Niclosamide Is Active In Vitro against Mycetoma Pathogens
Abstract
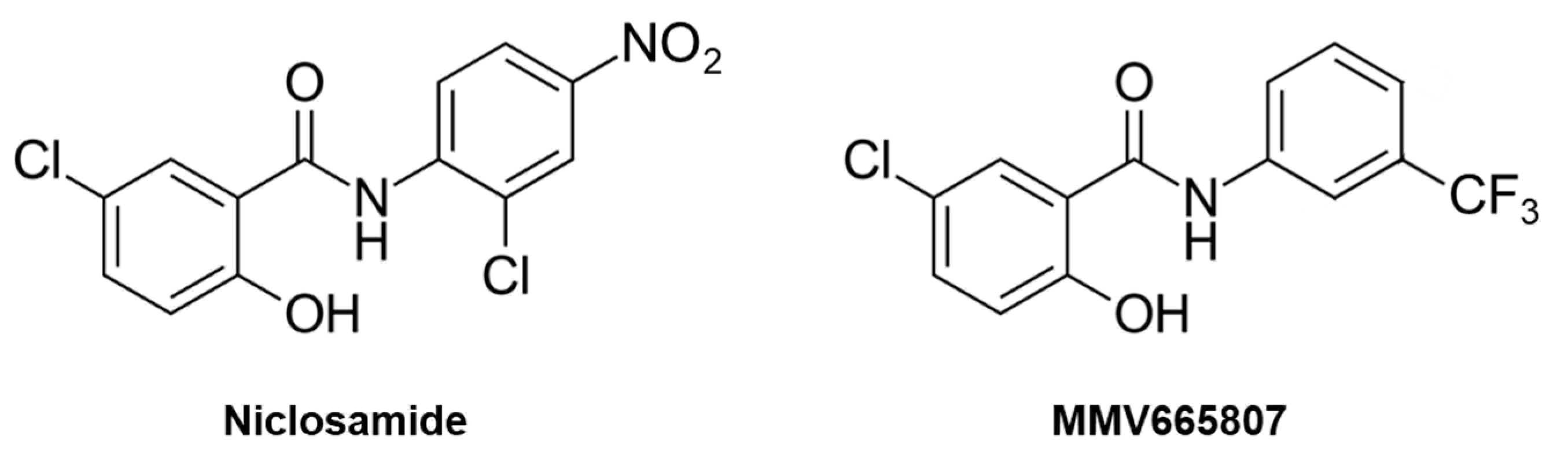
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Strains
4.3. In Vitro Drug Efficacy Testing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nenoff, P.; van de Sande, W.W.; Fahal, A.H.; Reinel, D.; Schofer, H. Eumycetoma and actinomycetoma--an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, E.E.; van de Sande, W.W.J.; Welsh, O.; Mahgoub, E.S.; Goodfellow, M.; Fahal, A.H. Mycetoma: A unique neglected tropical disease. Lancet Infect. Dis. 2016, 16, 100–112. [Google Scholar] [CrossRef]
- Ahmed, S.A.; van de Sande, W.W.; Stevens, D.A.; Fahal, A.; van Diepeningen, A.D.; Menken, S.B.; de Hoog, G.S. Revision of agents of black-grain eumycetoma in the order Pleosporales. Persoonia 2014, 33, 141–154. [Google Scholar] [CrossRef] [PubMed]
- van de Sande, W.; Fahal, A.; Ahmed, S.A.; Serrano, J.A.; Bonifaz, A.; Zijlstra, E. eumycetoma working, g. Closing the mycetoma knowledge gap. Med. Mycol. 2018, 56, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, S.H.; Wadaella, E.S.; Fahal, A.H. The Surgical Treatment of Mycetoma. PLoS Negl. Trop. Dis. 2016, 10, e0004690. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Andricopulo, A.D. Drug repositioning approaches to parasitic diseases: A medicinal chemistry perspective. Drug Discov. Today 2016, 21, 1699–1710. [Google Scholar] [CrossRef]
- Lim, W.; Melse, Y.; Konings, M.; Phat Duong, H.; Eadie, K.; Laleu, B.; Perry, B.; Todd, M.H.; Ioset, J.R.; van de Sande, W.W.J. Addressing the most neglected diseases through an open research model: The discovery of fenarimols as novel drug candidates for eumycetoma. PLoS Negl. Trop. Dis. 2018, 12, e0006437. [Google Scholar] [CrossRef]
- Pal, C.; Bandyopadhyay, U. Redox-active antiparasitic drugs. Antioxid. Redox Signal. 2012, 17, 555–582. [Google Scholar] [CrossRef]
- Mäser, P. Cherchez l’Electron. Mol. Microbiol. 2017, 106, 183–185. [Google Scholar] [CrossRef]
- Richle, R.; Hofheinz, W. Chemotherapeutische Wirksamkeit von 2 neuen 2-Nitroimidazolderivaten gegen Trypanosoma brucei rhodesiense bei der experimentellen Schlafkrankheit von Maus und Kaninchen. Mitt. Österr. Ges. Tropenmed. Parasitol. 1983, 5, 143–149. [Google Scholar]
- Trunz, B.B.; Jedrysiak, R.; Tweats, D.; Brun, R.; Kaiser, M.; Suwinski, J.; Torreele, E. 1-Aryl-4-nitro-1H-imidazoles, a new promising series for the treatment of human African trypanosomiasis. Eur. J. Med. Chem. 2011, 46, 1524–1535. [Google Scholar] [CrossRef]
- Abd Algaffar, S.O.; Verbon, A.; van de Sande, W.W.J.; Khalid, S.A. Development and Validation of an In Vitro Resazurin-Based Susceptibility Assay against Madurella mycetomatis. Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- van de Sande, W.W.; Fahal, A.H.; Riley, T.V.; Verbrugh, H.; van Belkum, A. In vitro susceptibility of Madurella mycetomatis, prime agent of Madura foot, to tea tree oil and artemisinin. J. Antimicrob. Chemother. 2007, 59, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Hecht, G.; Gloxhuber, C. Tolerance to 2′, 5-dichloro-4-nitrosalicylanilide ethanolamine salt. Z. Tropenmed. Parasit. 1962, 13, 1–8. [Google Scholar]
- Spangenberg, T.; Burrows, J.N.; Kowalczyk, P.; McDonald, S.; Wells, T.N.; Willis, P. The open access malaria box: A drug discovery catalyst for neglected diseases. PLoS ONE 2013, 8, e62906. [Google Scholar] [CrossRef]
- Zapotoczna, M.; Boksmati, N.; Donohue, S.; Bahtiar, B.; Boland, A.; Somali, H.A.; Cox, A.; Humphreys, H.; O’Gara, J.P.; Brennan, M.; et al. Novel anti-staphylococcal and anti-biofilm properties of two anti-malarial compounds: MMV665953 {1-(3-chloro-4-fluorophenyl)-3-(3,4-dichlorophenyl)urea} and MMV665807 {5-chloro-2-hydroxy-N-[3-(trifluoromethyl)phenyl]benzamide}. J. Med. Microbiol. 2017, 66, 377–387. [Google Scholar] [CrossRef]
- Aleman, A.; Guerra, T.; Maikis, T.J.; Milholland, M.T.; Castro-Arellano, I.; Forstner, M.R.; Hahn, D. The Prevalence of Trypanosoma cruzi, the Causal Agent of Chagas Disease, in Texas Rodent Populations. Ecohealth 2017, 14, 130–143. [Google Scholar] [CrossRef]
- Muller, J.; Winzer, P.A.; Samby, K.; Hemphill, A. In Vitro Activities of MMV Malaria Box Compounds against the Apicomplexan Parasite Neospora caninum, the Causative Agent of Neosporosis in Animals. Molecules 2020, 25, 1460. [Google Scholar] [CrossRef]
- Ritler, D.; Rufener, R.; Sager, H.; Bouvier, J.; Hemphill, A.; Lundstrom-Stadelmann, B. Development of a movement-based in vitro screening assay for the identification of new anti-cestodal compounds. PLoS Negl. Trop. Dis. 2017, 11, e0005618. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, B.; Rufener, R.; Aeschbacher, D.; Spiliotis, M.; Gottstein, B.; Hemphill, A. Screening of the Open Source Malaria Box Reveals an Early Lead Compound for the Treatment of Alveolar Echinococcosis. PLoS Negl. Trop. Dis. 2016, 10, e0004535. [Google Scholar] [CrossRef]
- Mook, R.A.; Jr Wang, J.; Ren, X.R.; Chen, M.; Spasojevic, I.; Barak, L.S.; Lyerly, H.K.; Chen, W. Structure-activity studies of Wnt/beta-catenin inhibition in the Niclosamide chemotype: Identification of derivatives with improved drug exposure. Bioorg. Med. Chem. 2015, 23, 5829–5838. [Google Scholar] [CrossRef]
- Alasadi, A.; Chen, M.; Swapna, G.V.T.; Tao, H.; Guo, J.; Collantes, J.; Fadhil, N.; Montelione, G.T.; Jin, S. Effect of mitochondrial uncouplers niclosamide ethanolamine (NEN) and oxyclozanide on hepatic metastasis of colon cancer. Cell Death Dis. 2018, 9, 215. [Google Scholar] [CrossRef]
- Kumar, R.; Coronel, L.; Somalanka, B.; Raju, A.; Aning, O.A.; An, O.; Ho, Y.S.; Chen, S.; Mak, S.Y.; Hor, P.Y.; et al. Mitochondrial uncoupling reveals a novel therapeutic opportunity for p53-defective cancers. Nat. Commun. 2018, 9, 3931. [Google Scholar] [CrossRef]
- Chen, W.; Mook, R.A.; Premont, R.T., Jr.; Wang, J. Niclosamide: Beyond an antihelminthic drug. Cell Signal 2018, 41, 89–96. [Google Scholar] [CrossRef]
- Kadri, H.; Lambourne, O.A.; Mehellou, Y. Niclosamide, a Drug with Many (Re)purposes. ChemMedChem 2018, 13, 1088–1091. [Google Scholar] [CrossRef]
- NIH National Center for Advancing Translational Sciences, Inxight: Drugs. Niclosamide. Available online: https://drugs.ncats.io/drug/8KK8CQ2K8G (accessed on 20 May 2021).
- Rajamuthiah, R.; Fuchs, B.B.; Conery, A.L.; Kim, W.; Jayamani, E.; Kwon, B.; Ausubel, F.M.; Mylonakis, E. Repurposing salicylanilide anthelmintic drugs to combat drug resistant Staphylococcus aureus. PLoS ONE 2015, 10, e0124595. [Google Scholar] [CrossRef]
- Imperi, F.; Massai, F.; Ramachandran Pillai, C.; Longo, F.; Zennaro, E.; Rampioni, G.; Visca, P.; Leoni, L. New life for an old drug: The anthelmintic drug niclosamide inhibits Pseudomonas aeruginosa quorum sensing. Antimicrob. Agents Chemother. 2013, 57, 996–1005. [Google Scholar] [CrossRef]
- Garcia, C.; Burgain, A.; Chaillot, J.; Pic, E.; Khemiri, I.; Sellam, A. A phenotypic small-molecule screen identifies halogenated salicylanilides as inhibitors of fungal morphogenesis, biofilm formation and host cell invasion. Sci. Rep. 2018, 8, 11559. [Google Scholar] [CrossRef]
- Jeon, S.; Ko, M.; Lee, J.; Choi, I.; Byun, S.Y.; Park, S.; Shum, D.; Kim, S. Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.J.; Jan, J.T.; Chen, C.M.; Hsieh, H.P.; Hwang, D.R.; Liu, H.W.; Liu, C.Y.; Huang, H.W.; Chen, S.C.; Hong, C.F.; et al. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob. Agents Chemother. 2004, 48, 2693–2696. [Google Scholar] [CrossRef]
- Barbosa, E.J.; Lobenberg, R.; de Araujo, G.L.B.; Bou-Chacra, N.A. Niclosamide repositioning for treating cancer: Challenges and nano-based drug delivery opportunities. Eur. J. Pharm. Biopharm. 2019, 141, 58–69. [Google Scholar] [CrossRef]
- Schultz, D.P.; Harman, P.D. Uptake, distribution, and elimination of the lampricide 2’,5-dichloro-4’-nitro[14C]salicylanilide (Bayer 2353) and its 2-aminoethanol salt (Bayer 73) by largemouth bass. J. Agric. Food Chem. 1978, 26, 1226–1230. [Google Scholar] [CrossRef]
- Guo, J.; Tao, H.; Alasadi, A.; Huang, Q.; Jin, S. Niclosamide piperazine prevents high-fat diet-induced obesity and diabetic symptoms in mice. Eat. Weight Disord. 2019, 24, 91–96. [Google Scholar] [CrossRef]
- Rehman, M.U.; Khan, M.A.; Khan, W.S.; Shafique, M.; Khan, M. Fabrication of Niclosamide loaded solid lipid nanoparticles: In vitro characterization and comparative in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1926–1934. [Google Scholar]
- Zhang, X.; Zhang, Y.; Zhang, T.; Zhang, J.; Wu, B. Significantly enhanced bioavailability of niclosamide through submicron lipid emulsions with or without PEG-lipid: A comparative study. J. Microencapsul. 2015, 32, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Zhan, H.; Shao, M.; Wang, W.; Song, G.; Yu, X.; Zhang, C.; Ge, N.; Yi, T.; Li, S.; et al. Niclosamide ethanolamine improves kidney injury in db/db mice. Diabetes Res. Clin. Pract. 2018, 144, 25–33. [Google Scholar] [CrossRef]
- Li, S.L.; Yan, J.; Zhang, Y.Q.; Zhen, C.L.; Liu, M.Y.; Jin, J.; Gao, J.L.; Xiao, X.L.; Shen, X.; Tai, Y.; et al. Niclosamide ethanolamine inhibits artery constriction. Pharmacol. Res. 2017, 115, 78–86. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, Y.S.; Lee, D.H.; Bae, S.H. Repositioning of niclosamide ethanolamine (NEN), an anthelmintic drug, for the treatment of lipotoxicity. Free Radic Biol. Med. 2019, 137, 143–157. [Google Scholar] [CrossRef]
- Tao, H.; Zhang, Y.; Zeng, X.; Shulman, G.I.; Jin, S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat. Med. 2014, 20, 1263–1269. [Google Scholar] [CrossRef]
- Lanteri, C.A.; Chaorattanakawee, S.; Lon, C.; Saunders, D.L.; Rutvisuttinunt, W.; Yingyuen, K.; Bathurst, I.; Ding, X.C.; Tyner, S.D. Ex vivo activity of endoperoxide antimalarials, including artemisone and arterolane, against multidrug-resistant Plasmodium falciparum isolates from Cambodia. Antimicrob. Agents Chemother. 2014, 58, 5831–5840. [Google Scholar] [CrossRef][Green Version]
- Vennerstrom, J.L.; Arbe-Barnes, S.; Brun, R.; Charman, S.A.; Chiu, F.C.; Chollet, J.; Dong, Y.; Dorn, A.; Hunziker, D.; Matile, H.; et al. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature 2004, 430, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Utzinger, J.; Tanner, M.; Dong, Y.; Vennerstrom, J.L. The synthetic peroxide OZ78 is effective against Echinostoma caproni and Fasciola hepatica. J. Antimicrob. Chemother. 2006, 58, 1193–1197. [Google Scholar] [CrossRef]
- Keiser, J.; Xiao, S.H.; Dong, Y.; Utzinger, J.; Vennerstrom, J.L. Clonorchicidal properties of the synthetic trioxolane OZ78. J. Parasitol. 2007, 93, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.H.; Keiser, J.; Chollet, J.; Utzinger, J.; Dong, Y.; Endriss, Y.; Vennerstrom, J.L.; Tanner, M. In vitro and in vivo activities of synthetic trioxolanes against major human schistosome species. Antimicrob. Agents Chemother. 2007, 51, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
| Compound | Class | Indication 1 | SO1 2 | CBS131320 2 |
|---|---|---|---|---|
| Niclosamide | Salicylanilide | Tapeworms | 0.78 | 1.6 |
| Secnidazole | Nitroimidazole | Bacterial vaginosis | >256 | >256 |
| Metronidazole | Nitroimidazole | Broad spectrum antibiotic | >256 | >256 |
| Fexinidazole | Nitroimidazole | Human African trypanosomiasis | >256 | >256 |
| RJ-164 | Nitroimidazole | (Human African trypanosomiasis) | >256 | >256 |
| RJ-55 | Nitroimidazole | (Human African trypanosomiasis) | >256 | >256 |
| Ro 15-6547 | Nitroimidazole | (Human African trypanosomiasis) | >256 | >256 |
| Nifurtimox | Nitrofuran | Chagas’ disease, HAT | >256 | >256 |
| Nifuroxazide | Nitrofuran | Colitis and diarrhea | >256 | >256 |
| Nitrofurantoine | Nitrofuran | Urinary tract infections | >256 | >256 |
| OZ 78 | Peroxide | (Malaria, trematodes) | >256 | >256 |
| Artemisinin | Peroxide | Malaria | 16 | 16 |
| Dihydroartemisinin | Peroxide | Malaria | >256 | >256 |
| Artesunate | Peroxide | Malaria | >256 | >256 |
| Artemether | Peroxide | Malaria | 64 | 64 |
| Itraconazole | Triazole | Antifungal | 0.13 | 0.25 |
| Compound | SO1 1 | CBS131320 1 | SAK-A05 2 | SAK-A08 2 |
|---|---|---|---|---|
| Niclosamide | 0.78 | 1.6 | 0.39 | 0.39 |
| Niclosamide-ethanolamine | 0.78 | 1.6 | 0.19 | 0.39 |
| MMV665807 | 1.6 | 1.6 | 0.39 | 0.39 |
| Itraconazole | 0.13 | 0.25 | n.d. | n.d. |
| Cotrimoxazole | n.d. | n.d. | 20 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, A.B.; Abd Algaffar, S.; van de Sande, W.; Khalid, S.; Kaiser, M.; Mäser, P. Niclosamide Is Active In Vitro against Mycetoma Pathogens. Molecules 2021, 26, 4005. https://doi.org/10.3390/molecules26134005
Mahmoud AB, Abd Algaffar S, van de Sande W, Khalid S, Kaiser M, Mäser P. Niclosamide Is Active In Vitro against Mycetoma Pathogens. Molecules. 2021; 26(13):4005. https://doi.org/10.3390/molecules26134005
Chicago/Turabian StyleMahmoud, Abdelhalim B., Shereen Abd Algaffar, Wendy van de Sande, Sami Khalid, Marcel Kaiser, and Pascal Mäser. 2021. "Niclosamide Is Active In Vitro against Mycetoma Pathogens" Molecules 26, no. 13: 4005. https://doi.org/10.3390/molecules26134005
APA StyleMahmoud, A. B., Abd Algaffar, S., van de Sande, W., Khalid, S., Kaiser, M., & Mäser, P. (2021). Niclosamide Is Active In Vitro against Mycetoma Pathogens. Molecules, 26(13), 4005. https://doi.org/10.3390/molecules26134005






