Promising Antimicrobial Properties of Bioactive Compounds from Different Honeybee Products
Abstract
:1. Introduction
2. Antimicrobial Properties of Bee Products
2.1. Honey
2.2. Propolis
2.3. Bee Venom
2.4. Royal Jelly
3. Comparison of Antimicrobial Properties of Bee Products
4. Prospective Use of Bee Products in the Treatment of Biofilm-Related Infections
5. Advances in the Characterization of Bee Products’ Antibacterial Properties
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Buchta, V.; Černý, J.; Opletalová, V. In Vitro antifungal activity of propolis samples of Czech and Slovak origin. Cent. Eur. J. Biol. 2011, 6, 160–166. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxidative Med. Cell. Longev. 2017, 1259510. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee Products in Dermatology and Skin Care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef] [Green Version]
- Kek, S.P.; Chin, N.L.; Yusof, Y.A.; Tan, S.W.; Chua, L.S. Classification of entomological origin of honey based on its physicochemical and antioxidant properties. Int. J. Food Prop. 2018, 20, 2723–2738. [Google Scholar] [CrossRef]
- Cebrero, G.; Sanhueza, O.; Pezoa, M.; Báez, M.E.; Martínez, J.; Báez, M.; Fuentes, E. Relationship among the minor constituents, antibacterial activity and geographical origin of honey: A multifactor perspective. Food Chem. 2020, 315, 126296. [Google Scholar] [CrossRef]
- Przybyłek, I.M.; Karpinski, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [Green Version]
- Bouacha, M.; Benbouzid, H. Antibacterial properties of honey from different Algerian regions against Staphylococcus aureus strains from wounds. J. Pure Appl. Microbiol. 2020, 14, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Kryłow, W.N.; Agafonow, A.W. Teorija i Sriedstwa Apiterapii; GNU: Moskwa, Russia, 2007; Volume 296, pp. 14–18. [Google Scholar]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Azevedo de Oliveira, M.L.F.; Teles da Silva, L.V.A.; do Nascimento, T.G.; de Almeida, L.M.; Calumby, R.J.N.; Nunes, Á.M.; de Magalhães Oliveira, L.M.T.; da Silva Fonseca, E.J. Antioxidant and antimicrobial activity of red propolis embedded mesoporous silica nanoparticles. Drug Dev. Ind. Pharm. 2020, 46, 1199–1208. [Google Scholar] [CrossRef]
- Okińczyc, P.; Paluch, R.F.; Widelski, J.; Wojtanowski, K.K.; Mroczek, T.; Krzyżanowska, B.; Skalicka-Woźniak, K.; Sroka, Z. Antimicrobial activity of Apis mellifera L. and Trigona sp. propolis from Nepal and its phytochemical analysis. Biomed. Pharmacother. 2020, 129, 110435. [Google Scholar] [CrossRef]
- Aytekin, A.A.; Tuncay Tanrıverdi, S.; Aydın Köse, F.; Kart, D.; Eroğlu, İ.; Özer, Ö. Propolis loaded liposomes: Evaluation of antimicrobial and antioxidant activities. J. Liposome Res. 2020, 30, 107–116. [Google Scholar] [CrossRef]
- Patel, S. Emerging Adjuvant Therapy for Cancer: Propolis and its Constituents. J. Diet. Suppl. 2016, 13, 245–268. [Google Scholar] [CrossRef]
- Kuo, Y.Y.; Jim, W.T.; Su, L.C.; Chung, C.J.; Lin, C.Y.; Huo, C.; Tseng, J.C.; Huang, S.H.; Lai, C.J.; Chen, B.C.; et al. Caffeic Acid phenethyl ester is a potential therapeutic agent for oral cancer. Int. J. Mol. Sci. 2015, 16, 10748–10766. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Bhargava, P.; Yu, Y.; Sari, A.N.; Zhang, H.; Ishii, N.; Yan, K.; Zhang, Z.; Ishida, Y.; Terao, K.; et al. Novel Caffeic Acid Phenethyl Ester-Mortalin Antibody Nanoparticles Offer Enhanced Selective Cytotoxicity to Cancer Cells. Cancers 2020, 12, 2370. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhang, L. Antibacterial Effect of Caffeic Acid Phenethyl Ester on Cariogenic Bacteria and Streptococcus mutans Biofilms. Antimicrob. Agents Chemother. 2020, 64, e00251-20. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCleskey, C.S.; Melampy, R.M. Bactericidal Properties of Royal Jelly of the Honeybee. J. Econ. Entomol. 1939, 32, 581–587. [Google Scholar] [CrossRef]
- Bellik, Y. Bee Venom: Its Potential Use in Alternative Medicine. Anti-Infect. Agents 2015, 13, 3–16. [Google Scholar] [CrossRef]
- El-Seedi, H.; Abd El-Wahed, A.; Yosri, N.; Musharraf, S.G.; Chen, L.; Moustafa, M.; Zou, X.; Al-Mousawi, S.; Guo, Z.; Khatib, A.; et al. Antimicrobial Properties of Apis mellifera’s Bee Venom. Toxins 2020, 12, 451. [Google Scholar] [CrossRef]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Jesus Simal-Gandara, J. Bee Venom: An Updating Review of Its Bioactive Molecules and Its Health Applications. Nutrients 2020, 12, 3360. [Google Scholar] [CrossRef]
- Ratajczak, M.; Kaminska, D.; Dlugaszewska, J.; Gajecka, M. Antibiotic Resistance, Biofilm Formation, and Presence of Genes Encoding Virulence Factors in Strains Isolated from the Pharmaceutical Production Environment. Pathogens 2021, 10, 130. [Google Scholar] [CrossRef]
- Majkut, M.; Kwiecińska-Piróg, J.; Wszelaczyńska, E.; Pobereżny, J.; Gospodarek-Komkowska, E.; Wojtacki, K.; Barczak, T. Antimicrobial activity of heat-treated Polish honeys. Food Chem. 2021, 343, 128561. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ren, P.; Wu, Y.; He, Q. Recent advances in analytical techniques for the detection of adulteration and authenticity of bee products—A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2021, 38, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Al-Waili, N.S. Investigating the antimicrobial activity of natural honey and its effects on the pathogenic bacterial infections of surgical wounds and conjunctiva. J. Med. Food. 2004, 7, 210–222. [Google Scholar] [CrossRef]
- Tan, H.T.; Rahman, R.A.; Gan, S.H.; Halim, A.S.; Hassan, S.A.; Sulaiman, S.A.; Kirnpal-kaur, B.S. The antibacterial properties of Malaysian tualang honey against wound and enteric microorganisms in comparison to manuka honey. BMC Complement. Altern. Med. 2009, 9, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.; Othman, N.H. Review of the medicinal effects of tualang honey and a comparison with manuka honey. Malays. J. Med. Sci. 2013, 20, 6–13. [Google Scholar]
- Boateng, J.; Diunase, K.N. Comparing the Antibacterial and Functional Properties of Cameroonian and Manuka Honeys for Potential Wound Healing-Have We Come Full Cycle in Dealing with Antibiotic Resistance? Molecules 2015, 20, 16068–16084. [Google Scholar] [CrossRef]
- Mullai, V.; Menon, T. Bactericidal Activity of Different Types of Honey against Clinical and Environmental Isolates of Pseudomonas aeruginosa. J. Altern. Complement. Med. 2007, 13, 439–442. [Google Scholar] [CrossRef] [Green Version]
- Ramón-Sierra, J.; Martínez-Guevara, J.L.; Pool-Yam, L.; Magaña-Ortiz, D.; Yam-Puc, A.; Ortiz-Vázquez, E. Effects of phenolic and protein extracts from Melipona beecheii honey on pathogenic strains of Escherichia coli and Staphylococcus aureus. Food Sci. Biotechnol. 2020, 29, 1013–1021. [Google Scholar] [CrossRef]
- Ramón-Sierra, J.M.; Villanueva, M.A.; Rodríguez-Mendiola, M.; Reséndez-Pérez, D.; Ortiz-Vázquez, E.; Arias-Castro, C. Characterization of a non-glycosylated fraction from honey proteins of Melipona beecheii with antimicrobial activity against Escherichia coli O157:H7. J. Appl. Microbiol. 2021, 130, 1913–1924. [Google Scholar] [CrossRef]
- Kędzia, B.; Hołderna-Kędzia, E. Działanie miodu na drobnoustroje wyizolowane z zakażonych ran. Postępy Fitoter. 2014, 1, 40–43. [Google Scholar]
- Majtan, J.; Majtanova, L.; Bohova, J.; Majtan, V. Honeydew honey as a potent antibacterial agent in eradication of multi-drug resistant Stenotrophomonas maltophilia isolates from cancer patients. Phytother. Res. 2011, 25, 584–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosravi, A.R.; Shokri, H.; Katiraee, F.; Ziglari, T.; Forsi, M. Fungicidal potential of different Iranian honeys against some pathogenic Candida species. J. Apic. Res. 2008, 47, 256–260. [Google Scholar] [CrossRef]
- Anand, S.; Deighton, M.; Livanos, G.; Pang, E.C.K.; Mantri, N. Agastache honey has superior antifungal activity in comparison with important commercial honeys. Sci. Rep. 2019, 9, 18197. [Google Scholar] [CrossRef] [Green Version]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Singh, N.P. Antibacterial activity of Manuka honey and its components: An overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef]
- Miorin, P.L.; Levy Junior, N.C.; Custodio, A.R.; Bretz, W.A.; Marcucci, M.C. Antibacterial activity of honey and propolis from Apis mellifera and Tetragonisca angustula against Staphylococcus aureus. J. Appl. Microbiol. 2003, 95, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Al-Naama, R.T. Evaluation of in-vitro inhibitory effect of honey on some microbial isolate. Afr. J. Bacteriol. Res. 2009, 1, 64–67. [Google Scholar]
- Chauhan, A.; Pandey, V.; Chacko, K.M.; Khandal, R.K. Antibacterial Efficacy of Raw and Processed Honey. Electron. J. Biol. 2010, 5, 58–66. [Google Scholar]
- Badawy, O.F.H.; Shafii, S.S.A.; Tharwat, E.E.; Kamal, A.M. Antibacterial activity of bee honey and its therapeutic usefulness against Escherichia coli O157:H7 and Salmonella typhimurium infection. OIE Rev. Sci. Tech. 2004, 23, 1011–1022. [Google Scholar] [CrossRef] [Green Version]
- Petruzzi, L.; Rosaria Corbo, M.; Campaniello, D.; Speranza, B.; Sinigaglia, M.; Bevilacqua, A. Antifungal and Antibacterial Effect of Propolis: A Comparative Hit for Food-Borne Pseudomonas, Enterobacteriaceae and Fungi. Foods 2020, 9, 559. [Google Scholar] [CrossRef]
- Silva, J.C.; Rodrigues, S.; Feás, X.; Estevinho, L.M. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem. Toxicol. 2012, 50, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.F.; dos Santos, U.P.; Macorini, L.F.B.; de Melo, A.M.M.F.; Balestieri, J.B.P.; Paredes-Gamero, E.J.; Cardoso, C.A.; de Picoli Souza, K.; dos Santos, E.L. Antimicrobial, antioxidant and cytotoxic activities of propolis from Melipona orbignyi (Hymenoptera, Apidae). Food Chem. Toxicol. 2014, 65, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.R.G.; Matias, T.M.S.; Souza, L.I.O.; Matos-Rocha, T.J.; Fonseca, S.A.; Mousinho, K.C.; Santos, A.F. Phytochemical screening and in vitro antibacterial, antifungal, antioxidant and antitumor activities of the red propolis Alagoas. Braz. J. Biol. 2019, 79, 452–459. [Google Scholar] [CrossRef]
- Kubiliene, L.; Laugaliene, V.; Pavilonis, A.; Maruska, A.; Majiene, D.; Barcauskaite, K.; Kubilius, R.; Kasparaviciene, G.; Savickas, A. Alternative preparation of propolis extracts: Comparison of their composition and biological activities. BMC Complement. Altern. Med. 2015, 15, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kędzia, B.; Hołderna-Kędzia, E. Aktywność antybiotyczna propolisu krajowego i europejskiego. Postępy Fitoter. 2013, 2, 97–107. [Google Scholar]
- Dantas Silva, R.P.; Machado, B.A.S.; Barreto, G.A.; Costa, S.S.; Andrade, L.N.; Amaral, R.G.; Carvalho, A.A.; Padilha, F.F.; Barbosa, J.D.; Umsza-Guez, M.A. Antioxidant, antimicrobial, antiparasitic, and cytotoxic properties of various Brazilian propolis extracts. PLoS ONE 2017, 12, e0172585. [Google Scholar] [CrossRef]
- AL-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines 2018, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Wojtyczka, R.D.; Kubina, R.; Kabała-Dzik, A.; Bułdak, R.J. Antibacterial activity of ethanol extract of propolis. Ann. Acad. Med. Siles. 2012, 66, 39–48. [Google Scholar]
- Cardoso, R.L.; Maboni, F.; Machado, G.; Alves, S.H.; de Vargas, A.C. Antimicrobial activity of propolis extract against Staphylococcus coagulase positive and Malassezia pachydermatis of canine otitis. Vet. Microbiol. 2010, 142, 432–434. [Google Scholar] [CrossRef]
- Popova, M.P.; Chinou, I.B.; Marekov, I.N.; Bankova, V.S. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry 2009, 70, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.F.; Dos Santos, U.P.; Rocha, P.d.S.d.; Damião, M.J.; Balestieri, J.B.; Cardoso, C.A.; Paredes-Gamero, E.J.; Estevinho, L.M.; de Picoli Souza, K.; Dos Santos, E.L. Antimicrobial, Antioxidant, Anti-Inflammatory, and Cytotoxic Activities of Propolis from the Stingless Bee Tetragonisca fiebrigi (Jataí). Evid. Based Complement. Altern. Med. 2015, 2015, 296186. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, E.M.; Stock, D.; Chada, F.J.; Finger, D.; Sawaya, A.C.; Eberlin, M.N.; Felsner, M.L.; Quináia, S.P.; Monteiro, M.C.; Torres, Y.R. A Comparison between Characterization and Biological Properties of Brazilian Fresh and Aged Propolis. BioMed Res. Int. 2014, 2014, 257617. [Google Scholar] [CrossRef] [Green Version]
- Righi, A.A.; Alves, T.R.; Negri, G.; Marques, L.M.; Breyer, H.; Salatino, A. Brazilian red propolis: Unreported substances, antioxidant and antimicrobial activities. J. Sci. Food Agric. 2011, 91, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Kędzia, B.; Hołderna-Kędzia, E. Badania nad skojarzonym działaniem antybiotyków i propolisu na Staphylococcus aureus. Herba Pol. 1986, 32, 187–195. [Google Scholar]
- Orsi, R.O.; Fernandes, A.; Bankova, V.; Sforcin, J.M. The effects of Brazilian and Bulgarian propolis in vitro against Salmonella Typhi and their synergism with antibiotics acting on the ribosome. Nat. Prod. Res. 2012, 26, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H.; Rinaldi, M.G.; Pfaller, M.A. Resistance of Candida species to fluconazole. Antimicrob. Agents Chemother. 1995, 39, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, C.; Unterkircher, C.; Fantinato, V.; Shimizu, M.T. Antifungal activity of propolis on different species of Candida. Mycoses 2001, 44, 375–378. [Google Scholar] [CrossRef]
- Mutlu Sariguzel, F.; Berk, E.; Koc, A.N.; Sav, H.; Demir, G. Antifungal Activity of Propolis Against Yeasts Isolated from Blood Culture: In Vitro Evaluation. J. Clin. Lab. Anal. 2016, 30, 513–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koç, A.N.; Silici, S.; Kasap, F.; Hörmet-Öz, H.T.; Mavus-Buldu, H.; Ercal, B.D. Antifungal Activity of the Honeybee Products Against Candida spp. and Trichosporon spp. J. Med. Food 2010, 14, 128–134. [Google Scholar] [CrossRef]
- Kosalec, I.; Pepeljnjak, S.; Kustrak, D. Antifungal activity of fluid extract and essential oil from anise fruits (Pimpinella anisum L., Apiaceae). Acta Pharm. 2005, 55, 377–385. [Google Scholar]
- Melliou, E.; Chinou, I. Chemical Analysis and Antimicrobial Activity of Greek Propolis. Planta Med. 2004, 70, 515–519. [Google Scholar] [CrossRef]
- Wożniak, M.; Kwiatkowska, A.; Hołderna-Kędzia, E.; Sosnowska, K.; Mrówczyńska, L.; Ratajczak, I. Biological activity of propolis extracts. Postępy Fitoter. 2021, 1, 8–13. [Google Scholar] [CrossRef]
- Veloz, J.J.; Alvear, M.; Salazar, L.A. Antimicrobial and Antibiofilm Activity against Streptococcus mutans of Individual and Mixtures of the Main Polyphenolic Compounds Found in Chilean Propolis. BioMed Res. Int. 2019, 2019, 7602343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, S.J.; Park, E.; Asandei, A.; Choi, J.Y.; Lee, S.C.; Seo, C.H.; Luchian, T.; Park, Y. Bee venom-derived antimicrobial peptide melectin has broad-spectrum potency, cell selectivity, and salt-resistant properties. Sci. Rep. 2020, 10, 10145. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.C.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Han, S.M.; Kim, J.M.; Hong, I.P.; Woo, S.O.; Kim, S.G.; Jang, H.R.; Pak, S.C. Antibacterial Activity and Antibiotic-Enhancing Effects of Honeybee Venom against Methicillin-Resistant Staphylococcus aureus. Molecules 2016, 21, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perumal Samy, R.; Gopalakrishnakone, P.; Thwin, M.M.; Chow, T.K.; Bow, H.; Yap, E.H.; Thong, T.W. Antibacterial activity of snake, scorpion and bee venoms: A comparison with purified venom phospholipase A2 enzymes. J. Appl. Microbiol. 2007, 102, 650–659. [Google Scholar] [CrossRef]
- Kim, S.-T.; Hwang, J.-Y.; Sung, M.-S.; Je, S.-Y.; Bae, D.-R.; Han, S.-M.; Lee, S.-H. The Minimum Inhibitory Concentration (MIC) of bee venom against bacteria isolated from pigs and chickens. Korean J. Vet. Serv. 2006, 29, 19–26. [Google Scholar]
- Leandro, L.F.; Mendes, C.A.; Casemiro, L.A.; Vinholis, A.H.C.; Cunha, W.R.; De Almeida, R.; Martins, C.H.G. Antimicrobial activity of apitoxin, melittin and phospholipase A2 of honey bee (Apis mellifera) venom against oral pathogens. Acad. Bras. Cienc. 2015, 87, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Zolfagharian, H.; Mohajeri, M.; Babaie, M. Bee Venom (Apis mellifera) an Effective Potential Alternative to Gentamicin for Specific Bacteria Strains: Bee Venom an Effective Potential for Bacteria. J. Pharmacopunct. 2016, 19, 225–230. [Google Scholar] [CrossRef]
- AL-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 2015, 22, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jang, A.Y.; Lin, S.; Lim, S.; Kim, D.; Park, K.; Han, S.M.; Yeo, J.H.; Seo, H.S. Melittin, a honeybee venom-derived antimicrobial peptide, may target methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 2015, 12, 6483–6490. [Google Scholar] [CrossRef] [Green Version]
- Dosler, S.; Gerceker, A.A. In Vitro activities of antimicrobial cationic peptides; melittin and nisin, alone or in combination with antibiotics against Gram-positive bacteria. J. Chemother. 2012, 24, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ebbensgaard, A.; Mordhorst, H.; Overgaard, M.T.; Nielsen, C.G.; Aarestrup, F.M.; Hansen, E.B. Comparative Evaluation of the Antimicrobial Activity of Different Antimicrobial Peptides against a Range of Pathogenic Bacteria. PLoS ONE 2015, 10, e0144611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques Pereira, A.F.; Albano, M.; Bérgamo Alves, F.C.; Murbach Teles Andrade, B.F.; Furlanetto, A.; Mores Rall, V.L.; Delazari Dos Santos, L.; de Oliveira Orsi, R.; Fernandes Júnior, A. Influence of apitoxin and melittin from Apis mellifera bee on Staphylococcus aureus strains. Microb. Pathog. 2020, 141, 104011. [Google Scholar] [CrossRef]
- Garcia, M.C.; Finola, M.S.; Marioli, J.M. Bioassay Directed Identification of Royal Jelly’s Active Compounds against the Growth of Bacteria Capable of Infecting Cutaneous Wounds. Adv. Microbiol. 2013, 03, 138–144. [Google Scholar] [CrossRef] [Green Version]
- García, M.C.; Finola, M.S.; Marioli, J.M. Antibacterial activity of Royal Jelly against bacteria capable of infecting cutaneous wounds. J. ApiProd. ApiMed. Sci. 2010, 2, 93–99. [Google Scholar] [CrossRef]
- Moselhy, W.A.; Fawzy, A.M.; Kamel, A.A. An evaluation of the potent antimicrobial effects and unsaponifiable matter analysis of the royal jelly. Life Sci. J. 2013, 10, 290–296. [Google Scholar]
- Ratanavalachai, T.; Wongchai, V. Antibacterial Activitv of Intact Roval Jelly, Its Lipid Extract and Its Defatted Extract. Thammasat Int. J. Sci. Technol. 2002, 7, 5–12. [Google Scholar]
- Bílikova, K.; Huang, S.-C.; Lin, I.-P.; Šimuth, J.; Peng, C.-C. Structure and antimicrobial activity relationship of royalisin, an antimicrobial peptide from royal jelly of Apis mellifera. Peptides 2015, 68, 190–196. [Google Scholar] [CrossRef]
- Shen, L.; Liu, D.; Li, M.; Jin, F.; Din, M.; Parnell, L.D.; Lai, C.Q. Mechanism of Action of Recombinant Acc-Royalisin from Royal Jelly of Asian Honeybee against Gram-Positive Bacteria. PLoS ONE 2012, 7, e47194. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Singh, A.K.; Wu, X.; Lyu, Y.; Bhunia, A.K.; Narsimhan, G. Characterization of antimicrobial activity against Listeria and cytotoxicity of native melittin and its mutant variants. Colloids Surf. 2016, 143, 194–205. [Google Scholar] [CrossRef]
- Shams Khozani, R.; Shahbaz Zadeh, D.; Harzandi, N.; Feizabadi, M.M.; Pooshang Bagheri, K. Kinetics Study of Antimicrobial Peptide, Melittin, in Simultaneous Biofilm Degradation and Eradication of Potent Biofilm Producing MDR Pseudomonas aeruginosa Isolates. Int. J. Pept. Res. 2018, 25, 329–338. [Google Scholar] [CrossRef]
- Karyne, R.; Curty Lechuga, G.; Almeida Souza, A.L.; Rangel da Silva Carvalho, J.P.; Simões Villas Bôas, M.H.; De Simone, S.G. Pan-Drug Resistant Acinetobacter baumannii, but Not Other Strains, Are Resistant to the Bee Venom Peptide Mellitin. Antibiotics 2020, 9, 178. [Google Scholar]
- Ali, E.M. Contributions of some biological activities of honey bee venom. J. Apic. Res. 2014, 53, 441–451. [Google Scholar] [CrossRef]
- Lee, S.B. Antifungal Activity of Bee Venom and Sweet Bee Venom against Clinically Isolated Candida albicans. J. Pharmacopunct. 2016, 1, 45–50. [Google Scholar] [CrossRef]
- Fennell, J.F.; Shipman, W.H.; Cole, L.J. Antibacterial action of a bee venom fraction (melittin) against a penicillin-resistant staphylococcus and other microorganisms. Res. Dev. Tech. Rep. 1967, 5, 1–13. [Google Scholar]
- Han, S.; Yeo, J.; Baek, H.; Lin, S.-M.; Meyer, S.; Molan, P. Postantibiotic effect of purified melittin from honeybee (Apis mellifera) venom against Escherichia coli and Staphylococcus aureus. J. Asian Nat. Prod. Res. 2009, 11, 796–804. [Google Scholar] [CrossRef]
- Lubke, L.L.; Garon, C.F. The Antimicrobial Agent Melittin Exhibits Powerful In Vitro Inhibitory Effects on the Lyme Disease Spirochete. Clin. Infect. Dis. 1997, 25, 48–51. [Google Scholar] [CrossRef] [Green Version]
- Lazarev, V.N.; Shkarupeta, M.M.; Kostryukova, E.S.; Levitskii, S.A.; Titova, G.A.; Akopian, T.A.; Govorun, V.M. Recombinant plasmid constructs expressing gene for antimicrobial peptide melittin for the therapy of mycoplasma and chlamydia infections. Bull. Exp. Biol. Med. 2007, 144, 452–456. [Google Scholar] [CrossRef]
- Lee, W.-R.; Kim, K.-H.; An, H.-J.; Kim, J.; Chang, Y.-C.; Chung, H.; Park, Y.-Y.; lee, M.-L.; Park, K.-K. The Protective Effects of Melittin on Propionibacterium acnes-Induced Inflammatory Responses in vitro and in vivo. J. Investig. Dermatol. 2014, 134, 1922–1930. [Google Scholar] [CrossRef] [Green Version]
- Pashaei, F.; Bevalian, P.; Akbari, R.; Pooshang, B.K. Single dose eradication of extensively drug resistant Acinetobacter spp. In a mouse model of burn infection by melittin antimicrobial peptide. Microb. Pathog. 2019, 127, 60–69. [Google Scholar] [CrossRef]
- Akbari, R.; Hakemi, V.M.; Hashemi, A.; Aghazadeh, H.; Sabatier, J.-M.; Pooshang Bagheri, K. Action mechanism of melittin-derived antimicrobial peptides, MDP1 and MDP2, de novo designed against multidrug resistant bacteria. Amino Acids 2018, 50, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Cerovský, V.; Hovorka, O.; Cvacka, J.; Voburka, Z.; Bednárová, L.; Borovicková, L.; Slaninová, J.; Fučík, V. Melectin: A novel antimicrobial peptide from the venom of the cleptoparasitic bee Melecta albifrons. ChemBioChem 2008, 9, 2815–2821. [Google Scholar] [CrossRef]
- Yu, A.R.; Kim, J.J.; Park, G.S.; Oh, S.M.; Han, C.S.; Lee, M.Y. The antifungal activity of Bee Venom against dermatophytes. J. Appl. Biol. Chem. 2012, 55, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Insects, arachnids and centipedes venom: A powerful weapon against bacteria. A literature review. Toxicon 2017, 130, 91–103. [Google Scholar] [CrossRef]
- Soman, N.R.; Baldwin, S.L.; Hu, G.; Marsh, J.N.; Lanza, G.M.; Heuser, J.E.; Arbeit, J.M.; Wickline, S.A.; Schlesinger, P.H. Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J. Clin. Investig. 2009, 119, 2830–2842. [Google Scholar] [CrossRef] [PubMed]
- Rajabnejad, S.H.; Mokhtarzadeh, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Razavi, B.M. Targeted delivery of melittin to cancer cells by AS1411 anti-nucleolin aptamer. Drug Dev. Ind. Pharm. 2018, 44, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Melliou, E.; Chinou, I. Chemistry and Bioactivity of Royal Jelly from Greece. J. Agric. Food Chem. 2005, 53, 8987–8992. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Yang, Y.C.; Chou, W.M.; Widowati, D.A.; Lin, I.P.; Peng, C.C. 10-Hydroxy-2-Decenoic Acid of Royal Jelly Exhibits Bactericide and Anti-Inflammatory Activity in Human Colon Cancer Cells. BMC Complement. Altern. Med. 2018, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef]
- Alreshoodi, F.; Sultanbawa, Y. Antimicrobial Activity of Royal Jelly. Anti-Infect. Agents 2015, 13, 50–59. [Google Scholar] [CrossRef]
- Fontana, R.; Mendes, M.A.; Souza, B.M.d.; Konno, K.; César, L.M.M.; Malaspina, O.; Palma, M.S. Jelleines: A family of antimicrobial peptides from the Royal Jelly of honeybees (Apis mellifera). Peptides 2004, 25, 919–928. [Google Scholar] [CrossRef]
- Romanelli, A.; Moggio, L.; Montella, R.C.; Campiglia, P.; Iannaccone, M.; Capuano, F.; Pedone, C.; Capparelli, R. Peptides from Royal Jelly: Studies on the antimicrobial activity of jelleins, jelleins analogs and synergy with temporins. J. Pept. Sci. 2011, 17, 348–352. [Google Scholar] [CrossRef]
- Capparelli, R.; De Chiara, F.; Nocerino, N.; Montella, R.C.; Iannaccone, M.; Fulgione, A.; Romanelli, A.; Avitabile, C.; Blaiotta, G.; Capuano, F. New perspectives for natural antimicrobial peptides: Application as antinflammatory drugs in a murine model. BMC Immunol. 2012, 13, 61. [Google Scholar] [CrossRef] [Green Version]
- Jia, F.; Wang, J.; Peng, J.; Zhao, P.; Kong, Z.; Wang, K.; Yan, W.; Wang, R. The in vitro, in vivo antifungal activity and the action mode of Jelleine-I against Candida species. Amino Acids 2018, 50, 229–239. [Google Scholar] [CrossRef]
- Jia, F.; Wang, J.; Zhang, L.; Zhou, J.; He, Y.; Lu, Y.; Liu, K.; Yan, W.; Wang, K. Multiple action mechanism and in vivo antimicrobial efficacy of antimicrobial peptide Jelleine-I. J. Pept. Sci. 2020, 27, e3294. [Google Scholar] [CrossRef] [PubMed]
- Gunaldi, O.; Daglioglu, Y.K.; Tugcu, B.; Kizilyildirim, S.; Postalci, L.; Ofluoglu, E.; Koksal, F. Antibacterial effect of royal jelly for preservation of implant-related spinal infection in rat. Turk. Neurosurg. 2014, 24, 249–252. [Google Scholar]
- Lu, J.; Cokcetin, N.N.; Burke, C.M.; Turnbull, L.; Liu, M.; Carter, D.A.; Whitchurch, C.B.; Harry, E.J. Honey can inhibit and eliminate biofilms produced by Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 18160. [Google Scholar] [CrossRef] [Green Version]
- Picoli, T.; Peter, C.M.; Zani, J.L.; Waller, S.B.; Lopes, M.G.; Boesche, K.N.; Vargas, G.D.A.; de Oliveira Hübner, S.; Fischer, G. Melittin and its potential in the destruction and inhibition of the biofilm formation by Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa isolated from bovine milk. Microb. Pathog. 2017, 112, 57–62. [Google Scholar] [CrossRef]
- Bardbari, A.M.; Arabestani, M.R.; Karami, M.; Keramat, F.; Aghazadeh, H.; Alikhani, M.Y.; Begheri, K.P. Highly synergistic activity of melittin with imipenem and colistin in biofilm inhibition against multidrug-resistant strong biofilm producer strains of Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Lee, J.H.; Kim, Y.G.; Kim, M.-S.; Seo, C.H.; Park, Y. Anti-microbial, anti-biofilm activities and cell selectivity of the NRC-16 peptide derived from which flounder, Glyptocephalus cynoglossus. Mar. Drugs 2013, 11, 1836–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Cunha, M.G.; Franchin, M.; de Carvalho Galvão, L.C.; de Ruiz, A.L.T.G.; de Carvalho, J.E.; Ikegaki, M.; de Alencar, M.S.H.; Rosalen, P.L. Antimicrobial and antiproliferative activities of stingless bee Melipona scutellaris geopropolis. BMC Complement. Altern. Med. 2013, 13, 23. [Google Scholar] [CrossRef] [Green Version]
- Zeghoud, S.; Rebiai, A.; Hemmami, H.; Ben Seghir, B.; Elboughdiri, N.; Ghareba, S.; Ghernaout, D.; Abbas, N. ATR-FTIR Spectroscopy, HPLC Chromatography, and Multivariate Analysis for Controlling Bee Pollen Quality in Some Algerian Regions. ACS Omega 2021, 6, 4878–4887. [Google Scholar] [CrossRef]
- Susilowati, H.; Murakami, K.; Yumoto, H.; Amoh, T.; Hirao, K.; Hirota, K.; Matsuo, T.; Miyake, Y. Royal Jelly Inhibits Pseudomonas aeruginosa Adherence and Reduces Excessive Inflammatory Responses in Human Epithelial Cells. BioMed Res. Int. 2017, 2017, 3191752. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Caravaca, A.M.; Gómez-Romero, M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Advances in the analysis of phenolic compounds in products derived from bees. J. Pharm. Biomed. Anal. 2006, 41, 1220–1234. [Google Scholar] [CrossRef]
- Da Silva, C.; Prasniewski, A.; Calegari, M.A.; de Lima, V.A.; Oldoni, T.L.C. Determination of Total Phenolic Compounds and Antioxidant Activity of Ethanolic Extracts of Propolis Using ATR–FT-IR Spectroscopy and Chemometrics. Food Anal. Methods 2018, 11, 2013–2021. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Çakir, H.E.; Şirin, Y.; Kolayli, S.; Can, Z. Validation Methods for Phenolic Components with RP-HPLC-UV in Various Bee Products. J. ApiTher. Nat. 2018, 1, 13–19. [Google Scholar]
- Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Gómez-Romero, M.; Aboud, F.; Giuseppe, A.; Fernández-Gutiérrez, A.; Segura-Carreteroc, A. Characterisation of phenolic compounds in Algerian honeys by RP-HPLC coupled to electrospray time-of-flight mass spectrometry. LWT Food Sci. Technol. 2017, 85, 460–469. [Google Scholar] [CrossRef]
- Kozłowicz, K.; Różyło, R.; Gładyszewska, B.; Matwijczuk, A.; Gładyszewski, G.; Chocyk, D.; Samborska, K.; Piekut, J.; Smolewska, M. Identification of sugars and phenolic compounds in honey powders with the use of GC–MS, FTIR spectroscopy, and X-ray diffraction. Sci. Rep. 2020, 10, 16269. [Google Scholar] [CrossRef]
- Ristivojević, P.; Trifković, J.; Gašić, U.; Andrić, F.; Nedić, N.; Tešić, Ž.; Milojković-Opsenica, D. Ultrahigh-performance Liquid Chromatography and Mass Spectrometry (UHPLC–LTQ/Orbitrap/MS/MS) Study of Phenolic Profile of Serbian Poplar Type Propolis. Phytochem. Anal. 2015, 26, 127–136. [Google Scholar] [CrossRef]
- Chernetsova, E.S.; Bromirski, M.; Scheibner, O.; Morlock, G.E. DART-Orbitrap MS: A novel mass spectrometric approach for the identification of phenolic compounds in propolis. Anal. Bioanal. Chem. 2012, 403, 2859–2867. [Google Scholar] [CrossRef]
- Castro, C.; Mura, F.; Valenzuela, G.; Figueroa, C.; Salinas, R.; Zuñiga, M.C.; Torres, J.L.; Fuguet, E.; Delporte, C. Identification of phenolic compounds by HPLC-ESI-MS/MS and antioxidant activity from Chilean propolis. Food Res. Int. 2014, 64, 873–879. [Google Scholar] [CrossRef] [PubMed]
- López-Gutiérrez, N.; Aguilera-Luiz, M.d.M.; Romero-González, R.; Vidal, J.L.M.; Garrido Frenich, A. Fast analysis of polyphenols in royal jelly products using automated TurboFlowTM-liquid chromatography–Orbitrap high resolution mass spectrometry. J. Chromatogr. B 2014, 973, 17–28. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef]
- Sultanbawa, Y.; Cozzolino, D.; Fuller, S.; Cusack, A.; Currie, M.; Smyth, H. Infrared spectroscopy as a rapid tool to detect methylglyoxal and antibacterial activity in Australian honeys. Food Chem. 2015, 172, 207–212. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Wang, L.; Li, L.; Guo, L.; Yang, M.; Huang, F.; Zhao, H. Determination of 10-HDA in royal jelly by ATR-FTMIR and NIR spectral combining with data fusion strategy. Optik 2020, 203, 164052. [Google Scholar] [CrossRef]
- Packer, J.M.; Irish, J.; Herbert, B.R.; Hill, C.; Padula, M.; Blair, S.E.; Cartel, D.A.; Harry, E.J. Specific non-peroxide antibacterial effect of manuka honey on the Staphylococcus aureus proteome. Int. J. Antimicrob. Agents 2012, 40, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Honey, Wound Repair and Regenerative Medicine. J. Funct. Biomater. 2018, 9, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brudzynski, K.; Abubaker, K.; Castle, A. Re-Examining the Role of Hydrogen Peroxide in Bacteriostatic and Bactericidal Activities of Honey. Front. Microbiol. 2011, 2, 213. [Google Scholar] [CrossRef] [Green Version]
- Majtan, J. Honey: An immunomodulator in wound healing. Wound Repair Regen. 2014, 22, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.; Yun, H.; Park, H.; Kim, S.Y.; Lee, K.-G.; Han, S.-M.; Cho, Y. Royal jelly enhances migration of human dermal fibroblasts and alters the levels of cholesterol and sphinganine in an in vitro wound healing model. Nutr. Res. Pract. 2010, 4, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
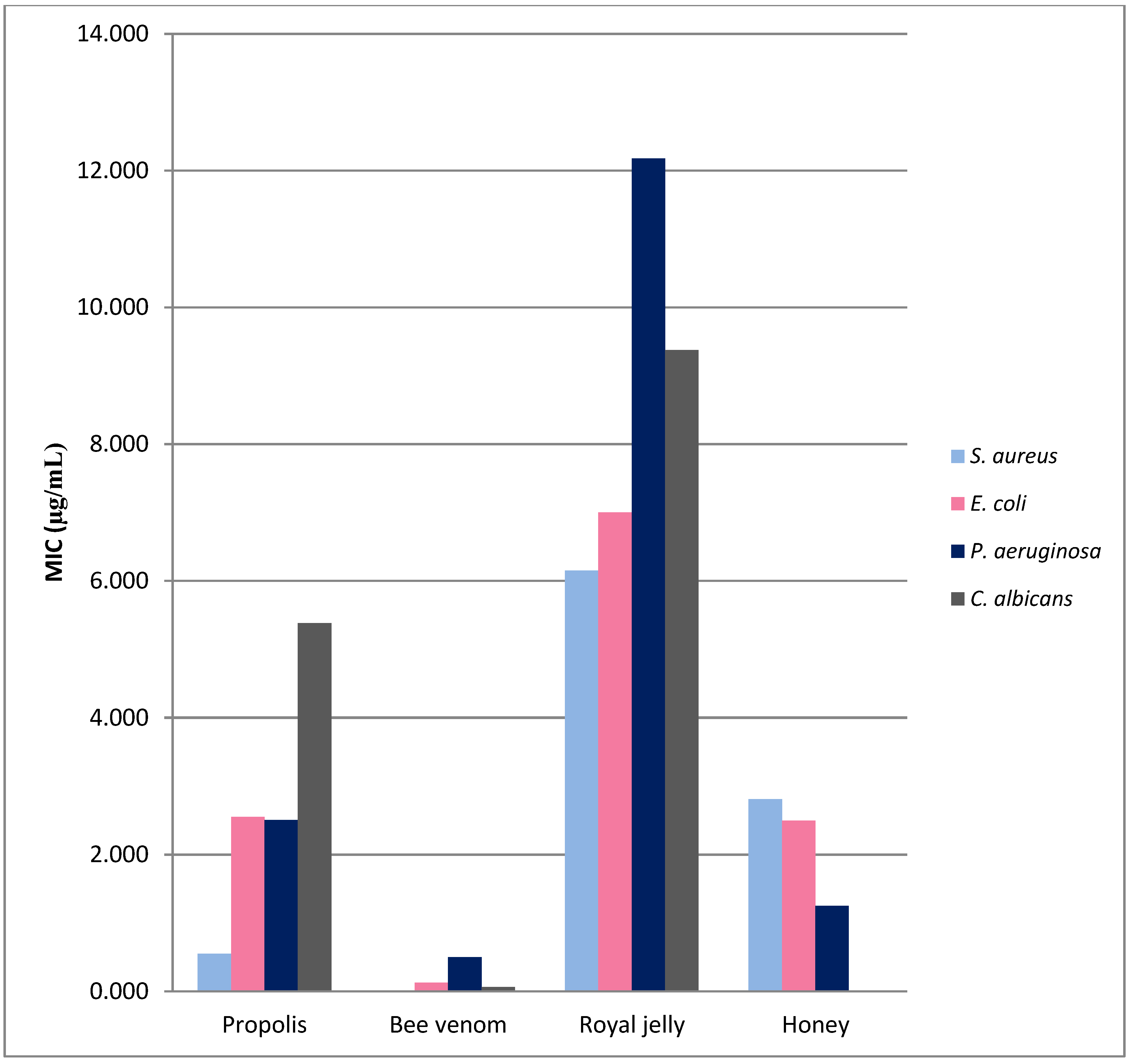
| Microorganism | MIC | Honey Samples | Reference |
|---|---|---|---|
| S. aureus | 126–185 mg/mL | Apis mallifera honey | [37] |
| 12.5 mg/mL | Honey from Basrah region/Iraq | [38] | |
| 0.625–500 mg/mL | Honey from India | [39] | |
| 10% (v/v) | Honey from the Adamawa region of Cameroon. | [28] | |
| 10–20% (v/v) | Manuka | [36] | |
| 142.87–214.33 mg/mL | Tetragonisca angustula honey | [37] | |
| 190 ± 10 mg/mL | Melipona honey | [30] | |
| ≤6.25% (v/v) | Multifloral | [23] | |
| E. coli | 2.500 mg/mL | Honey from India | [19] |
| 6.25 mg/mL | Honey from Basrah region/Iraq | [18] | |
| 100 mg/mL | Egyptian clover honey | [20] | |
| 150 ± 10 mg/mL | Melipona honey | [30] | |
| 25% (v/v) | Multifloral | [23] | |
| P. aeruginosa | 1.250 mg/mL | Honey from India | [19] |
| 1.5 mg/mL | Honey from Basrah region/Iraq | [18] | |
| 10–20% (v/v) | Manuka honey | [11] | |
| ≤6.25% (v/v) | Multifloral | [23] | |
| C. albicans | 40% (v/v) | Agastache | [15] |
| 40% (v/v) | Manuka | [15] | |
| 25–47% (v/v) | Honeys from southern Iran | [14] |
| Microorganism | MIC Value mg/mL (Min.–Max.) | Geographical Origin | Reference |
|---|---|---|---|
| S. aureus | 0.08–1.2 | Europe | [48] |
| 0.12 | Greece | [51] | |
| 0.59–1.72 | Portugal | [42] | |
| 0.25 | Poland | [46] | |
| 0.55 | Brazil | [52] | |
| 0.382 | Brazil | [53] | |
| 0.062–>1.0 | Brazil | [47] | |
| S. epidermidis | 0.05 | Greece | [51] |
| 0.77 | Brazil | [52] | |
| 0.6 | Europe | [48] | |
| 0.1 | Poland | [46] | |
| S. pyogenes | 0.51 | Brazil | [54] |
| 0.08–0.6 | Europe | [48] | |
| 0.05 | Poland | [46] | |
| E. faecalis | 0.88 | Brazil | [52] |
| 1.352 | Brazil | [53] | |
| 0.512 | Brazil | [54] | |
| 0.5 | Poland | [46] | |
| 0.031–>1.0 | Brazil | [47] | |
| E. coli | 0.40 | Greece | [51] |
| 0.512 | Brazil | [54] | |
| 0.6–5.0 | Europe | [48] | |
| 3.19–4.94 | Portugal | [42] | |
| 5.0 | Poland | [46] | |
| P. aeruginosa | 0.24 | Greece | [51] |
| 5.83 | Brazil | [52] | |
| 1.56–2.81 | Portugal | [42] | |
| 0.25 | Brazil | [54] | |
| 0.6–2.5 | Europe | [48] | |
| 5.0 | Poland | [46] | |
| E. cloace | 0.30 | Greece | [51] |
| >5.0 | Europe | [48] | |
| K. pneumoniae | 3.33 | Brazil | [52] |
| 0.512 | Brazil | [54] | |
| 5.0 | Poland | [46] | |
| 0.6–>5 | Europe | [48] | |
| P. mirabilis | 2.25 | Brazil | [52] |
| 0.512 | Brazil | [54] | |
| C. albicans | 13.19–13.90 | Portugal | [42] |
| 7.90–9.25 | Brazil | [52] | |
| 6.25 | Poland | [49] | |
| 0.3–5.0 | Europe | [48] | |
| 0.256 | Brazil | [54] | |
| >1.0 | Brazil | [47] |
| Microorganism | Bee Venom | Melittin | Royal Jelly | Royalisin | Reference |
|---|---|---|---|---|---|
| S. aureus | 10–60 µg/mL | 6–10 µg/mL | [72] | ||
| 6.25 µg/mL | [73] | ||||
| 2 µM | [65] | ||||
| 0.5–4 µg/mL | [74] | ||||
| 2–4 µg/mL | [75] | ||||
| 0.7 µg/mL | 3.6–57.3 µg/mL | [76] | |||
| 20–80 w/w | [77] | ||||
| 3.4–9.0 mg/mL | [78] | ||||
| 15.63–500 µg/mL | [79] | ||||
| 12.5 mg/mL | [80] | ||||
| 7.5 µg/mL | [81] | ||||
| <250 µg/mL | [82] | ||||
| MRSA | 60 µg/mL | 10–100 µg/mL | [72] | ||
| 0.78–3.12 µg/mL | [73] | ||||
| 1–4 µM | [65] | ||||
| 0.085–0.11 µg/mL | [67] | ||||
| 0.5–4 µg/mL | [74] | ||||
| 7.2 µg/mL | 6.7 µg/mL | [76] | |||
| 30–70 w/w | [77] | ||||
| 8.0–14.5 mg/mL | [78] | ||||
| S. epidermidis | 60 µg/mL | 10 µg/mL | [72] | ||
| 0.78 µg/mL | [73] | ||||
| 40–80 w/w | [77] | ||||
| 8.7–10.3 mg/mL | [78] | ||||
| S. saprophiticus | 10 µg/mL | 10 µg/mL | [72] | ||
| S. pyogenes | 100 µg/mL | 10 µg/mL | [72] | ||
| S. pneumoniae | 3.12 µg/mL | [73] | |||
| S. bovis | 1.56 µg/mL | [73] | |||
| S. oralis | 100 µg/mL | 200 µg/mL | [72] | ||
| S. salivarius | 10 µg/mL | [70] | |||
| S. sanguinis | 10 µg/mL | [70] | |||
| S. sorbinus | 10 µg/mL | [70] | |||
| S. mitis | 10 µg/mL | [70] | |||
| S. mutans | 40 µg/mL | [70] | |||
| S. agalactiae | 40 µg/mL | 30 µg/mL | [72] | ||
| 6.25 µg/mL | [72] | ||||
| 50–100 w/w | [77] | ||||
| E. faecalis | 100–200 µg/mL | 30–50 µg/mL | [72] | ||
| 1–8 µg/mL | [74] | ||||
| 2–4 µg/mL | [75] | ||||
| 6 µg/mL | [70] | ||||
| 40–100 w/w | [77] | ||||
| 3.7–13.7 mg/mL | [78] | ||||
| E. faecium | 50–70 w/w | [77] | |||
| E. casseliflavus | 10 µg/mL | 8 µg/mL | [72] | ||
| VRE | 200 µg/mL | 50 µg/mL | [72] | ||
| L. monocytogenes | 2–4 µg/mL | [77] | |||
| 0.315 µg/mL | [83] | ||||
| E. coli | 60–200 µg/mL | 30 µg/mL | [72] | ||
| 1–2 µM | [65] | ||||
| 16 µg/mL | [75] | ||||
| 60–100 w/w | [77] | ||||
| 7.0–7.1 mg/mL | [78] | ||||
| 500 µg/mL | [79] | ||||
| 13.5 mg/mL | [80] | ||||
| NI | [81] | ||||
| >2000 µg/mL | [82] | ||||
| K. pneumoniae | 30–500 µg/mL | [72] | |||
| 2 µM | [65] | ||||
| 80–100 w/w | [77] | ||||
| 8.0–8.1 mg/mL | [78] | ||||
| S. choleraesuis | 500 µg/mL | [72] | |||
| 9 µg/mL | [81] | ||||
| S. flexneri | 60 µg/mL | [72] | |||
| 14.5 mg/mL | [80] | ||||
| P. aeruginosa | 500–>500 µg/mL | 100 µg/mL | [72] | ||
| 2 µM | [65] | ||||
| ≥64 µg/mL | [75] | ||||
| 0.125–4 µg/mL | [84] | ||||
| 60–100 w/w | [77] | ||||
| 3.3–14.4 mg/mL | [78] | ||||
| 15.5 mg/mL | [80] | ||||
| 10 µg/mL | [81] | ||||
| A. baumannii | 30 µg/mL | 30 µg/mL | [72] | ||
| 17–20 µg/mL | [84] | ||||
| A. baumannii (XDR) | 31–45.4 µg/mL | [85] | |||
| A. baumannii (PDR) | >284 µg/mL | [85] | |||
| C. albicans | 60 µg/mL | 100 µg/mL | [48] | ||
| 40 µg/mL | [86] | ||||
| 62.5–125 µg/mL | [87] | ||||
| 62.5–125 µg/mL | [79] | ||||
| C. glabrata | >500 µg/mL | 300 µg/mL | [48] | ||
| C. parapsilosis | 60 µg/mL | 100 µg/mL | [48] | ||
| C. tropicalis | 300 µg/mL | [48] | |||
| C. krusei | 60 µg/mL | 30 µg/mL | [48] |
| Type of Bee Product | Group/Bioactive Compound | Chemical Structure/Amino Acid Sequence |
|---|---|---|
| Honey | Flavonoid: luteolin | 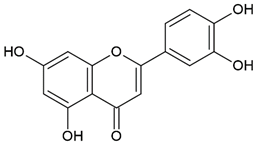 |
| Flavonoid: pinobanksin |  | |
| Propolis | Phenolic compound: 2,2-dimethyl-8-prenylchromene | 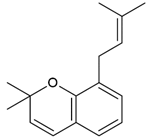 |
| Phenolic compound: 4-hydroxy-3,5-diprenyl cinnamic acid (artepillin C) | 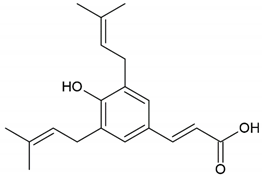 | |
| Phenolic compound: 3-prenyl cinnamic acid allyl ester | 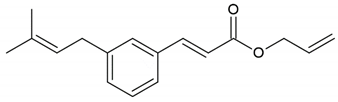 | |
| Terpenoid: isocupressic acid, a labdane diterpenoid | 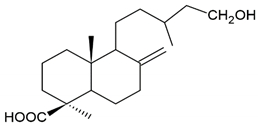 | |
| Honey, propolis | Flavonoid: apigenin | 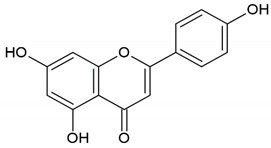 |
| Flavonoid: pinocembrin | 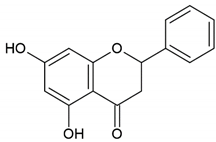 | |
| Flavonoid: quercetin | 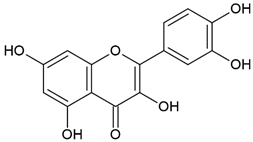 | |
| Flavonoid: chrysin | 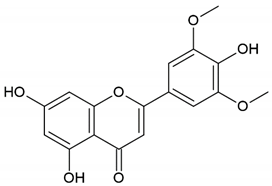 | |
| Flavonoid: fisetin | 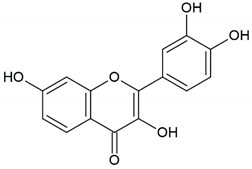 | |
| Flavonoid: caffeic acid phenethyl ester | 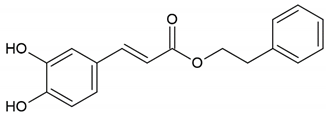 | |
| Propolis, royal jelly | 10-Hydroxyl-2-decenoic acid | 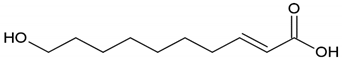 |
| Bee venom | Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ |
| Apamin | CNCKAPETALCARRCQQH | |
| Melectin | GFLSILKKVLPKVMAHMK-N |
| Bee Products | Studied Compounds | Applied Methods | Reference |
|---|---|---|---|
| Honey | Phenolic and flavonoid fraction, proline | Spectrophotometry | [120] |
| Honey | Phenolic fraction | Reversed-phase high-performance liquid chromatography–electrospray ionization time-of-flight mass spectrometry (RP-HPLC-ESI-TOF MS) | [124] |
| Honey | Phenolic fraction and sugars | Gas chromatography–mass spectrometry (GC-MS), Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction | [126] |
| Honey | Phenolic fraction | Ultrahigh-pressure liquid chromatography–tandem quadrupole mass spectrometry (UHPLC-Q MS/MS) | [127] |
| Honey | Methylglyoxal | Liquid chromatography–electrospray ionization time-of-flight mass spectrometry (LC-ESI-TOF MS) | [128] |
| Honey | Methylglyoxal | Infrared (IR) spectroscopy | [127] |
| Propolis, honey, bee pollen | Phenolic fraction | Reversed-phase high-performance liquid chromatography with UV detection (RP-HPLC-UV) | [123] |
| Propolis | Phenolic fraction | Fourier transform infrared attenuated total reflection spectroscopy (FTIR–ATR) | [119] |
| Propolis | Phenolic fraction | Ultrahigh-pressure liquid chromatography with a linear ion trap–high-resolution Orbitrap mass spectrometry (UHPLC–LTQ/Orbitrap MS/MS) | [127] |
| Propolis | Phenolic fraction | Direct analysis in real time–Orbitrap mass spectrometry (DART-Orbitrap MS) | [128] |
| Propolis | Phenolic fraction | High-performance liquid chromatography–electrospray ionization mass spectrometry (HPLC-ESI-MS/MS) | [129] |
| Propolis | Phenolic fraction | High-performance liquid chromatography with UV detection (HPLC-UV) | [125] |
| Propolis | Phenolic and flavonoid fraction | Spectrophotometry and colorimetry | [121] |
| Royal jelly | Polyphenols | Turbulent flow chromatography–Orbitrap mass spectrometry (TFC-Orbitrap MS) | [130] |
| Royal jelly | 10-Hydroxy-2-decenoic acid (10-HDA) | Attenuated total reflectance Fourier transform mid-infrared (ATR-FTMIR) and near-infrared (NIR) spectroscopy | [134] |
| Royal jelly | Phenolic and flavonoid fraction, 10-HDA | Spectrophotometry | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratajczak, M.; Kaminska, D.; Matuszewska, E.; Hołderna-Kedzia, E.; Rogacki, J.; Matysiak, J. Promising Antimicrobial Properties of Bioactive Compounds from Different Honeybee Products. Molecules 2021, 26, 4007. https://doi.org/10.3390/molecules26134007
Ratajczak M, Kaminska D, Matuszewska E, Hołderna-Kedzia E, Rogacki J, Matysiak J. Promising Antimicrobial Properties of Bioactive Compounds from Different Honeybee Products. Molecules. 2021; 26(13):4007. https://doi.org/10.3390/molecules26134007
Chicago/Turabian StyleRatajczak, Magdalena, Dorota Kaminska, Eliza Matuszewska, Elżbieta Hołderna-Kedzia, Jarosław Rogacki, and Jan Matysiak. 2021. "Promising Antimicrobial Properties of Bioactive Compounds from Different Honeybee Products" Molecules 26, no. 13: 4007. https://doi.org/10.3390/molecules26134007
APA StyleRatajczak, M., Kaminska, D., Matuszewska, E., Hołderna-Kedzia, E., Rogacki, J., & Matysiak, J. (2021). Promising Antimicrobial Properties of Bioactive Compounds from Different Honeybee Products. Molecules, 26(13), 4007. https://doi.org/10.3390/molecules26134007






