Discovery of New Pyrazolopyridine, Furopyridine, and Pyridine Derivatives as CDK2 Inhibitors: Design, Synthesis, Docking Studies, and Anti-Proliferative Activity
Abstract
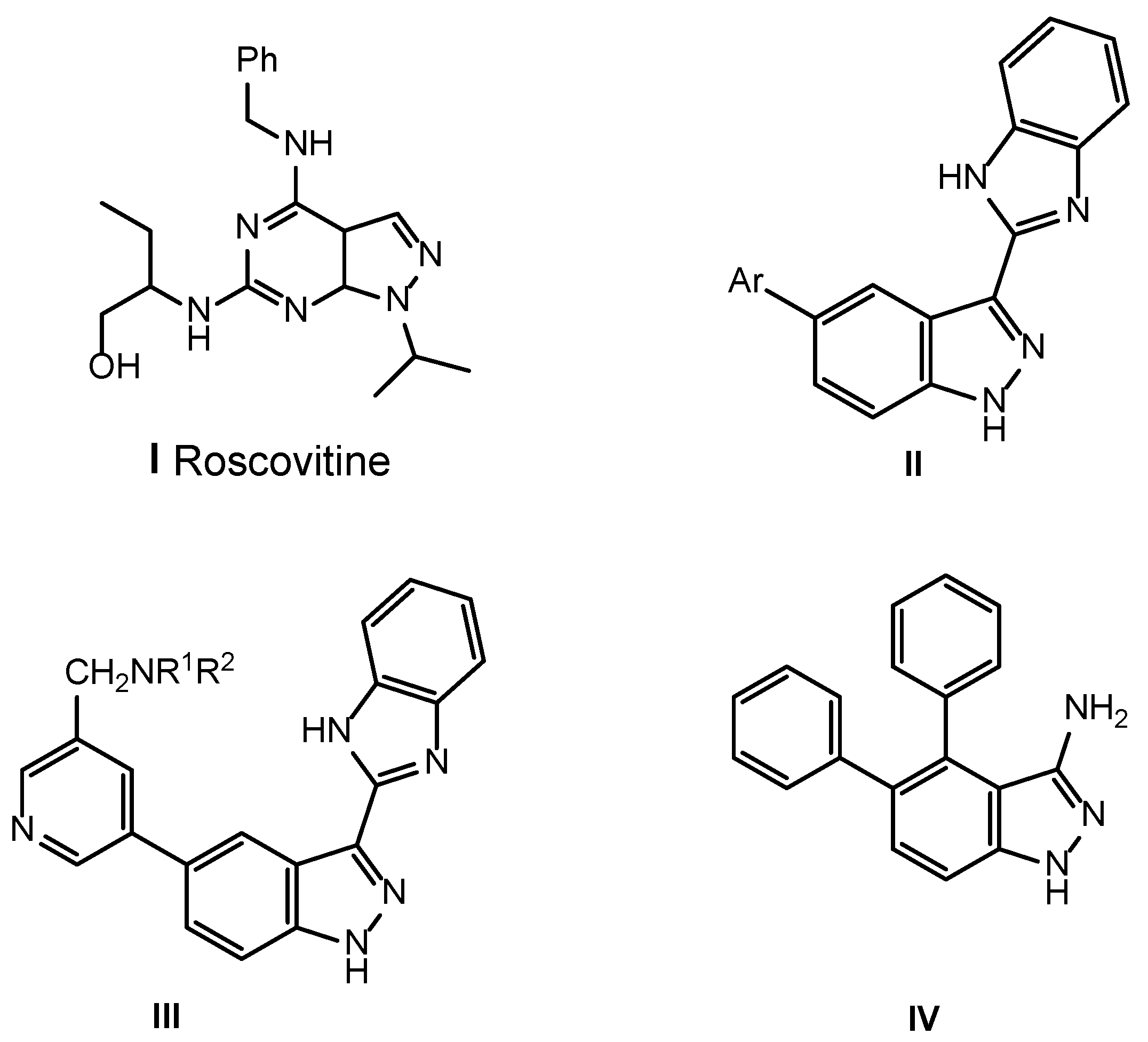
1. Introduction
2. Results and Discussion
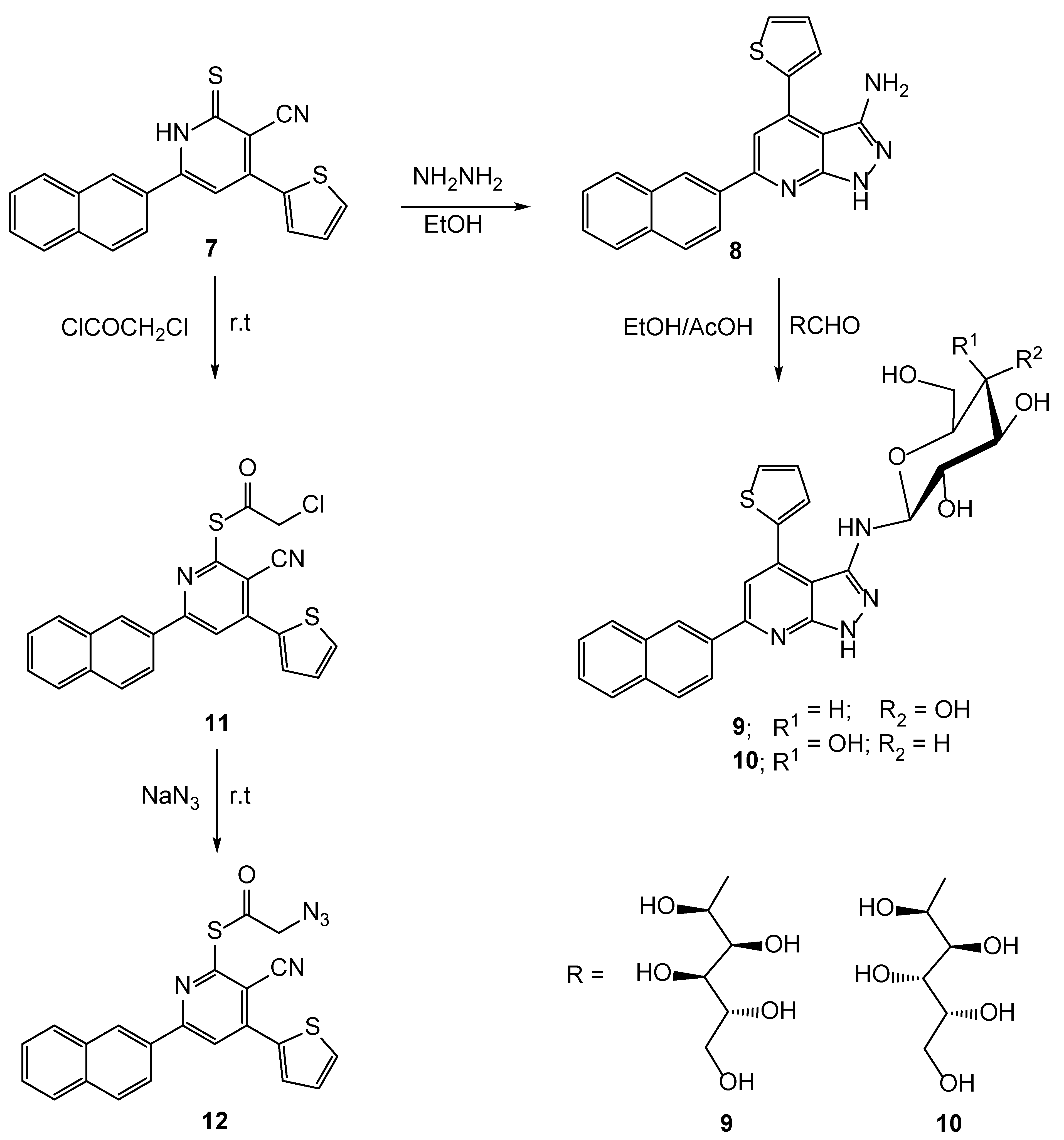
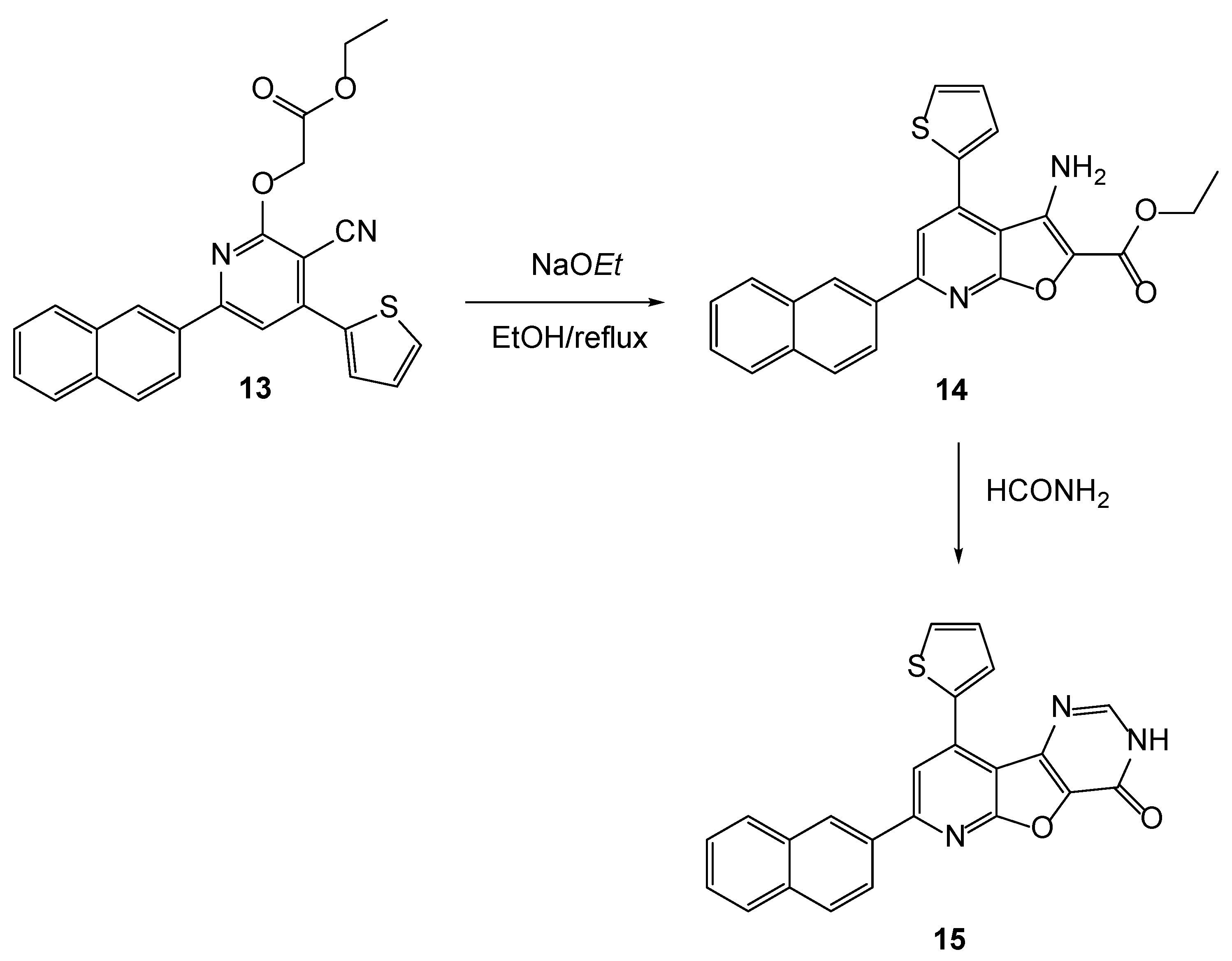
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. CDK2/Cyclin A2 Activity
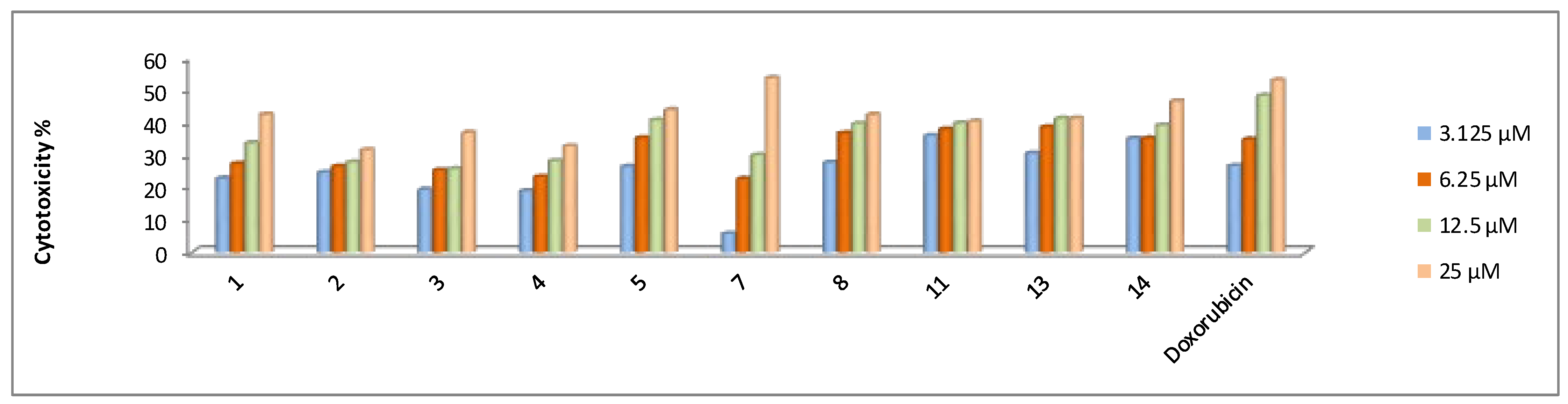
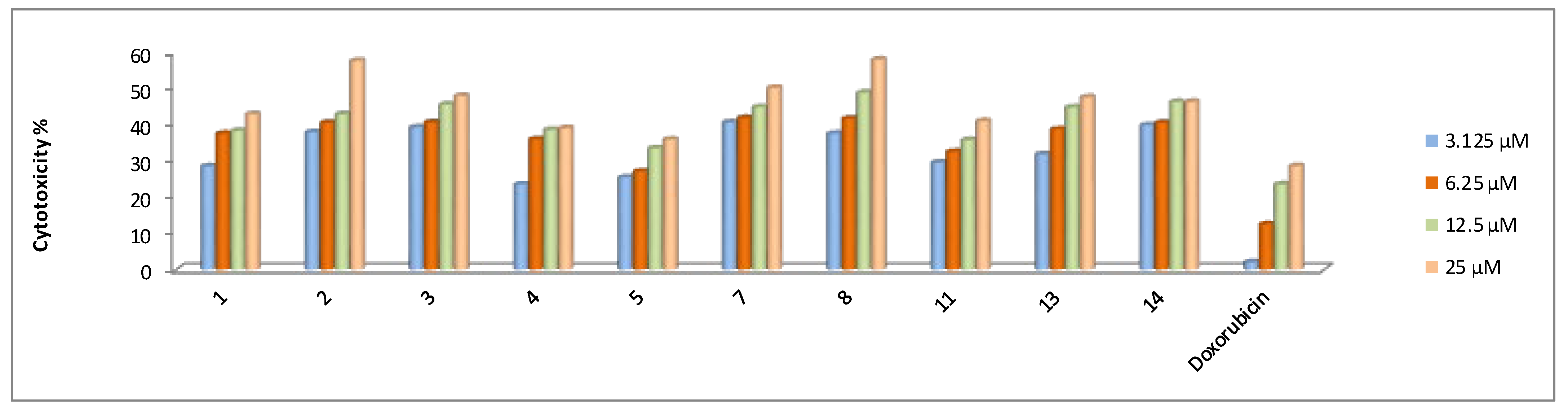
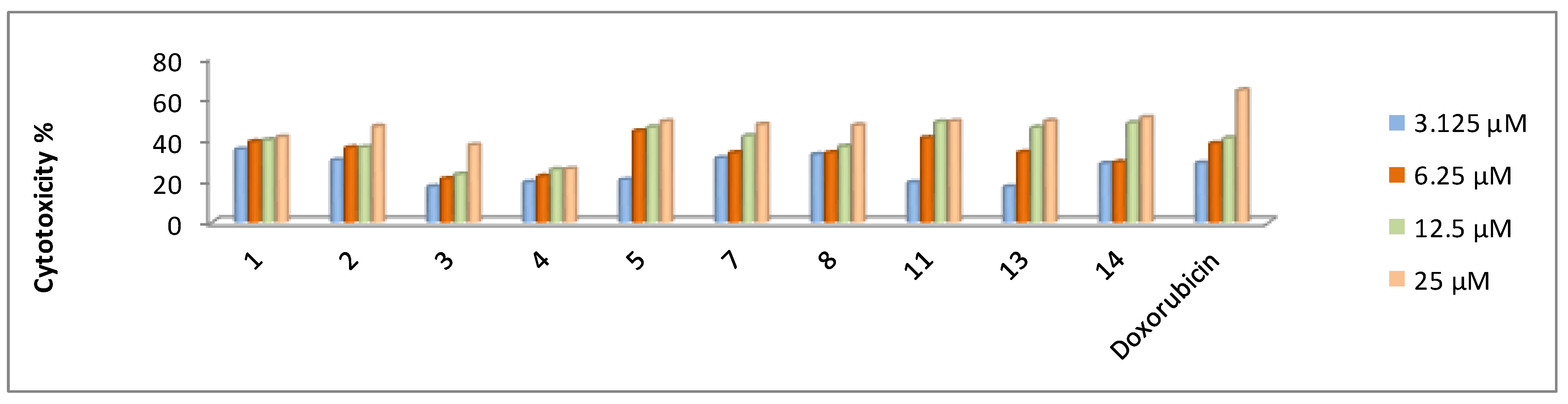
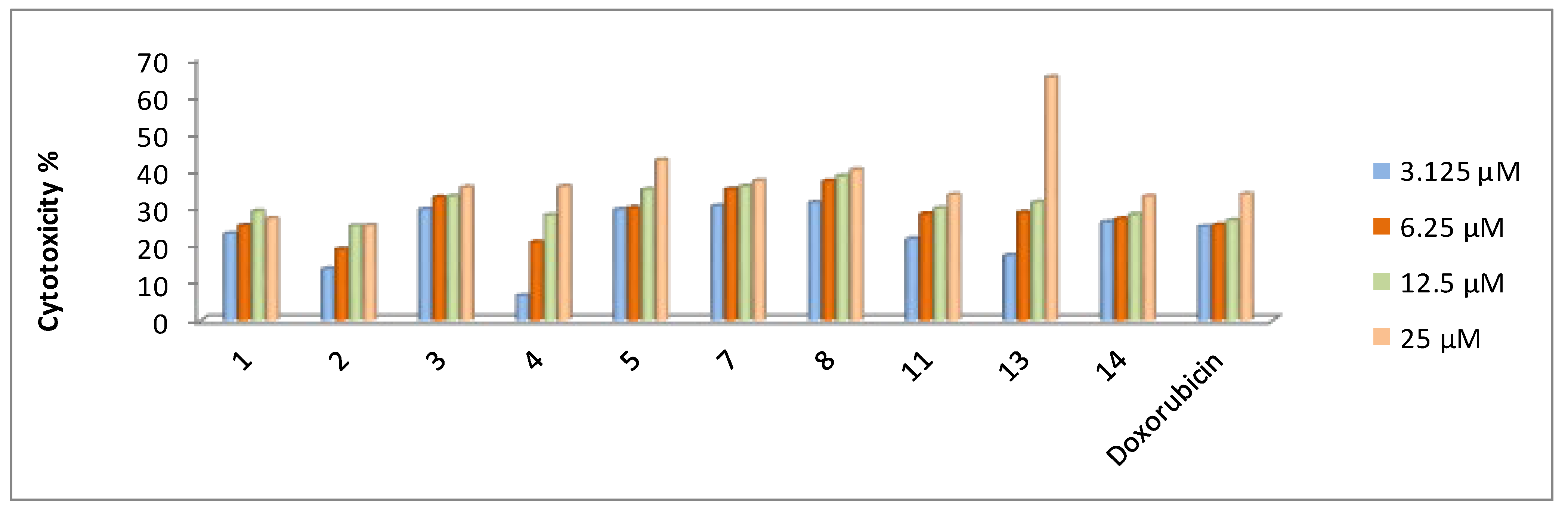
2.2.2. In Vitro Cytotoxicity Activity
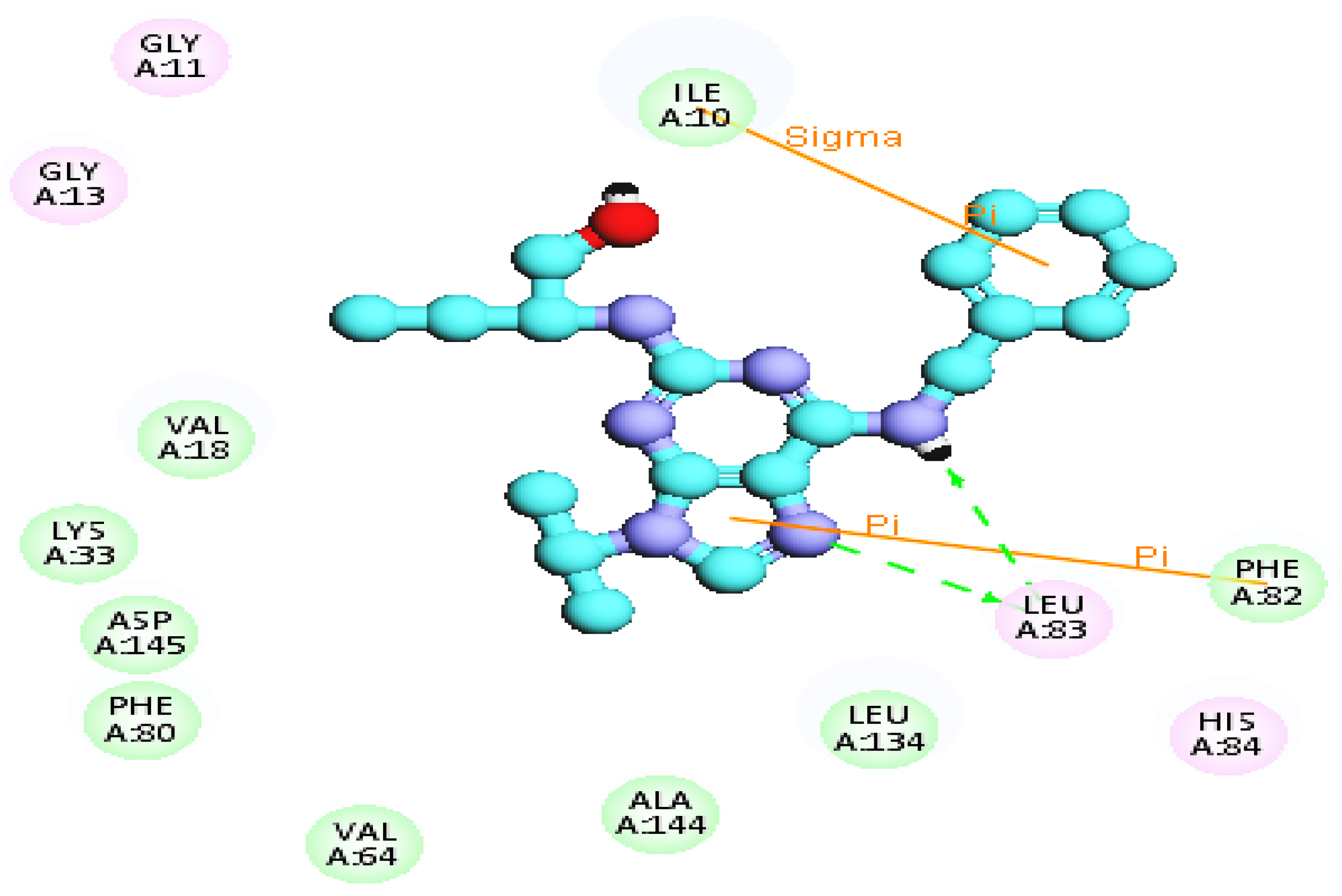
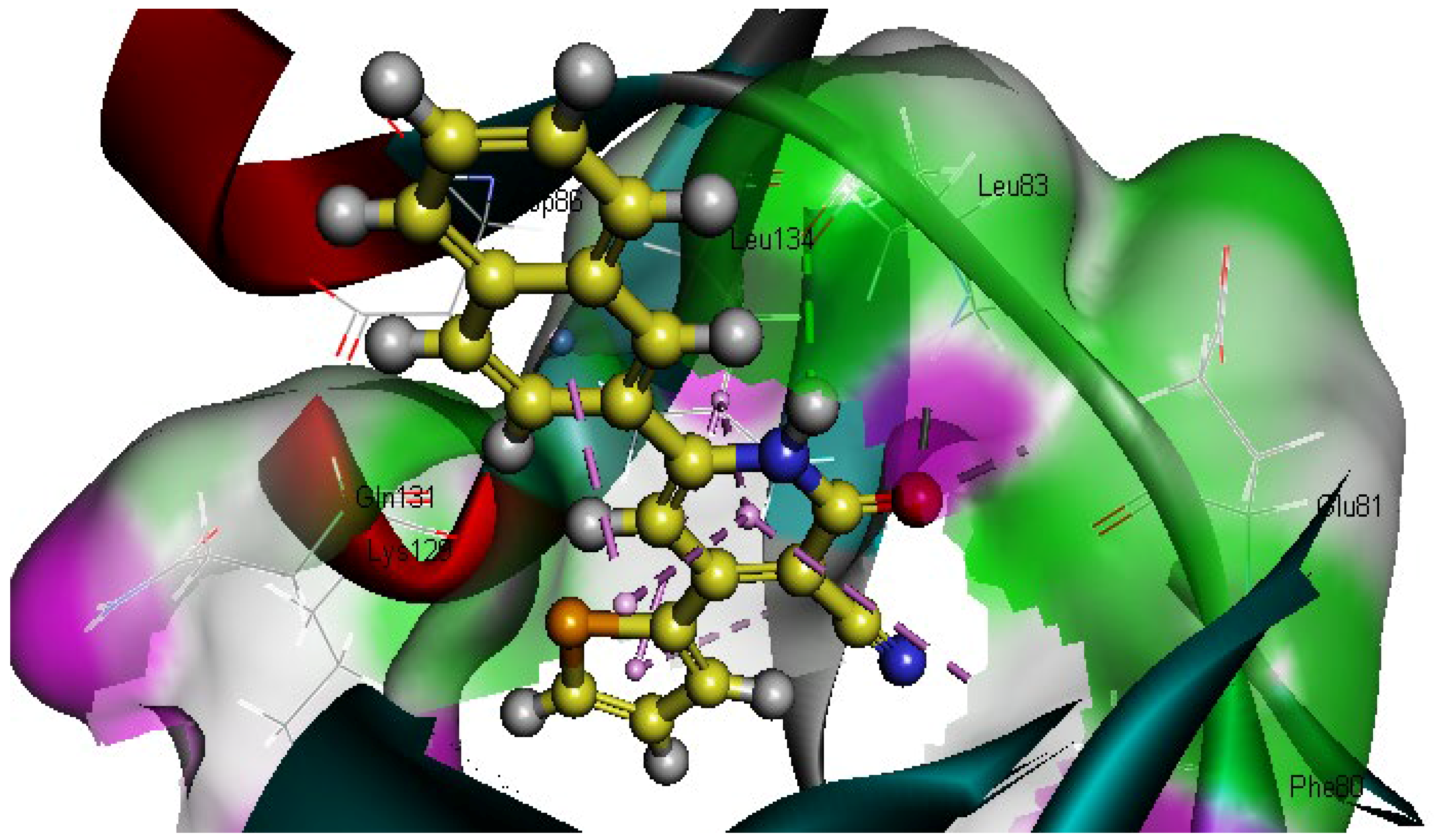
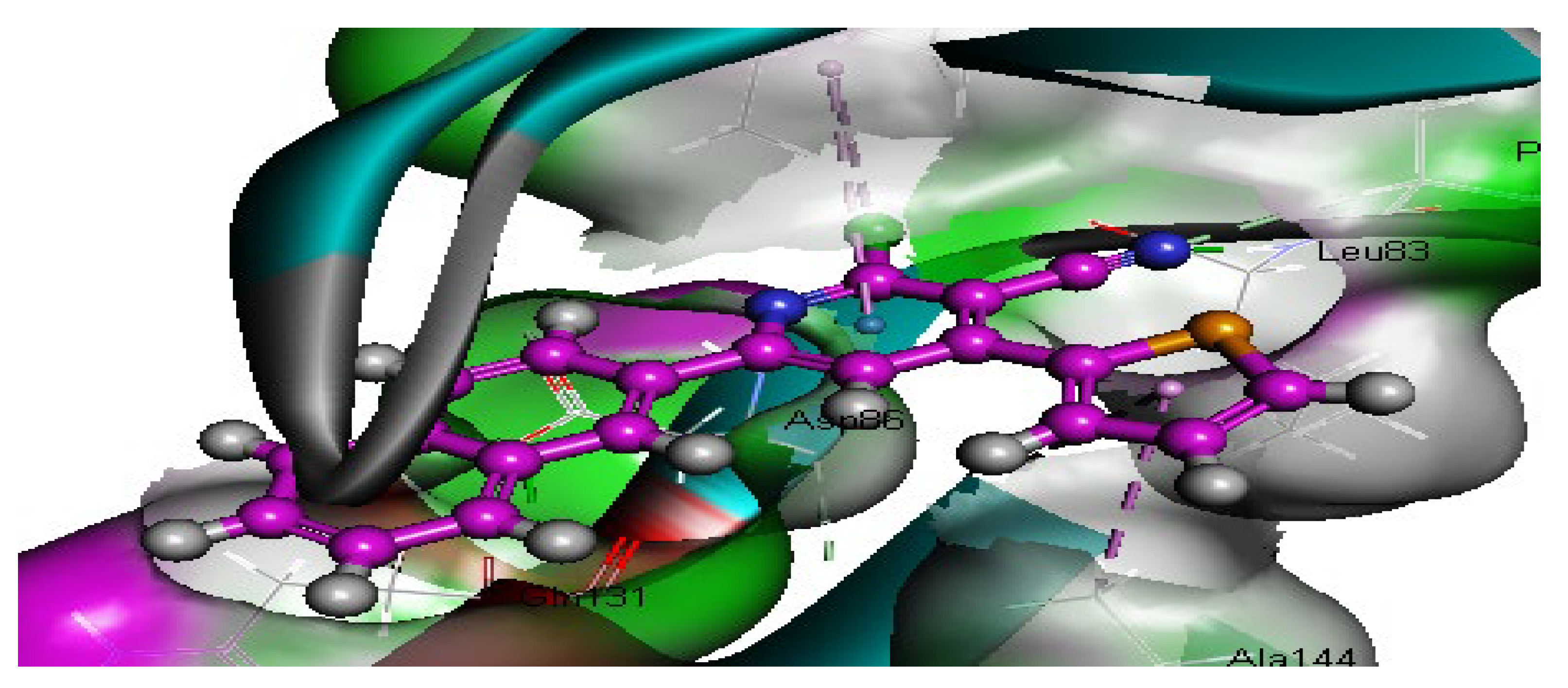
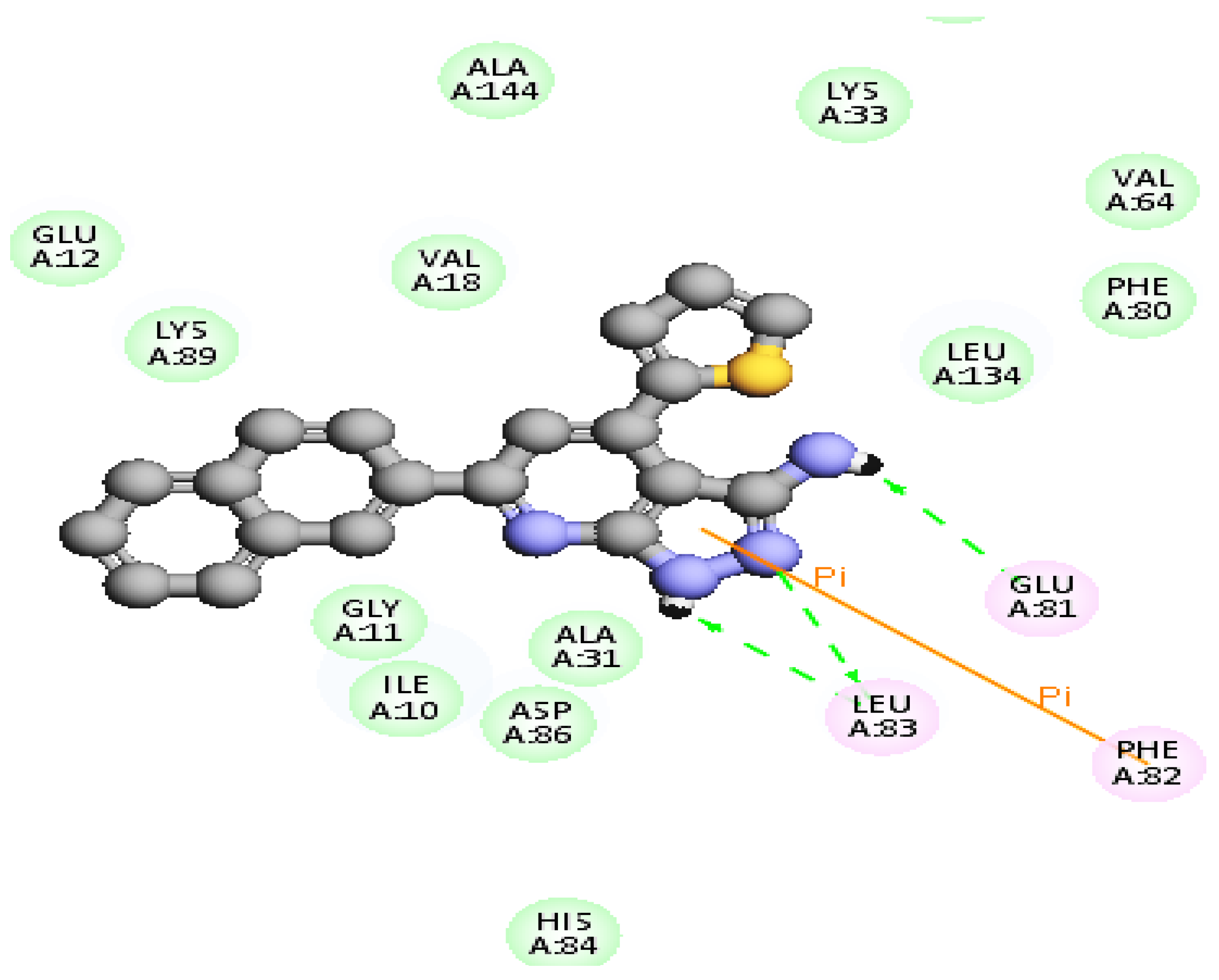
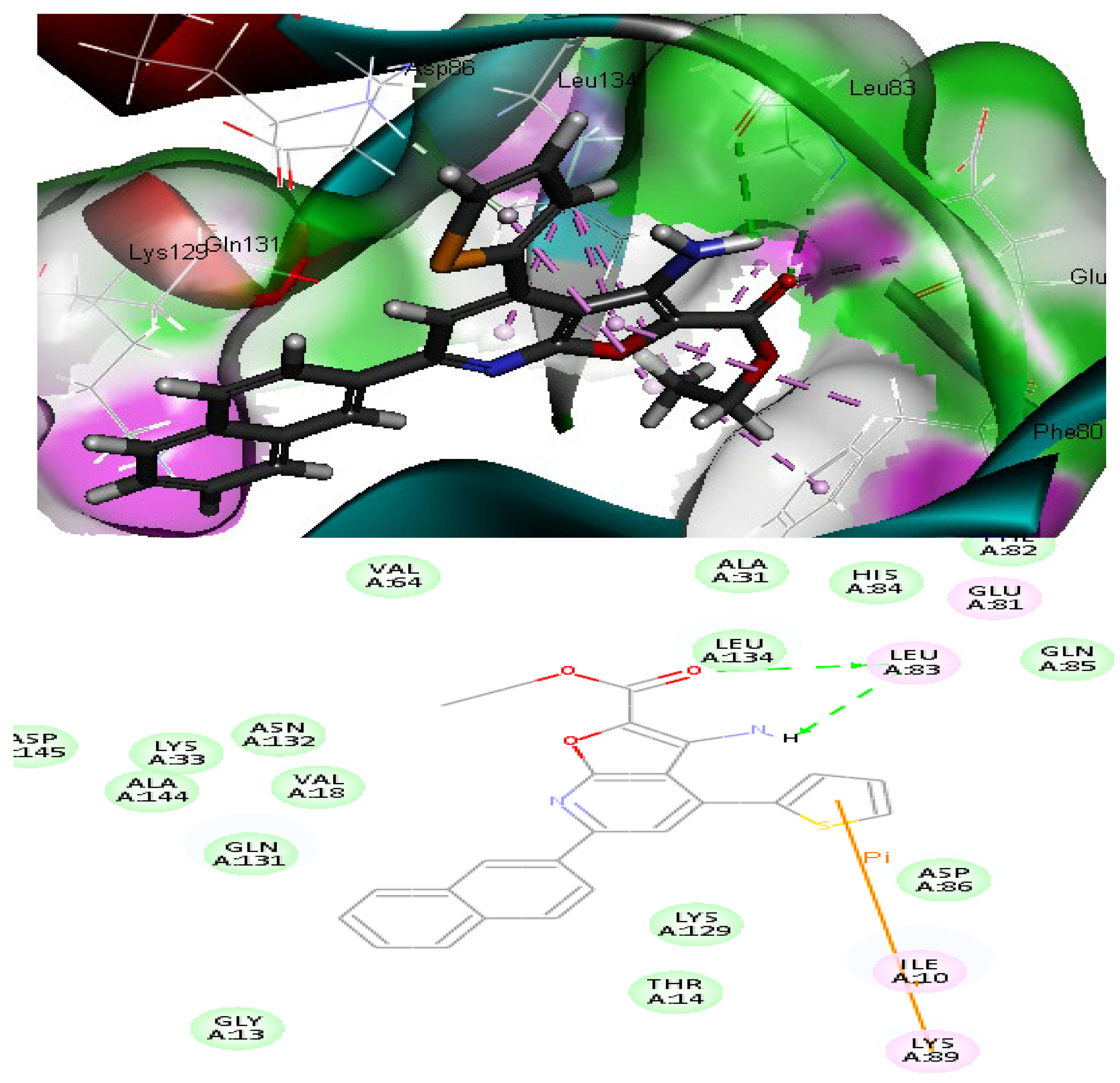
2.2.3. Molecular Docking Study
D-QSAR Studies
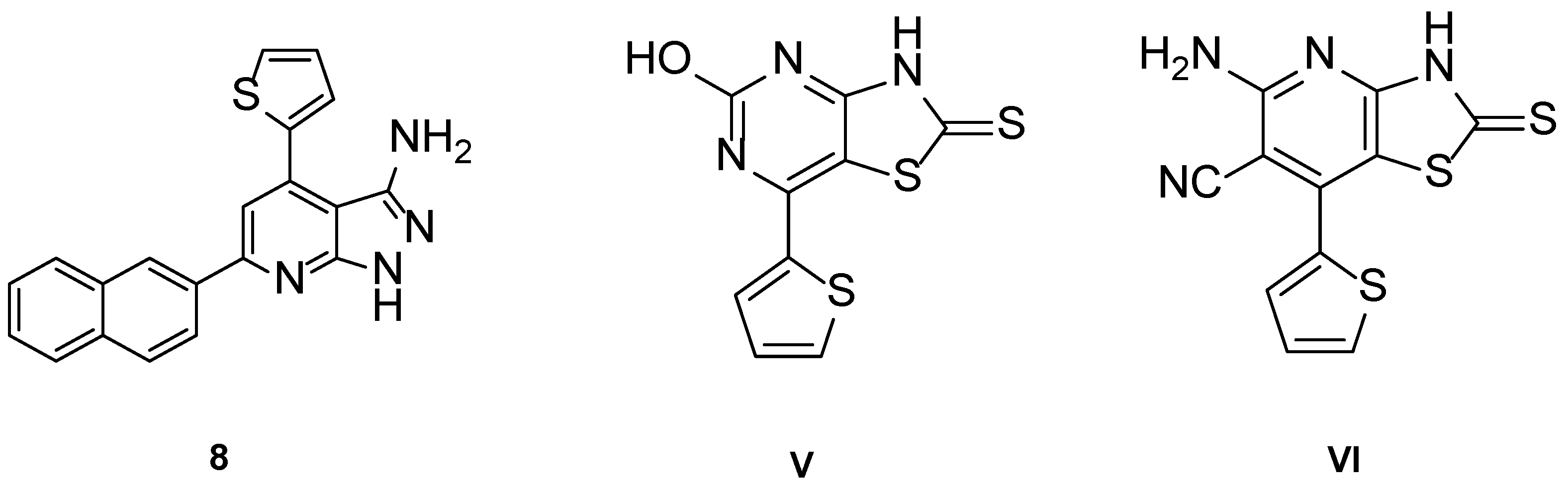
Validation of QSAR
2.2.4. Structure–Activity Relationship (SAR)
3. Materials and Methods
3.1. Chemistry
3.1.1. 3-Cyano-6-(naphthalen-2-yl)-4-(thiophen-2-yl)pyridin-2-yl 2-chloroacetate (2)
3.1.2. 6-(Naphthalen-2-yl)-4-(thiophen-2-yl)-2-((2-thioxo-2,5-dihydro-1H-imidazol-4-yl)oxy)nicotinonitrile (3)
3.1.3. 2-Methoxy-6-(naphthalen-2-yl)-4-(thiophen-2-yl)nicotinonitrile (5)
3.1.4. 6-(Naphthalen-2-yl)-2-(phenylamino)-4-(thiophen-2-yl)nicotinonitrile (6)
3.1.5. 6-(Naphthalen-2-yl)-4-(thiophen-2-yl)-1H-pyrazolo[3,4-b]pyridin-3-amine (8)
3.1.6. General Procedure for Preparing Amino Sugar Derivatives 9 and 10
3.1.7. S-(3-Cyano-6-(naphthalen-2-yl)-4-(thiophen-2-yl)pyridin-2-yl)2-chloroethanethioate (11)
3.1.8. S-(3-Cyano-6-(naphthlean-2-yl)-4-(thiophen-2-yl)pyridin-2-yl)-2-azidoethanethioate (12)
3.1.9. Ethyl-3-amino-6-(naphthalen-2-yl)-4-(thiophen-2-yl)furo[2,3-b]pyridine-2-carboxylate (14)
3.1.10. 7-(Naphthalen-2-yl)-9-(thiophen-2-yl)pyrido[3′,2′:4,5]furo[3,2-d]pyrimidin-4(3H)-one (15)
3.2. Anti-Cancer Activity
3.2.1. Cell Culture and Maintenance
3.2.2. Cytotoxicity Measurement
3.3. In Silico Studies
Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Alghassimou, D.; Claude, P. The serine/threonine kinases that control cell cycle progression as therapeutic targets. Bull. Cancer 2011, 98, 1335–1345. [Google Scholar]
- Johnsona, L.N.; De Moliner, E.; Browna, N.R.; Songa, H.; Barfordc, D.; Endicotta, J.A.; Noble, M.E. structure studies with inhibitors of the cell cycle regulatory kinase cyclin-dependent protein kinase 2. Pharmacol. Ther. 2002, 93, 113–124. [Google Scholar] [CrossRef]
- Chen, J.; Pang, L.; Wang, W.; Wang, L.; Zhang, J.Z.; Zhu, T. Decoding molecular mechanism of inhibitor bindings to CDK2 using molecular dynamics simulations and binding free energy calculations. J. Biomol. Struct. Dyn. 2020, 38, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Zhang, J.Z.; Zhu, T. effect of substitution in different positions of aminothiazole hing-binding scaffolds on inhibitor-CDK2 association probed by interaction entropy method. ACS Omega 2018, 3, 18052–18064. [Google Scholar] [CrossRef]
- Kontopidis, G.; McInnes, C.; Pandalaneni, S.R.; McNae, I.; Gibson, D.; Mezna, M.; Thomas, M.; Wood, G.; Wang, S.; Walkinshaw, M.D.; et al. Differential binding of inhibitors to active and inactive CDK2 provides insights for drug design. Chem. Biol. 2006, 13, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Abd El-sattar, N.E.A.; Badawy, E.H.K.; AbdEl-Hady, W.H.; Abo-Alkasem, M.I.; Mandour, A.A.; Ismail, N.S.M. Design and synthesis of new CDK2 inhibitors containing thiazolone and thiazolthione scaffold with apoptotic activity. Chem. Pharm. Bull. 2021, 69, 106–117. [Google Scholar] [CrossRef]
- Peyressatre, M.; Prével, C.; Pellerano, M.; Morris, M.C. Targeting cyclin-dependent kinase in human cancers: From small molecules to peptide inhibitors. Cancers 2015, 7, 179–237. [Google Scholar] [CrossRef]
- Eren, G.; Unlu, S.; Nunez, M.T.; Labeaga, L.; Ledo, F.; Entrena, A.; Banoglu, E.; Costantino, G.; Sahin, M.F. Synthesis, biological evaluation, and docking studies of novel heterocyclic diaryl compounds as selective COX-2 inhibitors. Bioorg. Med. Chem. 2010, 18, 6367–6376. [Google Scholar] [CrossRef]
- Matthew, E.W.; Scott, A.S.; Brent, R.S. Privileged scaffolds for librarydesign and drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar]
- Fortin, S.; Berube, G. Advances in the development of hybrid anticancer drugs. Expert Opin. Drug Discov. 2013, 8, 1029–1047. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; Khalaf, H.S.; Mohamed, S.F.; Hussien, H.A.; Kutkat, O.M.; Amr, A.E. Synthesis and antiviral activity of 1, 2, 3-triazole glycosides based substituted pyridine via click cycloaddition. Russ. J. Gen. Chem. 2017, 87, 2444–2453. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Al-Qalawi, H.R.M.; El-Sayed, W.A.; Arafa, W.A.A. Anticancer activity of newly synthesized triazolopyrimidine derivatives and their nucleoside analogs. Acta Pol. Pharm. 2015, 72, 307–318. [Google Scholar]
- Khalaf, H.S.; Tolana, H.E.; El-Bayaaa, M.N.; Radwan, M.A.; El-Manawaty, M.; El-Sayed, W.A. Synthesis and Anticancer Activity of New Pyridine-Thiophene and Pyridine-Furan Hybrid Compounds, Their Sugar Hydrazone, and Glycosyl Derivatives. Russ. J. Gen. Chem. 2020, 90, 1706–1715. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Pingaew, R.; Worachartcheewan, A.; Sinthupoom, N.; Prachayasittikul, V.; Ruchirawat, S.; Prachayasittikul, V. Roles of Pyridine and Pyrimidine Derivatives as Privileged Scaffolds in Anticancer Agents. Mini Rev. Med. Chem. 2017, 17, 869–901. [Google Scholar] [CrossRef]
- Rashad, A.E.; AMahmoud, A.E.; Ali, M.M. synthesis and anticancer effects of some novel pyrazolo[3,4-d]pyrimidine derivatives by generating reactive oxygen species in human breast adenocarcinoma cells. Eur. J. Med. Chem. 2011, 46, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Schenone, S.; Bruno, O.; Bondavalli, F.; Ranise, A.; Mosti, L.; Menozzi, G.; Fossa, P.; Donnini, S.; Santoro, A.; Ziche, M.; et al. Antiproliferative activity of new 1-aryl-4-amino-1H-pyrazolo[3,4-d]pyrimidine derivatives toward the human epidermoid carcinoma A431 cell line. Eur. J. Med. Chem. 2004, 39, 939–946. [Google Scholar] [CrossRef]
- Devesa, I.; Alcaraz, M.J.; Riguera, R.; Ferrándiz, M.L. Anew pyrazolo pyrimidine derivative inhibitor of cyclooxygenase-2 with anti-angiogenic activity. Eur. J. Pharmacol. 2004, 488, 225–230. [Google Scholar] [CrossRef]
- Abbas, H.A.S.; El-Sayed, W.A.; Fathy, N.M. Synthesis and antitumor activity of new dihydropyridine thioglycosides and their corresponding dehydrogenated forms. Eur. J. Med. Chem. 2010, 45, 973–982. [Google Scholar] [CrossRef]
- Abdel-Latif, E.; Abdel-Fattah, S.; Gaffer, H.E.; Etman, H.A. Synthesis and antitumor activity of some new pyrazolo[3,4-d]pyrimidine and pyrazolo[3,4-b]pyridine derivatives. Egypt. J. Basic Appl. Sci. 2016, 3, 118–124. [Google Scholar] [CrossRef]
- Eissa, I.H.; El-Naggar, A.M.; El-Hashash, M.A. Design, synthesis, molecular modeling and biological evaluation of novel 1H-pyrazolo [3,4-b] pyridine derivatives as potential anticancer agents. Bioorg. Chem. 2016, 67, 43–56. [Google Scholar] [CrossRef]
- Witherington, J.; Bordas, V.; Gaiba, A.; Garton, N.S.; Naylor, A.; Rawlings, A.D.; Slingsby, B.P.; Smith, D.G.; Takle, A.K.; Ward, R.W. 6-heteroaryl-pyrazolo[3,4-b]pyridines: Potent and selective inhibitors of glycogen synthase kinase-3 (GSK-3). Bioorg. Med. Chem. Lett. 2003, 13, 3059. [Google Scholar] [CrossRef]
- Mourad, C.; Elena, S.; Abdelouahid, S.; Marco-Contelles, J. Studies on the acetylation of 3,6-diamino-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile derivatives. J. Heterocycl. Chem. 2010, 47, 861–872. [Google Scholar]
- Mohamed, A.E.; Hala, F.R.; Doha, M.B.; Ibrahim, Y.E. Microwave assisted synthesis of some new pyrazolopyridines and theirantioxidant, antitumor and antimicrobial activities. Eur. J. Med. Chem. 2013, 66, 415–422. [Google Scholar]
- Altalbawy, F.M. Synthesis and antimicrobial evaluation of somenovel Bis-α,β-unsaturated ketones, nicotinonitrile, 1,2-dihydropyridine-3-carbonitrile, fused thieno[2,3-b] pyridine and pyrazolo[3,4-b]pyridine derivatives. Int. J. Mol. Sci. 2013, 14, 2967–2979. [Google Scholar] [CrossRef]
- Wenglowsky, S.; Ahrendt, K.A.; Buckmelter, A.J.; Feng, B.; Gloor, S.L.; Gradl, S.; Grina, J.; Hansen, J.D.; Laird, E.R.; Lunghofer, P.; et al. Pyrazolopyridine inhibitors of B-Raf (V600E). Part 2: Structure–activity relationships. Bioorg. Med. Chem. Lett. 2011, 21, 5533–5537. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zhou, W.; Huang, X.; Dhondi, P.; Palani, A.; Aslanian, R.; Zhu, Z.; Greenlee, W.; Cohen-Williams, M.; Jones, N.; et al. Discovery of a potent pyrazolopyridine series of γ-secretase modulators. ACS Med. Chem. Lett. 2011, 2, 471–476. [Google Scholar] [CrossRef][Green Version]
- Lin, R.; Connolly, P.J.; Lu, Y.; Chiu, G.; Li, S.; Yu, Y.; Huang, S.; Li, X.; Emanuel, S.L.; Middleton, S.A.; et al. Synthesis and evaluation of pyrazolo[3,4-b]pyridine CDK1 inhibitors as anti-tumor agents. Bioorg. Med. Chem. Lett. 2007, 17, 4297–4302. [Google Scholar] [CrossRef]
- Braña, M.F.; Cacho, M.; Garcı´a, M.L.; Mayo Lo’pez, E.P.; de Pascual-Teresa, B.; Ramos, A.; Acero, N.; Llinares, F.; Muñoz-Mingarro, D.; Lozach, O.; et al. Pyrazolo[3,4-c]pyridazines as Novel and Selective In-hibitors of Cyclin-Dependent Kinases. J. Med. Chem. 2005, 48, 6843–6854. [Google Scholar] [CrossRef]
- Meijer, L.; Raymond, E. Roscovitine and Purines as Kinase in Clinical Trials. Acc. Chem. Res. 2003, 36, 417–425. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, R.W.; Currie, K.S.; Mitchell, S.A.; Darrow, J.W.; Pippin, D.A. Privileged structures: Applications in drug discovery. Com. Chem. High Throughput Screen 2004, 7, 473–494. [Google Scholar] [CrossRef] [PubMed]
- Flynn, B.L.; Chaplin, J.H.; Paul, D.; Grobelny, D.W.; Kelly, B.B. Chemical Compounds and Processes. WO 2008070908 A1, 2008. [Google Scholar]
- Martin, M.W.; Newcomb, J.; Nunes, J.J.; Bemis, J.E.; McGowan, D.C.; White, R.D.; Buchanan, J.L.; DiMauro, E.F.; Boucher, C.; Faust, T.; et al. Discovery of novel 2,3-diarylfuro[2,3-b]pyridine-4-amines as potent and selective inhibitors of lck: Synthesis, SAR, and pharmacokinetic properties. Bioorg. Med. Chem. Lett. 2007, 17, 2299–2304. [Google Scholar] [CrossRef]
- Wu, Z.; Robinson, R.G.; Fu, S.; Barnett, S.F.; Defeo-Jones, D.; Jones, R.E.; Kral, A.M.; Huber, H.E.; Kohl, N.E.; Hartman, G.D.; et al. Rapid assembly of diverse and potent allosteric Akt inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 2211–2214. [Google Scholar] [CrossRef]
- Archna, S.P.; Pooja, A.C. Thiophene-based derivatives as anticancer agents: An overview on decade’s work. Bioorg. Chem. 2020, 101, 104026. [Google Scholar] [CrossRef] [PubMed]
- Blaszczak-Swiatkiewic, K.; Olszewska, P.; Mikiciuk-Olasik, E. Biological approach of anticancer activity of new benzimidazole derivatives. Pharmacol. Rep. 2014, 66, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ding, Y.; Qin, H.; Xu, Z.; Lan, H.; Yang, D.; Yi, C. One-pot synthesis of substituted pyrrole–imidazole derivatives with anticancer activity. Mol. Divers. 2020, 24, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Nassar, I.F.; Atta-Allah, S.R.; Abdel-Sattar, S.; Elgazwy, H.A. A convenient synthesis and molecular modeling study of novel pyrazolo[3,4-d]pyrimidine and pyrazole derivatives as anti-tumor agents. J. Enzym. Inhib. Med. Chem. 2015, 30, 396–405. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; Nassar, I.F.; Abdel Rahman, A.A.-H. Synthesis and Antitumor Activity of New 1,2,4-Triazine and [1,2,4]triazolo[4,3-b][1,2,4]triazine Derivatives and their Thioglycoside and Acyclic C-Nucleoside Analogous. J. Heterocycl. Chem. 2011, 4, 135. [Google Scholar] [CrossRef]
- Nassar, I.F.; El Farargy, A.F.; Abdelrazek, F.M. Synthesis and anticancer activity of some new fused pyrazoles and their glycoside derivatives. J. Heterocycl. Chem. 2018, 55, 1709–1718. [Google Scholar] [CrossRef]
- Kassem, A.F.; Nassar, I.F.; Abdel Aal, M.T.; Awad, H.M.; El-Sayed, W.A. Synthesis and Anticancer Activity of New ((Furan-2-yl)-1,3,4-thiadiazolyl)-1,3,4-Oxadiazole Acyclic Sugar Derivatives. J. Heterocycl. Chem. 2019, 67, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Nassar, I.F.; EL-Kady, D.S.; Awad, H.M.; El-Sayed, W.A. Design, synthesis and anticancer activity of new oxadiazolyl-linked and thiazolyl-linked benzimidazole arylidines, thioglycoside and acyclic analogs. J. Heterocycl. Chem. 2019, 56, 1086–1100. [Google Scholar] [CrossRef]
- Nassar, I.F.; El-Sayed, W.A.; Ragab, T.I.M.; Shalaby, A.S.G.; Mehany, A. Design, Synthesis of New Pyridine and Pyrimidine Sugar Compounds as Antagonists Targeting the ERα via Structure-Based Virtual Screening. Mini Rev. Med. Chem. 2019, 19, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Abdel Rahman, A.A.-H.; Nassar, I.F.; Shaban, A.K.F.; EL-Kady, D.S.; Awad, H.M.; El-Sayed, W.A. Synthesis, Docking Studies into CDK2 and Anticancer Activity of New Derivatives Based Pyrimidine Scaffold and Their Derived Glycosides. Mini Rev. Med. Chem. 2019, 19, 1093–1110. [Google Scholar] [CrossRef] [PubMed]
- Eman, R.K.; Mowafea, A.S.; Hemat, S.K.; Naiera, A.M. Synthesis and Reactions of Some Novel Nicotinonitrile Derivatives for Anticancer and Antimicrobial Evaluation. Acta Chim. Slov. 2009, 56, 908–919. [Google Scholar]
- Nora, M.R.; Sherif, M.E.; Hussein, F.Z. Eco-friendly, green synthesis and antimicrobial evaluation of 4,6-disubstituted-2-(6’-acetyl-O-β-D-glucopyranosyl sulfanyl)-nicotinonitrile. Int. J. Adv. Res. 2014, 2, 355–364. [Google Scholar]
- Ali, G.M.; Ibrahim, D.A.; Elmetwali, A.M.; Ismail, N.S. Design, synthesis and biological evaluation of certain CDK2 inhibitors based on pyrazole and pyrazolo[1,5-a] pyrimidine scaffold with apoptotic activity. Bioorg. Chem. 2019, 86, 1–14. [Google Scholar]
- De Azevedo, W.F.; Leclerc, S.; Meijer, L.; Havlicek, L.; Strnad, M.; Kim, S.H. Inhibition of cyclin-dependent kinases by purine analogues: Crystal structure of human CDK2 complexed with roscovitine. Eur. J. Biochem. 1997, 243, 518–526. [Google Scholar] [CrossRef]
- Adel, S.G.; Jacek, S.; Nasser, S.M.I.; Hanaa, F. Synthesis and QSAR study of novel cytotoxic spiro[3H-indole-3,2’ (1’ H)-pyrrolo[3,4-c]pyrrole]-2,3’,5’ (1H,2’ aH,4’ H)-triones. Eur. J. Med. Chem. 2012, 47, 312–322. [Google Scholar]
- Alminderej, F.M.; Elganzory, H.H.; El-Bayaa, M.N.; Awad, H.M.; Hanem, M.; El-Sayed, W.A. Synthesis and Cytotoxic Activity of New 1,3,4-Thiadiazole Thioglycosides and 1,2,3-Triazolyl-1,3,4-Thiadiazole N-glycosides. Molecules 2019, 24, 3738. [Google Scholar] [CrossRef]
- Emam, A.N.; Samah, A.L.; Mostafa, A.A.; Awad, H.M.; Mohamed, M.B. Cyto-toxicity, biocompatibility and cellular response of carbon dots–plasmonic based nano-hybrids for bio imaging. RSC Adv. 2017, 7, 23502–23514. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; El-Sofany, W.I.; Hussein, H.A.R.; Fathy, N.M. Synthesis and anticancer activity of new [(Indolyl) pyrazolyl]-1,3,4-oxadiazole thioglycosides and acyclic nucleoside analogs. Nucleos. Nucleot. Nucleic Acids. 2017, 36, 474–495. [Google Scholar] [CrossRef]
- Eman, M.F.; El-Sayed, W.A.; Ashraf, M.M.; Walaa, I.E.-S.; Hanem, M.A. Synthesis and Anticancer Activity of New 1-Thia-4-azaspiro[4.5]decane, Their Derived Thiazolopyrimidine and 1,3,4-Thiadiazole Thioglycosides. Molecules 2017, 22, 1–13. [Google Scholar]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Swaminathan, S.; Karplus, M. CHARMM: A program for macromolecular energy minimization and dynamics calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]




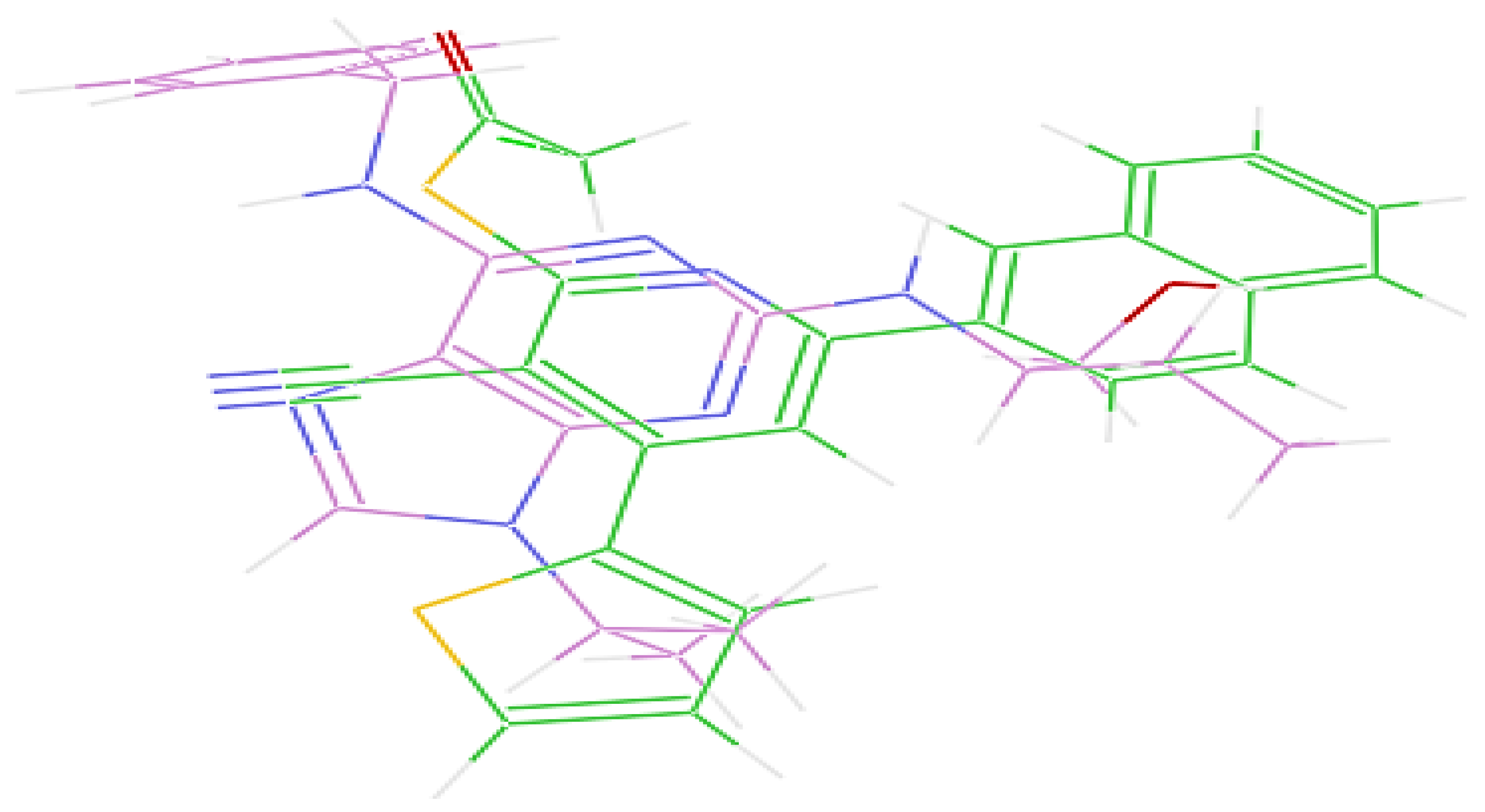
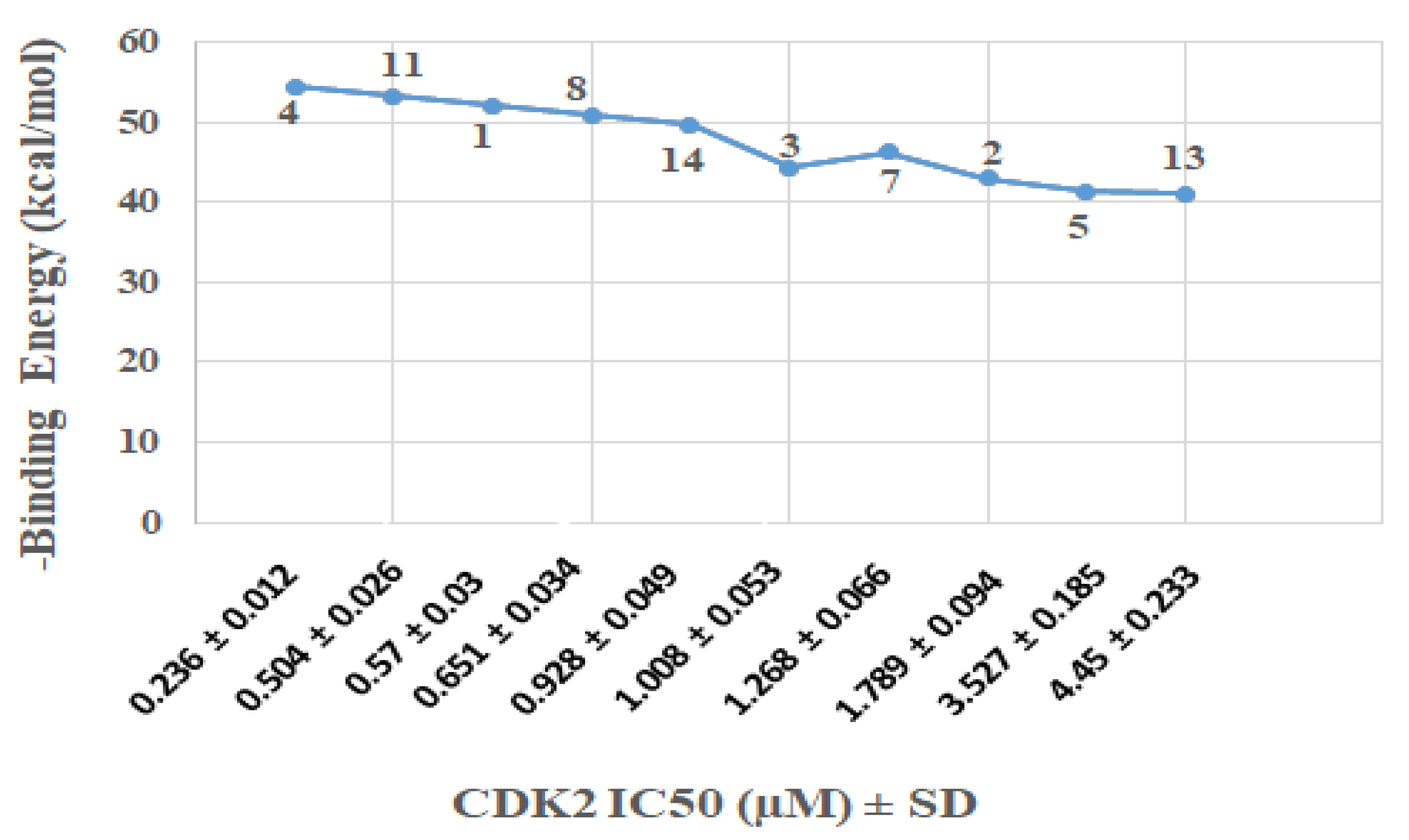
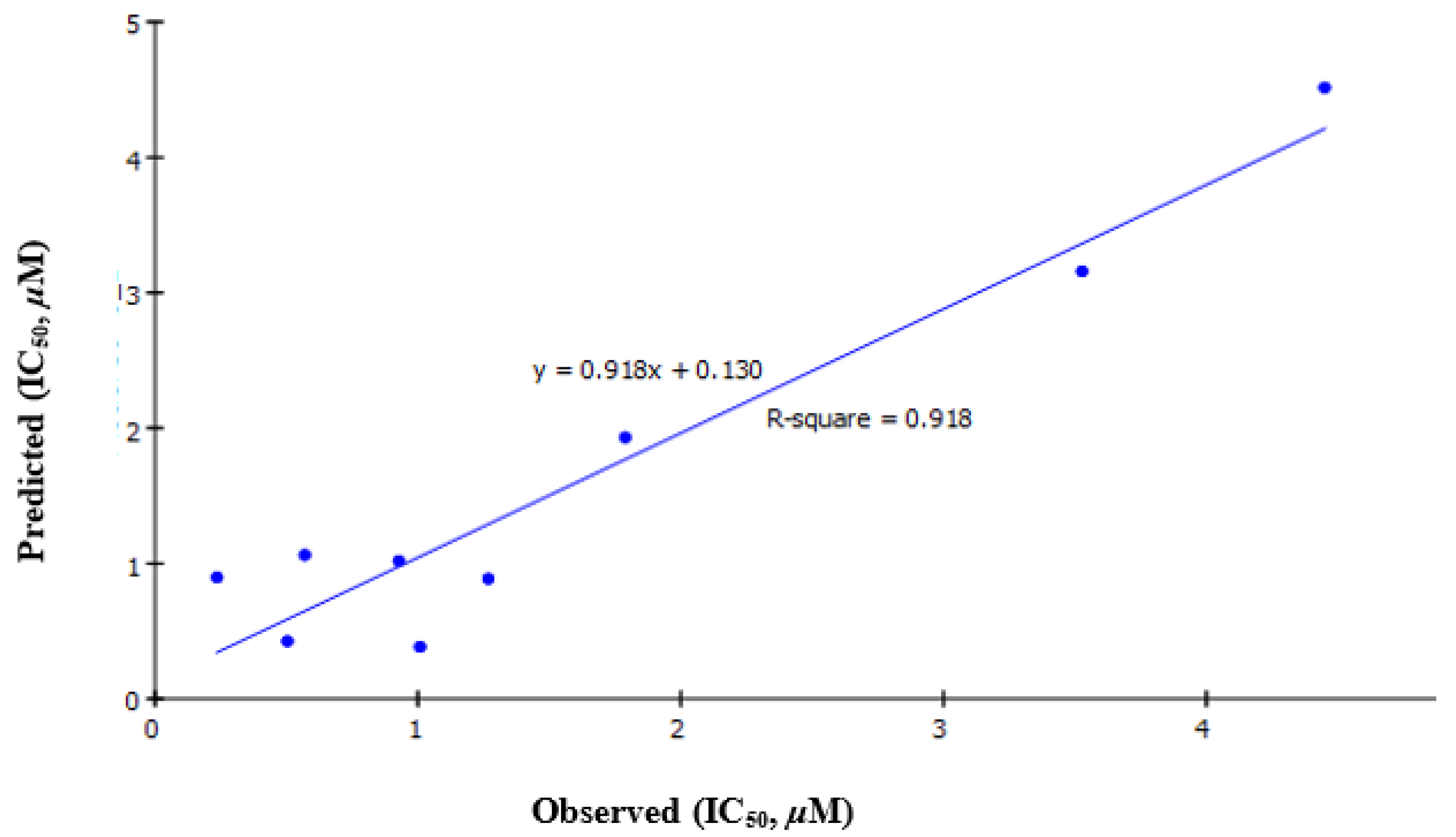
| Entry | Name | Results CDK2 IC50 (µM) ± SD |
|---|---|---|
| 1 | 1 | 0.57 ± 0.1 |
| 2 | 2 | 1.789 ± 0.1 |
| 3 | 3 | 1.008 ± 0.1 |
| 4 | 4 | 0.236 ± 0.1 |
| 5 | 5 | 3.527 ± 0.2 |
| 6 | 7 | 1.268 ± 0.1 |
| 7 | 8 | 0.651 ± 0.1 |
| 8 | 11 | 0.504 ± 0.1 |
| 9 | 13 | 4.45 ± 0.2 |
| 10 | 14 | 0.928 ± 0.1 |
| 11 | Roscovitine | 0.394 ± 0.1 |
| Compound Code | IC50 (µM) ± SD | |||
|---|---|---|---|---|
| HCT-116 | MCF-7 | HepG2 | A549 | |
| 1 | 39.1 ± 4.2 | 47.0 ± 4.5 | 27.9 ± 3.1 | 60.6 ± 4.8 |
| 2 | 43.5 ± 4.5 | 21.3 ± 3.1 | 29.4 ± 3.5 | 70.7 ± 4.1 |
| 3 | 49.0 ± 5.1 | 37.3 ± 4.1 | 44.8 ± 4.1 | 57.4 ± 4.5 |
| 4 | 45.9 ± 4.1 | 46.2 ± 4.1 | 42.9 ± 3.9 | 67.2 ± 5.1 |
| 5 | 33.8 ± 3.5 | 55.5 ± 4.5 | 27.2 ± 3.1 | 47.6 ± 4.5 |
| 7 | 37.8 ± 3.9 | 27.4 ± 2.4 | 22.7 ± 2.9 | 36.8 ± 4.1 |
| 8 | 33.7 ± 3.3 | 19.3 ± 2.1 | 29.1 ± 3.5 | 43.8 ± 4.1 |
| 11 | 31.7 ± 3.5 | 46.4 ± 4.3 | 27.6 ± 3.3 | 56.9 ± 4.6 |
| 13 | 32.4 ± 3.1 | 37.6 ± 3.5 | 30.2 ± 3.7 | 45.9 ± 4.3 |
| 14 | 31.3 ± 3.3 | 26.1 ± 3.9 | 27.9 ± 3.5 | 56.5 ± 4.8 |
| Doxorubicin | 40.0 ± 3.9 | 64.8 ± 4.1 | 24.7 ± 3.2 | 58.1 ± 4.1 |
| Compounds NO. | Binding Energy (K.cal/mol−1) | Hydrogen Bonding Interaction with the Key Amino Acids | Distance A° |
|---|---|---|---|
| 1 | −51.95 | 1 HBD through NH group with Leu83 | 2.84 |
| 1 HBA through an oxygen atom of the carbonyl group with Leu83 | 2.51 | ||
| 2 | −42.93 | 1HBA through an oxygen atom of C=O group with Lys89 | 2.47 |
| 1HBA through nitrogen atom of CN group with Leu83 | 2.91 | ||
| 3 | −44.24 | 1HBA through sulfur atom of thiophene ring with Leu 83 | 2.79 |
| 4 | −54.33 | 2HBA through nitrogen atom of CN group with Leu83 | 2.53 |
| 2.67 | |||
| 5 | −41.36 | 1HBA through nitrogen atom of pyridine with Lys89 | 3.29 |
| 1HBA through nitrogen atom of CN group with Leu83 | 3.45 | ||
| 7 | −46.25 | 1 HBD through NH group with Leu83 | 2.84 |
| 2 HBA through sulfur atom of thione and CN group with Asp89 | 2.69 | ||
| 8 | −50.9 | 1HBD through hydrogen atom of NH group of pyrazole ring with Leu83 | 2.38 |
| 1HBA through nitrogen atom of pyrazole ring with Leu83 | 2.80 | ||
| 1HBD through hydrogen atom of NH2 group with Glu81 | 2.85 | ||
| 11 | −53.17 | 1HBA through nitrogen atom of CN group with Leu83 | 2.54 |
| 2HBA 1HBA through an oxygen atom C=O group with Asp86 | 2.87, 2.95 | ||
| 13 | −41.07 | 1HBA through nitrogen atom of CN group with Leu83 | 2.94 |
| 3.03 | |||
| 14 | −49.69 | 1 HBD through NH2 group with Leu83 | 2.84 |
| 1HBA through oxygen atom of C=O group with Leu83 | 2.87 | ||
| Roscovitine | −55.75 | 1HBD through hydrogen atom of NH group with Leu83 | 2.33 |
| 1HBA through nitrogen atom of Imidazole ring with Leu83 | 2.87 |
| Model | r2 | r2 (Adj) | r2 (Pred) | RMS Residual Error | Friedman L.O.F. | S.O.R. p-Value |
|---|---|---|---|---|---|---|
| IC50 = −1.0859 + 1.294 [Dipole_X] + 0.013735 [Jurs_DPSA_1] | 0.9179 | 0.8905 | 0.8121 | 0.4811 | 0.9889 | 0.0005543 |
| Compound No. | Experimental Activity (IC50/μM) | Predicted Activity (IC50/μM) |
|---|---|---|
| 8 | 0.651 | 1.020 |
| V | 0.743 | 0.947 |
| VI | 0.139 | 0.425 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Rahman, A.A.-H.; Shaban, A.K.F.; Nassar, I.F.; EL-Kady, D.S.; Ismail, N.S.M.; Mahmoud, S.F.; Awad, H.M.; El-Sayed, W.A. Discovery of New Pyrazolopyridine, Furopyridine, and Pyridine Derivatives as CDK2 Inhibitors: Design, Synthesis, Docking Studies, and Anti-Proliferative Activity. Molecules 2021, 26, 3923. https://doi.org/10.3390/molecules26133923
Abdel-Rahman AA-H, Shaban AKF, Nassar IF, EL-Kady DS, Ismail NSM, Mahmoud SF, Awad HM, El-Sayed WA. Discovery of New Pyrazolopyridine, Furopyridine, and Pyridine Derivatives as CDK2 Inhibitors: Design, Synthesis, Docking Studies, and Anti-Proliferative Activity. Molecules. 2021; 26(13):3923. https://doi.org/10.3390/molecules26133923
Chicago/Turabian StyleAbdel-Rahman, Adel A.-H., Amira K. F. Shaban, Ibrahim F. Nassar, Dina S. EL-Kady, Nasser S. M. Ismail, Samy F. Mahmoud, Hanem M. Awad, and Wael A. El-Sayed. 2021. "Discovery of New Pyrazolopyridine, Furopyridine, and Pyridine Derivatives as CDK2 Inhibitors: Design, Synthesis, Docking Studies, and Anti-Proliferative Activity" Molecules 26, no. 13: 3923. https://doi.org/10.3390/molecules26133923
APA StyleAbdel-Rahman, A. A.-H., Shaban, A. K. F., Nassar, I. F., EL-Kady, D. S., Ismail, N. S. M., Mahmoud, S. F., Awad, H. M., & El-Sayed, W. A. (2021). Discovery of New Pyrazolopyridine, Furopyridine, and Pyridine Derivatives as CDK2 Inhibitors: Design, Synthesis, Docking Studies, and Anti-Proliferative Activity. Molecules, 26(13), 3923. https://doi.org/10.3390/molecules26133923






