Resveratrol Analogues as Dual Inhibitors of Monoamine Oxidase B and Carbonic Anhydrase VII: A New Multi-Target Combination for Neurodegenerative Diseases?
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biology
2.2.1. MAO Inhibition Study
2.2.2. CA Inhibition Study
2.3. Computational Studies
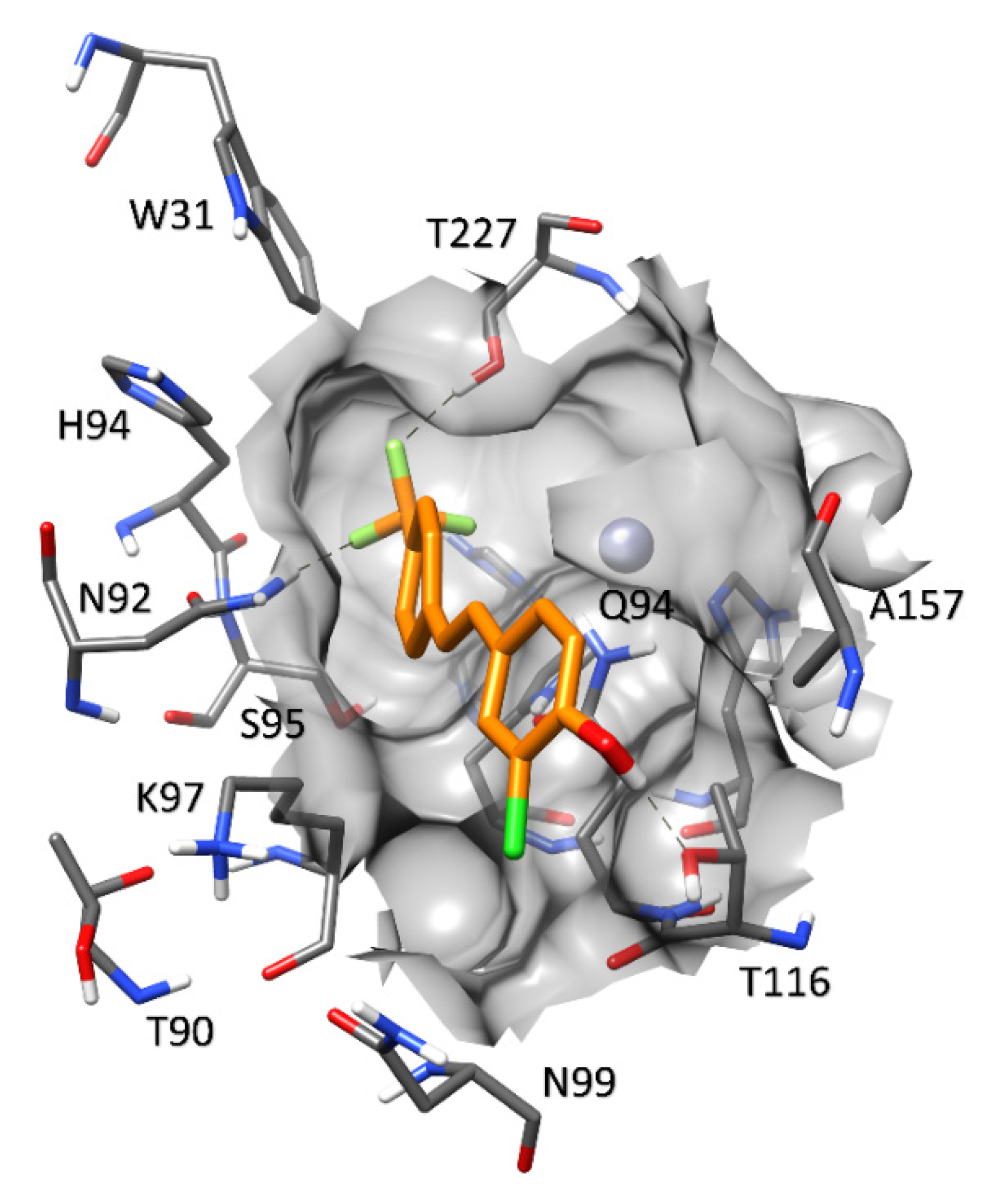
2.3.1. Monoamine Oxidase and Compounds 1–9
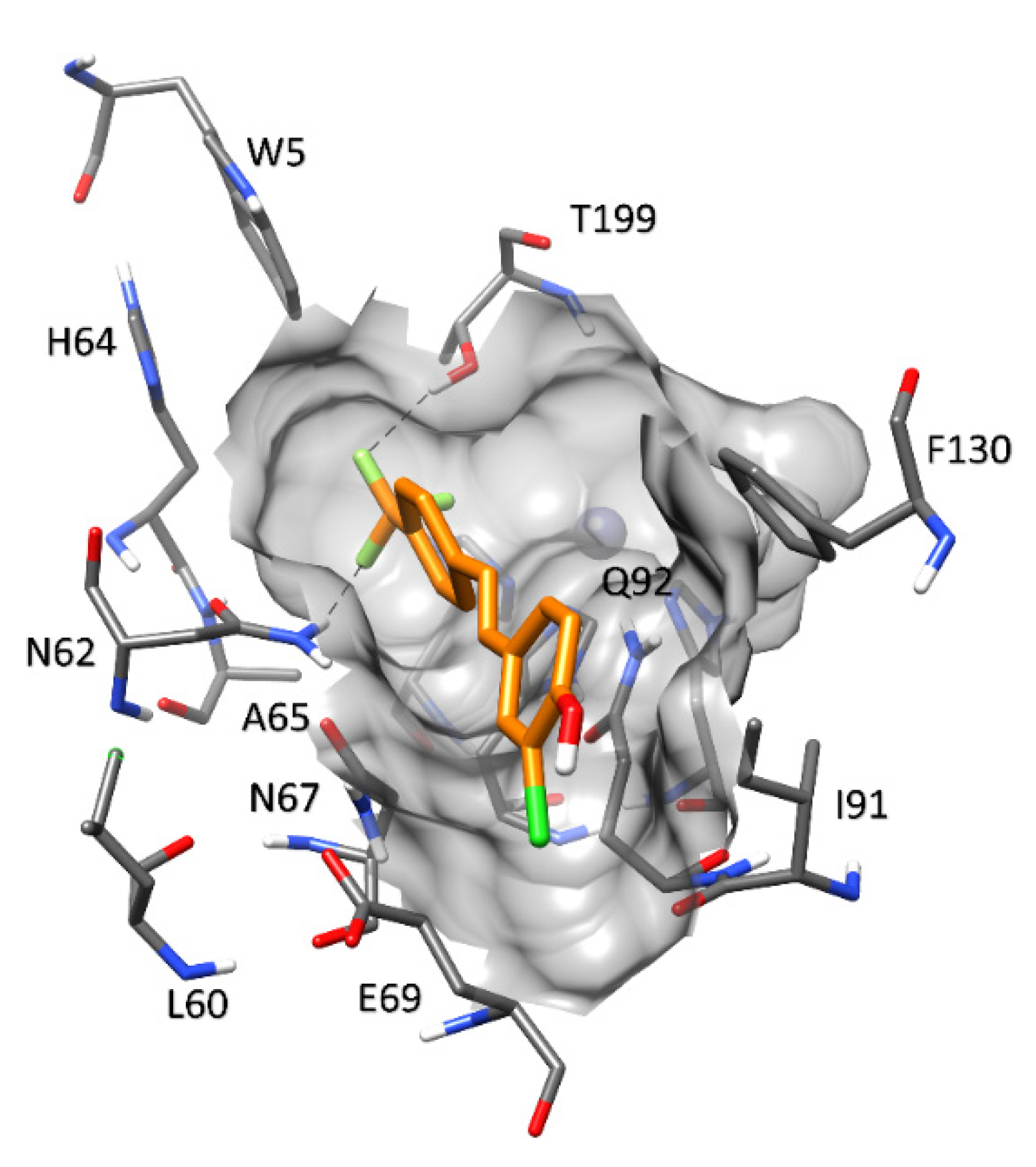
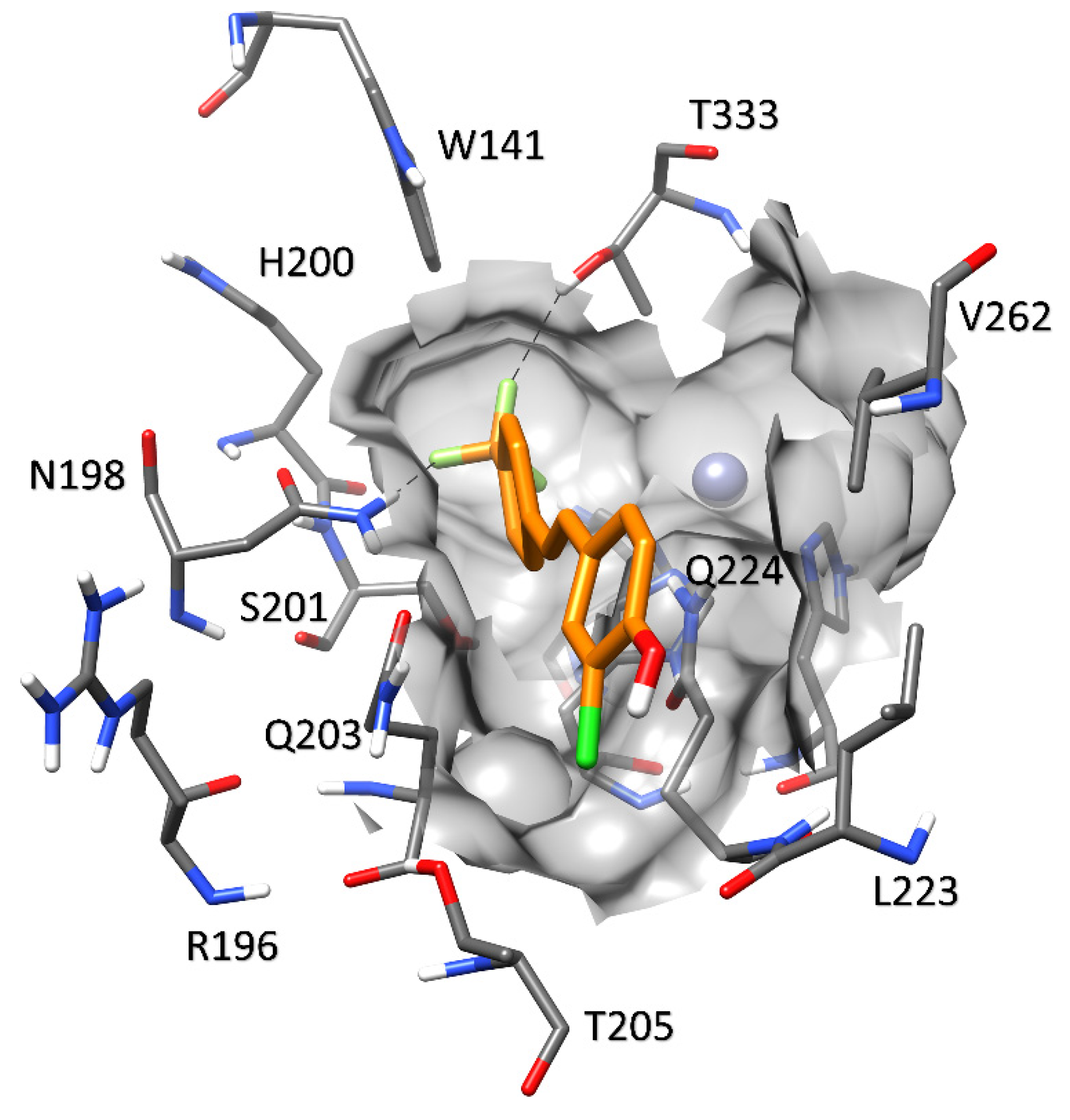
2.3.2. Carbonic Anhydrases and Compounds 1–9
2.3.3. Physicochemical and Pharmacokinetic Property Calculations
3. Materials and Methods
3.1. Chemistry
3.2. Biology
3.2.1. In Vitro MAO Inhibition Assay
3.2.2. In Vitro CA Inhibition Assay
3.3. Computational Studies
3.3.1. In Silico Studies on Monoamine Oxidases
Molecular Docking
Molecular Dynamics and MM-GBSA Calculation
3.3.2. In Silico Studies on Carbonic Anhydrases
Molecular Docking
Molecular Dynamics Simulations
Binding Energy Evaluation
3.3.3. Physicochemical and Pharmacokinetic Property Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving Neurodegeneration: Common Mechanisms and Strategies for New Treatments. Mol. Neurodegener. 2022, 17, 23–59. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.B.; Sanusi, K.O.; Ugusman, A.; Mohamed, W.; Kamal, H.; Ibrahim, N.H.; Khoo, C.S.; Kumar, J. Alzheimer’s Disease: An Update and Insights into Pathophysiology. Front. Aging Neurosci. 2022, 14, 742408. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. General Aspects of Neurodegeneration. Adv. Res. Neurodegener. 2003, 65, 101–144. [Google Scholar] [CrossRef]
- Maresova, P.; Hruska, J.; Klimova, B.; Barakovic, S.; Krejcar, O. Activities of Daily Living and Associated Costs in the Most Widespread Neurodegenerative Diseases: A Systematic Review. Clin. Interv. Aging 2020, 15, 1841–1862. [Google Scholar] [CrossRef]
- Yin, P.; Li, S.; Li, X.J.; Yang, W. New Pathogenic Insights from Large Animal Models of Neurodegenerative Diseases. Protein Cell 2022, 13, 707–720. [Google Scholar] [CrossRef]
- Martemucci, G.; Portincasa, P.; Di Ciaula, A.; Mariano, M.; Centonze, V.; D’Alessandro, A.G. Oxidative Stress, Aging, Antioxidant Supplementation and Their Impact on Human Health: An Overview. Mech. Ageing Dev. 2022, 206, 111707. [Google Scholar] [CrossRef]
- Tipton, K.F. 90 Years of Monoamine Oxidase: Some Progress and Some Confusion. J. Neural Transm. 2018, 125, 1519–1551. [Google Scholar] [CrossRef]
- Tipton, K.F.; Boyce, S.; O’Sullivan, J.; Davey, G.P.; Healy, J. Monoamine Oxidases: Certainties and Uncertainties. Curr. Med. Chem. 2004, 11, 1965–1982. [Google Scholar] [CrossRef]
- Manzoor, S.; Hoda, N. A Comprehensive Review of Monoamine Oxidase Inhibitors as Anti-Alzheimer’s Disease Agents: A Review. Eur. J. Med. Chem. 2020, 206, 112787. [Google Scholar] [CrossRef]
- Ostadkarampour, M.; Putnins, E.E. Monoamine Oxidase Inhibitors: A Review of Their Anti-Inflammatory Therapeutic Potential and Mechanisms of Action. Front. Pharmacol. 2021, 12, 676239. [Google Scholar] [CrossRef]
- Duarte, P.; Cuadrado, A.; León, R. Monoamine Oxidase Inhibitors: From Classic to New Clinical Approaches. Handb. Exp. Pharmacol. 2021, 264, 229–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lv, Y.; Bai, R.; Xie, Y. Structural Exploration of Multifunctional Monoamine Oxidase B Inhibitors as Potential Drug Candidates Against Alzheimer’s Disease. Bioorg. Chem. 2021, 114, 105070. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, P.; Carradori, S.; Ammazzalorso, A.; Secci, D. Novel Approaches to The Discovery of Selective Human Monoamine Oxidase-B Inhibitors: Is There Room for Improvement? Expert Opin. Drug Discov. 2019, 14, 995–1035. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.K.P.; Ayyannan, S.R. Monoamine Oxidase-B Inhibitors as Potential Neurotherapeutic Agents: An Overview and Update. Med. Res. Rev. 2019, 39, 1603–1706. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Jenner, P.; Chen, S.D. Monoamine Oxidase-B Inhibitors for the Treatment of Parkinson’s Disease: Past, Present, and Future. J. Park. Dis. 2022, 12, 477–493. [Google Scholar] [CrossRef]
- Lemon, N.; Canepa, E.; Ilies, M.A.; Fossati, S. Carbonic Anhydrases as Potential Targets Against Neurovascular Unit Dysfunction in Alzheimer’s Disease and Stroke. Front. Aging Neurosci. 2021, 13, 772278. [Google Scholar] [CrossRef]
- Supuran, C.T. Emerging Role of Carbonic Anhydrase Inhibitors. Clin. Sci. 2021, 135, 1233–1249. [Google Scholar] [CrossRef]
- Monti, D.M.; de Simone, G.; Langella, E.; Supuran, C.T.; Di Fiore, A.; Monti, S.M. Insights into The Role of Reactive Sulfhydryl Groups of Carbonic Anhydrase III and VII During Oxidative Damage. J. Enzyme Inhib. Med. Chem. 2017, 32, 5–12. [Google Scholar] [CrossRef]
- Alterio, V.; Di Fiore, A.; D’Ambrosio, K.; Supuran, C.T.; de Simone, G. Multiple Binding Modes of Inhibitors to Carbonic Anhydrases: How to Design Specific Drugs Targeting 15 Different Isoforms? Chem. Rev. 2012, 112, 4421–4468. [Google Scholar] [CrossRef]
- Schmidt, S.D.; Costa, A.; Rani, B.; Godfried Nachtigall, E.; Passani, M.B.; Carta, F.; Nocentini, A.; de Carvalho Myskiw, J.; Furini, C.R.G.; Supuran, C.T.; et al. The Role of Carbonic Anhydrases in Extinction of Contextual Fear Memory. Proc. Natl. Acad. Sci. USA 2020, 117, 16000–16008. [Google Scholar] [CrossRef]
- Sun, M.K.; Alkon, D.L. Pharmacological Enhancement of Synaptic Efficacy, Spatial Learning, and Memory Through Carbonic Anhydrase Activationi in Rats. J. Pharmacol. Exp. Ther. 2001, 297, 961–967. [Google Scholar]
- Yang, M.T.; Chien, W.L.; Lu, D.H.; Liou, H.C.; Fu, W.M. Acetazolamide Impairs Fear Memory Consolidation in Rodents. Neuropharmacology 2013, 67, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Canto de Souza, L.; Provensi, G.; Vullo, D.; Carta, F.; Scozzafava, A.; Costa, A.; Schmidt, S.D.; Passani, M.B.; Supuran, C.T.; Blandina, P. Carbonic Anhydrase Activation Enhances Object Recognition Memory in Mice Through Phosphorylation of the Extracellular Signal-Regulated Kinase in the Cortex and the Hippocampus. Neuropharmacology 2017, 118, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Ozsoy, H.Z. Anticonvulsant Effects of Carbonic Anhydrase Inhibitors: The Enigmatic Link Between Carbonic Anhydrases and Electrical Activity of the Brain. Neurochem. Res. 2021, 46, 2783–2799. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.D.; Nachtigall, E.G.; Marcondes, L.A.; Zanluchi, A.; Furini, C.R.G.; Passani, M.B.; Supuran, C.T.; Blandina, P.; Izquierdo, I.; Provensi, G.; et al. Modulation of Carbonic Anhydrases Activity in the Hippocampus or Prefrontal Cortex Differentially Affects Social Recognition Memory in Rats. Neuroscience 2022, 497, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Provensi, G.; Carta, F.; Nocentini, A.; Supuran, C.T.; Casamenti, F.; Passani, M.B.; Fossati, S. A New Kid on the Block? Carbonic Anhydrases as Possible New Targets in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 4724. [Google Scholar] [CrossRef]
- Blandina, P.; Provensi, G.; Passani, M.B.; Capasso, C.; Supuran, C.T. Carbonic Anhydrase Modulation of Emotional Memory. Implications For the Treatment of Cognitive Disorders. J. Enzyme Inhib. Med. Chem. 2020, 35, 1206–1214. [Google Scholar] [CrossRef]
- Akgül, Ö.; Lucarini, E.; Mannelli, L.D.C.; Ghelardini, C.; D’Ambrosio, K.; Buonanno, M.; Monti, S.M.; de Simone, G.; Angeli, A.; Supuran, C.T.; et al. Sultam based Carbonic Anhydrase VII Inhibitors for the Management of Neuropathic Pain. Eur. J. Med. Chem. 2022, 227, 113956. [Google Scholar] [CrossRef]
- Ghiasi, M.; Shahabi, P.; Supuran, C.T. Quantum Mechanical Study on the Activation Mechanism of Human Carbonic Anhydrase VII Cluster Model with Bis-Histamine Schiff Bases and Bis-Spinaceamine Derivatives. Bioorg. Med. Chem. 2021, 44, 116276. [Google Scholar] [CrossRef]
- Nocentini, A.; Cuffaro, D.; Ciccone, L.; Orlandini, E.; Nencetti, S.; Nuti, E.; Rossello, A.; Supuran, C.T. Activation of Carbonic Anhydrases from Human Brain by Amino Alcohol Oxime Ethers: Towards Human Carbonic Anhydrase VII Selective Activators. J. Enzyme Inhib. Med. Chem. 2021, 36, 48–57. [Google Scholar] [CrossRef]
- Angeli, A.; Carta, F.; Supuran, C.T. Carbonic Anhydrases: Versatile and Useful Biocatalysts in Chemistry and Biochemistry. Catalysts 2020, 10, 1008. [Google Scholar] [CrossRef]
- Solesio, M.E.; Peixoto, P.M.; Debure, L.; Madamba, S.M.; de Leon, M.J.; Wisniewski, T.; Pavlov, E.V.; Fossati, S. Carbonic Anhydrase Inhibition Selectively Prevents Amyloid β Neurovascular Mitochondrial Toxicity. Aging Cell 2018, 17, e12787. [Google Scholar] [CrossRef] [PubMed]
- Tatulian, S.A. Challenges and Hopes for Alzheimer’s Disease. Drug Discov. Today 2022, 27, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R.; Majekova, M.; Medina, M.; Valoti, M. Key Targets for Multi-Target Ligands Designed to Combat Neurodegeneration. Front. Neurosci. 2016, 10, 375. [Google Scholar] [CrossRef]
- Katselou, M.G.; Matralis, A.N.; Kourounakis, A.P. Multi-target Drug Design Approaches for Multifactorial Diseases: From Neurodegenerative to Cardiovascular Applications. Curr. Med. Chem. 2014, 21, 2743–2787. [Google Scholar] [CrossRef]
- Decker, M. Hybrid Molecules Incorporating Natural Products: Applications in Cancer Therapy, Neurodegenerative Disorders and Beyond. Curr. Med. Chem. 2011, 18, 1464–1475. [Google Scholar] [CrossRef]
- Rodríguez-Vera, D.; Abad-García, A.; Vargas-Mendoza, N.; Pinto-Almazán, R.; Farfán-García, E.D.; Morales-González, J.A.; Soriano-Ursúa, M.A. Polyphenols as Potential Enhancers of Stem Cell Therapy Against Neurodegeneration. Neural Regen. Res. 2022, 17, 2093–2101. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, T.; Mołdoch, J.; Kowalska, I.; Szponar, J.; Oniszczuk, A. Selected Natural Products in Neuroprotective Strategies for Alzheimer’s Disease—A Non-Systematic Review. Int. J. Mol. Sci. 2022, 23, 1212. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Frémont, L. Biological Effects of Resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Riccio, B.V.F.; Spósito, L.; Carvalho, G.C.; Ferrari, P.C.; Chorilli, M. Resveratrol Isoforms and Conjugates: A Review from Biosynthesis in Plants to Elimination from the Human Body. Arch. Pharm. 2020, 353, e2000146. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-C.; Ho, C.-T.; Pan, M.-H. Stilbenes: Chemistry and Molecular Mechanisms of Anti-obesity. Curr. Pharmacol. Rep. 2018, 4, 202–209. [Google Scholar] [CrossRef]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A Systemic Review on the Antioxidant and Anti-Inflammatory Effects of Resveratrol, Curcumin, and Dietary Nitric Oxide Supplementation on Human Cardiovascular Health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol Stilbenes: Molecular Mechanisms of Defence Against Oxidative Stress and Aging-Related Diseases. Oxid. Med. Cell. Longev. 2015, 2015, 340520. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.S.L.; Tan, L.T.-H.; Chan, K.-G.; Yap, W.H.; Pusparajah, P.; Chuah, L.-H.; Ming, L.C.; Khan, T.M.; Lee, L.H.; Goh, B.H. Resveratrol-Potential Antibacterial Agent Against Foodborne Pathogens. Front. Pharmacol. 2018, 9, 102–118. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and Antifungal Properties of Resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Mendonsa, R.; Koli, M.; Subramanian, M.; Nayak, S.K. Antibacterial Activity of Resveratrol Structural Analogues: A Mechanistic Evaluation of the Structure-Activity Relationship. Toxicol. Appl. Pharmacol. 2019, 367, 23–32. [Google Scholar] [CrossRef]
- Adnan, M.; Rasul, A.; Shah, M.A.; Hussain, G.; Asrar, M.; Riaz, A.; Sarfraz, I.; Hussain, A.; Khorsandi, K.; Lai, N.S.; et al. Radioprotective Role of Natural Polyphenols: From Sources to Mechanisms. Anticancer Agents Med. Chem. 2022, 22, 30–39. [Google Scholar] [CrossRef]
- Abbas, S.R.; Khan, R.T.; Shafique, S.; Mumtaz, S.; Khan, A.A.; Khan, A.M.; Hassan, Z.; Hussain, S.A.; Abbas, S.; Abbas, M.R.; et al. Study of Resveratrol Against Bone Loss by Using In-Silico and In-Vitro Methods. Braz. J. Biol. 2021, 83, e248024. [Google Scholar] [CrossRef]
- dos Santos, M.G.; Schimith, L.E.; André-Miral, C.; Muccillo-Baisch, A.L.; Arbo, B.D.; Hort, M.A. Neuroprotective Effects of Resveratrol in in Vivo and in Vitro Experimental Models of Parkinson’s Disease: A Systematic Review. Neurotox. Res. 2022, 40, 319–345. [Google Scholar] [CrossRef]
- Freyssin, A.; Page, G.; Fauconneau, B.; Bilan, R.A. Natural Stilbenes Effects in Animal Models of Alzheimer’s Disease. Neural Regen. Res. 2020, 15, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Rahman, H.; Behl, T.; Chowdhury, M.A.R.; Manirujjaman, M.; Bulbul, I.J.; Elshenaw, S.E.; Tit, D.M.; Bungau, S. Prospective Role of Polyphenolic Compounds in the Treatment of Neurodegenerative Diseases. CNS Neurol. Disord.-Drug Targets 2021, 20, 430–450. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, X.; Zeng, Q.; Ren, X.; Kong, Q. Exploring the mechanism of stilbenes to quench singlet oxygen based on the key structures of resveratrol and its analogue. Food Chem. 2023, 403, 134350. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health Benefits of Resveratrol: Evidence from Clinical Studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef] [PubMed]
- Wicinski, M.; Domanowska, A.; Wodkiewicz, E.; Malinowski, B. Neuro-Protective Properties of Resveratrol and its Derivatives-Influence on Potential Mechanisms Leading to the Development of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2749. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Parambi, D.G.T.; Mathew, G.E.; Uddin, M.S.; Inasu, S.T.; Kim, H.; Marathakam, A.; Unnikrishnan, M.K.; Carradori, S. Emerging Therapeutic Potentials of Dual-Acting MAO and Ache Inhibitors in Alzheimer’s and Parkinson’s Diseases. Arch. Pharm. 2019, 352, e1900177. [Google Scholar] [CrossRef]
- Blaikie, L.; Kay, G.; Lin, P.K.T. Current and Emerging Therapeutic Targets of Alzheimer’s Disease for the Design of Multi-Target Directed Ligands. Med. Chem. Commun. 2019, 10, 2052–2072. [Google Scholar] [CrossRef]
- Pecyna, P.; Wargula, J.; Murias, M.; Kucinska, M. More Than Resveratrol: New Insights into Stilbene-Based Compounds. Biomolecules 2020, 10, 1111. [Google Scholar] [CrossRef]
- Li, Q.-S.; Li, Y.; Deora, G.S.; Ruan, B.-F. Derivatives and Analogues of Resveratrol: Recent Advances in Structural Modification. Mini Rev. Med. Chem. 2019, 19, 809–825. [Google Scholar] [CrossRef]
- Agis-Torres, A.; Sölhuber, M.; Fernandez, M.; Sanchez-Montero, J.M. Multi-Target-Directed Ligands and other Therapeutic Strategies in the Search of a Real Solution for Alzheimer’s Disease. Curr. Neuropharmacol. 2014, 12, 2–36. [Google Scholar] [CrossRef]
- Tang, W.; Shi, C.J.; Yang, H.L.; Cai, P.; Liu, Q.H.; Yang, X.L.; Kong, L.Y.; Wang, X.B. Synthesis and Evaluation of Isoprenylation-Resveratrol Dimer Derivatives Against Alzheimer’s Disease. Eur. J. Med. Chem. 2019, 163, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Carradori, S.; D’Ascenzio, M.; Chimenti, P.; Secci, D.; Bolasco, A. Selective MAO-B Inhibitors: A Lesson from Natural Products. Mol. Divers. 2014, 18, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, P.; Carradori, S.; Poli, G.; Secci, D.; Cirilli, R.; Rotondi, G.; Chimenti, P.; Petzer, A.; Petzer, J.P. Design, Synthesis, Docking Studies and Monoamine Oxidase Inhibition of a Small Library of 1-acetyl- and 1-thiocarbamoyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazoles. Molecules 2019, 24, 484. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Uddin, M.S.; Rahman, M.A.; Samsuzzaman, M.; Behl, T.; Hafeez, A.; Perveen, A.; Barreto, G.E.; Ashraf, G.M. Exploring the Role of Monoamine Oxidase Activity in Aging and Alzheimer’s Disease. Curr. Pharm. Des. 2021, 27, 4017–4029. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and Pharmacokinetics of Resveratrol and Pterostilbene. Biofactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Khatoon, S.; Kalam, N.; Shaikh, M.F.; Hasnain, M.S.; Hafiz, A.K.; Ansari, M.T. Nanoencapsulation of Polyphenols as Drugs and Supplements for Enhancing Therapeutic Profile—A Review. Curr. Mol. Pharmacol. 2022, 15, 77–107. [Google Scholar] [CrossRef]
- De Filippis, B.; Ammazzalorso, A.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R. Anticancer Activity of Stilbene-Based Derivatives. ChemMedChem 2017, 12, 558–570. [Google Scholar] [CrossRef]
- Giacomini, E.; Rupiani, S.; Guidotti, L.; Recanatini, M.; Roberti, M. The Use of Stilbene Scaffold in Medicinal Chemistry and Multi-Target Drug Design. Curr. Med. Chem. 2016, 23, 2439–2489. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Mukazhanova, Z.; Knut, E.; Turgumbayeva, A.; Kipchakbayeva, A.; Seitimova, G.; Mahomoodally, M.F.; Lobine, D.; Koay, A.; et al. Resveratrol-Based Nanoformulations as an Emerging Therapeutic Strategy for Cancer. Front. Mol. Biosci. 2021, 8, 649395. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update. Antioxidants 2019, 8, 244. [Google Scholar] [CrossRef]
- Poltronieri, P.; Xu, B.; Giovinazzo, G. Resveratrol and other Stilbenes: Effects on Dysregulated Gene Expression in Cancers and Novel Delivery Systems. Anticancer Agents Med. Chem. 2021, 21, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Fantacuzzi, M.; Amoroso, R.; Carradori, S.; de Filippis, B. Resveratrol-Based Compounds and Neurodegeneration: Recent Insight in Multitarget Therapy. Eur. J. Med. Chem. 2022, 233, 114242. [Google Scholar] [CrossRef] [PubMed]
- Giampietro, L.; Gallorini, M.; de Filippis, B.; Amoroso, R.; Cataldi, A.; di Giacomo, V. PPAR-γ Agonist GL516 Reduces Oxidative Stress and Apoptosis Occurrence in a Rat Astrocyte Cell Line. Neurochem. Int. 2019, 126, 239–245. [Google Scholar] [CrossRef]
- Mishraa, P.; Kumar, A.; Panda, G. Anti-Cholinesterase Hybrids as Multi-Target Directed Ligands Against Alzheimer’s Disease (1998–2018). Bioorg. Med. Chem. 2019, 27, 895–930. [Google Scholar] [CrossRef]
- Queiroz, A.N.; Gomes, B.A.; Moraes, W.M., Jr.; Borges, R.S. A Theoretical Antioxidant Pharmacophore for Resveratrol. Eur. J. Med. Chem. 2009, 44, 1644–1649. [Google Scholar] [CrossRef]
- De Filippis, B.; de Lellis, L.; Florio, R.; Ammazzalorso, A.; Amoia, P.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R.; Veschi, S.; et al. Synthesis and Cytotoxic Effects on Pancreatic Cancer Cells of Resveratrol Analogs. Med. Chem. Res. 2019, 28, 984–991. [Google Scholar] [CrossRef]
- Fantacuzzi, M.; Gallorini, M.; Gambacorta, N.; Ammazzalorso, A.; Aturki, Z.; Balaha, M.; Carradori, S.; Giampietro, L.; Maccallini, C.; Cataldi, A.; et al. Design, Synthesis and Biological Evaluation of Aromatase Inhibitors based on Sulfonates and Sulfonamides of Resveratrol. Pharmaceuticals 2021, 14, 984. [Google Scholar] [CrossRef]
- Di Fermo, P.; Di Lodovico, S.; Amoroso, R.; de Filippis, B.; D’Ercole, S.; Di Campli, E.; Cellini, L.; Di Giulio, M. Searching for New Tools to Counteract the Helicobacter Pylori Resistance: The Positive Action of Resveratrol Derivatives. Antibiotics 2020, 9, 891. [Google Scholar] [CrossRef]
- di Filippo, E.S.; Giampietro, L.; de Filippis, B.; Balaha, M.; Ferrone, V.; Locatelli, M.; Pietrangelo, T.; Tartaglia, A.; Amoroso, A.; Fulle, S. Synthesis and Biological Evaluation of Halogenated E-Stilbenols as Promising Antiaging Agents. Molecules 2020, 25, 5770. [Google Scholar] [CrossRef] [PubMed]
- Orgován, G.; Gonda, I.; Noszál, B. Biorelevant physicochemical profiling of (E)-and (Z)-resveratrol determined from isomeric mixtures. J. Pharm. Biomed. Anal. 2017, 138, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.-S.; Liu, Y.; Hou, J.; Yang, J.; Zhang, X.-Y.; Zhao, Y.; Xie, S.-S.; Ding, Y.; Zhang, T. Design, Synthesis and Evaluation of Resveratrol-Indazole Hybrids as Novel Monoamine Oxidases Inhibitors with Amyloid-B Aggregation Inhibition. Bioorg. Chem. 2018, 76, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Saha, S.C.; Sunita, K.; Majumder, M.; Ghorai, M.; Mane, B.A.; Prasanth, D.A.; Kumar, P.; Kumar Pandey, D.; Al-Tawaha, A.R.; et al. Promising Botanical-Derived Monoamine Oxidase (MAO) Inhibitors: Pharmacological Aspects and Structure-Activity Studies. S. Afr. J. Bot. 2022, 146, 127–145. [Google Scholar] [CrossRef]
- Yáñez, M.; Fraiz, N.; Cano, E.; Orallo, F. Inhibitory effects of cis- and trans-resveratrol on noradrenaline and 5-hydroxytryptamine uptake and on monoamine oxidase activity. Biochem. Biophys. Res. Commun. 2006, 344, 688–695. [Google Scholar] [CrossRef]
- Herraiz, T.; Flores, A.; Fernández, L. Analysis of monoamine oxidase (MAO) enzymatic activity by high-performance liquid chromatography-diode array detection combined with an assay of oxidation with a peroxidase and its application to MAO inhibitors from foods and plants. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1073, 136–144. [Google Scholar] [CrossRef]
- Li, W.; Yang, X.; Song, Q.; Cao, Z.; Shi, Y.; Deng, Y.; Zhang, L. Pyridoxine-resveratrol hybrids as novel inhibitors of MAO-B with antioxidant and neuroprotective activities for the treatment of Parkinson’s disease. Bioorg. Chem. 2020, 97, 103707. [Google Scholar] [CrossRef]
- Provensi, G.; Costa, A.; Rani, B.; Becagli, M.V.; Vaiano, F.; Passani, M.B.; Tanini, D.; Capperucci, A.; Carradori, S.; Petzer, J.P.; et al. New β-Arylchalcogeno Amines with Procognitive Properties Targeting Carbonic Anhydrases and Monoamine Oxidases. Eur. J. Med. Chem. 2022, 244, 114828–114839. [Google Scholar] [CrossRef]
- Reniers, J.; Robert, S.; Frederick, R.; Masereel, B.; Vincent, S.; Wouters, J. Synthesis and Evaluation of beta-Carboline Derivatives as Potential Monoamine Oxidase Inhibitors. Bioorg. Med. Chem. 2011, 19, 134–144. [Google Scholar] [CrossRef]
- Zhang, Z.; Hamada, H.; Gerk, P.M. Selectivity of Dietary Phenolics for Inhibition of Human Monoamine Oxidases A and B. Biomed. Res. Int. 2019, 2019, 8361858. [Google Scholar] [CrossRef]
- Sentürk, M.; Gülçin, I.; Beydemir, S.; Küfrevioğlu, O.İ.; Supuran, C.T. In Vitro Inhibition of Human Carbonic Anhydrase I and II Isozymes with Natural Phenolic Compounds. Chem. Biol. Drug Des. 2011, 77, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Gülçin, I.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Antioxidant polyphenols effectively inhibit mammalian isoforms I–XV. Bioorg. Med. Chem. Lett. 2010, 20, 5050–5053. [Google Scholar] [CrossRef] [PubMed]
- Son, S.-Y.; Ma, J.; Kondou, Y.; Yoshimura, M.; Yamashita, E.; Tsukihara, T. Structure of Human Monoamine Oxidase A at 2.2-Å Resolution: The Control of Opening the Entry for Substrates/Inhibitors. Proc. Natl. Acad. Sci. USA 2008, 105, 5739–5744. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Wang, J.; Pisani, L.; Caccia, C.; Carotti, A.; Salvati, P.; Edmondson, D.E.; Mattevi, A. Structures of Human Monoamine Oxidase B Complexes with Selective Noncovalent Inhibitors: Safinamide and Coumarin Analogs. J. Med. Chem. 2007, 5848–5852. [Google Scholar] [CrossRef]
- Binda, C.; Li, M.; Hubálek, F.; Restelli, N.; Edmondson, D.E.; Mattevi, A. Insights into the Mode of Inhibition of Human Mitochondrial Monoamine Oxidase B from High-Resolution Crystal Structures. Proc. Natl. Acad. Sci. USA 2003, 100, 9750–9755. [Google Scholar] [CrossRef] [PubMed]
- Strolin Benedetti, M.S.; Marrari, P.; Colombo, M.; Castelli, M.G.; Arand, M.; Oesch, F.; Dostert, P. The Anticonvulsant FCE 26743 is a Selective and Short-Acting MAO-B Inhibitor Devoid of Inducing Properties Towards Cytochrome P450-Dependent Testosterone Hydroxylation in Mice and Rats. J. Pharm. Pharmacol. 1994, 46, 814–819. [Google Scholar] [CrossRef]
- Carotti, A.; Altomare, C.; Catto, M.; Gnerre, C.; Summo, L.; de Marco, A.; Rose, S.; Jenner, P.; Testa, B. Lipophilicity Plays a Major Role in Modulating the Inhibition of Monoamine Oxidase B by 7-Substituted Coumarins. Chem. Biodivers. 2006, 3, 134–149. [Google Scholar] [CrossRef]
- Tandarić, T.; Vianello, R. Computational Insight into the Mechanism of the Irreversible Inhibition of Monoamine Oxidase Enzymes by the Antiparkinsonian Propargylamine Inhibitors Rasagiline and Selegiline. ACS Chem. Neurosci. 2019, 10, 3532–3542. [Google Scholar] [CrossRef]
- Tandarić, T.; Prah, A.; Stare, J.; Mavri, J.; Vianello, R. Hydride Abstraction as the Rate-Limiting Step of the Irreversible Inhibition of Monoamine Oxidase B by Rasagiline and Selegiline: A Computational Empirical Valence Bond Study. Int. J. Mol. Sci. 2020, 21, 6151. [Google Scholar] [CrossRef]
- Martin, D.P.; Cohen, S.M. Nucleophile Recognition as an Alternative Inhibition Mode for Benzoic Acid Based Carbonic Anhydrase Inhibitors. Chem. Commun. 2012, 48, 5259–5261. [Google Scholar] [CrossRef]
- Güzel-Akdemir, Ö.; Demir-Yazıcı, K.; Vullo, D.; Supuran, C.T.; Akdemir, A. New Pyridinium Salt Derivatives of 2-(Hydrazinocarbonyl)-3-phenyl-1H-indole-5-sulfonamide as Selective Inhibitors of Tumour-Related Human Carbonic Anhydrase Isoforms IX and XII. Anticancer Agents Med. Chem. 2022, 22, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Temperini, C.; Pochet, L.; Masereel, B.; Scozzafava, A.; Supuran, C.T. Deciphering the Mechanism of Carbonic Anhydrase Inhibition with Coumarins and Thiocoumarins. J. Med. Chem. 2010, 53, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2021-4: Maestro, Glide, Protein Preparation Wizard, Epik, SiteMap, QikProp, MacroModel, Desmond, Prime; Schrödinger, LLC.: New York, NY, USA, 2021.
- Weissbach, H.; Smith, T.E.; Daly, J.W.; Witko, B.; Udenfriend, S. A rapid Spectrophotometric Assay of Mono-Amine Oxidase Based on the Rate of Disappearance of Kynuramine. J. Biol. Chem. 1960, 235, 1160–1163. [Google Scholar] [CrossRef]
- Mostert, S.; Petzer, A.; Petzer, J.P. Indanones as High-Potency Reversible Inhibitors of Monoamine Oxidase. ChemMedChem 2015, 10, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Khalifah, R.G. The Carbon Dioxide Hydration Activity of Carbonic Anhydrase. I. Stop-Flow Kinetic Studies on the Native Human Isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [CrossRef]
- Thacker, P.S.; Mohammed, A.; Supuran, C.T.; Tiwari, P.L.; Goud, N.S.; Srikanth, D.; Angeli, A. Synthesis and Biological Evaluation of Coumarin Carboxamides as Selective and Potent Inhibitors of Carbonic Anhydrases IX and XII. Anticancer Agents Med. Chem. 2022, 22, 2647–2654. [Google Scholar] [CrossRef]
- Carradori, S.; de Monte, C.; D’Ascenzio, M.; Secci, D.; Celik, G.; Ceruso, M.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Salen and Tetrahydrosalen Derivatives Act as Effective Inhibitors of The Tumor-Associated Carbonic Anhydrase XII—A New Scaffold for Designing Isoform-Selective Inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 6759–6763. [Google Scholar] [CrossRef]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and Ligand Preparation: Parameters, Protocols, and Influence on Virtual Screening Enrichments. J. Comput.-Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Halgren, T. Identifying and Characterizing Binding Sites and Assessing Druggability. J. Chem. Inf. Model. 2009, 49, 377–389. [Google Scholar] [CrossRef]
- Halgren, T. New Method for Fast and Accurate Binding-site Identification and Analysis. Chem. Biol. Drug Des. 2007, 69, 146–148. [Google Scholar] [CrossRef]
- Greenwood, J.R.; Calkins, D.; Sullivan, A.P.; Shelley, J.C. Towards the comprehensive, rapid, and accurate prediction of the favorable tautomeric states of drug-like molecules in aqueous solution. J. Comput. Aided Mol. Des. 2010, 24, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Shelley, J.C.; Cholleti, A.; Frye, L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A Software Program for Pka Prediction and Protonation State Generation for Drug-Like Molecules. J. Comput.-Aided Mol. Design 2007, 21, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 2. Enrichment Factors in Database Screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shaw, D.E.; Shelley, M.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.; Kannan, K.K. Drug-Protein Interactions. Refined Structures of Three Sulfonamide Drug Complexes of Human Carbonic Anhydrase I Enzyme. J. Mol. Biol. 1994, 243, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Leitans, J.; Kazaks, A.; Balod, A.; Ivanova, J.; Zalubovskis, R.; Supuran, C.T.; Tars, K. Efficient Expression and Crystallization System of Cancer-Associated Carbonic Anhydrase Isoform IX. J. Med. Chem. 2015, 25, 9004–9009. [Google Scholar] [CrossRef] [PubMed]
- Whittington, D.A.; Waheed, A.; Ulmasov, B.; Shah, G.N.; Grubb, J.H.; Sly, W.S.; Christianson, D.W. Crystal Structure of the Dimeric Extracellular Domain of Human Carbonic Anhydrase XII, a Bitopic Membrane Protein Overexpressed in Certain Cancer Tumor Cells. Proc. Natl. Acad. Sci. USA 2001, 98, 9545–9550. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Poli, G.; Bozdag, M.; Berrino, E.; Angeli, A.; Tuccinardi, T.; Carta, F.; Supuran, C.T. N-Aryl-N′-Ureido-O-Sulfamates as Potent and Selective Inhibitors of HCA VB over HCA VA: Deciphering the Binding Mode of New Potential Agents in Mitochondrial Dysfunctions. Bioorg. Chem. 2020, 100, 103896. [Google Scholar] [CrossRef]
- Bozdag, M.; Poli, G.; Angeli, A.; Lucarini, E.; Tuccinardi, T.; Di Cesare Mannelli, L.; Selleri, S.; Ghelardini, C.; Winum, J.-Y.; Carta, F.; et al. N-Aryl-N′-Ureido-O-Sulfamates: Potent and Selective Inhibitors of the Human Carbonic Anhydrase VII Isoform with Neuropathic Pain Relieving Properties. Bioorg. Chem. 2019, 89, 103033. [Google Scholar] [CrossRef]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved Protein–Ligand Docking Using GOLD. Proteins Struct. Funct. Bioinform. 2003, 52, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Tuccinardi, T.; Nuti, E.; Ortore, G.; Supuran, C.T.; Rossello, A.; Martinelli, A. Analysis of Human Carbonic Anhydrase II: Docking Reliability and Receptor-Based 3D-QSAR Study. J. Chem. Inf. Model. 2007, 47, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Berryman, J.T.; Betz, R.M.; Cerutti, D.S.; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Homeyer, N.; et al. AMBER, Version 20; University of California: San Francisco, CA, USA, 2020. [Google Scholar]
- D’Ascenzio, M.; Secci, D.; Carradori, S.; Zara, S.; Guglielmi, P.; Cirilli, R.; Pierini, M.; Poli, G.; Tuccinardi, T.; Angeli, A.; et al. 1,3-Dipolar Cycloaddition, HPLC Enantioseparation, and Docking Studies of Saccharin/Isoxazole and Saccharin/Isoxazoline Derivatives as Selective Carbonic Anhydrase IX and XII Inhibitors. J. Med. Chem. 2020, 63, 2470–2488. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Li Puma, S.; Landini, L.; Tuccinardi, T.; Poli, G.; Preti, D.; de Siena, G.; Patacchini, R.; Tsagareli, M.G.; Geppetti, P.; et al. The Acyl-Glucuronide Metabolite of Ibuprofen Has Analgesic and Anti-inflammatory Effects via the TRPA1 Channel. Pharmacol. Res. 2019, 142, 127–139. [Google Scholar] [CrossRef]
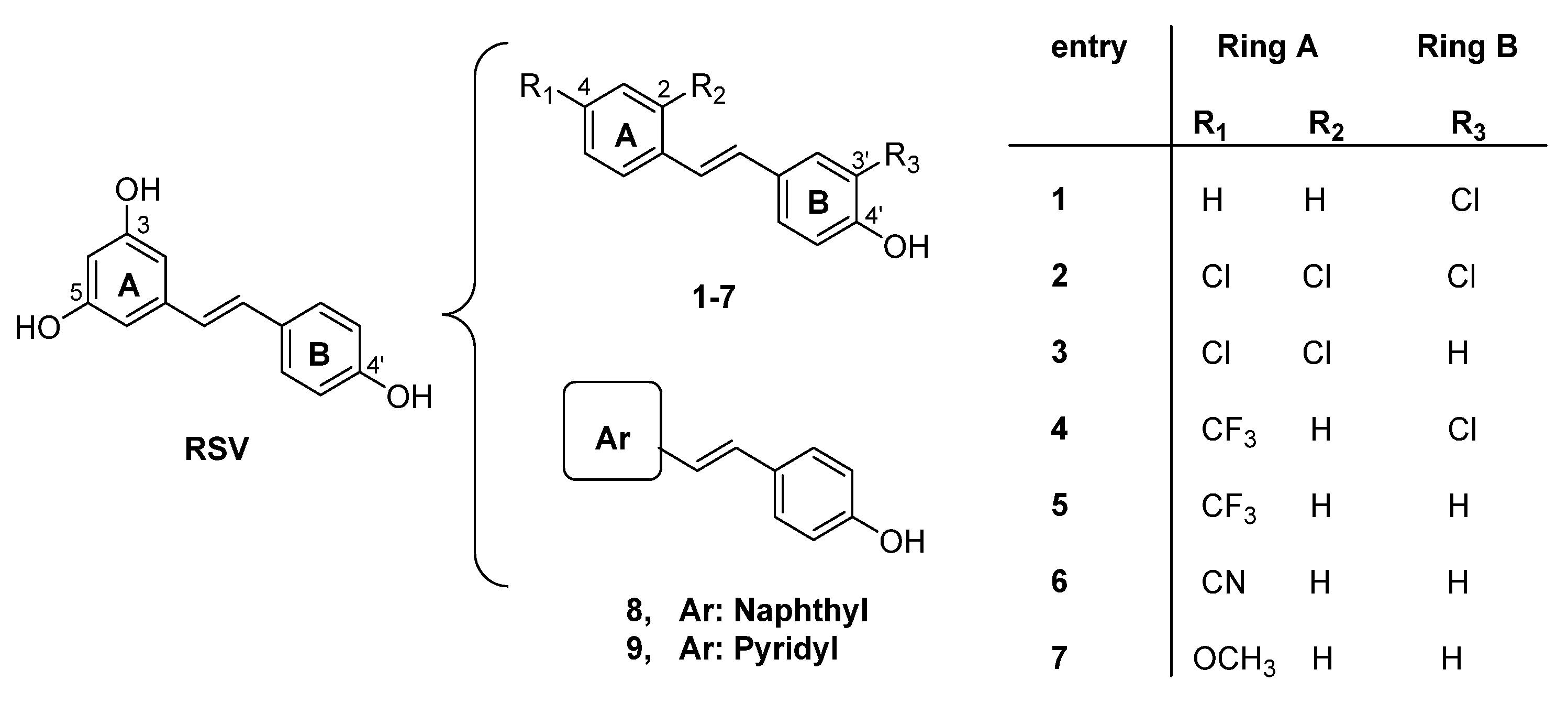
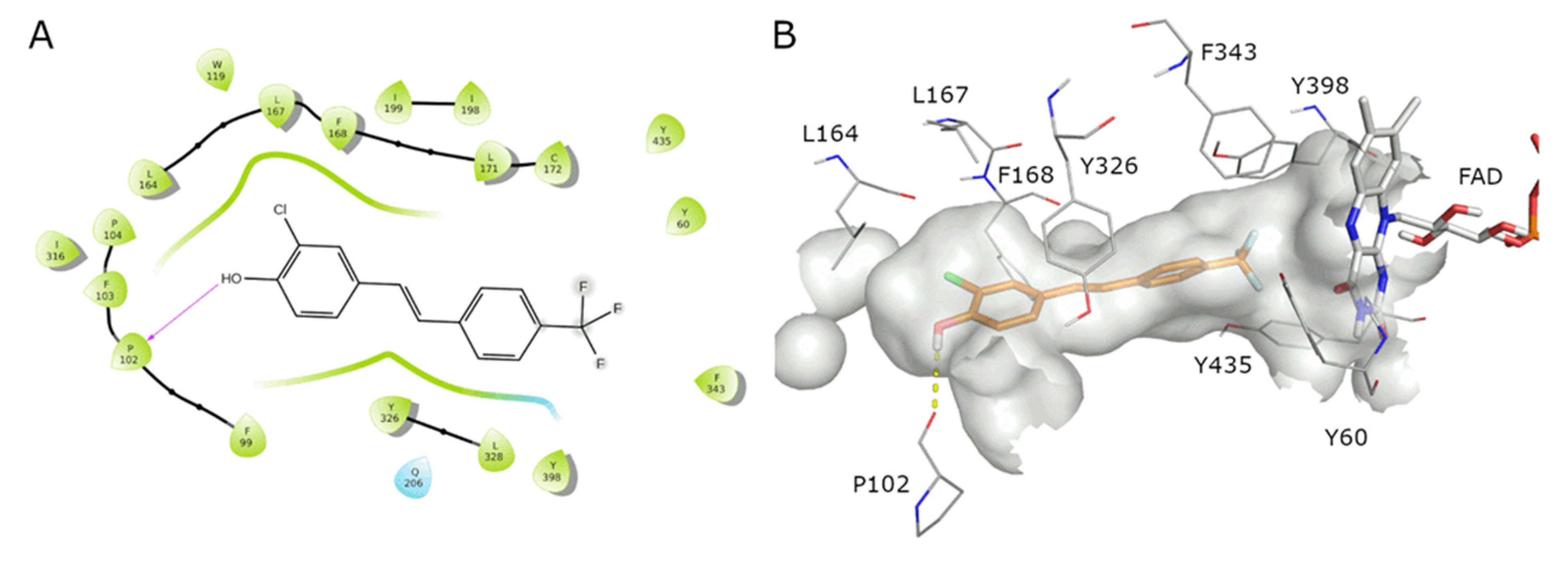
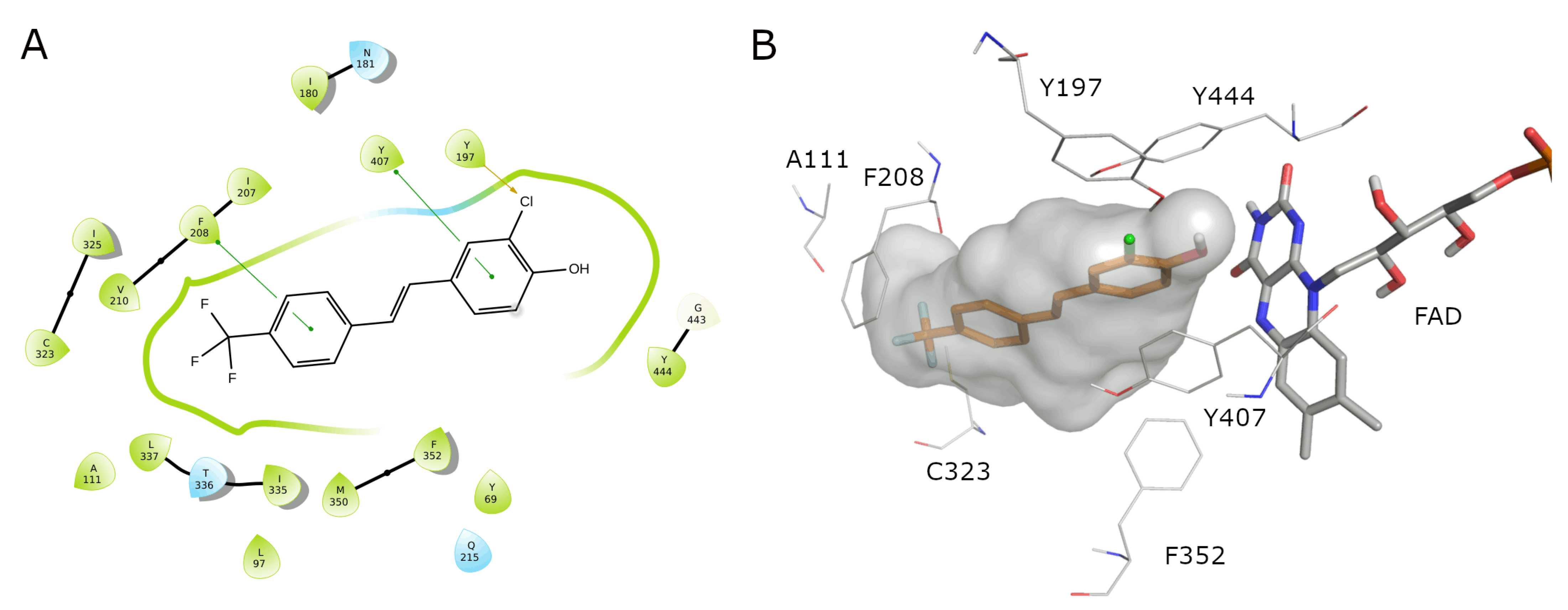


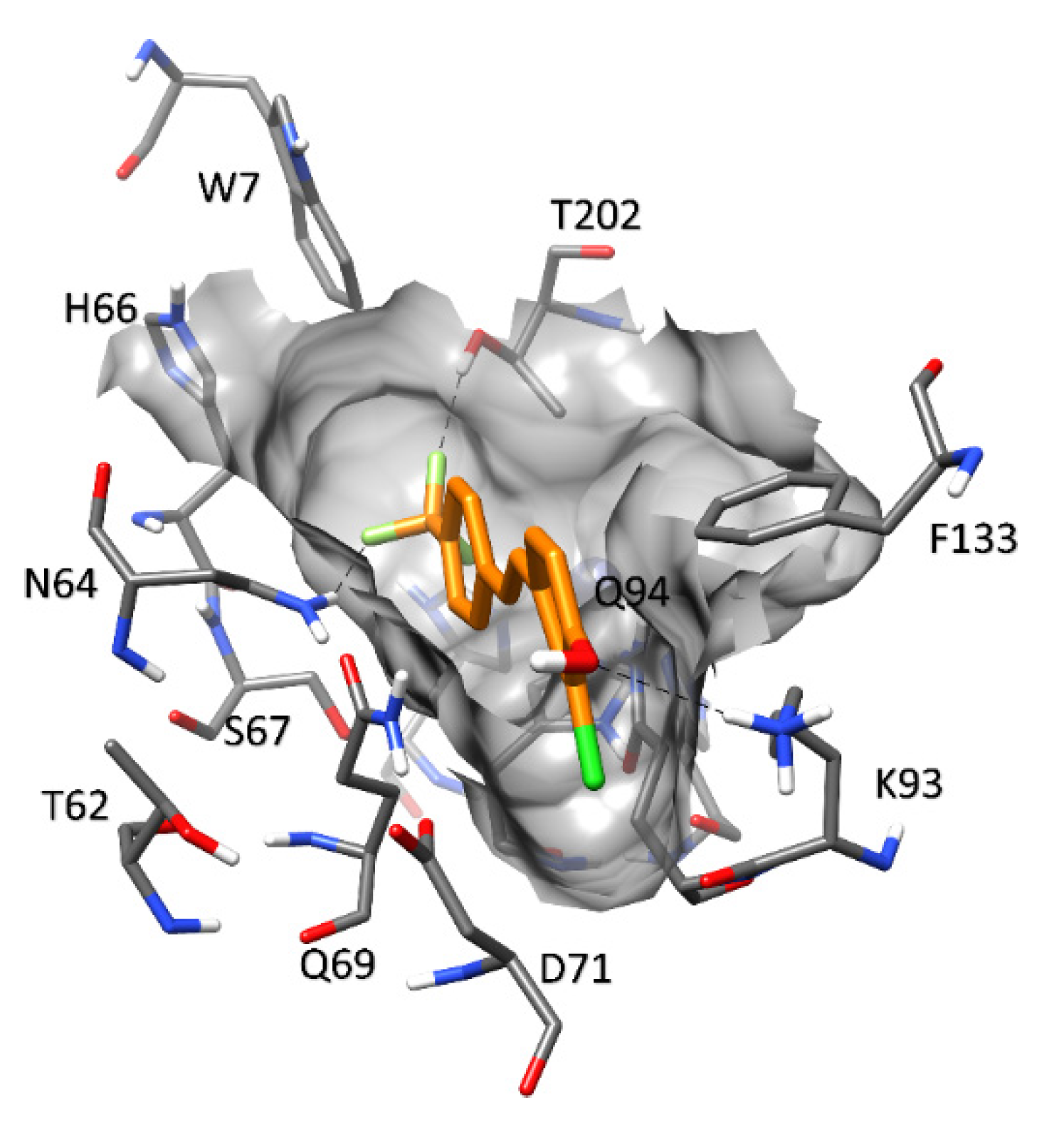
| Compound | MAO-A IC50 ± S.D. (µM) | MAO-B IC50 ± S.D. (µM) |
|---|---|---|
| 1 | 3.06 ± 0.100 | 0.156 ± 0.009 |
| 2 | 2.02 ± 0.175 | 1.44 ± 0.035 |
| 3 | 1.79 ± 0.095 | 1.52 ± 0.067 |
| 4 | 0.433 ± 0.127 | 0.011 ± 0.0065 |
| 5 | 9.77 ± 0.553 | 14.2 ± 1.82 |
| 6 | 2.01 ± 0.033 | 0.103 ± 0.003 |
| 7 | 2.71 ± 0.016 | 0.185 ± 0.009 |
| 8 | 2.20 ± 0.093 | 0.387 ± 0.022 |
| 9 | 21.3 ± 7.30 | 11.9 ± 1.51 |
| RSV | 13.5 ± 1.28 | >100 |
| Harmine | 0.0041 ± 0.00007 | - |
| Isatin | 8.43 ± 0.245 | 3.90 ± 0.792 |
| KI (µM) * | |||||||
|---|---|---|---|---|---|---|---|
| Compound | hCA I | hCA II | hCA VA | hCA VB | hCA VII | hCA IX | hCA XII |
| 1 | 89.5 | 35.8 | 9.0 | 84.5 | >100 | 23.6 | 2.7 |
| 2 | >100 | >100 | 37.2 | 32.2 | 4.2 | 81.1 | 4.5 |
| 3 | >100 | >100 | 86.0 | >100 | 7.5 | >100 | >100 |
| 4 | 86.8 | 15.6 | 70.9 | 36.2 | 0.7 | 17.4 | 6.9 |
| 5 | >100 | 43.4 | 83.0 | 8.7 | >100 | 94.6 | >100 |
| 6 | >100 | 75.3 | >100 | 9.5 | 7.4 | >100 | >100 |
| 7 | >100 | >100 | >100 | 9.7 | 9.4 | 81.8 | >100 |
| 8 | >100 | 77.5 | 98.2 | 21.1 | >100 | 47.7 | >100 |
| 9 | 78.4 | 11.8 | >100 | >100 | >100 | 75.3 | >100 |
| RSV | 2.2 | 2.8 | 4.7 | 4.6 | 4.3 | 0.8 | 0.9 |
| AAZ | 0.250 | 0.012 | 0.063 | 0.054 | 0.002 | 0.026 | 0.006 |
| Protein | SiteScore | Dscore | Size | Volume | Phobic | Philic | Balance |
|---|---|---|---|---|---|---|---|
| MAO-A | 1.17 | 1.191 | 130 | 240.958 | 3.474 | 0.882 | 3.939 |
| MAO-B | 1.209 | 1.231 | 163 | 270.37 | 3.12 | 0.913 | 3.418 |
| Complex | MMGBSA dG Bind a | MMGBSA dG Bind Coulomb b | MMGBSA dG Bind Covalent c | MMGBSA dG Bind Hbond d | MMGBSA dG Bind Lipo e | MMGBSA dG Bind Packing f | MMGBSA dG Bind Solv GB g | MMGBSA dG Bind vdW h |
|---|---|---|---|---|---|---|---|---|
| MAO-B:4 | −24.09 | −12.15 | 2.01 | −0.64 | −28.66 | −5.49 | 69.83 | −49.00 |
| MAO-B:5 | −21.00 | −16.04 | 2.01 | −0.61 | −25.35 | −3.51 | 64.79 | −42.29 |
| MAO-A:4 | −23.89 | −12.55 | 2.00 | −0.61 | −24.84 | −4.24 | 59.68 | −43.33 |
| ID | mol MW | accptHB | donorHB | CIQPlogS | Human Oral Absorption | Percent Human Oral Absorption | QPPCaco | QPlogHERG | QPPMDCK | QPlogBB | CNS | QPlogKhsa | # metab |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 230.693 | 0.75 | 1 | −4.217 | 3 | 100 | 3434.609 | −5.475 | 4398.946 | 0.049 | 1 | 0.395 | 1 |
| 2 | 299.583 | 0.75 | 1 | −5.662 | 3 | 100 | 3434.792 | −5.324 | 10,000 | 0.357 | 1 | 0.634 | 1 |
| 3 | 265.138 | 0.75 | 1 | −4.938 | 3 | 100 | 3014.508 | −5.372 | 8693.74 | 0.146 | 1 | 0.513 | 1 |
| 4 | 298.692 | 0.75 | 1 | −5.643 | 3 | 100 | 3434.791 | −5.506 | 10,000 | 0.314 | 1 | 0.673 | 1 |
| 5 | 264.247 | 0.75 | 1 | −4.920 | 3 | 100 | 3014.399 | −5.557 | 7150.372 | 0.102 | 1 | 0.552 | 1 |
| 6 | 221.258 | 2.25 | 1 | −4.491 | 3 | 94.309 | 625.083 | −5.691 | 297.703 | −0.916 | −1 | 0.149 | 1 |
| 7 | 226.274 | 1.5 | 1 | −3.858 | 3 | 100 | 3014.378 | −5.471 | 1630.445 | −0.236 | 0 | 0.314 | 2 |
| 8 | 246.308 | 0.75 | 1 | −4.783 | 3 | 100 | 3014.399 | −6.226 | 1630.457 | −0.187 | 0 | 0.667 | 1 |
| 9 | 197.236 | 2.25 | 1 | −3.011 | 3 | 100 | 1629.035 | −5.308 | 838.343 | −0.407 | 0 | −0.015 | 3 |
| RSV | 228.247 | 2.25 | 3 | −3.396 | 3 | 82.354 | 280.757 | −5.277 | 125.332 | −1.28 | −2 | −0.172 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carradori, S.; Fantacuzzi, M.; Ammazzalorso, A.; Angeli, A.; De Filippis, B.; Galati, S.; Petzer, A.; Petzer, J.P.; Poli, G.; Tuccinardi, T.; et al. Resveratrol Analogues as Dual Inhibitors of Monoamine Oxidase B and Carbonic Anhydrase VII: A New Multi-Target Combination for Neurodegenerative Diseases? Molecules 2022, 27, 7816. https://doi.org/10.3390/molecules27227816
Carradori S, Fantacuzzi M, Ammazzalorso A, Angeli A, De Filippis B, Galati S, Petzer A, Petzer JP, Poli G, Tuccinardi T, et al. Resveratrol Analogues as Dual Inhibitors of Monoamine Oxidase B and Carbonic Anhydrase VII: A New Multi-Target Combination for Neurodegenerative Diseases? Molecules. 2022; 27(22):7816. https://doi.org/10.3390/molecules27227816
Chicago/Turabian StyleCarradori, Simone, Marialuigia Fantacuzzi, Alessandra Ammazzalorso, Andrea Angeli, Barbara De Filippis, Salvatore Galati, Anél Petzer, Jacobus P. Petzer, Giulio Poli, Tiziano Tuccinardi, and et al. 2022. "Resveratrol Analogues as Dual Inhibitors of Monoamine Oxidase B and Carbonic Anhydrase VII: A New Multi-Target Combination for Neurodegenerative Diseases?" Molecules 27, no. 22: 7816. https://doi.org/10.3390/molecules27227816
APA StyleCarradori, S., Fantacuzzi, M., Ammazzalorso, A., Angeli, A., De Filippis, B., Galati, S., Petzer, A., Petzer, J. P., Poli, G., Tuccinardi, T., Agamennone, M., & Supuran, C. T. (2022). Resveratrol Analogues as Dual Inhibitors of Monoamine Oxidase B and Carbonic Anhydrase VII: A New Multi-Target Combination for Neurodegenerative Diseases? Molecules, 27(22), 7816. https://doi.org/10.3390/molecules27227816















