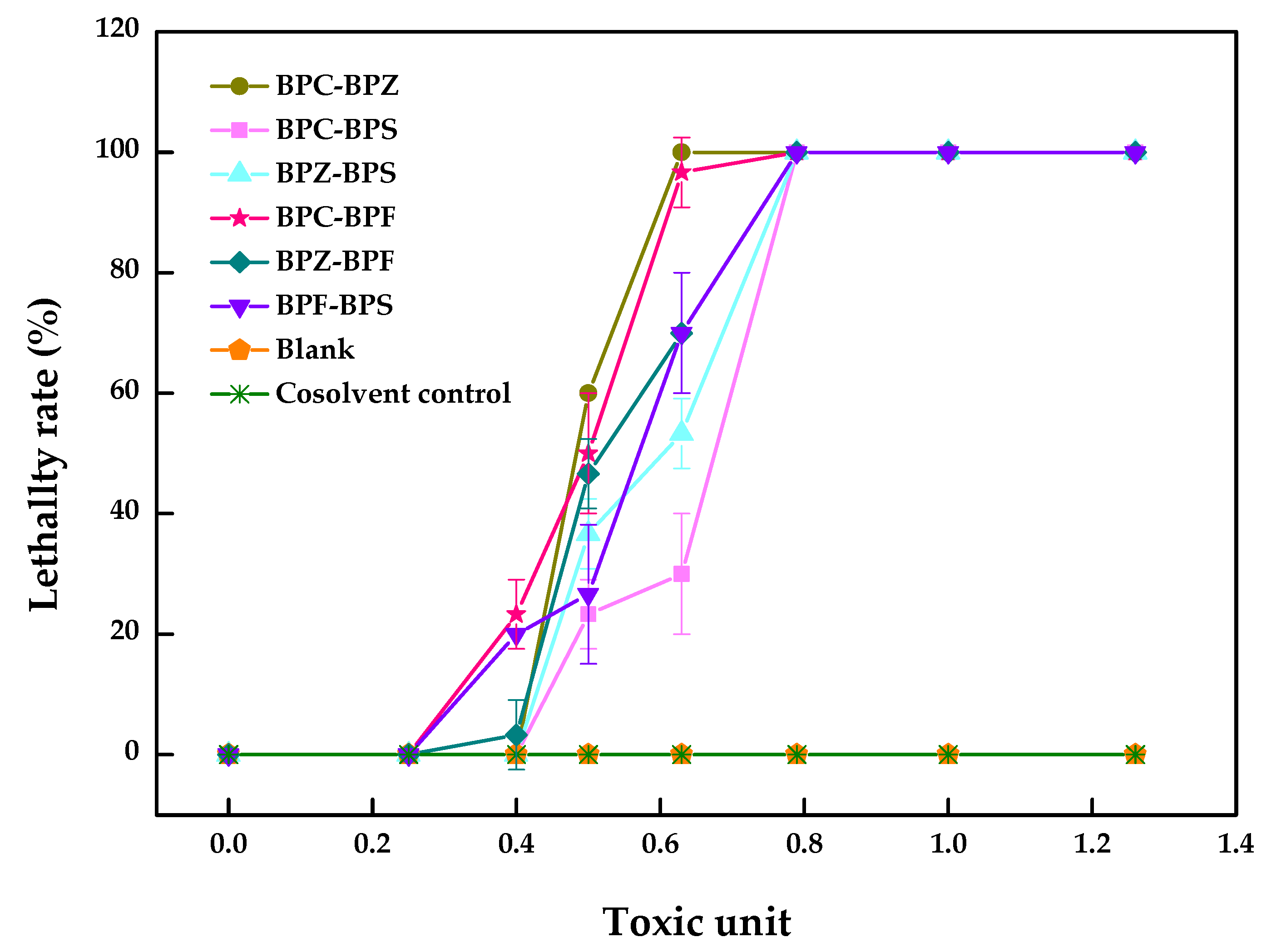
2.2. Dual Joint Acute Toxicity of BPZ, BPC, BPF and BPS to Zebrafish
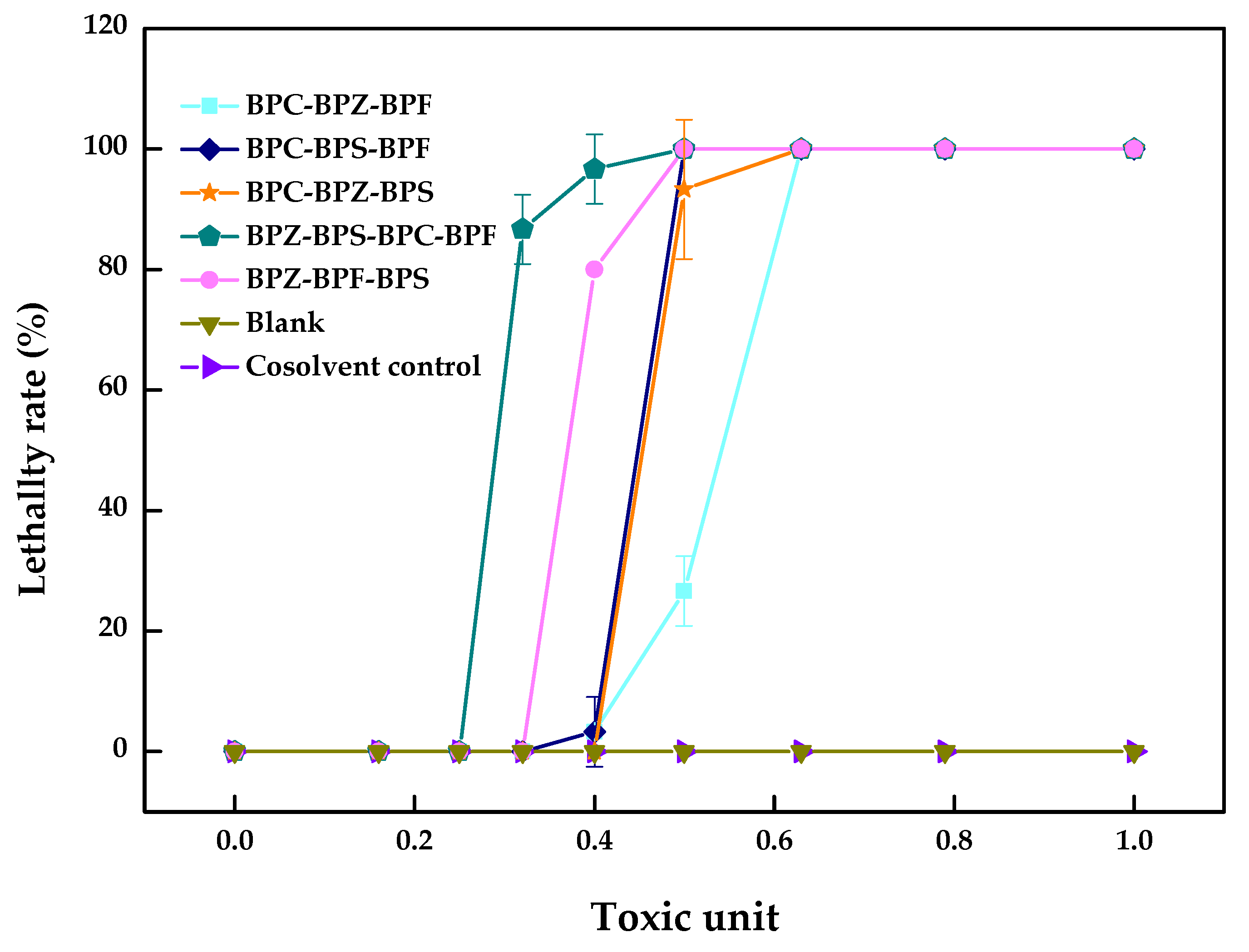
2.3. Multi-Joint Acute Toxicity of BPZ, BPC, BPF and BPS to Zebrafish
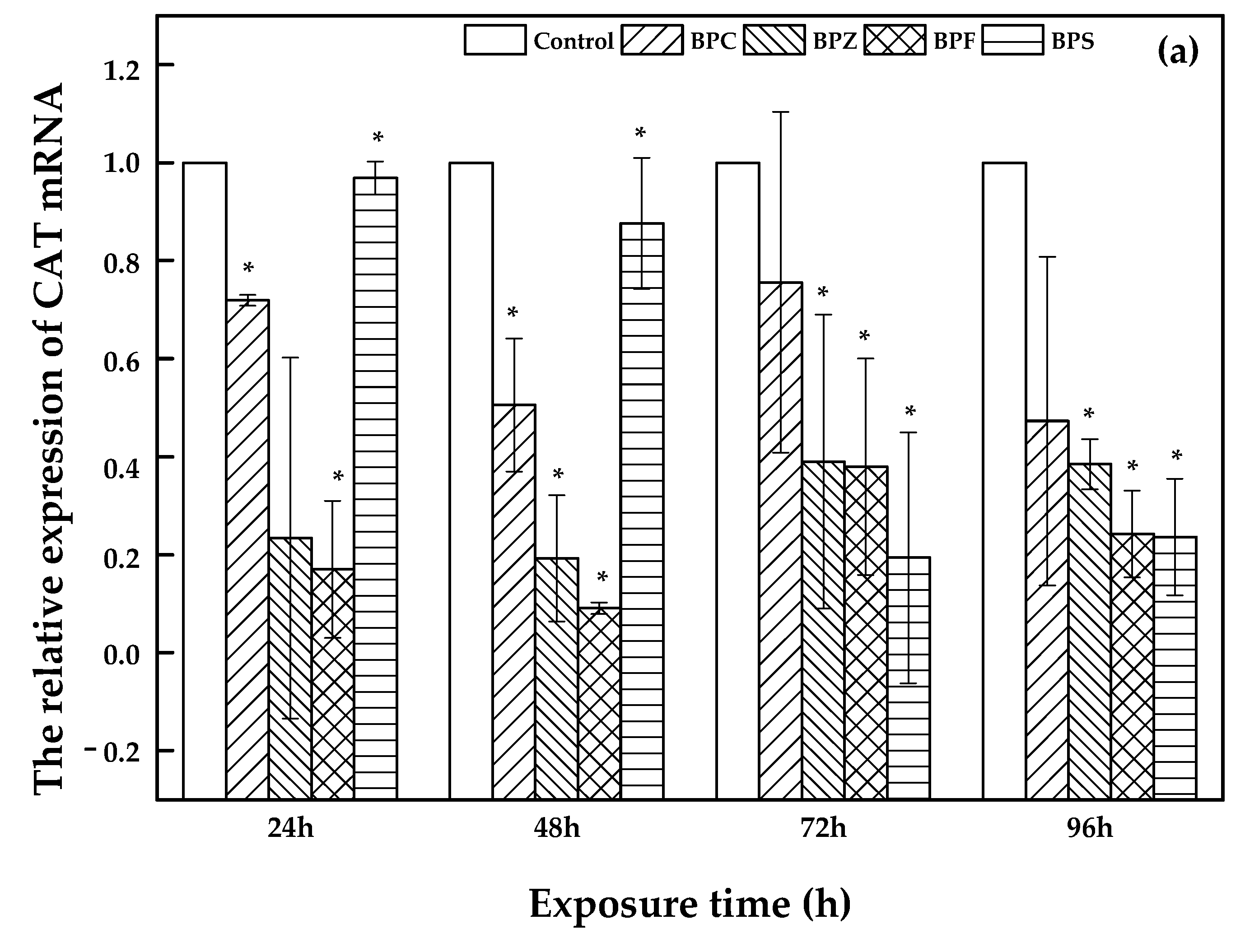
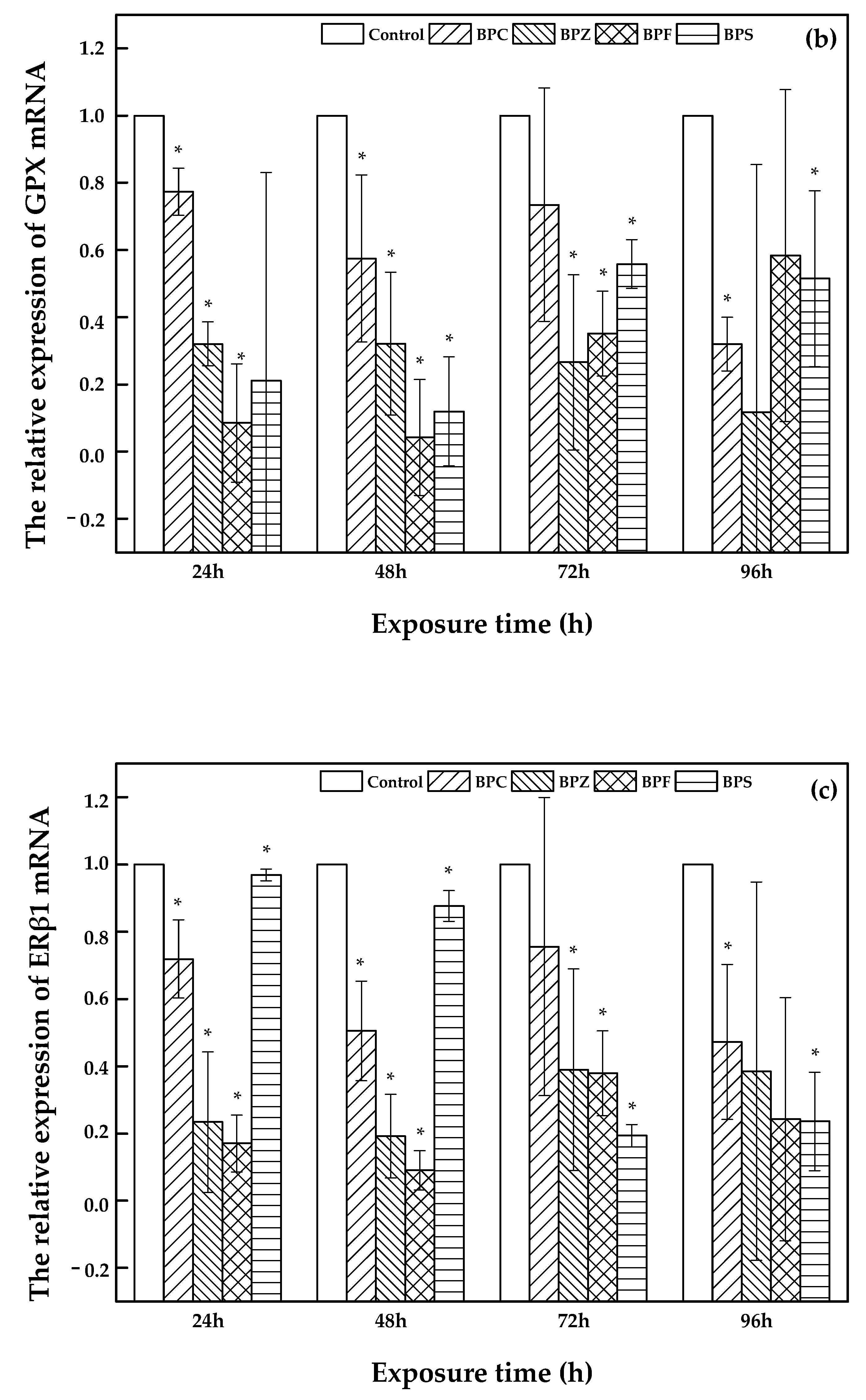
2.4. Effects of BPZ, BPC, BPF and BPS Exposure on Relevant Gene Expression
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Experimental Instruments
3.3. Experiment Design
3.3.1. Toxicity Test
3.3.2. Single Toxicity Test
3.3.3. Joint Acute Toxicity Test
3.3.4. Joint Toxicity Assessment Method
3.3.5. Gene Expression Analysis
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shigeyuki, K.; Tomoharu, S.; Seigo, S.; Ryuki, K.; Norimasa, J.; Kazumi, S.; Shin’Ichi, Y.; Nariaki, F.; Hiromitsu, W.; Shigeru, O. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. Off. J. Soc. Toxicol. 2005, 84, 249–259. [Google Scholar]
- Ruth, R.J.; Louise, B.A. Bisphenol S and F: A Systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar]
- Becerra, V.; Odermatt, J. Interferences in the direct quantification of bisphenol S in paper by means of thermochemolysis. J. Chromatogr. A 2013, 1275, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.Y.; Ren, X.M.; Li, C.H.; Zhang, J.; Qin, W.P.; Yang, Y.; Wan, B.; Guo, L.H. Bisphenol AF and bisphenol B exert higher estrogenic effects than bisphenol A via g protein-coupled estrogen receptor pathway. Environ. Sci. Technol. 2017, 51, 11423–11430. [Google Scholar] [CrossRef]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.G.; Widelka, M. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity—A review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Liao, C.; Liu, F.; Ying, G.; Moon, H.B.; Nakata, H.; Wu, Q.; Kannan, K. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: Implications for human exposure. Environ. Sci. Technol. 2012, 46, 9138–9145. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Y.; Yan, K.; Wu, S.; Han, Z.; Guo, R.; Chen, M.; Yang, Q.; Zhang, S.; Chen, J. Bisphenol analogues in surface water and sediment from the shallow Chinese freshwater lakes: Occurrence, distribution, source apportionment, and ecological and human health risk. Chemosphere 2017, 184, 318–328. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, J.; Ren, L.; Fan, R.; Zhang, J.; Liu, G.; Zhou, L.; Chen, D.; Yu, Y.; Lu, S. Urinary bisphenol analogues and triclosan in children from south China and implications for human exposure. Environ. Pollut. 2018, 238, 299. [Google Scholar] [CrossRef]
- Kha, B.; Smc, D.; Mf, A.; Sd, E.; Lka, E.; Be, A. Genotoxic activity of bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF and their mixtures in human hepatocellular carcinoma (HepG2) cells. Sci. Total Environ. 2019, 687, 267–276. [Google Scholar]
- Mu, X.; Huang, Y.; Li, X.; Lei, Y.; Teng, M.; Li, X.; Wang, C.; Li, Y. Developmental effects and estrogenicity of bisphenol A alternatives in a zebrafish embryo model. Environ. Sci. Technol. 2018, 52, 3222–3231. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.M.; Liu, H.L.; Wei, S.; Wei, S.; Giesy, J.P.; Yu, H.X. Effects of perfluorinated compounds on development of zebrafish embryos. Environ. Sci. Pollut. Res. 2011, 19, 2498–2505. [Google Scholar] [CrossRef]
- Khan, A.; Zaman, T.; Fahad, T.M.; Akther, T.; Bari, M. Perspectives of zebrafish as an animal model in the biomedical science. In Proceedings of the International Conference on Science and Technology for Celebrating the Birth Centenary of Bangabandhu (ICSTB-2021), Dhaka, Bangladesh, 11–13 March 2021. [Google Scholar]
- Zhao, F.; Jiang, G.; Wei, P.; Wang, H.; Ru, S. Bisphenol S exposure impairs glucose homeostasis in male zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2017, 147, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, C.; Shin, H.; Kho, Y.; Choi, K. Comparison of thyroid hormone disruption potentials by bisphenols A, S, F, and Z in embryo-larval zebrafish. Chemosphere 2019, 221, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Wong, M.; Gholami, F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat. Toxicol. 2014, 148, 195–203. [Google Scholar] [CrossRef]
- Ji, K.; Hong, S.; Kho, Y.; Choi, K. Effects of bisphenol s exposure on endocrine functions and reproduction of zebrafish. Environ. Sci. Technol. 2013, 47, 8793–8800. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, C. Re: A new chapter in the bisphenol a story: Bisphenol S and bisphenol F are not safe alternatives to this compound editorial comment. J. Urol. 2015, 194, 1368–1370. [Google Scholar] [CrossRef]
- Chen, M.Y. Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ. Toxicol. 2010, 17, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Busquet, F.; Halder, B.T.; Braunbeck, T.; Gourmelon, L.A.; Kleensang, A.; Belanger, S.; Carr, G.; Walter-rohde, S. OECD Guidlines for the Testing of Chemicals 236—Fish Embryo Acute Toxicity (FET) Test; The OECD Observer; Organisation for Economic Co-operation and Development: Paris, France, 2013. [Google Scholar]
- Ren, W.J.; Wang, Z.; Yang, X.H.; Liu, J.N.; Yang, Q.; Chen, Y.W.; Shen, S.B. Acute toxicity effect of bisphenol A and its analogues on adult and embryo of zebrafish. J. Ecol. Rural Environ. 2017, 33, 372–378. [Google Scholar]
- Hernández, A.F.; Gil, F.; Lacasaña, M. Toxicological interactions of pesticide mixtures: An update. Arch. Toxicol. 2017, 91, 3211–3223. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Sun, Z.H.; Yan, Z.L.; Yuan, C.X. Joint toxicity of bisphenol A and p-Nitrophenol on Misgurnus anguillicadatus. J. Northwest A F Univ. 2012, 40, 93–1600. [Google Scholar]
- Fang, N.; Zhang, S.L.; Guo-Peng, J.U. Joint toxicity effect of cresol isomers on zebrafish (Brachydanio rerio). J. Baoji Univ. Arts Sci. (Nat. Sci. Ed.) 2017, 37, 43–46. [Google Scholar]
- Fol, V.L.; Ait-Aissa, S.; Sonavane, M.; Porcher, J.M.; Balaguer, P.; Cravedi, J.P.; Zalko, D.; Brion, F. In vitro and in vivo estrogenic activity of BPA, BPF and BPS in zebrafish-specific assays. Ecotoxicoogyl. Environ. Saf. 2017, 142, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Shao, H.; Lei, P.; Zheng, C.; Qiu, C.; Ming, Y.; Yi, Z. Immunotoxicity of bisphenol S and F are similar to that of bisphenol A during zebrafish early development. Chemosphere 2017, 194, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wu, J.; Xu, S.; Zhang, L.; Fan, D.; Shi, L.; Wang, J.; Ji, G. Bisphenol F exposure impairs neurodevelopment in zebrafish larvae (Danio rerio). Ecotoxicol. Environ. Saf. 2020, 188, 109870.109871–109870.109877. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Dong, X.; Zhang, Z.; Yang, M.; Wu, X.; Liu, H.; Lao, Q.; Li, C. Assessment of immunotoxicity of dibutyl phthalate using live zebrafish embryos. Fish Shellfish Immunol. 2015, 45, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Salahinejad, A.; Attaran, A.; Naderi, M.; Meuthen, D.; Niyogi, S.; Chivers, D.P. Chronic exposure to bisphenol S induces oxidative stress, abnormal anxiety, and fear responses in adult zebrafish (Danio rerio). Sci. Total Environ. 2020, 750, 141633. [Google Scholar] [CrossRef]
- Pikulkaew, S.; Nadai, A.D.; Belvedere, P.; Colombo, L.; Valle, L.D. Expression analysis of steroid hormone receptor mRNAs during zebrafish embryogenesis. Gen. Comp. Endocrinol. 2010, 165, 215–220. [Google Scholar] [CrossRef]
- Gyimah, E.; Dong, X.; Qiu, W.; Zhang, Z.; Xu, H. Sublethal concentrations of triclosan elicited oxidative stress, DNA damage, and histological alterations in the liver and brain of adult zebrafish. Environ. Sci. Pollut. Res. 2020, 27, 17329–17338. [Google Scholar] [CrossRef] [PubMed]
- Xiu, R.Q.; Xu, Y.X.; Gao, S.R. Joint toxicity test of arsenic with cadmium and zinc ions to zebrafish, brachynanio rerio. China Environ. Sci. 1998, 18, 349–352. [Google Scholar]
- Marking, L.L. Method for assessing additive toxicity of chemical mixtures. In Aquatic Toxicology and Hazard Evaluation; ASTM International: West Conshohocken, PA, USA, 1977. [Google Scholar]
- Schmittgen, T.D. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]

| Analytes | Molecular Weight | CAS | Log Kow | Log BAF | Structures |
|---|---|---|---|---|---|
| BPC | 256.34 | 79-97-0 | 4.74 | 2.79 |  |
| BPZ | 268.35 | 843-55-0 | 5.48 | 3.28 | 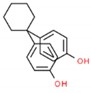 |
| BPF | 200.23 | 620-92-8 | 3.06 | 1.59 | 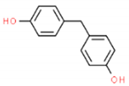 |
| BPS | 250.27 | 80-09-1 | 1.65 | 0.75 | 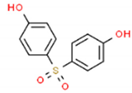 |
| Target | LC50/(µM) | S 1 | AI | Action |
|---|---|---|---|---|
| BPF–BPS | 21.7 (19.0~24.2)–3.3 × 102 (2.9 × 102~3.7 × 102) | 1.07 | −0.07 | antagonism |
| BPF–BPZ | 21.7 (19.2~24.0)–5.1 (4.6~5.7) | 1.08 | −0.08 | antagonism |
| BPF–BPC | 19.3 (15.9~21.3)–5.2 (4.2~5.7) | 0.95 | 0.05 | synergism |
| BPS–BPZ | 3.6 × 102 (3.2 × 102~4.0 × 102)–5.5 (5.0~6.2) | 1.15 | −0.15 | antagonism |
| BPS–BPC | 3.9 × 102 (3.5 × 102~4.5 × 102)–6.8 (6.1~7.9) | 1.25 | −0.25 | antagonism |
| BPZ–BPC | 4.7–5.3 | 0.99 | 0.01 | synergism |
| Target | LC50/(µM) | S | AI | Action |
|---|---|---|---|---|
| BPF–BPS–BPZ | 15.0–2.3 × 102–3.5 | 1.11 | −0.11 | antagonism |
| BPF–BPZ–BPC | 16.4 (8.2~18.9)–3.9 (1.9~4.5)–4.4 (2.2~5.0) | 1.22 | −0.22 | antagonism |
| BPS–BPZ–BPC | 2.2 × 102–3.4–3.8 | 1.05 | −0.05 | antagonism |
| BPS–BPC–BPF | 2.7 × 102–4.6–17.4 | 1.30 | −0.30 | antagonism |
| BPF–BPS–BPZ–BPC | 11.7 (6.1~13.8)–1.8 × 102 (94.2~2.1 × 102)–2.8 (1.5~3.3)–3.2 (1.6~3.7) | 1.18 | −0.18 | antagonism |
| Gene Symbol | Accession NO. | Primer Sequence(5′-3′) | Product Length (bp) |
|---|---|---|---|
| β-actin | AF025305.1 | F: CGAGCTGTCTTCCCATCCA R: TCACCAACGTAGCTGTCTTTCTG | 86 |
| rp17 | NM_213213644.2 | F: CAGAGGTATCAATGGTGTCAGCCC R: TTCGGAGCATGTTGATGGAGGC | 119 |
| CAT | AF170069.1 | F: CTCCTGATGTGGCCCGATAC R: TCAGATGCCCGGCCATATTC | 126 |
| SOD | BX055516 | F: GTCCGCACTTCAACCCTCA R: TCCTCATTGCCACCCTTCC | 217 |
| GPX | AW232474 | F: AGATGTCATTCCTGCACACG R: AAGGAGAAGCTTCCTCAGCC | 94 |
| ERα | AF268283 | F: CCC ACA GGA CAA GAG GAA GA R: CCT GGT CAT GCA GAG ACA GA | 250 |
| ERβ1 | AJ414566 | F: GGG GAG AGT TCA ACC ACG GAG R: GCT TTC GGA CAC AGG AGG ACG | 89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Fei, Y.; Wang, M.; Xue, Y.; Chen, H.; Liu, Y. Study on the Joint Toxicity of BPZ, BPS, BPC and BPF to Zebrafish. Molecules 2021, 26, 4180. https://doi.org/10.3390/molecules26144180
Han Y, Fei Y, Wang M, Xue Y, Chen H, Liu Y. Study on the Joint Toxicity of BPZ, BPS, BPC and BPF to Zebrafish. Molecules. 2021; 26(14):4180. https://doi.org/10.3390/molecules26144180
Chicago/Turabian StyleHan, Ying, Yumeng Fei, Mingxin Wang, Yingang Xue, Hui Chen, and Yuxuan Liu. 2021. "Study on the Joint Toxicity of BPZ, BPS, BPC and BPF to Zebrafish" Molecules 26, no. 14: 4180. https://doi.org/10.3390/molecules26144180
APA StyleHan, Y., Fei, Y., Wang, M., Xue, Y., Chen, H., & Liu, Y. (2021). Study on the Joint Toxicity of BPZ, BPS, BPC and BPF to Zebrafish. Molecules, 26(14), 4180. https://doi.org/10.3390/molecules26144180





