Synthesis and Bioinformatic Characterization of New Schiff Bases with Possible Applicability in Brain Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
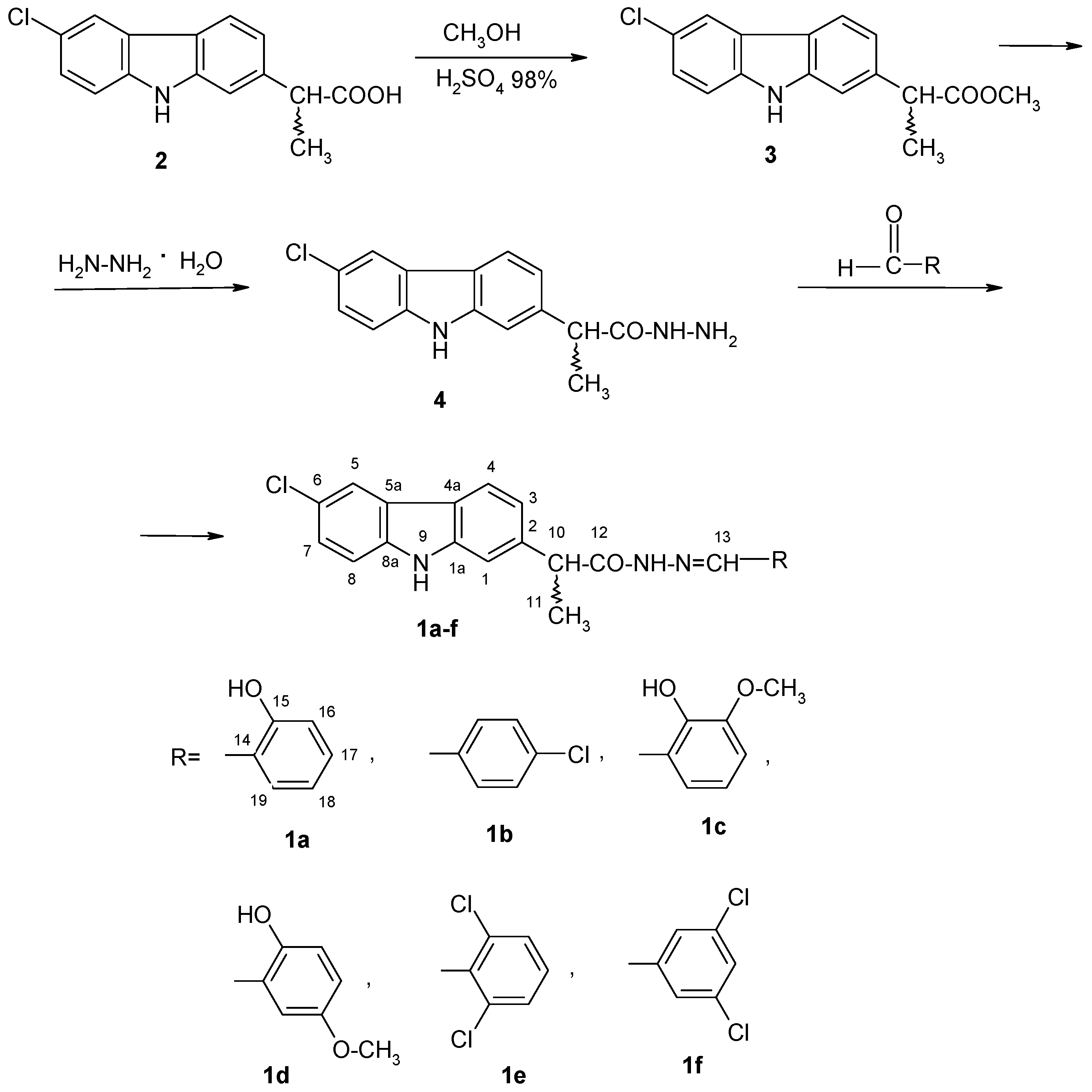
2.1.1. General Procedure for the Synthesis of (EZ)-N′-Benzylidene-(2RS)-2-(6-chloro-9H-carbazol-2-yl)propanehydrazide Derivatives
2.1.2. Spectral Data
2.2. Bioinformatic Procedure
2.2.1. Molecular Modelling of (EZ)-N′-Benzylidene-(2RS)-2-(6-chloro-9H-carbazol-2-yl)propanehydrazide Derivatives and Drug-Like Character Evaluation
2.2.2. Computational Pharmacokinetics and Pharmacogenomics Profiles of (EZ)-N′-Benzylidene-(2RS)-2-(6-chloro-9H-carbazol-2-yl)propanehydrazide Derivatives
2.2.3. Computational Pharmacodynamic Profiles of (EZ)-N′-Benzylidene-(2RS)-2-(6-chloro-9H-carbazol-2-yl)propanehydrazide Derivatives and Their Predicted ICD-10 Association
3. Results
3.1. Chemistry
3.2. Bioinformatics
3.2.1. Drug Likeness Evaluation of (EZ)-N′-Benzylidene-(2RS)-2-(6-chloro-9H-carbazol-2-yl)propanohydrazide Derivatives
3.2.2. Computational Pharmacokinetics and Pharmacogenomics Profiles of (EZ)-N′-Benzylidene-(2RS)-2-(6-chloro-9H-carbazol-2-yl)propanohydrazide Derivatives
3.2.3. Computational Pharmacodynamic Profiles of (EZ)-N′-Benzylidene-(2RS)-2-(6-chloro-9H-carbazol-2-yl)propanehydrazide Derivatives and Their Predicted ICD-10 Association
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Byman, E.; Schultz, N.; Bank, N.B.; Fex, M.; Wennström, M. Brain alpha-amylase: A novel energy regulator important in Alzheimer disease? Brain Pathol. 2018, 28, 920–932. [Google Scholar] [CrossRef] [Green Version]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.P.; Obrenovich, M.E.; Atwood, C.S.; Perry, G.; Smith, M.A. Involvement of Maillard reactions in Alzheimer disease. Neurotox. Res. 2002, 4, 191–209. [Google Scholar] [CrossRef]
- Sun, X.; Jin, L.; Ling, P. Review of drugs for Alzheimer’s disease. Drug Discov. Ther. 2012, 6, 285–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saengkhae, C.; Salerno, M.; Adès, D.; Siove, A.; Le Moyec, L.; Migonney, V.; Garnier-Suillerot, A. Ability of carbazole salts, inhibitors of Alzheimer beta-amyloid fibril formation, to cross cellular membranes. Eur. J. Pharmacol. 2007, 559, 124–131. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, M.; Li, M.; Luo, B.; Zhao, Y.; Huang, P.; Xue, F.; Rapposelli, S.; Pi, R.; Wen, S. Discovery of novel N-substituted carbazoles as neuroprotective agents with potent anti-oxidative activity. Eur. J. Med. Chem. 2013, 68, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.; Tsao, L.T.; Chang, C.S.; Chen, C.J.; Huang, L.J.; Kuo, S.C.; Lin, R.H.; Wang, J.P. Inhibition of nitric oxide production by the carbazole compound LCY-2-CHO via blockade of activator protein-1 and CCAAT/enhancer-binding protein activation in microglia. Biochem. Pharmacol. 2008, 76, 507–519. [Google Scholar] [CrossRef]
- Bordei (Telehoiu), A.T.; Nuță, D.C.; Mușat, D.G.; Missir, A.V.; Căproiu, M.T.; Dumitrașcu, F.; Zarafu, I.; Ioniță, P.; Bădiceanu, C.D.; Limban, C.; et al. Microwave assisted synthesis and spectroscopic characterization of some novel Schiff bases of carprofen hydrazide. Farmacia 2019, 67, 955–962. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 13, gkab225. [Google Scholar] [CrossRef]
- Udrea, A.-M.; Mernea, M.; Buiu, C.; Avram, S. Scutellariabaicalensis Flavones as Potent Drugs against Acute Respiratory Injury during SARS-CoV-2 Infection: Structural Biology Approaches. Processes 2020, 8, 1468. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Vogt, I.; Haque, T.; Campillos, M. HitPick: A web server for hit identification and target prediction of chemical screenings. Bioinformatics 2013, 29, 1910–1912. [Google Scholar] [CrossRef] [PubMed]
- Gallo, K.; Goede, A.; Eckert, A.; Moahamed, B.; Preissner, R.; Gohlke, B.O. PROMISCUOUS 2.0: A resource for drug-repositioning. Nucleic Acids Res. 2021, 49, D1373–D1380. [Google Scholar] [CrossRef]
- Avram, S.; Udrea, A.M.; Negrea, A.; Ciopec, M.; Duteanu, N.; Postolache, C.; Duda-Seiman, C.; Duda-Seiman, D.; Shaposhnikov, S. Prevention of Deficit in Neuropsychiatric Disorders through Monitoring of Arsenic and Its Derivatives as Well as Through Bioinformatics and Cheminformatics. Int. J. Mol. Sci. 2019, 20, 1804. [Google Scholar] [CrossRef] [Green Version]
- Jonker, J.W.; Schinkel, A.H. Pharmacological and physiological functions of the polyspecific organic cation transporters: OCT1, 2, and 3 (SLC22A1-3). J. Pharmacol. Exp. Ther. 2004, 308, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Solvo Biotechnology. Available online: https://www.solvobiotech.com/transporters/oct2 (accessed on 18 May 2021).
- Ingelman-Sundberg, M.; Sim, S.C.; Gomez, A.; Rodriguez-Antona, C. Influence of cytochrome P450 polymorphisms on drug therapies: Pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol. Ther. 2007, 496–526. [Google Scholar] [CrossRef]
- Nakajima, M.; Yokoi, T. Handbook of Pharmacogenomics and Stratified Medicine, Chapter 19—MicroRNA: Regulation of P450 and Pharmacogenetics. Fundam. Pharm. 2014, 385–401. [Google Scholar] [CrossRef]
- Tornio, A.; Backman, J.T. Advances in Pharmacology Chapter One—Cytochrome P450 in Pharmacogenetics: An Update. Adv. Pharmacol. 2018, 83, 3–32. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [Green Version]
- Palla, G.; Predieri, G.; Domano, P. Conformational Behaviour and E/Z Izomerization of N-Acyl and N-Aroylhydrazones. Tetrahedron 1986, 42, 3649–3654. [Google Scholar] [CrossRef]
- Patorski, P.; Wyrzykiewicz, E.; Bartkowiak, G. Synthesis and Conformational Assignment of N--Stilbenyloxymethylenecarbonyl-Substituted Hydrazones of Acetone and o-(m- and p-) Chloro- (nitro-) benzaldehydes by Means of 1H and 13C NMR Spectroscopy. J. Spectrosc. 2013, 197475. [Google Scholar] [CrossRef] [Green Version]
- Lopes, A.B.; Miguez, E.; Kümmerle, A.E.; Rumjanek, V.M.; Fraga, C.A.M.; Barreiro, E.J. Characterization of Amide Bond Conformers for a Novel Heterocyclic Template of N-acylhydrazone Derivatives. Molecules 2013, 18, 1683. [Google Scholar] [CrossRef] [PubMed]
- Leigh, D.A.; Marcos, V.; Nalbantoglu, T.; Vitorica-Yrezabal, I.J.; Yasar, F.T.; Xiaokang Zhu, F.T. Pyridyl-Acyl Hydrazone Rotaxanes and Molecular Shuttles. J. Am. Chem. Soc. 2017, 139, 7104–7109. [Google Scholar] [CrossRef] [Green Version]
- Lacerda, R.B.; Sales, N.M.; da Silva, L.L.; Tesch, R.; Miranda, A.P.; Barreiro, E.J.; Fernandes, P.D.; Fraga, C.A.M. Novel Potent Imidazo[1,2-a]pyridine-N-Glycinyl Hydrazone Inhibitors of TNF-a Production: In Vitro and In Vivo Studies. PLoS ONE 2014, 9, E91660. [Google Scholar] [CrossRef] [PubMed]
- Eliel, E.L.; Wilen, S.H. Stereochemistry of alkene. In Stereochemistry of Organic Compounds; John Wiley & Sons, Inc.: New York, NY, USA, 1994; pp. 539–596. [Google Scholar]
- Vlad, I.M.; Nuta, D.C.; Chirita, C.; Caproiu, M.T.; Draghici, C.; Dumitrascu, F.; Bleotu, C.; Avram, S.; Udrea, A.M.; Missir, A.V.; et al. In Silico and In Vitro Experimental Studies of New Dibenz[b,e]oxepin-11(6H)one O-(arylcarbamoyl)-oximes Designed as Potential Antimicrobial Agents. Molecules 2020, 25, 321. [Google Scholar] [CrossRef] [Green Version]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef] [Green Version]
- Nagao, M.; Sato, Y.; Yamauchi, A. A meta-analysis of PTGS1 and PTGS2 polymorphisms and NSAID intake on the risk of developing cancer. PLoS ONE 2013, 8, e71126. [Google Scholar] [CrossRef] [PubMed]

| Compound | Lipinski | Ghose | Veber | Egan | Bioavailability Score |
|---|---|---|---|---|---|
| 1a | yes | yes | yes | yes | 0.55 |
| 1b | yes | no | yes | no | 0.55 |
| 1c | yes | yes | yes | yes | 0.55 |
| 1d | yes | yes | yes | yes | 0.55 |
| 1e | yes | no | yes | no | 0.55 |
| 1f | yes | no | yes | no | 0.55 |
| ADME-Tox Features | 1a | 1b | 1c | 1d | 1e | 1f | Unit |
|---|---|---|---|---|---|---|---|
| Intestinal absorption (human) | 87.95 | 86.98 | 89.11 | 90.46 | 86.88 | 88.06 | % Absorbed |
| BBB permeability | −0.93 | 0.13 | −0.92 | −1.12 | 0.07 | 0.12 | log BB |
| CNS permeability | −1.69 | −1.43 | −1.91 | −1.93 | −1.33 | −1.31 | log PS |
| Renal OCT2 substrate | no | no | no | no | no | no | yes/no |
| AMES toxicity | yes | yes | yes | yes | yes | yes | yes/no |
| Max. tolerated dose (human) | 0.34 | 0.43 | 0.05 | 0.68 | 0.41 | 0.23 | log mg/kg/day |
| hERG I inhibitor | no | no | no | no | no | no | yes/no |
| hERG II inhibitor | yes | yes | yes | yes | yes | yes | yes/no |
| Oral Rat Acute Toxicity (LD50) | 2.17 | 1.79 | 1.29 | 1.90 | 1.84 | 1.99 | mol/kg |
| Hepatotoxicity | yes | yes | yes | yes | yes | yes | yes/no |
| P450 Cytocroms | 1a | 1b | 1c | 1d | 1e | 1f |
|---|---|---|---|---|---|---|
| CYP2D6 substrate | yes | no | no | no | no | yes |
| CYP3A4 substrate | yes | yes | yes | yes | yes | yes |
| CYP1A2 inhibitior | yes | yes | yes | yes | yes | yes |
| CYP2C19 inhibitior | yes | yes | yes | yes | yes | yes |
| CYP2C9 inhibitior | yes | yes | yes | yes | yes | yes |
| CYP2D6 inhibitior | no | no | no | no | no | no |
| CYP3A4 inhibitior | no | yes | yes | yes | yes | yes |
| Enzyme | 1a | 1b | 1c | 1d | 1e | 1f |
|---|---|---|---|---|---|---|
| Microtubule-associated protein tausubstrate similarity | 0.33 | 0.27 | 0.20 | 0.20 | 0.24 | 0.26 |
| PTGS1/PTGS 2 substrate similarity | 0.44 | 0.50 | 0.41 | 0.40 | 0.47 | 0.47 |
| ICD-10 substance use Probability | ||||||
| F10-F19:Mental and behavioural disorders due to psychoactive | - | 0.85 | - | - | 0.86 | 0.85 |
| F30-F39: Mood (affective) disorders | - | 0.83 | - | - | 0.84 | 0.83 |
| G20-G26: Extrapyramidal and movement disorders | - | 0.80 | 0.81 | - | 0.82 | 0.80 |
| G30-G32: Other degenerative diseases of the nervous system | - | - | - | - | 0.83 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avram, S.; Udrea, A.M.; Nuta, D.C.; Limban, C.; Balea, A.C.; Caproiu, M.T.; Dumitrascu, F.; Buiu, C.; Bordei, A.T. Synthesis and Bioinformatic Characterization of New Schiff Bases with Possible Applicability in Brain Disorders. Molecules 2021, 26, 4160. https://doi.org/10.3390/molecules26144160
Avram S, Udrea AM, Nuta DC, Limban C, Balea AC, Caproiu MT, Dumitrascu F, Buiu C, Bordei AT. Synthesis and Bioinformatic Characterization of New Schiff Bases with Possible Applicability in Brain Disorders. Molecules. 2021; 26(14):4160. https://doi.org/10.3390/molecules26144160
Chicago/Turabian StyleAvram, Speranta, Ana Maria Udrea, Diana Camelia Nuta, Carmen Limban, Adrian Cosmin Balea, Miron Teodor Caproiu, Florea Dumitrascu, Cătălin Buiu, and Alexandra Teodora Bordei. 2021. "Synthesis and Bioinformatic Characterization of New Schiff Bases with Possible Applicability in Brain Disorders" Molecules 26, no. 14: 4160. https://doi.org/10.3390/molecules26144160
APA StyleAvram, S., Udrea, A. M., Nuta, D. C., Limban, C., Balea, A. C., Caproiu, M. T., Dumitrascu, F., Buiu, C., & Bordei, A. T. (2021). Synthesis and Bioinformatic Characterization of New Schiff Bases with Possible Applicability in Brain Disorders. Molecules, 26(14), 4160. https://doi.org/10.3390/molecules26144160









