Zuccagnia punctata Cav. Essential Oil into Poly(ε-caprolactone) Matrices as a Sustainable and Environmentally Friendly Strategy Biorepellent against Triatoma infestans (Klug) (Hemiptera, Reduviidae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Essential Oil Composition, Yield, and Spectroscopy Characterization
2.2. Morphological Characterization
2.3. Thermal Properties and Crystallinity
2.4. ZEO Loading Capacity and Encapsulation Efficiency
2.5. Repellent Activity against Triatoma Infestans Nymphs
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Essential Oil Extraction and Chemical Analysis
3.4. Preparation of Zuccagnia punctata Essential Oil Loaded Polymeric Systems
3.5. PCL, ZEO, and ZEOP Matrices Characterization
3.6. Repellent Activity against Triatoma infestans Nymphs Fifth Instars
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- WHO. La Enfermedad de Chagas (Tripanosomiasis Americana). 2020. Available online: http://www.who.int/mediacentre/factsheets/fs340/es/ (accessed on 20 January 2020).
- Zerba, E.N. Susceptibility and resistance to insecticides of Chagas disease vectors. Medicina 1999, 59, 41–46. [Google Scholar]
- Rojas de Arias, A.; Lehane, M.J.; Schofield, C.J.; Maldonado, M. Pyrethroid insecticide evaluation on different house structures in a Chagas disease endemic area of the Paraguayan Chaco. Mem. Inst. Oswaldo Cruz 2004, 99, 657–662. [Google Scholar] [CrossRef]
- Vassena, C.V.; Cueto, G.M.; Audino, P.G.; Alzogaray, R.; Picollo, M.I.; Zerba, E.N. Prevalence and Levels of Permethrin Resistance in Pediculus humanus capitis De Geer (Anoplura: Pediculidae) from Buenos Aires, Argentina. J. Med. Emtomol. 2003, 40, 447–450. [Google Scholar] [CrossRef] [Green Version]
- Picollo, M.I.; Vassena, C.V.; Santo Orihuela, P.; Barrios, S.; Zerba, E.N. High resistance to pyrethroid insecticides associated with ineffective field treatments in Triatoma infestans (Hemiptera, Reduvidae) from the north of Argentina. J. Med. Entomol. 2005, 42, 637–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santo Orihuela, P.L.; Vassena, C.V.; Zerba, E.N.; Picollo, M.I. Relative contribution of monooxygenase and esterase to pyrethroid resistance in Triatoma infestans (Hemiptera: Reduviidae) from Argentina and Bolivia. J. Med. Entomol. 2008, 45, 298–306. [Google Scholar] [CrossRef]
- Cycon, M.; Piotrowska-Seget, Z. Pyrethroid-Degrading Microorganisms and Their Potential for the Bioremediation of Contaminated Soils: A Review. Front. Microbiol. 2016, 7, 1463. [Google Scholar] [CrossRef] [Green Version]
- Chiriac, A.P.; Rusu, A.G.; Nita, L.E.; Chiriac, V.M.; Neamtu, I.; Sandu, A. Polymeric Carriers Designed for Encapsulation of Essential Oils with Biological Activity. Pharmaceutics 2021, 13, 631. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, V.; Buffa, F.; Molinari, F.; Hermida, L.G.; García, J.J.; Abraham. G.A. Electrospun ethyl cellulose-based mats with insect-repellent activity. Mater. Lett. 2019, 253, 289–292. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Dhiman, S.; Borah, S.; Rabha, B. Essential oil based polymeric patch development and evaluating its repellent activity against mosquitoes. Acta Trop. 2015, 147, 45–53. [Google Scholar] [CrossRef]
- Toloza, A.C.; Zygadlo, J.; Cueto, G.; Biurrun, F.; Zerba, E.; Picollo, M.I. Fumigant and Repellent Properties of Essential Oils and Component Compounds Against Permethrin-Resistant Pediculus humanus capitis (Anoplura: Pediculidae). J. Med. Entomol. 2006, 43, 889–895. [Google Scholar] [CrossRef]
- Lima, B.; López, S.; Luna, L.; Agüero, M.B.; Aragón, L.; Tapia, A.; Zacchino, S.; López, M.L.; Zygadlo, J.; Feresin, G.E. Essential oils of medicinal plants from the Central Andes of Argentina: Chemical composition, and antifungal, antibacterial, and insect-repellent activities. Chem. Biodivers. 2011, 8, 924–936. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Lima, B.; Aragón, L.; Ariza-Espinar, L.; Tapia, A.; Zacchino, S.; Zygadlo, J.; Feresin, G.E.; López, M.L. Essential Oil of Azorella cryptantha collected in two different locations from San Juan Province, Argentina: Chemical variability and anti-insect and antimicrobial activities. Chem. Biodivers. 2012, 9, 1452–1464. [Google Scholar] [CrossRef]
- López, S.; Lima, B.; Agüero, M.B.; López, M.L.; Hadad, M.; Zygadlo, J.; Caballero, D.; Stariolo, R.; Suero, E.; Feresin, G.E.; et al. Chemical composition, antibacterial and repellent activities of Azorella trifurcata, Senecio pogonias, and Senecio oreophyton essential oils. Arab. J. Chem. 2018, 11, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Barradas, T.N.; Senna, J.P.; Júnior, E.R.; Mansur, C.R.E. Polymer-based drug delivery systems applied to insects repellents devices: A review. Curr. Drug Deliv. 2016, 13, 221–235. [Google Scholar] [CrossRef]
- Gómez, J.; Simirgiotis, M.; Manrique, S.; Lima, B.; Bórquez, J.; Feresin, G.E.; Tapia, A. UHPLC-HESI-OT-MS-MS biomolecules profiling, antioxidant and antibacterial activity of the “orange-yellow resin” from Zuccagnia punctata Cav. Antioxidants 2020, 9, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paneva, D.; Manolova, N.; Argirova, M.; Rashkov, I. Antibacterial electrospun poly(ɛ-caprolactone)/ascorbylpalmitate nanofibrous materials. Int. J. Pharm. 2011, 416, 346–355. [Google Scholar] [CrossRef]
- Seremeta, K.A.; Höcht, C.; Taira, C.; Tornello, P.R.C.; Abraham, G.A.; Sosnik, A. Didanosine-loaded poly(epsilon-caprolactone) microparticles by a coaxial electrohydrodynamic atomization (CEHDA) technique. J. Mater. Chem. B 2015, 3, 102–111. [Google Scholar] [CrossRef]
- Tornello, P.R.C.; Feresin, G.E.; Tapia, A.; Veiga, I.G.; Moraes, A.M.; Abraham, G.A.; Cuadrado, T.R. Dispersion and release of embelin from electrospun biodegradable, polymeric, membranes. Polym. J. 2012, 44, 1105–1111. [Google Scholar] [CrossRef] [Green Version]
- Tornello, P.R.C.; Feresin, G.E.; Tapia, A.; Cuadrado, T.R.; Abraham, G.A. Multilayered electrospun nanofibrous scaffolds for tailored controlled release of embelin. Soft Mater. 2018, 16, 51–61. [Google Scholar] [CrossRef]
- Peres, M.C.; de Souza Costa, G.C.; dos Reis, L.E.L.; da Silva, L.D.; Peixoto, M.F.; Alves, C.C.F.; Forim, M.R.; Quintela, E.D.; Araújo, W.L.; de Melo Cazal, C. In natura and nanoencapsulated essential oils from Xylopia aromatica reduce oviposition of Bemisia tabaci in Phaseolus vulgaris. J. Pest Sci. 2020, 93, 807–821. [Google Scholar] [CrossRef]
- De Ávila, D.S.C.; Holz, J.P.; Carone, C.L.P.; Morisso, F.D.; Ligabue, R.A.; Pighinelli, L. Microcapsules PCL with essential oil citronella. Adv. Tissue Eng. Regen. 2017, 2, 159–162. [Google Scholar]
- Akolade, J.O.; Balogun, M.; Swanepoel, A.; Ibrahim, R.B.; Yusuf, A.A.; Labuschagne, P. Microencapsulation of eucalyptol in polyethylene glycol and polycaprolactone using particles from gas-saturated solutions. RSC Adv. 2019, 9, 34039. [Google Scholar] [CrossRef] [Green Version]
- Unalan, I.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Frank, G.; Boccaccini, A.R. Physical and antibacterial properties of Peppermint essential oil loaded poly(e-caprolactone) (PCL) electrospun fiber matts for wound healing. Front. Bioeng. Biotechnol. 2019, 7, 346. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Adams, R.P., Ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 1932633219. [Google Scholar]
- Stein, S.; Mirokhin, D.; Tchekhovskoi, D.; Mallard, G.; Mikaia, A.; Zaikin, V.; Sparkmanm, D. The NIST Mass Spectral Search Program for the Nist/Epa/Nih Mass Spectra Library; Standard Reference Data Program of the National Institute of Standards and Technology; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2011. [Google Scholar]
- Alvarez, S.L.; Cortadi, A.; Juarez, M.A.; Petenatti, E.; Tomi, F.; Casanova, J.; Van Baren, C.M.; Zacchino, S.; Vila, R. (−)-5, 6-Dehydrocamphor from the antifungal essential oil of Zuccagnia punctata. Phytochem. Lett. 2012, 5, 194–199. [Google Scholar] [CrossRef]
- Alves, S.F.; Borges, L.L.; de Paula, J.A.M.; Vieira, R.F.; Ferri, P.H.; do Couto, R.O.; de Paula, J.R.; Bara, M.T.F. Chemical variability of the essential oils from fruits of Pterodon emarginatus in the Brazilian Cerrado. Rev. Bras. Farmacogn. 2013, 23, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem. 2003, 54, 4364–4370. [Google Scholar] [CrossRef]
- Masotti, V.; Juteau, F.; Bessie’re, J.M.; Viano, J. Seasonal and phenological variations of the essential oil from the narrow endemic species Artemisia molinieri and its biological activities. J. Agric. Food Chem. 2003, 51, 7115–7121. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Waomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Paumgartten, F.J.R.; Delgado, I.F. Mosquito repellents, effectiveness in preventing diseases and safety during pregnancy. Vigil. Sanit. Debate 2016, 4, 97–104. [Google Scholar]
- Kurdelas, R.R.; López, S.; Lima, B.; Feresin, G.E.; Zygadlo, J.; Zacchino, S.; López, M.L.; Tapia, A.; Freile, M.L. Chemical composition, anti-insect and antimicrobial activity of the Baccharis darwinii essential oil from Argentine Patagonia. Ind. Crops Prod. 2012, 40, 261–267. [Google Scholar] [CrossRef]
- Lima, L.A.; Ferreira-Sá, P.S.; Garcia, M.D.N., Jr.; Pereira, V.L.P.; Carvalho, J.C.T.; Rocha, L.; Fernandes, C.P.; Souto, R.N.P.; Araújo, R.S.; Botas, G.; et al. Nano-emulsions of the essential oil of Baccharis reticularia and its constituents as eco-friendly repellents against Tribolium castaneum. Ind. Crops Prod. 2021, 162, 113282. [Google Scholar] [CrossRef]
- Tavares, M.; da Silva, M.R.M.; de Siqueira, L.B.O.; Rodrigues, R.A.S.; Bodjolle-d’Almeidab, L.; dos Santos, P.E.; Ricci-Júnior, E. Trends in insectrepellent formulations: A review. Int. J. Pharm. 2018, 539, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.; Caldas, A.L.; Tohidi, S.D.; Molina, J.; Souto, A.P.; Fangueiro, R.; Zille, A. Properties and controlled release of chitosan microencapsulated limonene oil. Braz. J. Pharmacogn. 2014, 24, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Van Krevelen, W.; teNijenhuis, K. Properties of Polymers, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; p. 121. [Google Scholar]
- Talukder, F.A.; Howse, P.E. Laboratory evaluation of toxic and repellent properties of the pithraj tree, Aphanamixis polystachya Wall & Parker, against Sitophilus oryzae (L.). Int. J. Pest Manag. 1994, 40, 274–279. [Google Scholar]
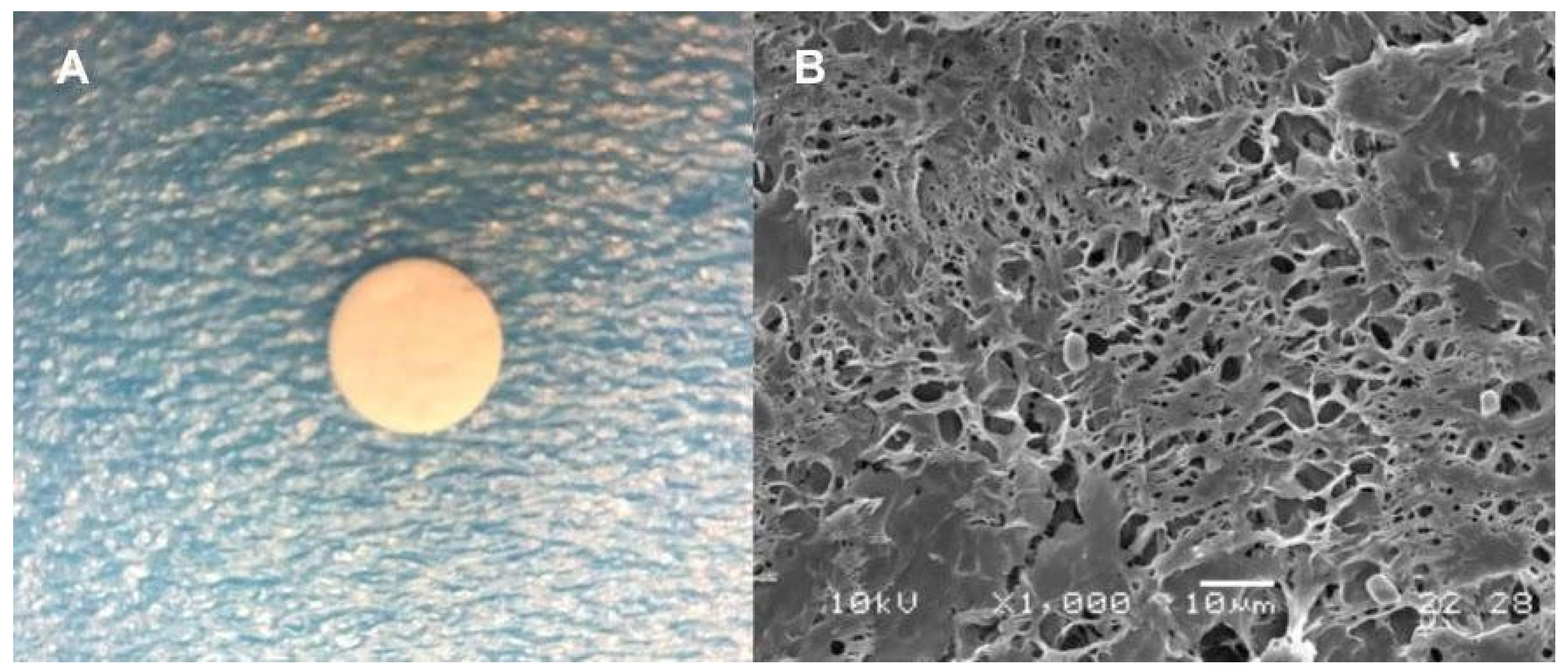

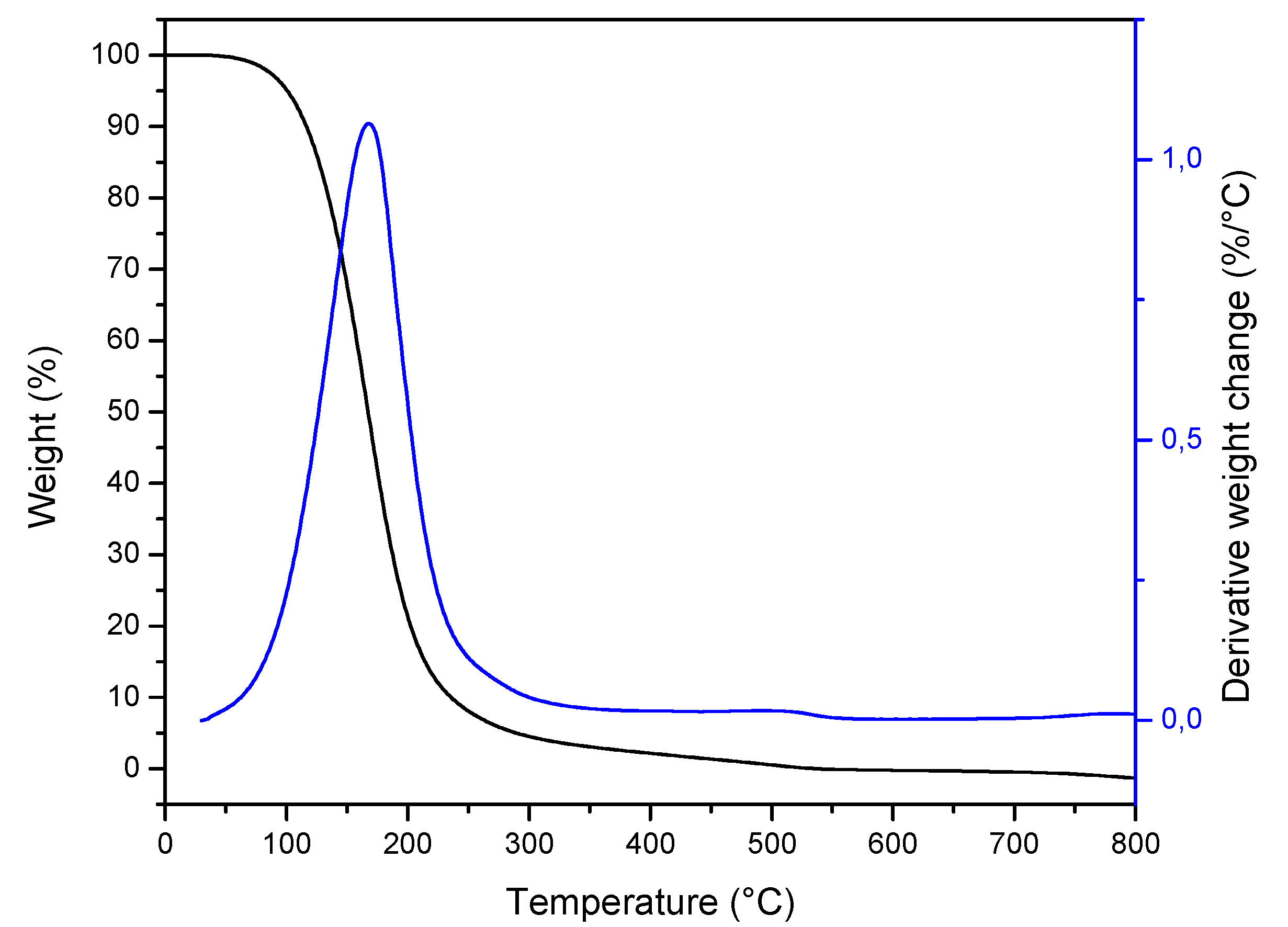
| Peak | Component | RI | Area (%) | Identification Method |
|---|---|---|---|---|
| 1 | Alpha-thujene | 928 | t | 1 |
| 2 | Alpha-pinene | 936 | 9.1 | 1, 2 |
| 3 | Alpha-fenchene | 950 | 0.3 | 1 |
| 4 | Camphene | 951 | t | 1, 2 |
| 5 | Thuja-2,4(10)-diene | 956 | 4.6 | 1 |
| 6 | Myrcene | 990 | t | 1, 2 |
| 7 | Alpha-terpinene | 1016 | 0.6 | 1 |
| 8 | p-cymene | 1025 | t | 1, 2 |
| 9 | Limonene | 1030 | 0.8 | 1, 2 |
| 10 | Gamma-terpinene | 1059 | 1.6 | 1 |
| 11 | p-cymenene | 1091 | 2.3 | 1 |
| 12 | (−)-5,6-dehydrocamphor | 1097 | 62.4 | 1 |
| 13 | Terpinen-4-ol | 1178 | 4.4 | 1, 2 |
| 14 | Verbenone | 1206 | 3.1 | 1 |
| 15 | (E)-Cinnamyl alcohol | 1304 | 0.4 | 1 |
| 16 | Piperitenone | 1343 | t | 1, 2 |
| 17 | Eugenol (dihydro) | 1369 | 4.5 | 1 |
| 18 | (E)- caryophyllene | 1419 | 0.8 | 1 |
| 19 | Epi-beta-santalene | 1447 | 2.1 | 1 |
| 20 | Delta-amorphene | 1512 | 0.6 | 1 |
| 21 | (Z)-gamma-bisabolene | 1515 | 0.1 | 1 |
| 22 | Beta-curcumene | 1516 | 0.2 | 1 |
| 23 | Delta-cadinene | 1523 | 0.4 | 1 |
| 24 | (E)-gamma-bisabolene | 1531 | 0.5 | 1 |
| 25 | Epi-alpha-cadinol | 1640 | 0.7 | 1 |
| Monoterpene hydrocarbons | 19.3 | |||
| Oxygenated monoterpenes | 69.9 | |||
| Phenylpropanoids | 4.9 | |||
| Sesquiterpenes hydrocarbons | 4.7 | |||
| Oxygenated sesquiterpenes | 0.7 | |||
| Total | 99.5 |
| Sample | Mc (±s.d.) (mg/g) | LC (±s.d.) (%) | EE (±s.d.) (%) |
|---|---|---|---|
| ZEOP 0.5% | 4.87 ± 0.30 | 0.48 ± 0.08 | 98.45 ± 0.03 |
| ZEOP 1% | 9.75 ± 0.20 | 0.97 ± 0.10 | 98.51 ± 0.02 |
| Repellency (%) at 0.5 % (wt./wt.) | ||||
|---|---|---|---|---|
| Treatments | ||||
| Time (h) | ZEO | ZEOP | Control 3) | DEET 4) |
| 1 | 97.0 ± 2.0 | 33.0 ± 11.1 | −12.0 ± 5.4 | 100.0 ± 0.0 |
| 24 | 92.0 ± 10.4 | 60.0± 24.0 | −20.0 ± 32.0 | 100.0 ± 0.0 |
| 72 | 76.0 ± 5.3 | 60.0 ± 24.0 | −100.0 ± 0.0 | 100.0 ± 0.0 |
| Average repellency 1) | 88.3 ± 5.0 a | 51.0 ± 8.1 a | −44.0 ± 17.8 b | 100.0 ± 0.0 a |
| Class 2) | V | III | - | V |
| 96 | 33.0 ± 23.1 a | 73.0 ± 12.1 a | −100.0 ± 0.0 b | 100.0 ± 0.0 a |
| Class 2) | II | IV | - | V |
| Repellency (%) at 1 % (wt./wt.) | ||||
|---|---|---|---|---|
| Treatments | ||||
| Time (h) | ZEO | ZEOP | Control 3) | DEET 4) |
| 1 | 100.0 ± 0.0 | 46.6 ± 11.1 | −12.0 ± 34.4 | 100.0 ± 0.0 |
| 24 | 93.0 ± 11.5 | 60.0 ± 6.0 | −28.0 ± 32.0 | 100.0 ± 0.0 |
| 72 | 73.3 ± 23.1 | 66.0 ± 23.0 | −100.0 ± 0.0 | 100.0 ± 0.0 |
| Average repellency 1) | 88.8 ± 5.0 a | 55.5 ± 7.0 a | −46.7 ± 17.8 b | 100.0 ± 0.0 c |
| Class 2) | V | III | - | V |
| 96 | 40.0 ± 13.1 | 66.6 ± 6.7 a | −100.0 ± 0.0 b | 100.0 ± 0.0 |
| Class 2) | II | IV | V | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, S.; Tapia, A.; Zygadlo, J.; Stariolo, R.; Abraham, G.A.; Cortez Tornello, P.R. Zuccagnia punctata Cav. Essential Oil into Poly(ε-caprolactone) Matrices as a Sustainable and Environmentally Friendly Strategy Biorepellent against Triatoma infestans (Klug) (Hemiptera, Reduviidae). Molecules 2021, 26, 4056. https://doi.org/10.3390/molecules26134056
López S, Tapia A, Zygadlo J, Stariolo R, Abraham GA, Cortez Tornello PR. Zuccagnia punctata Cav. Essential Oil into Poly(ε-caprolactone) Matrices as a Sustainable and Environmentally Friendly Strategy Biorepellent against Triatoma infestans (Klug) (Hemiptera, Reduviidae). Molecules. 2021; 26(13):4056. https://doi.org/10.3390/molecules26134056
Chicago/Turabian StyleLópez, Sandra, Alejandro Tapia, Julio Zygadlo, Raúl Stariolo, Gustavo A. Abraham, and Pablo R. Cortez Tornello. 2021. "Zuccagnia punctata Cav. Essential Oil into Poly(ε-caprolactone) Matrices as a Sustainable and Environmentally Friendly Strategy Biorepellent against Triatoma infestans (Klug) (Hemiptera, Reduviidae)" Molecules 26, no. 13: 4056. https://doi.org/10.3390/molecules26134056
APA StyleLópez, S., Tapia, A., Zygadlo, J., Stariolo, R., Abraham, G. A., & Cortez Tornello, P. R. (2021). Zuccagnia punctata Cav. Essential Oil into Poly(ε-caprolactone) Matrices as a Sustainable and Environmentally Friendly Strategy Biorepellent against Triatoma infestans (Klug) (Hemiptera, Reduviidae). Molecules, 26(13), 4056. https://doi.org/10.3390/molecules26134056








