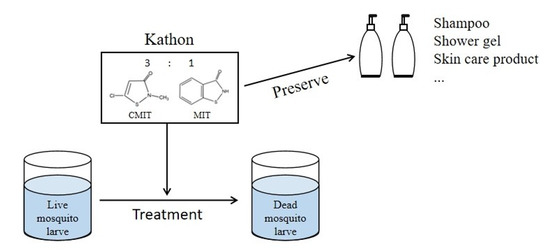
Effects of Kathon, a Chemical Used Widely as a Microbicide, on the Survival of Two Species of Mosquitoes
Abstract
:1. Introduction
2. Results
2.1. The Effects of Kathon on the Survival of C. quinquefasciatus Larvae
2.2. The Effects of Kathon on the Survival of A. albopictus Larvae
2.3. The Effects of Sublethal Concentrations of Kathon on the Larval Development Duration of C. quinquefasciatus
2.4. The Effects of CMIT and MIT on the Survival of C. quinquefasciatus Larvae
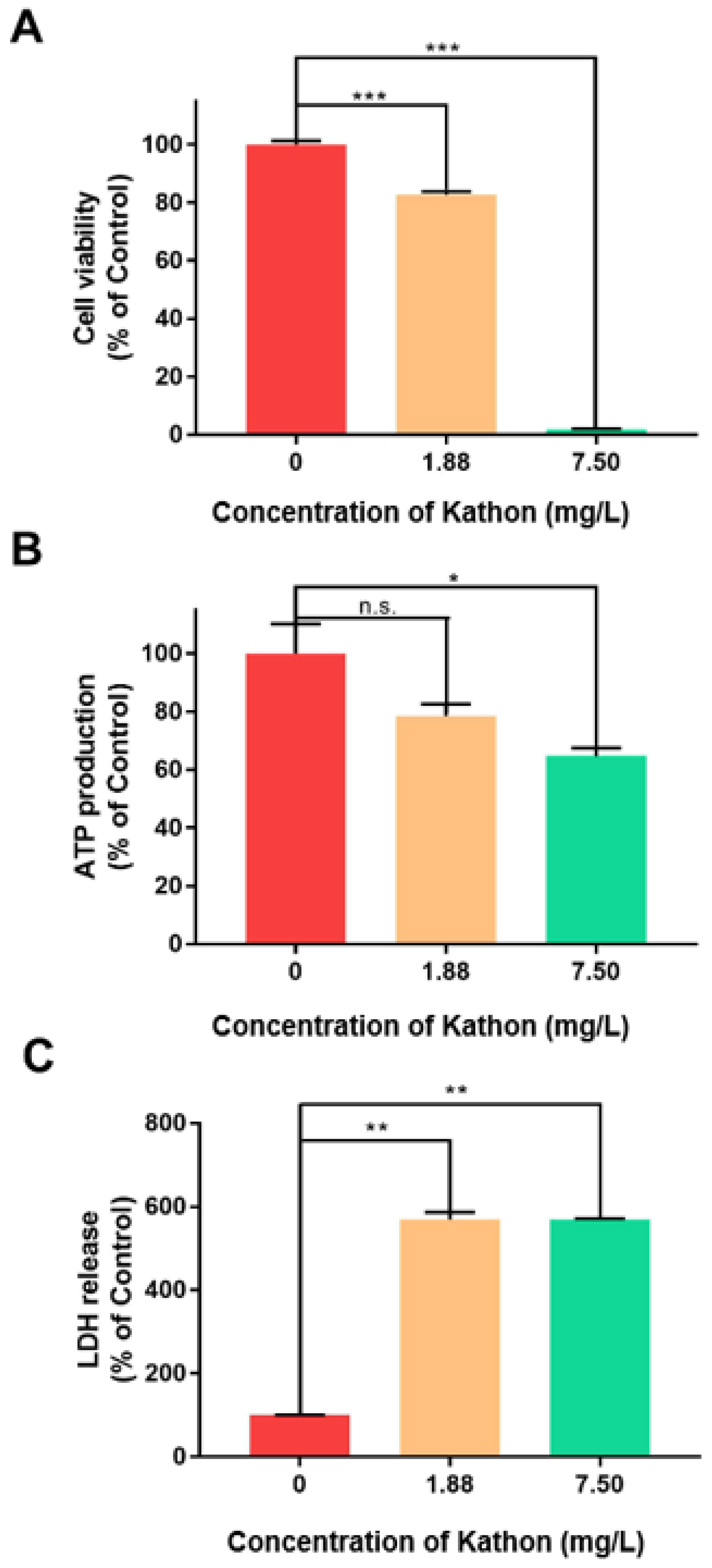
2.5. Effects of Kathon on Cell Viability, Adenosine Triphosphate (ATP) Production and Lactate Dehydrogenase Release of S2 Cells
3. Discussion and Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Mosquitoes
4.3. Effects of Kathon on Mosquito Larval Survival
4.4. Effects of Sublethal Concentrations of Kathon on the Larval Development Duration of C. quinquefasciatus
4.5. Effects of CMIT and MIT on the Survival of C. quinquefasciatus Larvae
4.6. Effect on Insect Cells
4.6.1. Cell Culture
4.6.2. Cell Viability
4.6.3. Adenosine Triphosphate (ATP) Assay
4.6.4. Lactate Dehydrogenase Assay
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Caraballo, H.; King, K. Emergency department management of mosquito-borne illness: Malaria, dengue, and West Nile virus. Emerg. Med. Pract. 2014, 16, 1–23. [Google Scholar]
- Dahmana, H.; Mediannikov, O. Mosquito-borne diseases emergence/resurgence and how to effectively control it biologically. Pathogens 2020, 9, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girarda, M.; Nelson, C.B.; Picot, V.; Gublerd, D.J. Arboviruses: A global public health threat. Vaccine 2020, 38, 3989–3994. [Google Scholar] [CrossRef] [PubMed]
- Tolle, M.A. Mosquito-borne Diseases. Curr. Probl. Pediatr. Adolesc. Health Care 2009, 39, 97–140. [Google Scholar] [CrossRef] [PubMed]
- Katureebe, A.; Zinszer, K.; Arinaitwe, E.; Rek, J.; Kakande, E.; Charland, K.; Kigozi, R.; Kilama, M.; Nankabirwa, J.; Yeka, A.; et al. Measures of malaria burden after long-lasting insecticidal net distribution and indoor residual spraying at three sites in Uganda: A prospective observational study. PLoS Med. 2016, 13, e1002167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, J.H. Chemical and plant-based insect repellents: Efficacy, safety, and toxicity. Wilderness Environ. Med. 2016, 27, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fillinger, U.; Lindsay, S.W. Larval source management for malaria control in Africa: Myths and reality. Malar. J. 2011, 10, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparks, T.C. Insecticide discovery: An evaluation and analysis. Pestic. Biochem. Physiol. 2013, 107, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Andersen, F.A. Final report of the safety assessment of methylisothiazolinone. Int. J. Toxicol. 2010, 29, 187S–213S. [Google Scholar] [CrossRef]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics preservation: A review on present strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef] [Green Version]
- Özkaya, E.; Sayar, S.K.; Kobaner, G.B.; Pehlivan, G. Methylchloroisothiazolinone/methylisothiazolinone and methylisothiazolinone contact allergy: A 24-year, single-center, retrospective cohort study from turkey. Contact Dermat. 2021, 84, 24–33. [Google Scholar] [CrossRef]
- Williams, T.M. The Mechanism of action of isothiazolone biocides. PowerPlant Chem. 2007, 9, 14–22. [Google Scholar]
- He, W.; Pan, L.; Han, W.; Wang, X. Isothiazolinones as novel candidate insecticides for the control of hemipteran insects. Antibiotics 2021, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, S.; Chang, J. Methylisothiazolinone may induce cell death and inflammatory response through DNA damage in human liver epithelium cells. Environ. Toxicol. 2018, 33, 156–166. [Google Scholar] [CrossRef]
- Raghavendra, K.; Barik, T.K.; Reddy, B.P.N.; Sharma, P.; Dash, A.P. Malaria vector control: From past to future. Parasitol. Res. 2011, 108, 757–779. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Para, J.A.; Romero Cortés, T.; Ramirez-Lepe, M. Mosquito-Borne Diseases, Pesticides Used for Mosquito Control, and Development of Resistance to Insecticides. In Insecticides Resistance; Trdan, S., Ed.; IntechOpen: London, UK, 2016; pp. 111–134. [Google Scholar]
- Marina, C.F.; Bond, J.G.; Muñoz, J.; Valle, J.; Novelo-Gutiérrez, R.; Williams, T. Efficacy and non-target impact of spinosad, Bti and temephos larvicides for control of Anopheles spp. in an endemic malaria region of southern Mexico. Parasites Vectors 2014, 7, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Amer, A.; Mehlhorn, H. Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol. Res. 2006, 99, 466–472. [Google Scholar] [CrossRef]
- Tong, F.; Bloomquist, J.R. Plant essential oils affect the toxicities of carbaryl and permethrin against Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2021, 50, 826–832. [Google Scholar] [CrossRef]
- Elder, R.L. Final report on the safety assessment of methylisothiazolinone and methylchloroisothiazolinone. JACT 1992, 11, 75–128. [Google Scholar]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.; Schneider, K.; Gartiser, S.; Heger, W.; Mangelsdorf, I. Consumer exposure to biocides—Identification of relevant sources and evaluation of possible health effects. Environ. Health 2010, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Hidalgo, E.; Schneider, D.; von Goetz, N.; Delmaarc, C.; Siegrist, M.; Hungerbühler, K. Aggregate consumer exposure to isothiazolinones via household care and personal care products: Probabilistic modelling and benzisothiazolinone risk assessment. Environ. Int. 2018, 118, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Kim, K.B.; Lee, J.Y.; Kwack, S.J.; Kwon, Y.C.; Kang, J.S.; Kim, H.S.; Lee, B.M. Risk Assessment of 5-Chloro-2-Methylisothiazol-3(2H)-One/2-Methylisothiazol-3(2H)-One (CMIT/MIT) used as a preservative in cosmetics. Toxicol. Res. 2019, 35, 103–117. [Google Scholar] [CrossRef]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Coon, K.; Vogel, K.; Brown, M.R.; Strand, M.R. Mosquitoes rely on their gut microbiota for development. Mol. Ecol. 2014, 23, 2727–2739. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, T.; Wu, Y.; Zhong, D.; Zhou, G.; Su, X.; Xu, J.; Sotero, C.F.; Sadruddin, A.A.; Wu, K.; et al. Bacterial microbiota assemblage in Aedes albopictus mosquitoes and its impacts on larval development. Mol. Ecol. 2018, 27, 2972–2985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal. Commun. 1999, 36, 47–50. [Google Scholar] [CrossRef]
| Instars | Concentration (mg/L) | Mortality (%) † | |
|---|---|---|---|
| 24 h | 48 h | ||
| 1 | 0.00 | 0.00 ± 0.00 d | 0.00 ± 0.00 c |
| 1.88 | 53.80 ± 6.70 c | 68.40 ± 6.76 b | |
| 3.75 | 79.00 ± 1.18 b | 94.40 ± 3.92 a | |
| 7.50 | 83.80 ± 5.54 b | 100.00 ± 0.00 a | |
| 15.00 | 95.80 ± 2.58 a | 97.60 ± 2.40 a | |
| 2 | 0.00 | 0.00 ± 0.00 d | 0.00 ± 0.00 d |
| 1.88 | 0.00 ± 0.00 d | 0.00 ± 0.00 d | |
| 3.75 | 31.70 ± 8.38 c | 34.63 ± 9.17 c | |
| 7.50 | 66.68 ± 3.74 b | 68.58 ± 3.53 b | |
| 15.00 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | |
| 3 | 0.00 | 0.00 ± 0.00 e | 0.00 ± 0.00 d |
| 1.88 | 70.76 ± 5.51 d | 62.62 ± 3.80 c | |
| 3.75 | 83.85 ± 1.52 c | 81.40 ± 3.54 b | |
| 7.50 | 94.43 ± 1.69 b | 100.00 ± 0.00 a | |
| 15.00 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | |
| 4 | 0.00 | 0.00 ± 0.00 d | 0.00 ± 0.00 d |
| 1.88 | 58.16 ± 2.08 c | 73.41 ± 1.65 c | |
| 3.75 | 59.86 ± 2.08 c | 85.98 ± 1.42 b | |
| 7.50 | 90.53 ± 1.09 b | 100.00 ± 0.00 a | |
| 15.00 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | |
| Instars | Concentration (mg/L) | Mortality (%) † | |
|---|---|---|---|
| 24 h | 48 h | ||
| 1 | 0.00 | 0.00 ± 0.00 e | 0.00 ± 0.00 e |
| 1.88 | 18.64 ± 10.79 d | 32.26 ± 3.02 d | |
| 3.75 | 37.37 ± 2.01 c | 42.85 ± 2.24 c | |
| 7.50 | 71.22 ± 0.51 b | 84.37 ± 3.19 b | |
| 15.00 | 90.56 ± 1.58 a | 92.35 ± 2.09 a | |
| 2 | 0.00 | 0.00 ± 0.00 e | 0.00 ± 0.00 e |
| 1.88 | 15.81 ± 9.13 d | 15.81 ± 9.13 d | |
| 3.75 | 30.57 ± 0.66 c | 36.83 ± 3.54 c | |
| 7.50 | 70.66 ± 1.86 b | 72.81 ± 1.05 b | |
| 15.00 | 89.01 ± 1.11 a | 91.31 ± 1.80 a | |
| 3 | 0.00 | 0.00 ± 0.00 d | 0.00 ± 0.00 e |
| 1.88 | 15.81 ± 9.13 c | 15.81 ± 9.13 d | |
| 3.75 | 22.66 ± 7.57 c | 32.87 ± 2.71 c | |
| 7.50 | 49.75 ± 2.90 b | 52.26 ± 2.51 b | |
| 15.00 | 79.44 ± 3.16 a | 84.23 ± 3.01 a | |
| 4 | 0.00 | 0.00 ± 0.00 d | 0.00 ± 0.00 d |
| 1.88 | 0.00 ± 0.00 d | 0.00 ± 0.00 d | |
| 3.75 | 34.90 ± 3.28 c | 34.90 ± 3.28 c | |
| 7.50 | 43.96 ± 4.75 b | 47.23 ± 2.51 b | |
| 15.00 | 85.00 ± 2.86 a | 89.35 ± 2.29 a | |
| Instars | Concentration (mg/L) | Development Time (Days) † |
|---|---|---|
| 1 | 0.00 | 3.78 ± 0.05 b |
| 0.57 | 4.41 ± 0.13 a | |
| 0.98 | 4.62 ± 0.16 a | |
| 2 | 0.00 | 3.77 ± 0.16 b |
| 3.90 | 4.12 ± 0.21 ab | |
| 5.52 | 4.35 ± 0.23 a | |
| 3 | 0.00 | 4.09 ± 0.09 c |
| 0.65 | 4.53 ± 0.12 b | |
| 1.13 | 4.88 ± 0.05 a | |
| 4 | 0.00 | 7.29 ± 0.22 c |
| 0.47 | 8.15 ± 0.21 b | |
| 1.10 | 8.79 ± 0.17 a |
| Treatment | Concentration (mg/L) | Mortality (%) † | |
|---|---|---|---|
| 24 h | 48 h | ||
| CMIT | 0.00 | 0.00 ± 0.00 c | 0.00 ± 0.00 c |
| 5.63 | 14.77 ± 3.02 b | 46.59 ± 3.41 b | |
| 11.25 | 57.50 ± 5.13 a | 69.32 ± 3.86 a | |
| MIT | 0.00 | 0.00 ± 0.00 a | 0.00 ± 0.00 b |
| 1.88 | 0.00 ± 0.00 a | 0.00 ± 0.00 b | |
| 3.75 | 0.00 ± 0.00 a | 28.44 ± 2.77 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.-Z.; Pan, L.-L.; Han, W.-H.; Abd-Rabou, S.; Liu, S.-S.; Wang, X.-W. Effects of Kathon, a Chemical Used Widely as a Microbicide, on the Survival of Two Species of Mosquitoes. Molecules 2021, 26, 4177. https://doi.org/10.3390/molecules26144177
He W-Z, Pan L-L, Han W-H, Abd-Rabou S, Liu S-S, Wang X-W. Effects of Kathon, a Chemical Used Widely as a Microbicide, on the Survival of Two Species of Mosquitoes. Molecules. 2021; 26(14):4177. https://doi.org/10.3390/molecules26144177
Chicago/Turabian StyleHe, Wen-Ze, Li-Long Pan, Wen-Hao Han, Shaaban Abd-Rabou, Shu-Sheng Liu, and Xiao-Wei Wang. 2021. "Effects of Kathon, a Chemical Used Widely as a Microbicide, on the Survival of Two Species of Mosquitoes" Molecules 26, no. 14: 4177. https://doi.org/10.3390/molecules26144177
APA StyleHe, W.-Z., Pan, L.-L., Han, W.-H., Abd-Rabou, S., Liu, S.-S., & Wang, X.-W. (2021). Effects of Kathon, a Chemical Used Widely as a Microbicide, on the Survival of Two Species of Mosquitoes. Molecules, 26(14), 4177. https://doi.org/10.3390/molecules26144177