ILs and MMPs Levels in Inflamed Human Dental Pulp: A Systematic Review
Abstract
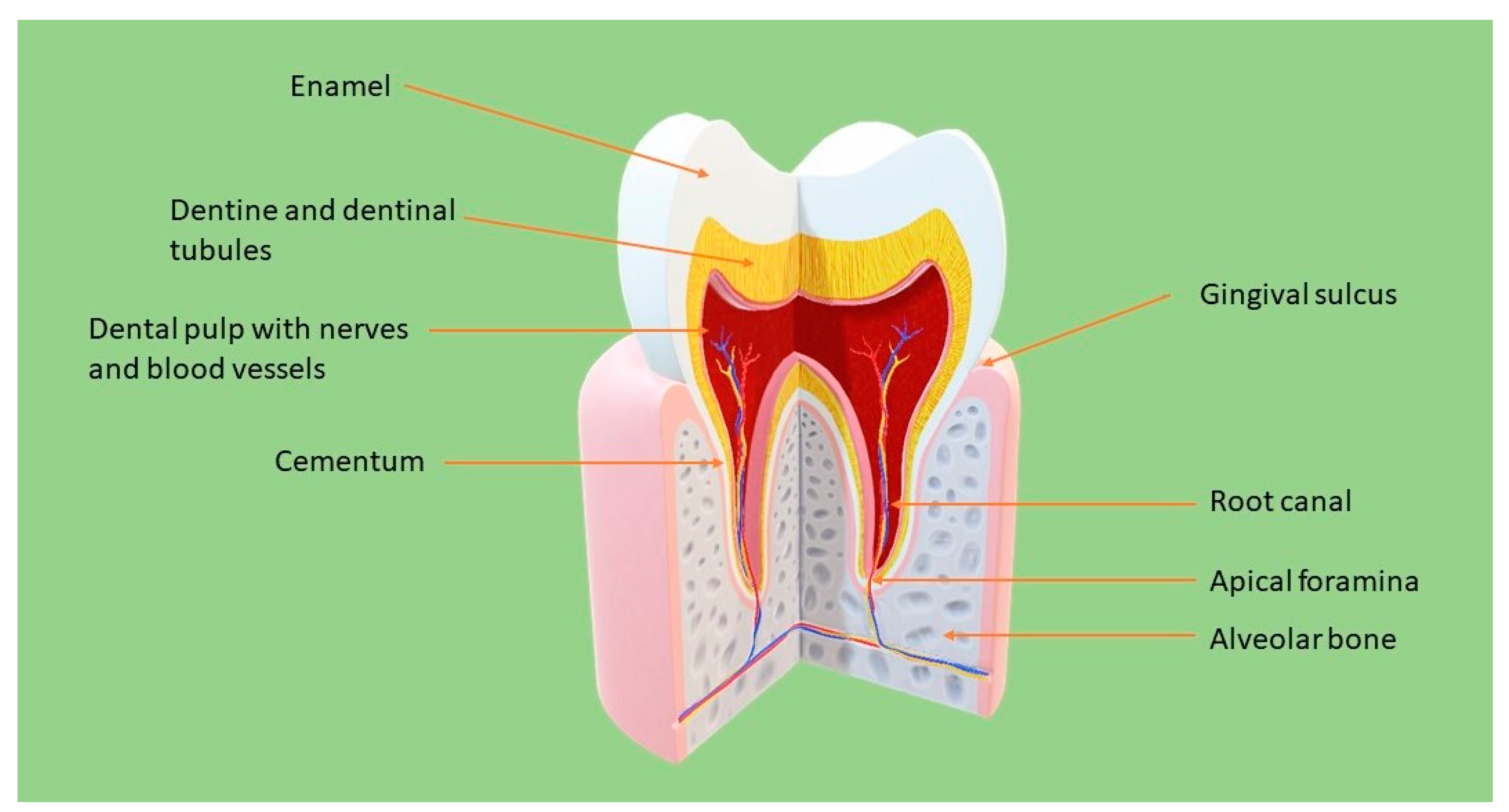
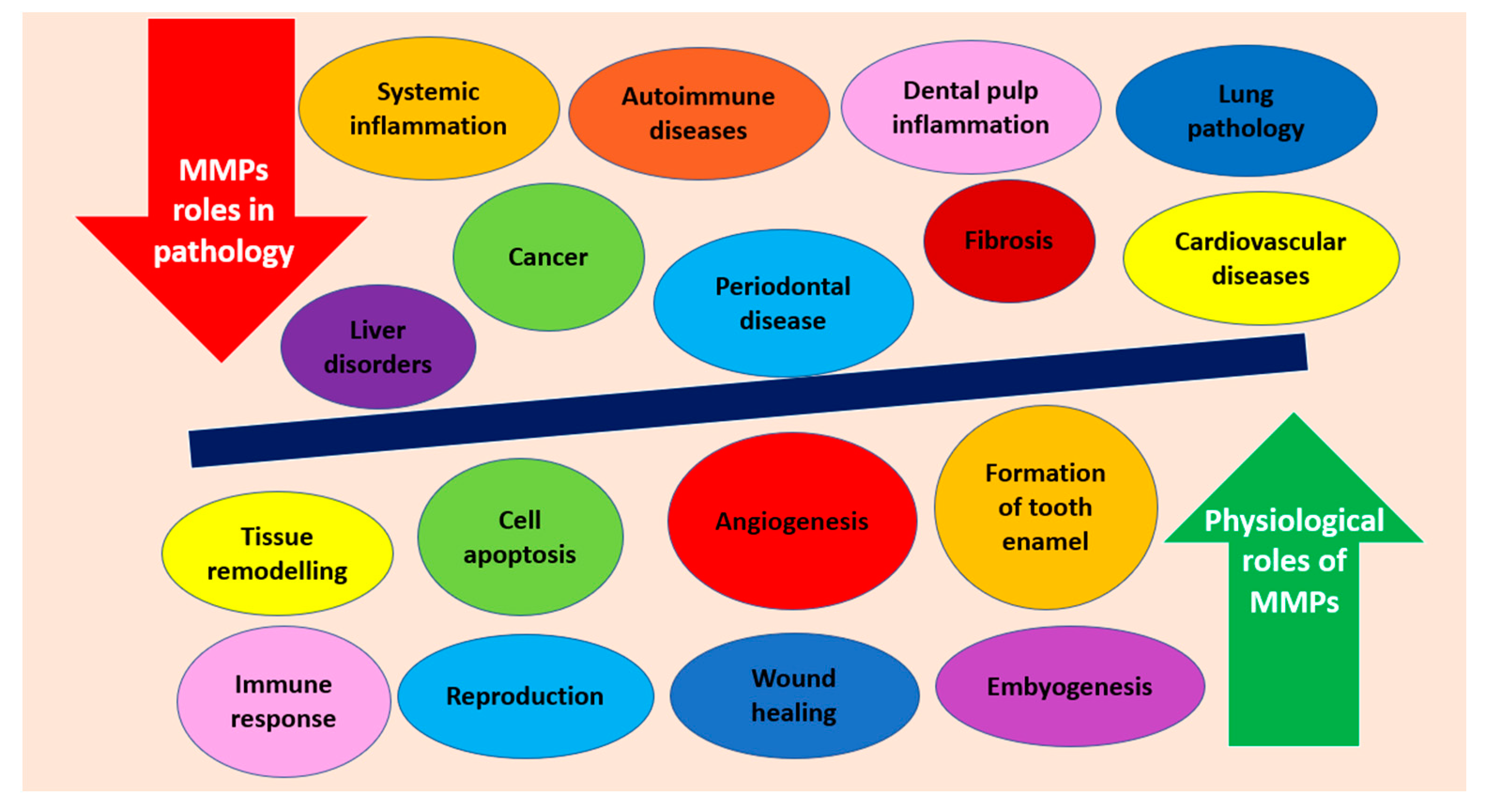
:1. Introduction
2. Materials and Methods
2.1. Selection Criteria
2.2. Literature Search
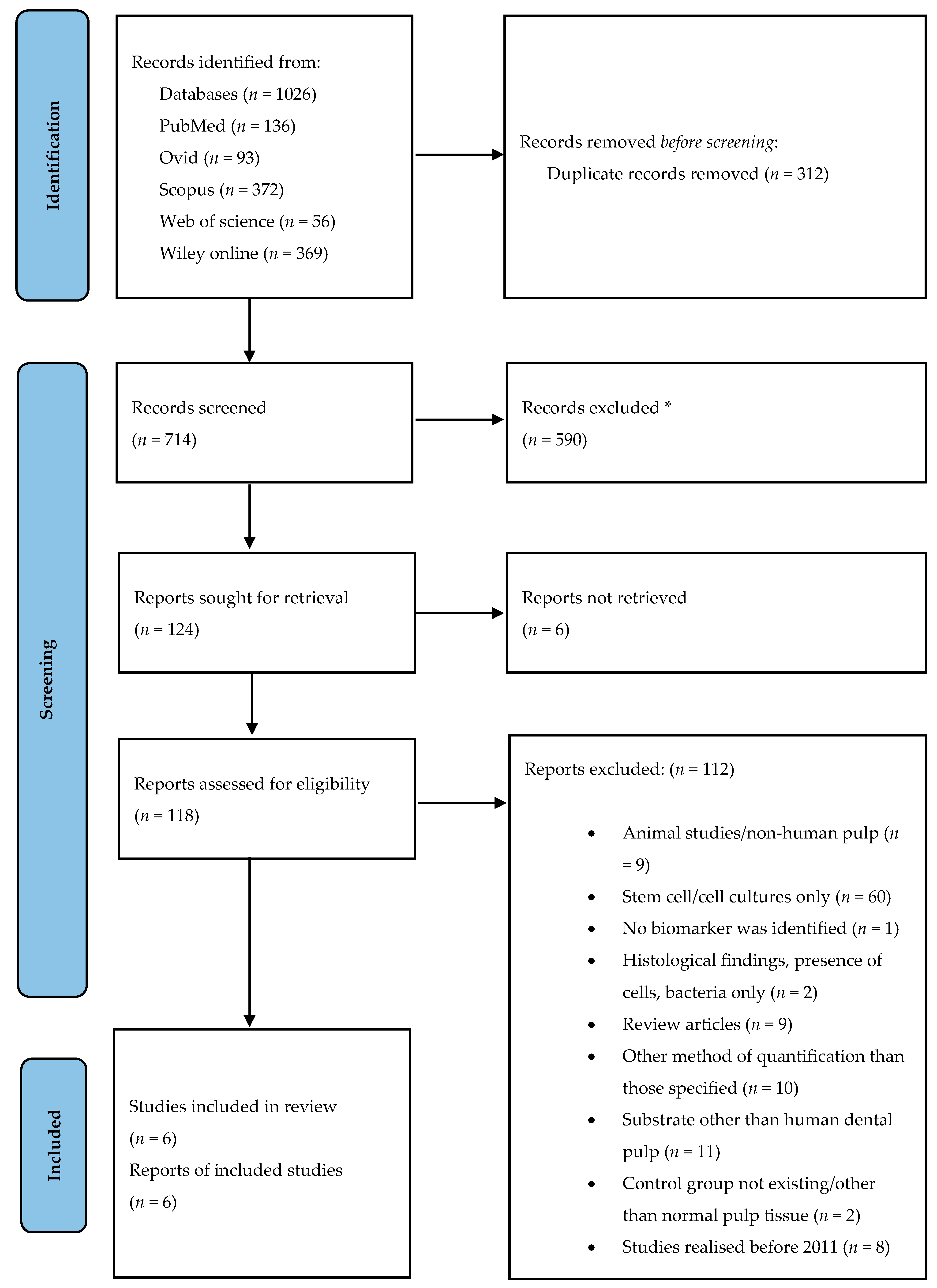
2.3. Study Selection
2.4. Data Collection and Quality Assessment
3. Results
3.1. Studies Included
3.2. Main Analyte
3.3. Diagnostic Tests
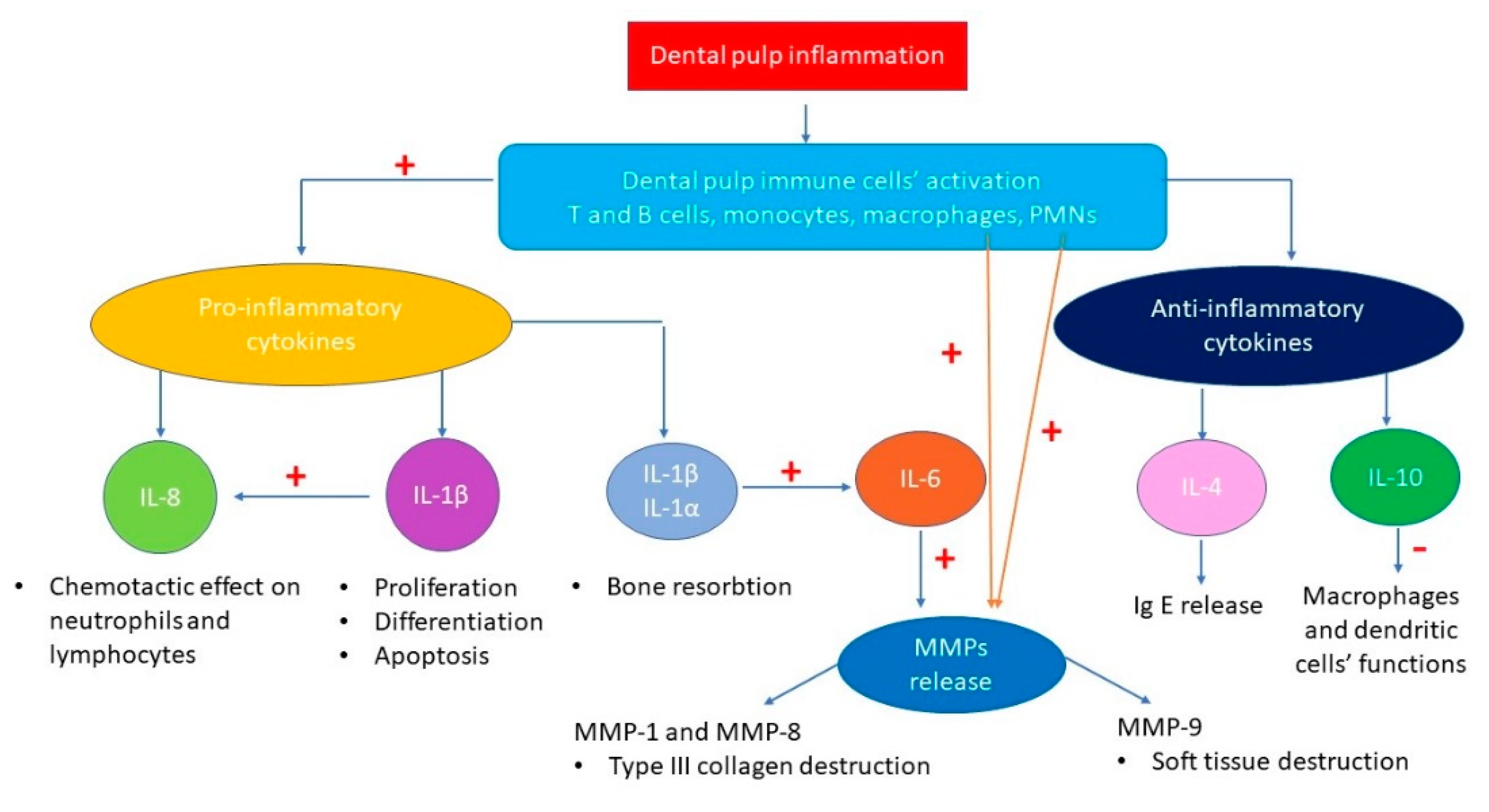
3.4. Biomarkers Identified and Types of Inflammation
3.5. Comparison and Statistical Significance
3.6. Quality of the Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yu, C.; Abbott, P.V. An overview of the dental pulp: Its functions and responses to injury. Aust. Dent. J. 2007, 52, S4–S6. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D. Dynamics of the Pulpo-Dentin Complex. Crit. Rev. Oral Biol. Med. 1996, 7, 104–133. [Google Scholar] [CrossRef]
- Bergenholtz, G.; Mjör, I.; Cotton, W.; Hanks, C.; Kim, S.; Torneck, C.; Trowbridge, H. Consensus Report. J. Dent. Res. 1985, 64, 631–633. [Google Scholar] [CrossRef]
- Conrads, G.; About, I. Pathophysiology of Dental Caries. Monogr. Oral Sci. 2018, 27, 1–10. [Google Scholar] [CrossRef]
- Giuroiu, C.L.; Căruntu, I.-D.; Lozneanu, L.; Melian, A.; Vataman, M.; Andrian, S. Dental Pulp: Correspondences and Contradictions between Clinical and Histological Diagnosis. BioMed Res. Int. 2015, 2015, 960321. [Google Scholar] [CrossRef] [PubMed]
- Subaric, L.; Mitic, A.; Matvijenko, V.; Jovanovic, R.; Zivkovic, D.; Peric, D.; Vlahovic, Z. Interleukin 1-beta analysis in chronically inflamed and healthy human dental pulp. Vojn. Pregl. 2017, 74, 256–260. [Google Scholar] [CrossRef] [Green Version]
- Abbott, P.V.; Yu, C. A clinical classification of the status of the pulp and the root canal system. Aust. Dent. J. 2007, 52, S17–S31. [Google Scholar] [CrossRef] [PubMed]
- AAE. Glossary of Endodontic Terms; AAE: Chicago, IL, USA, 2020; p. 60601. [Google Scholar]
- Silva, A.C.O.; Faria, M.R.; Fontes, A.; Campos, M.S.; Cavalcanti, B.N. Interleukin-1 beta and interleukin-8 in healthy and inflamed dental pulps. J. Appl. Oral Sci. 2009, 17, 527–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, L.M.; Dumsha, T.C.; McDonald, N.J.; Spitznagel, J.K. Evaluating IL-2 Levels in Human Pulp Tissue. J. Endod. 2002, 28, 651–655. [Google Scholar] [CrossRef]
- Hahn, C.-L.; Liewehr, F.R. Innate Immune Responses of the Dental Pulp to Caries. J. Endod. 2007, 33, 643–651. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and biological attributes of matrix metalloproteinases. In Computational Approaches for Understanding Dynamical Systems: Protein Folding and Assembly; Elsevier: New York, NY, USA, 2017; Volume 147, pp. 1–73. [Google Scholar]
- Bode, W.; Fernández-Catalán, C.; Tschesche, F.G.H.; Nagase, K.M.H.; Tschesche, H.; Grams, F.; Nagase, H.; Maskos, K. Structural properties of matrix metalloproteinases. Cell. Mol. Life Sci. 1999, 55, 639–652. [Google Scholar] [CrossRef]
- Leo Tjäderhane, S.P. Dentin-Pulp and Periodontal Anatomy and Physiology. In Essential Endodontology: Prevention and Treatment of Apical Periodontitis, 3rd ed.; Ørstavik, D., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2019; pp. 11–58. [Google Scholar]
- O’Boskey, F.J.; Panagakos, F.S. Cytokines stimulate matrix metalloproteinase production by human pulp cells during long-term culture. J. Endod. 1998, 24, 7–10. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, V.; Wolgin, M.; Mitronin, A.; Kielbassa, A. Inflammatory cytokines in normal and irreversibly inflamed pulps: A systematic review. Arch. Oral Biol. 2017, 82, 38–46. [Google Scholar] [CrossRef]
- Rechenberg, D.-K.; Galicia, J.; Peters, O. Biological Markers for Pulpal Inflammation: A Systematic Review. PLoS ONE 2016, 11, e0167289. [Google Scholar] [CrossRef] [Green Version]
- Zanini, M.; Meyer, E.; Simon, S. Pulp Inflammation Diagnosis from Clinical to Inflammatory Mediators: A Systematic Review. J. Endod. 2017, 43, 1033–1051. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.; Álvarez-Pasquin, M.J.; Díaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013, 13, 154. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.A.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 31 May 2021).
- Abd-Elmeguid, A.; Yu, D.C.; Kline, L.W.; Moqbel, R.; Vliagoftis, H. Dentin Matrix Protein-1 Activates Dental Pulp Fibroblasts. J. Endod. 2012, 38, 75–80. [Google Scholar] [CrossRef]
- Abd-Elmeguid, A.; Abdeldayem, M.; Kline, L.W.; Moqbel, R.; Vliagoftis, H.; Yu, D.C. Osteocalcin Expression in Pulp Inflammation. J. Endod. 2013, 39, 865–872. [Google Scholar] [CrossRef]
- Dincer, G.A.; Erdemir, A.; Kisa, U. Comparison of NKA, SP, IL-8, MMP-8 Changes in Pulp tissue and GCF Samples of Healthy and Acute Irreversible Pulpitis Teeth. J. Endod. 2020, 46, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Evrosimovska, B.; Dimova, C.; Kovacevska, I.; Panov, S. Concentration of collagenases (MMP-1, -8, -13) in patients with chronically inflamed dental pulp tissue. Prilozi 2012, 33, 191–204. [Google Scholar]
- Suwanchai, A.; Theerapiboon, U.; Chattipakorn, N.; Chattipakorn, S.C. NaV1.8, but not NaV1.9, is upregulated in the inflamed dental pulp tissue of human primary teeth. Int. Endod. J. 2011, 45, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, M.; Delaleu, N.; Du, Y.; Bickel, M. Cytokine gene expression—part of host defence in pulpitis. Cytokine 2003, 22, 84–88. [Google Scholar] [CrossRef]
- Sattari, M.; Haghighi, A.K.; Tamijani, H.D. The relationship of pulp polyp with the presence and concentration of immunoglobulin E, histamine, interleukin-4 and interleukin. Aust. Endod. J. 2009, 35, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Fedele, S.; D’Aiuto, F.; Donos, N. Interleukin-6 in oral diseases: A review. Oral Dis. 2011, 18, 236–243. [Google Scholar] [CrossRef]
- Barkhordar, R.A.; Hayashi, C.; Hussain, M.Z. Detection of interleukin-6 in human dental pulp and periapical lesions. Dent. Traumatol. 1999, 15, 26–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.-J.; Potente, A.P.; Kim, J.-W.; Chugal, N.; Zhang, X. Increased interleukin-8 expression in inflamed human dental pulps. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 88, 214–220. [Google Scholar] [CrossRef]
- Sambandam, V.; Neelakantan, P. Matrix Metalloproteinases (Mmp) in Restorative Dentistry and Endodontics. J. Clin. Pediatr. Dent. 2014, 39, 57–59. [Google Scholar] [CrossRef]
- Gusman, H.; Santana, R.; Zehnder, M. Matrix metalloproteinase levels and gelatinolytic activity in clinically healthy and inflamed human dental pulps. Eur. J. Oral Sci. 2002, 110, 353–357. [Google Scholar] [CrossRef]
- Chaussain-Miller, C.; Fioretti, F.; Goldberg, M.; Menashi, S. The Role of Matrix Metalloproteinases (MMPs) in Human Caries. J. Dent. Res. 2006, 85, 22–32. [Google Scholar] [CrossRef]
| Reference | Clinical Diagnosis & n Per Group | Subjects (n) & Age (Mean Age ± SE) | Biomarker | Focus of the Study | Method | Comparison & Significance |
|---|---|---|---|---|---|---|
| Abd-Elmeguid et al., (2012) [22] | H (n = 5) INF (n = 12) (IF without differentiation) H (n = 8) INF (n = 20) | no data | IL-6 | To examine the presence of dentin matrix protein-1 (DMP-1) in inflamed pulps; to define pulp inflammation in terms of cytokine expression. | ELISA | H/INF (R+IR): ↑ levels in INF; SS, p < 0.05 |
| IL-8 | H/INF (R+IR): ↑ levels in INF; SS, p < 0.05 | |||||
| Abd-Elmeguid et al., (2013) [23] | H (n = 30) R INF (n = 23) IR INF (n = 12) | no data | IL-1α | To localize osteocalcin (OCN) in inflamed pulps, to distinguish its different levels in 2 stages of pulp inflammation and to suggest its possible interactions in pulpal inflammation; to identify the presence of different inflammatory and remodeling mediators in pulp tissue and measure their levels. | Multiplex Assay | H/R INF: ↑ levels in R INF; SS, p < 0.01 H/IR INF: ↑ levels in IR INF; SS, p < 0.001 R INF/IR INF: ↑ levels in IR INF but NS |
| IL-1β | H/R INF: ↑ levels in R INF; SS, p < 0.01 H/IR INF: ↑ levels in IR INF; SS, p < 0.01 R INF/IR INF: ↑ levels in R INF but NS | |||||
| IL-1rα | H/R INF: ↑ levels in R INF; SS, p < 0.05 H/IR INF: ↑ levels in IR INF; SS, p < 0.05 R INF/IR INF: ↑ levels in IR INF but NS | |||||
| IL-4 | H/R INF: ↑ levels in R INF; SS, p < 0.05 H/IR INF: ↑ levels in IR INF; SS, p < 0.05 R INF/IR INF: ↑ levels in IR INF but NS | |||||
| IL-6 | H/R INF: ↑ levels in R INF; SS, p < 0.01 H/IR INF: ↑ levels in IR INF; SS, p < 0.05 R INF/IR INF: ↑ levels in R INF but NS | |||||
| IL-7 | H/R INF: ↑ levels in H; SS, p < 0.001 H/IR INF: ↑ levels in H; SS, p < 0.01 R INF/IR INF: ↑ levels in R INF but NS | |||||
| IL-8 | H/R INF: ↑ levels in H; SS, p < 0.05 H/IR INF: ↑ levels in H; SS, p < 0.01 R INF/IR INF: ↑ levels in R INF but NS | |||||
| IL-12 p40 | H/R INF: ↑ levels in R INF; SS, p < 0.05 H/IR INF: ↑ levels in IR INF; SS, p < 0.01 R INF/IR INF: ↑ levels in IR INF but NS | |||||
| IL-13 | H/R INF: ↑ levels in H; SS, p < 0.05 H/IR INF: ↑ levels in H; SS, p < 0.05 R INF/IR INF: ↑ levels in R INF but NS R INF/IR INF: ↑ levels in IR INF but NS | |||||
| IL-15 | H/R INF: ↑ levels in R INF; SS, p < 0.01 H/IR INF: ↑ levels in IR INF but NS R INF/IR INF: ↑ levels in R INF but NS | |||||
| Dincer at al., 2020 [24] | H (n = 20) IR INF (n = 20) | n = 40 16–50 yr. H:36.35 yr. IR INF: 22.50 yr. | IL-8 | To compare the changes in amounts of NKA, SP, IL-8 and MMP-8 in pulp tissue | ELISA | H/IR INF: ↑ levels in IR INF; SS, p = 0/001 |
| MMP-8 | H/IR INF: ↑ levels in IR INF; SS, p < 0.001 | |||||
| Evrosimovska et al., (2012) [25] | H (n = 10) CHR INF (n = 20) | n = 30 15–70 yr. | MMP-1 MMP-8 MMP-13 | To determinate the concentration of MMP-1, -8 and -13 in the healthy pulp tissue of impacted third molars and to compare the values of the concentration between healthy and pathologically changed tissue | ELISA | H/CHR INF: ↑ levels in CHR INF; SS, p < 0.01 |
| Subaric et al., (2017) [6] | H (n = 12) CHR AP INF (n = 22) CHR CL INF (n = 19) | no data | IL-1β | To determine the IL-1β concentrations in chronically inflamed and healthy dental pulp | ELISA | H/CHR INF (AP+CL): ↑ levels in CHR INF but NS, p = 0.590 H/CHR CL INF/CHR AP INF: ↑ levels in CHR CL INF; SS, p < 0.01 H/CHR CL INF: ↑ levels in CHR CL INF; SS, p < 0.01 H/CHR AP INF: ↑ levels in H but NS, p = 0.081 CHR CL INF/CHR AP INF: ↑ levels in CHR CL INF; SS, p < 0.01 |
| Suwanchai et al., (2011) [26] | H(P) (n = 18) H (T) (n = 7) IR INF (P) (n = 7) R INF (T) (n = 8) IR INF (T) (n = 8) | n = no data H(P): 17.3 ± 1.1 yr. IR INF(P): 35.4 ± 6.3 yr. H(T): 9.4 ± 0.9 yr. R + IR INF(T): 6.1 ± 0.7 yr. | MMP-9 | To investigate alterations in Nav1.8 and Nav1.9 expression within inflamed dental pulp tissue of human primary teeth | Western Blot | H(P)/IR INF(P): ↑ levels in IR INF; SS, p < 0.05 H(T)/R+IR INF (without differentiation): ↑ levels in R+IR INF; SS, p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kritikou, K.; Greabu, M.; Imre, M.; Miricescu, D.; Ripszky Totan, A.; Burcea, M.; Stanescu-Spinu, I.-I.; Spinu, T. ILs and MMPs Levels in Inflamed Human Dental Pulp: A Systematic Review. Molecules 2021, 26, 4129. https://doi.org/10.3390/molecules26144129
Kritikou K, Greabu M, Imre M, Miricescu D, Ripszky Totan A, Burcea M, Stanescu-Spinu I-I, Spinu T. ILs and MMPs Levels in Inflamed Human Dental Pulp: A Systematic Review. Molecules. 2021; 26(14):4129. https://doi.org/10.3390/molecules26144129
Chicago/Turabian StyleKritikou, Konstantina, Maria Greabu, Marina Imre, Daniela Miricescu, Alexandra Ripszky Totan, Marian Burcea, Iulia-Ioana Stanescu-Spinu, and Tudor Spinu. 2021. "ILs and MMPs Levels in Inflamed Human Dental Pulp: A Systematic Review" Molecules 26, no. 14: 4129. https://doi.org/10.3390/molecules26144129
APA StyleKritikou, K., Greabu, M., Imre, M., Miricescu, D., Ripszky Totan, A., Burcea, M., Stanescu-Spinu, I.-I., & Spinu, T. (2021). ILs and MMPs Levels in Inflamed Human Dental Pulp: A Systematic Review. Molecules, 26(14), 4129. https://doi.org/10.3390/molecules26144129








