Determination of Activation Energies and the Optimum Temperatures of Hydrolysis of Starch by α-Amylase from Porcine Pancreas
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Activation Energy Ea
2.2. The Activation Energy of the Deactivation Process Ed
2.3. Optimum Temperature Topt
3. Materials and Methods
3.1. The Effect of Temperature on α-Amylase Activity
3.2. Assay of α-Amylase Activity
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khlestkin, V.; Eltsov, I. Different reactivity of raw starch from diverse potato genotypes. Molecules 2021, 26, 226. [Google Scholar] [CrossRef]
- Albani, J.R. Starch hydrolysis by amylase. In Principles and Applications of Fluorescence Spectroscopy; Blackwell Publishing: Oxford, UK, 2007; pp. 59–78. [Google Scholar]
- Zhang, B.; Guo, K.; Lin, L.; Wei, C. Comparison of structural and functional properties of starches from the rhizome and bulbil of Chinese Yam (Dioscorea opposita Thunb.). Molecules 2018, 23, 427. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.J.; Husain, Q.; Azam, A. Immobilization of porcine pancreatic α-amylase on magnetic Fe2O3 nanoparticles: Applications to the hydrolysis of starch. Biotechnol. Bioprocess Eng. 2012, 17, 377–384. [Google Scholar] [CrossRef]
- Li, Z.; Guo, K.; Lin, L.; He, W.; Zhang, L.; Wei, C. Comparison of physicochemical properties of starches from flesh and peel of green banana fruit. Molecules 2018, 23, 2312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.; Lin, Q.; Liu, G.-Q. Effect of cross-linking and enzymatic hydrolysis composite modification on the properties of rice starches. Molecules 2012, 17, 8136–8146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhond, M.; Pashangeh, K.; Karbalaei-Heidari, H.R.; Absalan, G. Efficient immobilization of porcine pancreatic α-amylase on amino-functionalized magnetite nanoparticles: Characterization and stability evaluation of the immobilized enzyme. Appl. Biochem. Biotechnol. 2016, 180, 954–968. [Google Scholar] [CrossRef]
- Couto, S.R.; Sanromán, M.Á. Application of solid-state fermentation to food industry—A review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- Balakrishnan, D.; Kumar, S.S.; Sugathan, S. Amylases for food applications—Updated information. In Green Bioprocesses. Enzymes in Industrial Food Processing; Springer: Singapore, 2019; pp. 199–228. [Google Scholar]
- Azzopardi, E.; Lloyd, C.; Teixeira, S.R.; Conlan, R.S.; Whitaker, I. Clinical applications of amylase: Novel perspectives. Surgery 2016, 160, 26–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaidya, S.; Srivastava, P.K.; Rathore, P.; Pandey, A.K. Amylases: A prospective enzyme in the field of biotechnology. J. Appl. Biosci. 2015, 41, 1–18. [Google Scholar]
- Stotz, M.; Barth, D.A.; Riedl, J.M.; Asamer, E.; Klocker, E.V.; Kornprat, P.; Hutterer, G.C.; Prinz, F.; Lackner, K.; Stöger, H.; et al. The lipase/amylase ratio (LAR) in peripheral blood might represent a novel prognostic marker in patients with surgically resectable pancreatic cancer. Cancers 2020, 12, 1798. [Google Scholar] [CrossRef]
- Quek, A.; Kassim, N.K.; Ismail, A.; Latif, M.A.M.; Shaari, K.; Tan, D.C.; Lim, P.C. Identification of dipeptidyl peptidase-4 and α-amylase inhibitors from Melicope glabra (Blume) T. G. Hartley (Rutaceae) using liquid chromatography tandem mass spectrometry, in vitro and in silico methods. Molecules 2021, 26, 1. [Google Scholar] [CrossRef]
- Nazir, N.; Zahoor, M.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.E. Curative effect of catechin isolated from Elaeagnus umbellata Thunb. berries for diabetes and related complications in streptozotocin-induced diabetic rats model. Molecules 2021, 26, 137. [Google Scholar] [CrossRef] [PubMed]
- Gopal, B.A.; Muralikrishna, G. Porcine pancreatic α-amylase and its isoforms: Purification and kinetic studies. Int. J. Food Prop. 2009, 12, 571–586. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Nowicka, P.; Rubiński, P.; Cebulak, T. Evaluation of innovative dried purée from Jerusalem artichoke—In vitro studies of its physicochemical and health-promoting properties. Molecules 2021, 26, 2644. [Google Scholar] [CrossRef] [PubMed]
- Riyaphan, J.; Jhong, C.-H.; Lin, S.-R.; Chang, C.-H.; Tsai, M.-J.; Lee, D.-N.; Sung, P.-J.; Leong, M.K.; Weng, C.-F. Hypoglycemic efficacy of docking selected natural compounds against α-glucosidase and α-amylase. Molecules 2018, 23, 2260. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Shi, J.; Wang, L.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Inhibitory kinetics and mechanism of flavonoids from lotus (Nelumbo nucifera Gaertn.) leaf against pancreatic α-amylase. Int. J. Biol. Macromol. 2018, 120, 2589–2596. [Google Scholar] [CrossRef]
- Megazyme, Resistant Starch. Assay Procedure. K-RSTAR 05/19. (100 Assays per Kit). AOAC Method 2002.02. AACC Method 32-40.01. Codex Type II Method. Available online: https://www.megazyme.com/documents/Booklet/K-RSTAR_DATA.pdf (accessed on 27 May 2021).
- Aksoy, S.; Tumturk, H.; Hasirci, N. Stability of α-amylase immobilized on poly(methylmethacrylate-acrylic acid) microspheres. J. Biotechnol. 1998, 60, 37–46. [Google Scholar] [CrossRef]
- Louati, H.; Zouari, N.; Fendri, A.; Gargouri, Y. Digestive amylase of a primitive animal, the scorpion: Purification and biochemical characterization. J. Chromatogr. B 2010, 878, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Tang, Y.; Yu, Y.; Xue, L.; Qian, J.-Q. Covalent immobilization of α-amylase on magnetic particles as catalyst for hydrolysis of high-amylose starch. Int. J. Biol. Macromol. 2016, 87, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, A.; Delbrassine, F.; Da Lage, J.-L.; Feller, G. Temperature adaptations in psychrophilic, mesophilic and thermophilic chloride-dependent alpha-amylases. Biochimie 2012, 94, 1943–1950. [Google Scholar] [CrossRef]
- D’Amico, S.; Marx, J.-C.; Gerday, C.; Feller, G. Activity-Stability Relationships in Extremophilic Enzymes. J. Biol. Chem. 2003, 278, 7891–7896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, J.R.; Giordano, R.D.L.C.; Fernandez-Lafuente, R.; Tardioli, P.W. Evaluation of strategies to produce highly porous cross-linked aggregates of porcine pancreas lipase with magnetic properties. Molecules 2018, 23, 2993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haq, I.-U.; Javed, M.M.; Hameed, U.; Adnan, F. Kinetics and thermodynamic studies of alfa amylase from Bacillus licheniformis mutant. Pak. J. Bot. 2010, 42, 3507–3516. [Google Scholar]
- Ademakinwa, A.; Agunbiade, M.; Ayinla, Z.; Agboola, F. Optimization of aqueous two-phase partitioning of Aureobasidium pullulans α-amylase via response surface methodology and investigation of its thermodynamic and kinetic properties. Int. J. Biol. Macromol. 2019, 140, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.; Miłek, J. A new method to determine optimum temperature and activation energies for enzymatic reactions. Bioprocess Biosyst. Eng. 2016, 39, 1319–1323. [Google Scholar] [CrossRef] [Green Version]
- Miłek, J. Application of the new method to determine of the kinetic parameters of inulin hydrolysis by exo-inulinase Aspergillus niger. J. Therm. Anal. Calorim. 2021. [Google Scholar] [CrossRef]
- Miłek, J. Calculation of temperature optimum as well as activation and deactivation energy for the olive oil hydrolysis with porcine pancreas lipase. Przem. Chem. 2020, 99, 585–587. [Google Scholar] [CrossRef]
- Miłek, J. The effect of pH on determination of activation energies and the optimum temperatures of hydrolysis of olive oil by lipase from porcine pancreas. Acta Bioeng. Biomech. 2021, unpublished work. [Google Scholar] [CrossRef]
- Miłek, J. Determination of the activation energies and optimum temperature for the hydrolysis of p-nitrophenyl palmitate catalyzed by lipases. Przem. Chem. 2021, 100, 103–104. [Google Scholar] [CrossRef]
- Miłek, J. Determination the optimum temperature and activation energy for the hydrolysis of starch catalyzed by α-amylase Bacillus licheniformis. Przem. Chem. 2020, 99, 880–881. [Google Scholar] [CrossRef]
- Miłek, J. Determination the optimum temperatures and activation energies of inulin hydrolysis by endo-inulinase Aspergillus niger. Chem. Proc. Eng. 2020, 41, 229–236. [Google Scholar] [CrossRef]
- Miłek, J. Estimation of the kinetic parameters for h2o2 enzymatic decomposition and for catalase deactivation. Braz. J. Chem. Eng. 2018, 35, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Miłek, J. Thermodynamics and kinetics of thermal deactivation of catalase Aspergillus niger. Pol. J. Chem. Technol. 2020, 22, 67–72. [Google Scholar] [CrossRef]
- Apar, D.K.; Özbek, B. α-Amylase inactivation by temperature during starch hydrolysis. Process. Biochem. 2004, 39, 1137–1144. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
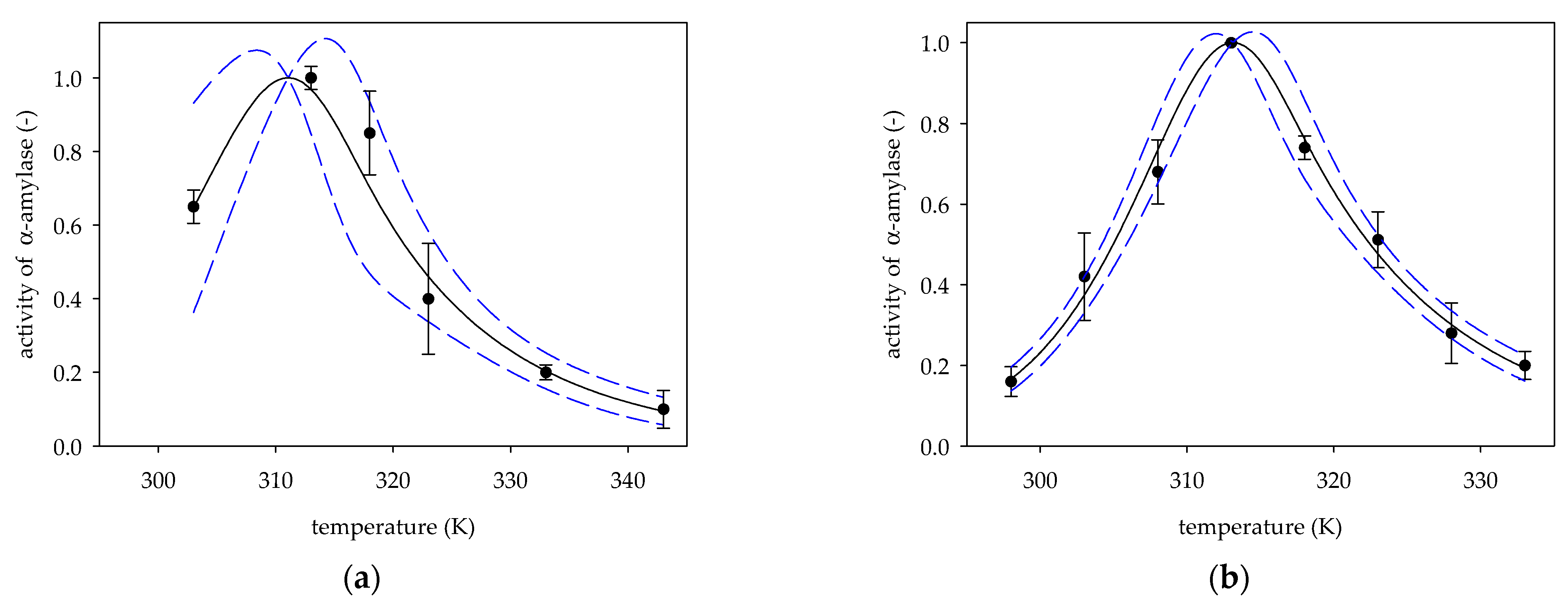
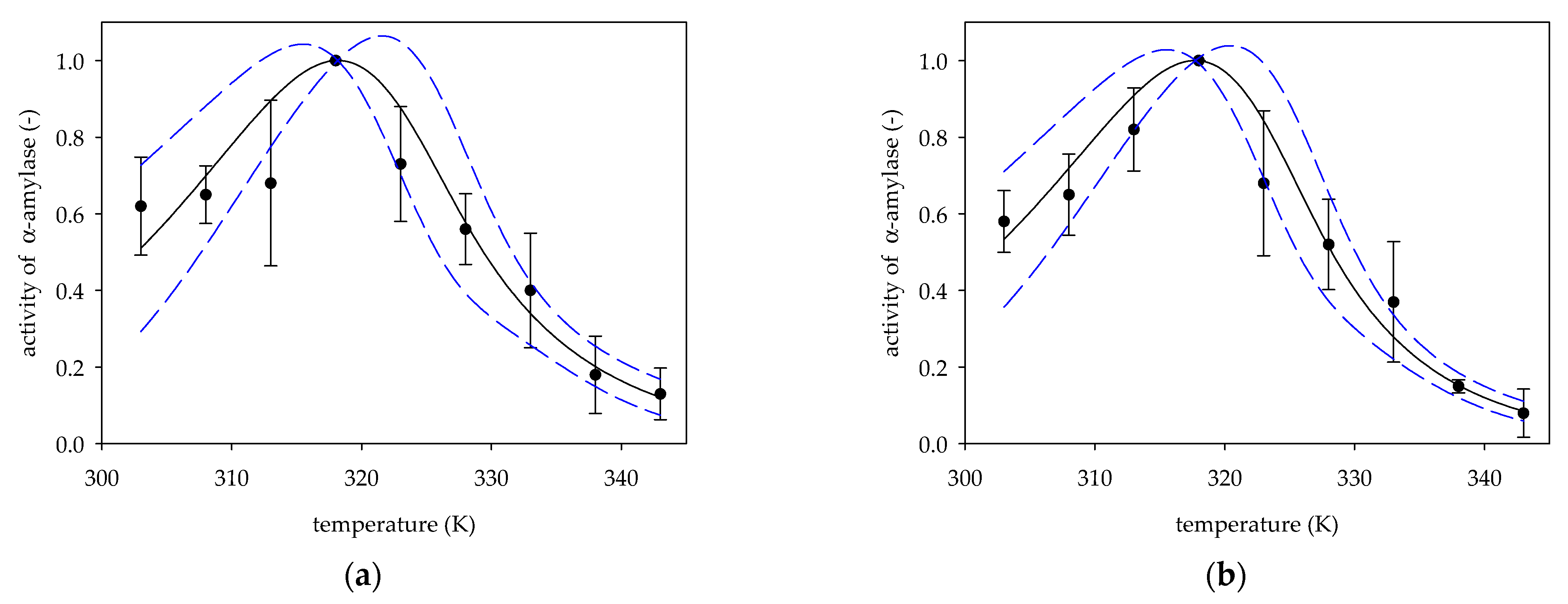
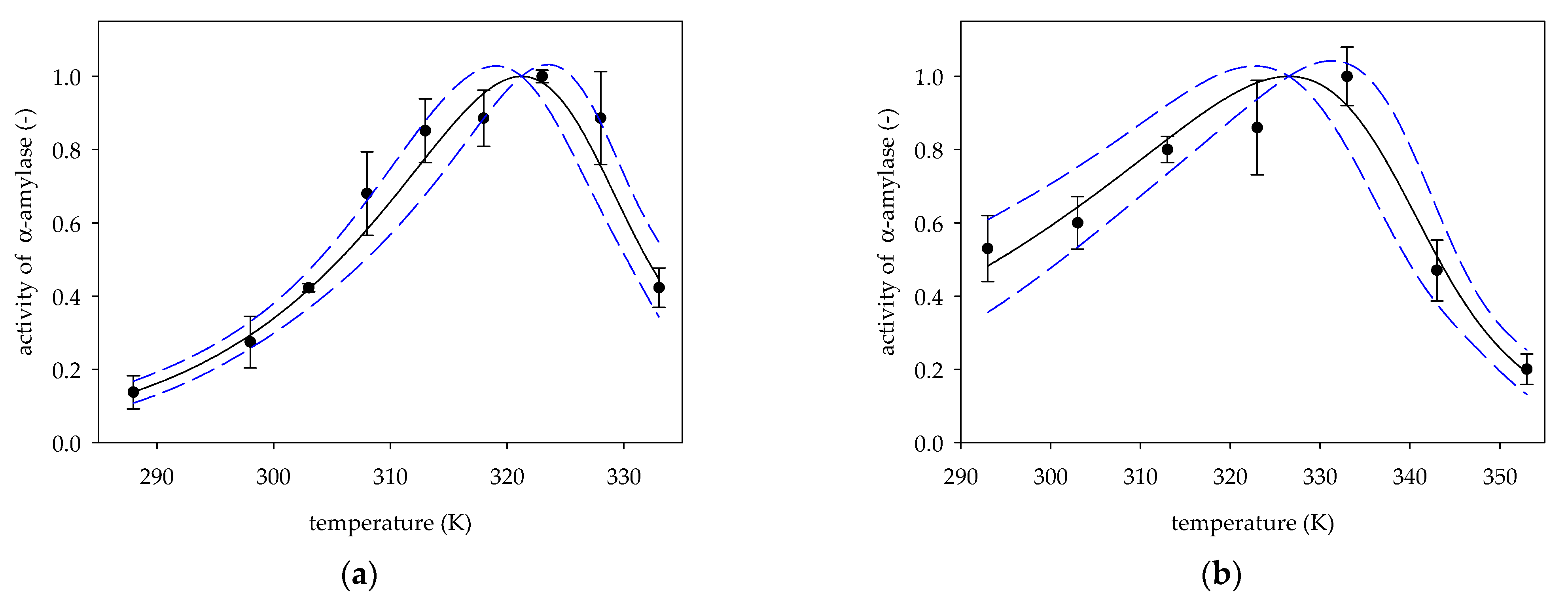
| Figure | t (min) | β | Ref. | ||||
|---|---|---|---|---|---|---|---|
| 1a | 3 | 311.06 ± 1.10 | 164.9 ± 19.14 | 1.46 ± 0.29 | 92.08 ± 23.07 | 1.79 | [7] |
| 1b | 5 | 313.12 ± 0.55 | 209.37 ± 5.17 | 1.68 ± 0.12 | 128.80 ± 9.27 | 1.63 | [20] |
| 2a | 60 | 318.17 ± 1.36 | 152.83 ± 11.06 | 0.83 ± 0.24 | 54.75 ± 17.02 | 2.79 | [15] |
| 2b | 60 | 317.74 ± 1.04 | 164.06 ± 9.23 | 0.71 ± 0.16 | 51.41 ± 12.71 | 3.19 | [15] |
| 3a | 15 | 321.24 ± 1.04 | 162.70 ± 19.21 | 0.76 ± 0.17 | 54.07 ± 15.88 | 3.01 | [21] |
| 3b | 30 | 326.52 ± 1.75 | 123.57 ± 14.17 | 0.34 ± 0.10 | 19.82 ± 7.22 | 6.23 | [22] |
| Figure | R2 | RSS | p | F | P | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| β | ||||||||
| 1a | 0.9789 | 0.1370 | 0.0033 | <0.0001 | 0.0151 | 69.56 | 0.0031 | [7] |
| 1b | 0.9856 | 0.0080 | <0.0001 | <0.0001 | <0.0001 | 170.77 | <0.0001 | [20] |
| 2a | 0.9374 | 0.1912 | <0.0001 | <0.0001 | 0.0132 | 44.93 | 0.0002 | [15] |
| 2b | 0.9679 | 0.1592 | <0.0001 | <0.0001 | 0.0041 | 90.43 | <0.0001 | [15] |
| 3a | 0.9817 | 0.1034 | 0.0001 | <0.0001 | 0.0044 | 160.97 | <0.0001 | [21] |
| 3b | 0.9751 | 0.1117 | 0.0010 | <0.0001 | 0.0185 | 78.41 | 0.0006 | [22] |
| BufferpH | t (min) | λ (nm) | Concentration of Starch | Source Inulinase | Ref. |
|---|---|---|---|---|---|
| 6.9 sodium phosphate | 3 | 540 | 0.5% | Sigma-Aldrich (St. Louis, MO, USA) | [7] |
| 6.9 phosphate | 5 | 600 | 2% | Merck AG (Germany) | [20] |
| 6.9 sodium phosphate | 60 | 540 | 1% | Sigma Chemical Company | [16] |
| 7.0 MOPS | 15 | 575 | 0.5% | Sigma | [21] |
| 7.0 MES | 30 | 520 | - | Shanghai Kaiyang BiologicalTechnology Co., Ltd. (Shanghai, China) | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miłek, J. Determination of Activation Energies and the Optimum Temperatures of Hydrolysis of Starch by α-Amylase from Porcine Pancreas. Molecules 2021, 26, 4117. https://doi.org/10.3390/molecules26144117
Miłek J. Determination of Activation Energies and the Optimum Temperatures of Hydrolysis of Starch by α-Amylase from Porcine Pancreas. Molecules. 2021; 26(14):4117. https://doi.org/10.3390/molecules26144117
Chicago/Turabian StyleMiłek, Justyna. 2021. "Determination of Activation Energies and the Optimum Temperatures of Hydrolysis of Starch by α-Amylase from Porcine Pancreas" Molecules 26, no. 14: 4117. https://doi.org/10.3390/molecules26144117
APA StyleMiłek, J. (2021). Determination of Activation Energies and the Optimum Temperatures of Hydrolysis of Starch by α-Amylase from Porcine Pancreas. Molecules, 26(14), 4117. https://doi.org/10.3390/molecules26144117






