Hairy Roots of Scutellaria spp. (Lamiaceae) as Promising Producers of Antiviral Flavones
Abstract
:1. Introduction
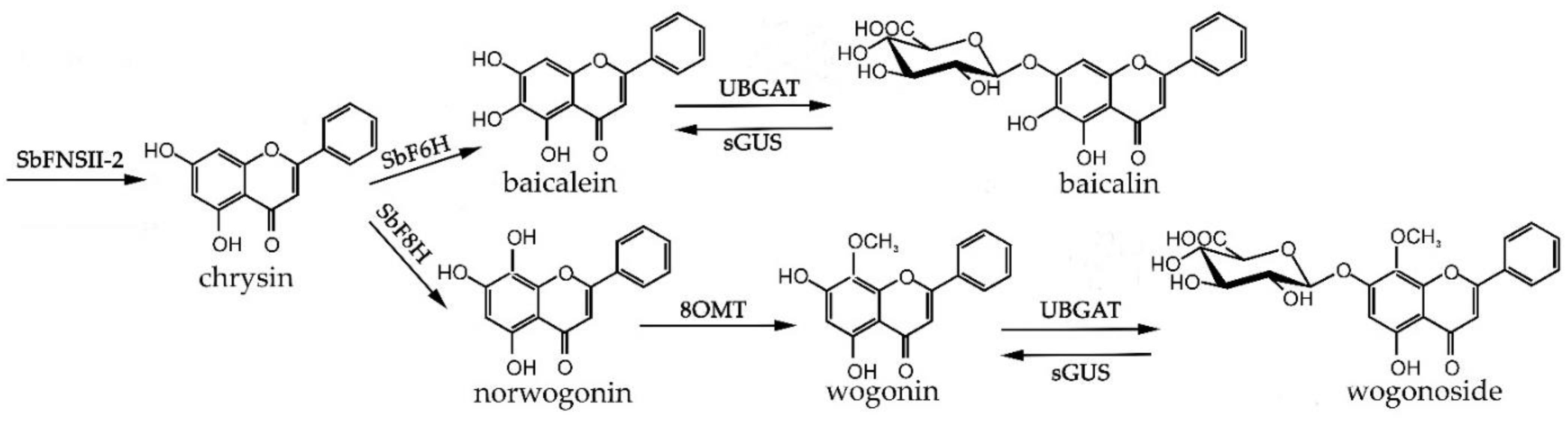
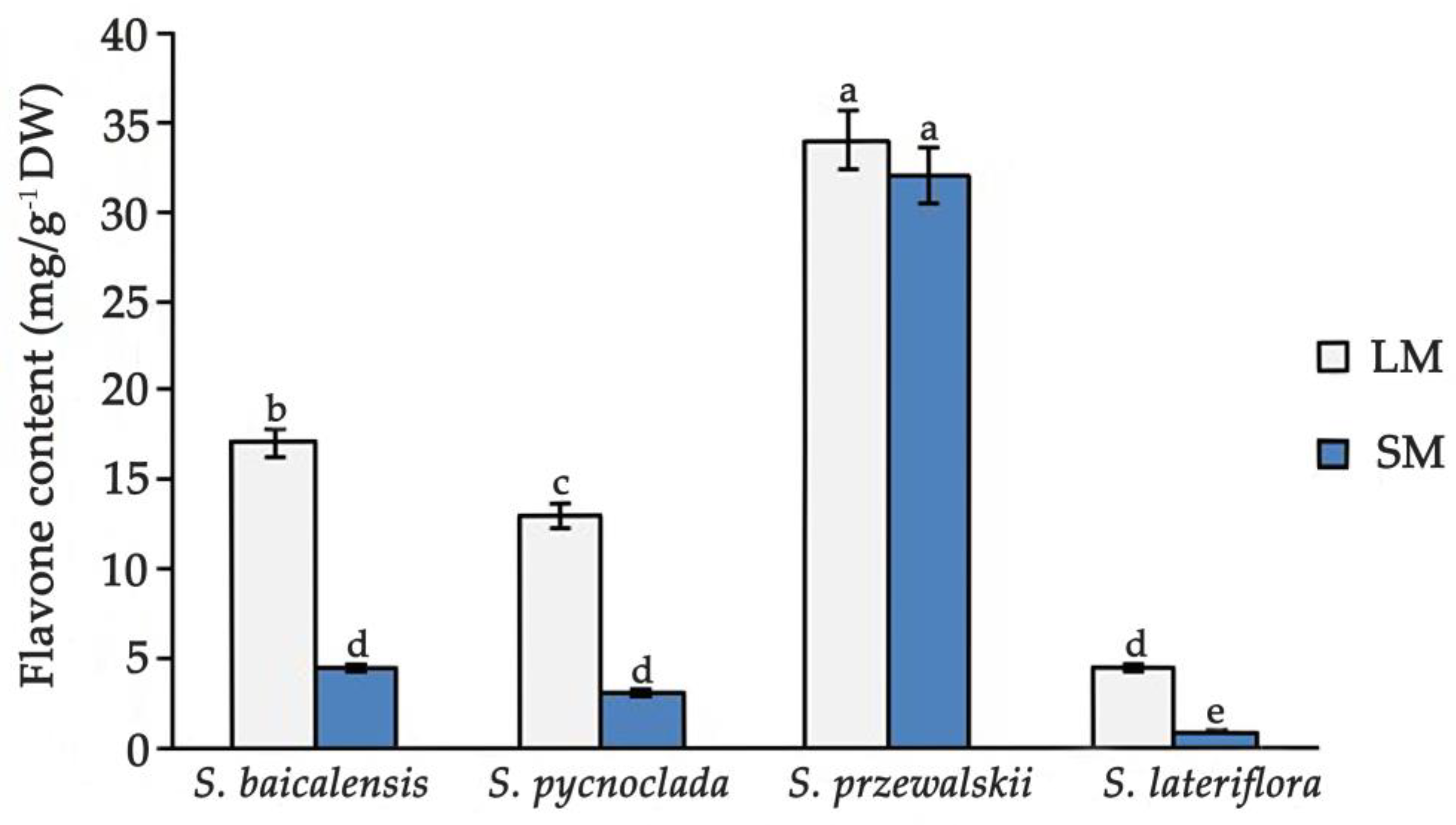
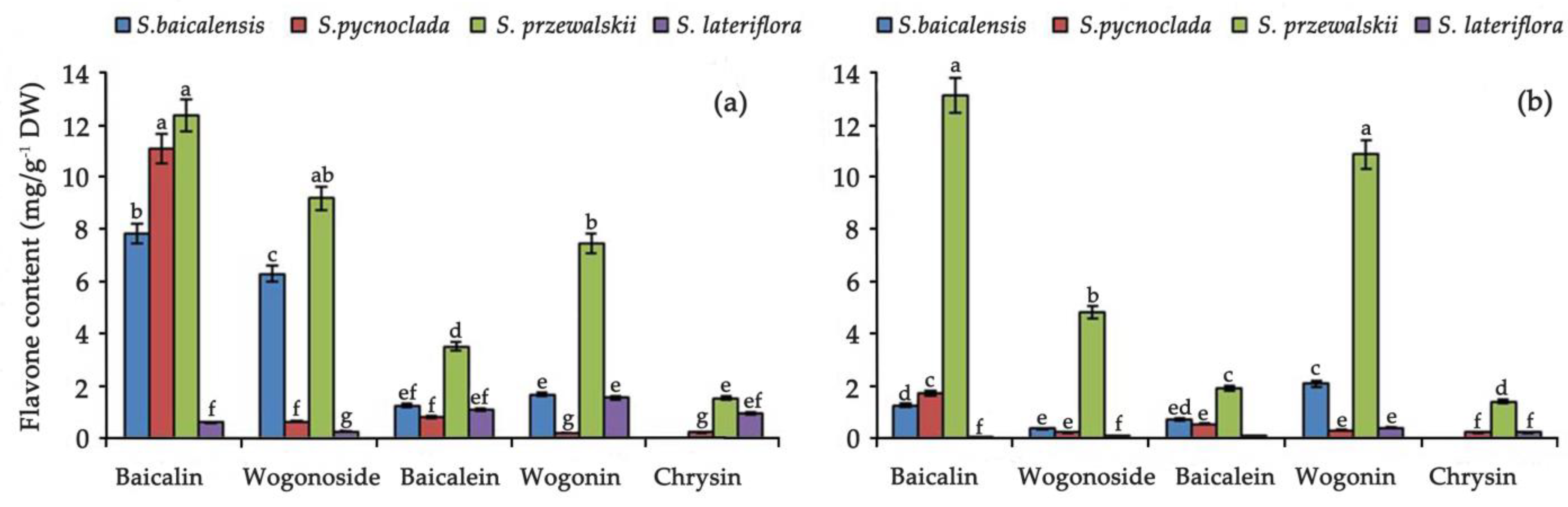
2. Results and Discussion
3. Materials and Methods
3.1. Obtaining the Hairy Roots of S. pycnoclada, S. lateriflora and S. przewalskii, and Determining the Growth Index
3.2. DNA Isolation and PCR Analysis
3.3. Preparation of the Extracts and Determination of Secondary Metabolite Contents
3.4. HPLC
3.5. HPLC-MS/MS
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Orhan, I.E.; Senol Deniz, F.S. Natural Products as Potential Leads Against Coronaviruses: Could They be Encouraging Structural Models Against SARS-CoV-2? Nat. Prod. Bioprospect. 2020, 10, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Khan, M.K.; Hamurcu, M.; Gezgin, S. Natural Plant Products: A Less Focused Aspect for the COVID-19 Viral Outbreak. Front. Plant Sci. 2020, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Shohag, M.J.I.; Khan, F.Z.; Tang, L.; Wei, Y.; He, Z.; Yang, X. COVID-19 Crisis: How Can Plant Biotechnology Help? Plants 2021, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Boozari, M.; Hosseinzadeh, H. Natural products for COVID-19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phyther. Res. 2021, 35, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Iauk, L.; Acquaviva, R.; Mastrojeni, S.; Amodeo, A.; Pugliese, M.; Ragusa, M.; Loizzo, M.R.; Menichini, F.; Tundis, R. Antibacterial, antioxidant and hypoglycaemic effects of Thymus capitatus (L.) Hoffmanns. et Link leaves’ fractions. J. Enzyme Inhib. Med. Chem. 2015, 30, 360–365. [Google Scholar] [CrossRef] [Green Version]
- Bekut, M.; Brkić, S.; Kladar, N.; Dragović, G.; Gavarić, N.; Božin, B. Potential of selected Lamiaceae plants in anti(retro)viral therapy. Pharmacol. Res. 2018, 133, 301–314. [Google Scholar] [CrossRef]
- Catella, C.; Camero, M.; Lucente, M.S.; Fracchiolla, G.; Sblano, S.; Tempesta, M.; Martella, V.; Buonavoglia, C.; Lanave, G. Virucidal and antiviral effects of Thymus vulgaris essential oil on feline coronavirus. Res. Vet. Sci. 2021, 137, 44–47. [Google Scholar] [CrossRef]
- Song, J.W.; Long, J.Y.; Xie, L.; Zhang, L.L.; Xie, Q.X.; Chen, H.J.; Deng, M.; Li, X.F. Applications, phytochemistry, pharmacological effects, pharmacokinetics, toxicity of Scutellaria baicalensis Georgi. And its probably potential therapeutic effects on COVID-19: A review. Chin. Med. (United Kingdom) 2020, 15, 1–26. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Rana, A.K.; Srivastava, Y.; Jha, S.K.; Jha, N.K. A drug repurposing approach towards elucidating the potential of flavonoids as covid-19 spike protein inhibitors. Biointerface Res. Appl. Chem. 2021, 11, 8482–8501. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhou, C.C.; Yu, L.Y.; Wang, L.; Deng, J.L.; Tao, Y.L.; Zhang, F.; Chen, W.S. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol. Res. 2021, 163, 105224. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Y.; Wang, G.; Hill, L.; Weng, J.K.; Chen, X.Y.; Xue, H.; Martin, C. A specialized flavone biosynthetic pathway has evolved in the medicinal plant, Scutellaria baicalensis. Sci. Adv. 2016, 2, e1501780. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, L.; Xu, Y.; Yang, D.; Zhang, L.; Yang, S.; Zhang, W.; Wang, J.; Tian, S.; Yang, S.; et al. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem. Pharmacol. 2021, 183, 114302. [Google Scholar] [CrossRef]
- Liu, H.; Ye, F.; Sun, Q.; Liang, H.; Li, C.; Li, S.; Lu, R.; Huang, B.; Tan, W.; Lai, L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J. Enzyme Inhib. Med. Chem. 2021, 36, 497–503. [Google Scholar] [CrossRef] [PubMed]
- XIA, L.; SHI, Y.; SU, J.; Friedemann, T.; TAO, Z.; Lu, Y.; LING, Y.; Lv, Y.; ZHAO, R.; GENG, Z.; et al. Shufeng Jiedu, a promising herbal therapy for moderate COVID-19:Antiviral and anti-inflammatory properties, pathways of bioactive compounds, and a clinical real-world pragmatic study. Phytomedicine 2021, 85. [Google Scholar] [CrossRef]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. Vitr. Cell. Dev. Biol. Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic Plant Species Conservation: Biotechnological Approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, L.; Tripathi, J.N. Role of biotechnology in medicinal plants. Trop. J. Pharm. Res. 2005, 2, 243–253. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, A.; Yadav, M.; Yadav, J.P. Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm.f. BMC Res. Notes 2017, 10, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, H.; Savita; Sharma, R.; Sinha, S.; Kumar, M.; Kumar, P.; Verma, A.; Sharma, S.K. Physiological functioning of Lagerstroemia speciosa L. under heavy roadside traffic: An approach to screen potential species for abatement of urban air pollution. 3 Biotech 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verpoorte, R.; Contin, A.; Memelink, J. Biotechnology for the production of plant secondary metabolites. Phytochem. Rev. 2002, 1, 13–25. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.M.; Jang, M.O.; Jin, Y.W.; Lee, E.K.; Loake, G.J. Plant cell culture strategies for the production of natural products. BMB Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojakowska, A.; Kisiel, W. Production of parthenolide in organ cultures of feverfew. Plant Cell. Tissue Organ Cult. 1997, 47, 159–162. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Gołąb, K.; Gburek, J.; Wysokińska, H.; Matkowski, A. Inhibition of Advanced Glycation End-Product Formation and Antioxidant Activity by Extracts and Polyphenols from Scutellaria alpina L. and S. altissima L. Molecules 2016, 21, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, I.B.; Saxena, P.K.; Murch, S.J. Medicinal biotechnology in the genus scutellaria. Vitr. Cell. Dev. Biol. Plant 2007, 43, 318–327. [Google Scholar] [CrossRef]
- Erdoğan, M.; Konya, R.; Özhan, Y.; Sipahi, H.; Çinbilgel, İ.; Masullo, M.; Piacente, S.; Kırmızıbekmez, H. Secondary metabolites from Scutellaria brevibracteata subsp. subvelutina and their in vitro anti-inflammatory activities. South African J. Bot. 2021, 139, 12–18. [Google Scholar] [CrossRef]
- Gharari, Z.; Bagheri, K.; Sharafi, A.; Danafar, H. Thidiazuron induced efficient in vitro organogenesis and regeneration of Scutellaria bornmuelleri: An important medicinal plant. Vitr. Cell. Dev. Biol. Plant 2019, 55, 133–138. [Google Scholar] [CrossRef]
- White, F.F.; Garfinkel, D.J.; Huffman, G.A.; Gordon, M.P.; Nester, E.W. Sequences homologous to Agrobacterium rhizogenes T-DNA in the genomes of uninfected plants. Nature 1983, 301, 348–350. [Google Scholar] [CrossRef]
- Hernández-Altamirano, J.M.; Ugidos, I.F.; Palazón, J.; Bonfill, M.; García-Angulo, P.; Álvarez, J.; Acebes, J.L.; Bye, R.; Encina, A. Production of encecalin in cell cultures and hairy roots of Helianthella quinquenervis (Hook.) A. Gray. Molecules 2020, 25, 3231. [Google Scholar] [CrossRef]
- Roy, A. Hairy Root Culture an Alternative for Bioactive Compound Production from Medicinal Plants. Curr. Pharm. Biotechnol. 2020, 22, 136–149. [Google Scholar] [CrossRef]
- Zhou, Y.; Hirotani, M.; Yoshikawa, T.; Furuya, T. Flavonoids and phenylethanoids from hairy root cultures of Scutellaria baicalensis. Phytochemistry 1997, 44, 83–87. [Google Scholar] [CrossRef]
- Stojakowska, A.; Malarz, J. Flavonoid production in transformed root cultures of Scutellaria baicalensis. J. Plant Physiol. 2000, 156, 121–125. [Google Scholar] [CrossRef]
- Kuzovkina, I.N.; Guseva, A.V.; Alterman, I.E.; Karnachuk, R.A. Flavonoid production in transformed scutellaria baicalensis roots and ways of its regulation. Russ. J. Plant Physiol. 2001, 48, 448–452. [Google Scholar] [CrossRef]
- Wilczańska-Barska, A.; Królicka, A.; Głód, D.; Majdan, M.; Kawiak, A.; Krauze-Baranowska, M. Enhanced accumulation of secondary metabolites in hairy root cultures of Scutellaria lateriflora following elicitation. Biotechnol. Lett. 2012, 34, 1757–1763. [Google Scholar] [CrossRef]
- Marsh, Z.; Yang, T.; Nopo-Olazabal, L.; Wu, S.; Ingle, T.; Joshee, N.; Medina-Bolivar, F. Effect of light, methyl jasmonate and cyclodextrin on production of phenolic compounds in hairy root cultures of Scutellaria lateriflora. Phytochemistry 2014, 107, 50–60. [Google Scholar] [CrossRef]
- Tuan, P.A.; Kim, Y.S.; Kim, Y.; Thwe, A.A.; Li, X.; Park, C.H.; Lee, S.Y.; Park, S.U. Molecular characterization of flavonoid biosynthetic genes and accumulation of baicalin, baicalein, and wogonin in plant and hairy root of Scutellaria lateriflora. Saudi J. Biol. Sci. 2018, 25, 1639–1647. [Google Scholar] [CrossRef] [Green Version]
- Stepanova, A.; Malunova, M.; Salamaikina, S.; Selimov, R.; Solov’eva, A. Establishment of Rhodiola quadrifida Hairy Roots and Callus Culture to Produce Bioactive Compounds. Phyton (B. Aires). 2021, 90, 543. [Google Scholar] [CrossRef]
- Solov’eva, A.I.; Evsyukov, S.V.; Sidorov, R.A.; Stepanova, A.Y. Correlation of endogenous β-glucuronidase activity with differentiation of in vitro cultures of Scutellaria baicalensis. Acta Physiol. Plant. 2020, 42, 1–9. [Google Scholar] [CrossRef]
- Zhao, Q.; Cui, M.Y.; Levsh, O.; Yang, D.; Liu, J.; Li, J.; Hill, L.; Yang, L.; Hu, Y.; Weng, J.K.; et al. Two CYP82D Enzymes Function as Flavone Hydroxylases in the Biosynthesis of Root-Specific 4′-Deoxyflavones in Scutellaria baicalensis. Mol. Plant 2018, 11, 135–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Yang, J.; Cui, M.Y.; Liu, J.; Fang, Y.; Yan, M.; Qiu, W.; Shang, H.; Xu, Z.; Yidiresi, R.; et al. The Reference Genome Sequence of Scutellaria baicalensis Provides Insights into the Evolution of Wogonin Biosynthesis. Mol. Plant 2019, 12, 935–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagashima, S.; Hirotani, M.; Yoshikawa, M. Purification and characterization of UDP-glucuronate: Baicalein 7-O-glucuronosyltransferase from Scutellaria baicalensis Georgi. cell suspension cultures. Phytochemistry 2000, 53, 533–538. [Google Scholar] [CrossRef]
- Levy, G.A. Baicalinase, a plant beta-glucuronidase. Biochem. J. 1954, 58, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, Y.S.; Kim, Y.J.; Uddin, M.R.; Kim, Y.B.; Kim, H.H.; Park, S.Y.; Lee, M.Y.; Chung, S.O.; Park, S.U. Comparative analysis of flavonoids and polar metabolites from hairy roots of Scutellaria baicalensis and Scutellaria lateriflora. World J. Microbiol. Biotechnol. 2014, 30, 887–892. [Google Scholar] [CrossRef]
- Olina, A.V.; Solovyova, A.I.; Solovchenko, A.E.; Orlova, A.V.; Stepanova, A.Y. Physiologically active flavones contentin Scutellaria baicalensis georgiinvitro cultures. Biotekhnologiya 2017, 33, 29–37. [Google Scholar] [CrossRef]
- Dikaya, V.S.; Solovyeva, A.I.; Sidorov, R.A.; Solovyev, P.A.; Stepanova, A.Y. The Relationship Between Endogenous β-Glucuronidase Activity and Biologically Active Flavones-Aglycone Contents in Hairy Roots of Baikal Skullcap. Chem. Biodivers. 2018, 15, e1700409. [Google Scholar] [CrossRef] [PubMed]
- Udrea, A.-M.; Mernea, M.; Buiu, C.; Avram, S. Scutellaria baicalensis Flavones as Potent Drugs against Acute Respiratory Injury during SARS-CoV-2 Infection: Structural Biology Approaches. Processes 2020, 8, 1468. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Vervliet, G.; Holsters, M.; Teuchy, H.; Van Montagu, M.; Schell, J. Characterization of different plaque forming and defective temperate phages in Agrobacterium strains. J. Gen. Virol. 1975, 26, 33–48. [Google Scholar] [CrossRef]
- Nunes, C.F.; Ferreira, J.L.; Fernandes, M.C.N.; de Breves, S.; Generoso, A.L.; Soares, B.D.F.; Dias, M.S.C.; Pasqual, M.; Borem, A.; de Cançado, G.M.A. Otimização de um método para extração de DNA genômico a partir de folhas de morangueiro. Cienc. Rural 2011, 41, 1383–1389. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Li, P.; He, C.; Liu, H.; Liu, Y.; Sun, X.; Xu, R.; Xiao, P. Simultaneous determination of 15 flavonoids from different parts of Scutellaria baicalensis and its chemometrics analysis. Chin. Herb. Med. 2019, 11, 20–27. [Google Scholar] [CrossRef]
- Li, K.; Fan, H.; Yin, P.; Yang, L.; Xue, Q.; Li, X.; Sun, L.; Liu, Y. Structure-activity relationship of eight high content flavonoids analyzed with a preliminary assign-score method and their contribution to antioxidant ability of flavonoids-rich extract from Scutellaria baicalensis shoots. Arab. J. Chem. 2018, 11, 159–170. [Google Scholar] [CrossRef]
- Tong, L.; Wan, M.; Zhang, L.; Zhu, Y.; Sun, H.; Bi, K. Simultaneous determination of baicalin, wogonoside, baicalein, wogonin, oroxylin A and chrysin of Radix scutellariae extract in rat plasma by liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 70, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Tong, C.; Guo, Y.; Xu, J.; Shi, F.; Shi, S.; Xiao, Y. Flavonoid aglycone–oriented data-mining in high-performance liquid chromatography–quadrupole time-of-flight tandem mass spectrometry: Efficient and targeted profiling of flavonoids in Scutellaria barbata. Anal. Bioanal. Chem. 2020, 412, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Human Metabolome Database. Available online: https://hmdb.ca/ (accessed on 5 May 2021).
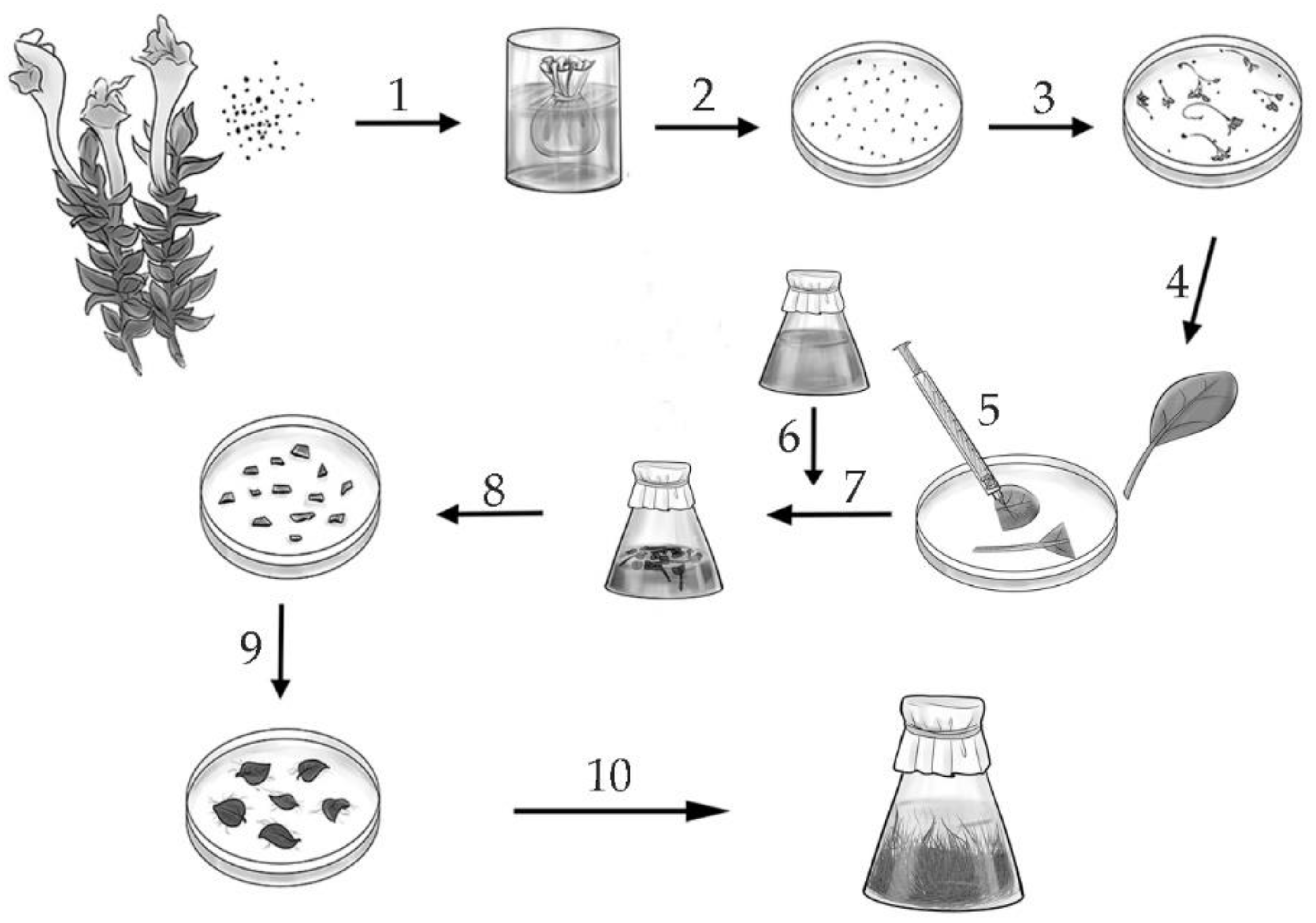

| Hairy Roots Growing Medium | S. baicalensis | S. lateriflora | S. przewalskii | S. pycnoclada |
|---|---|---|---|---|
| Liquid medium | 43 ± 3.8 a | 6.4 ± 2.7 c | 32 ± 1.6 b | 4.7 ± 0.5 c |
| Solid medium | 5.9 ± 0.7 c | 5.1 ± 1.2 c | 4.8 ± 0.8 c | 1.9 ± 0.1 d |
| № | Compounds | Molecular Mass | MRM Transition, m/z |
|---|---|---|---|
| 1 | baicalin | 446 | 447.1 → 271.1 |
| 2 | wogonoside | 460 | 461.1 → 285.1 |
| 3 | baicalein | 270 | 271.1 → 123.1 |
| 4 | wogonin | 284 | 285.1 → 270.1 |
| 5 | chrysin | 254 | 255.1 → 153.1 |
| 6 * | chrysin-6-C-β-d-glucoside | 416 | 417.1 → 281.1 |
| 7 * | chrysin-8-C-β-d-glucoside | ||
| 8 | tenaxin | 344 | 345.1 → 330.1 |
| 9 | viscudulin III 6-O-β-d-glucoside | 508 | 509.1 → 347.1 |
| 10 | apigenin | 270 | 271.1 → 153.1 |
| 11 | apigetrin (apigenin-7-O-β-d-glucopuranoside) | 432 | 433.1 → 271.1 |
| 12 * | isocarthamidin-7-O-β-d-glucuronide | 464 | 465.1 → 289.1 |
| 13 * | carthamidin-7-O-β-d-glucuronide | ||
| 14 | scutellarin | 462 | 463.1 → 287.1 |
| 15 | chrysin-7-O-β-d-glucuronide | 430 | 431.1 → 255.1 |
| 16 | baicalein-7-O-β-d-glucoside | 432 | 433.1 → 415.1 |
| 17 | naringenin | 272 | 273.1 → 153.1 |
| 18 | oroxylin A | 284 | 285.1 → 267.1 |
| 19 | scullcapflavone II | 374 | 375.1 → 345.1 |
| Genes | Nucleotide Sequence (nt) | Tm (°C) |
|---|---|---|
| rolA | 5′-ggaaatccgcaatcaac-3′ | 62 |
| 5′-tttgcacgcctaacaag-3′ | ||
| rolB | 5′-GCTCTTGCAGTGCTAGATTT-3′ | 60 |
| 5′-GAAGGTGCAAGCTACCTCTC-3′ | ||
| rolC | 5′-CTCCTGACATCAAACTCGTC-3′ | 60 |
| 5′-TGCTTCGAGTTATGGGTACA-3′ | ||
| rolD | 5′-CATCTGCAACTGAGCGTGTG-3′ | 62 |
| 5′-TGTCTGATAGGGAGGAACGA-3′ |
| Flavone | Calibration Range (µg/mL) | R2 |
|---|---|---|
| baicalin | 1–100 | 0.9997 |
| wogonoside | 1–100 | 0.9986 |
| baicalein | 1–100 | 0.9988 |
| wogonin | 1–100 | 0.9998 |
| chrysin | 1–50 | 0.9993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, A.Y.; Solov’eva, A.I.; Malunova, M.V.; Salamaikina, S.A.; Panov, Y.M.; Lelishentsev, A.A. Hairy Roots of Scutellaria spp. (Lamiaceae) as Promising Producers of Antiviral Flavones. Molecules 2021, 26, 3927. https://doi.org/10.3390/molecules26133927
Stepanova AY, Solov’eva AI, Malunova MV, Salamaikina SA, Panov YM, Lelishentsev AA. Hairy Roots of Scutellaria spp. (Lamiaceae) as Promising Producers of Antiviral Flavones. Molecules. 2021; 26(13):3927. https://doi.org/10.3390/molecules26133927
Chicago/Turabian StyleStepanova, Anna Yurievna, Aleksandra Ivanovna Solov’eva, Maria Victorovna Malunova, Svetlana Andreevna Salamaikina, Yury Mikhailovich Panov, and Andrey Aleksandrovich Lelishentsev. 2021. "Hairy Roots of Scutellaria spp. (Lamiaceae) as Promising Producers of Antiviral Flavones" Molecules 26, no. 13: 3927. https://doi.org/10.3390/molecules26133927
APA StyleStepanova, A. Y., Solov’eva, A. I., Malunova, M. V., Salamaikina, S. A., Panov, Y. M., & Lelishentsev, A. A. (2021). Hairy Roots of Scutellaria spp. (Lamiaceae) as Promising Producers of Antiviral Flavones. Molecules, 26(13), 3927. https://doi.org/10.3390/molecules26133927







