Novel Pyridyl–Oxazole Carboxamides: Toxicity Assay Determination in Fungi and Zebrafish Embryos
Abstract
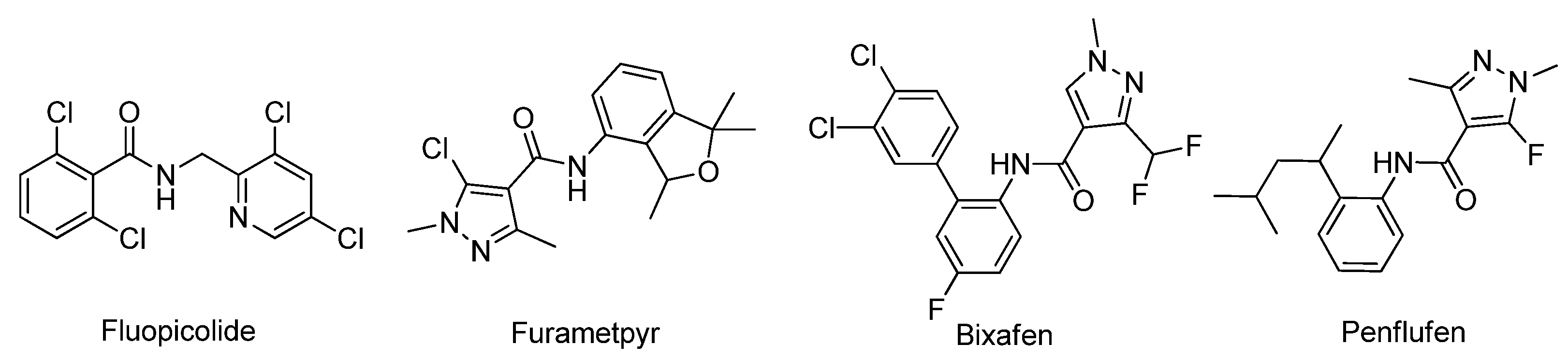
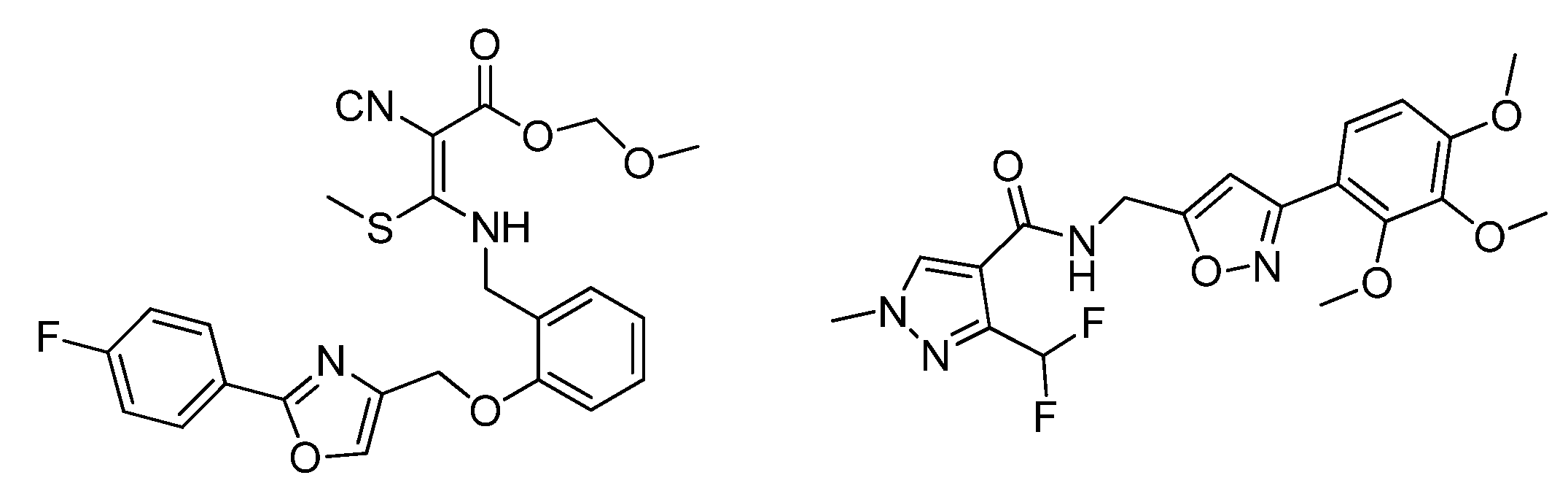
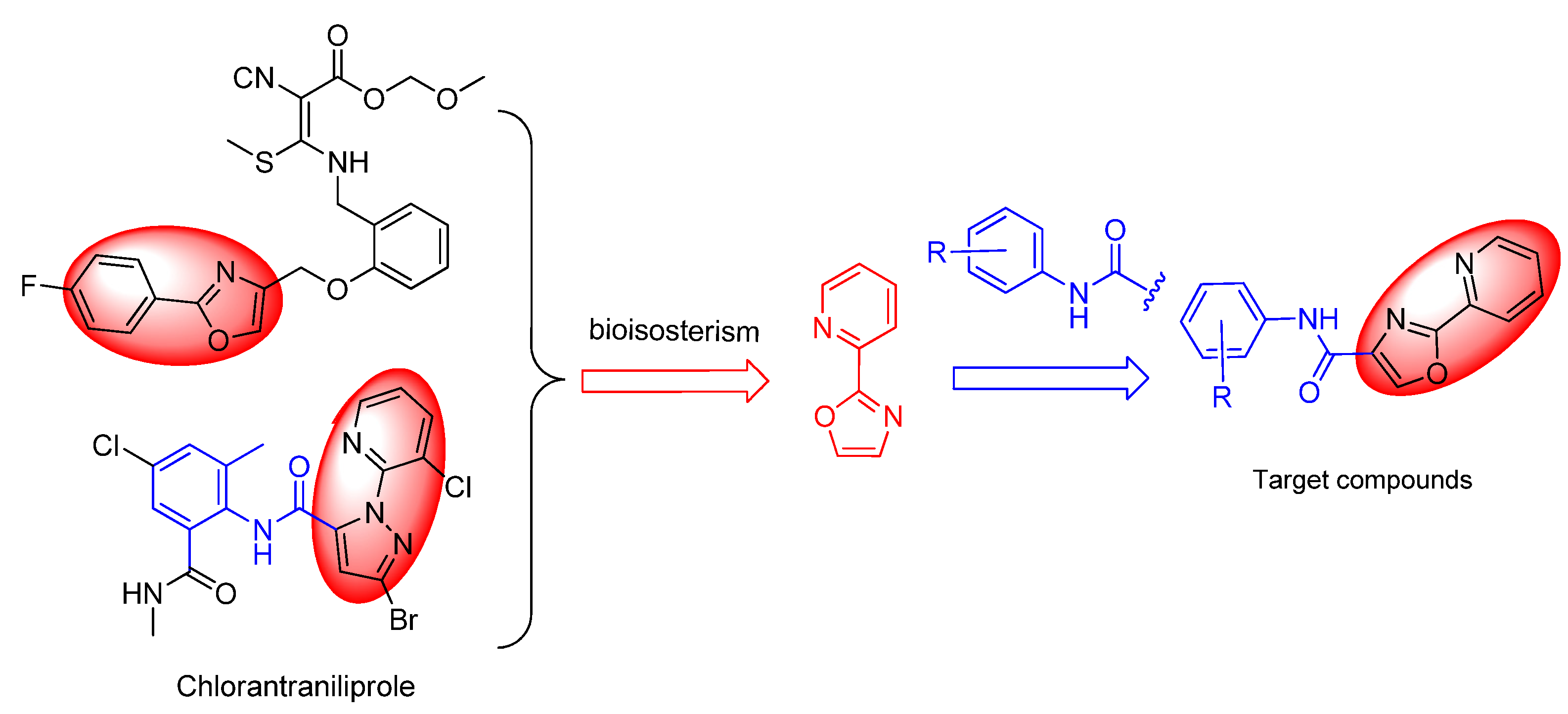
:1. Introduction
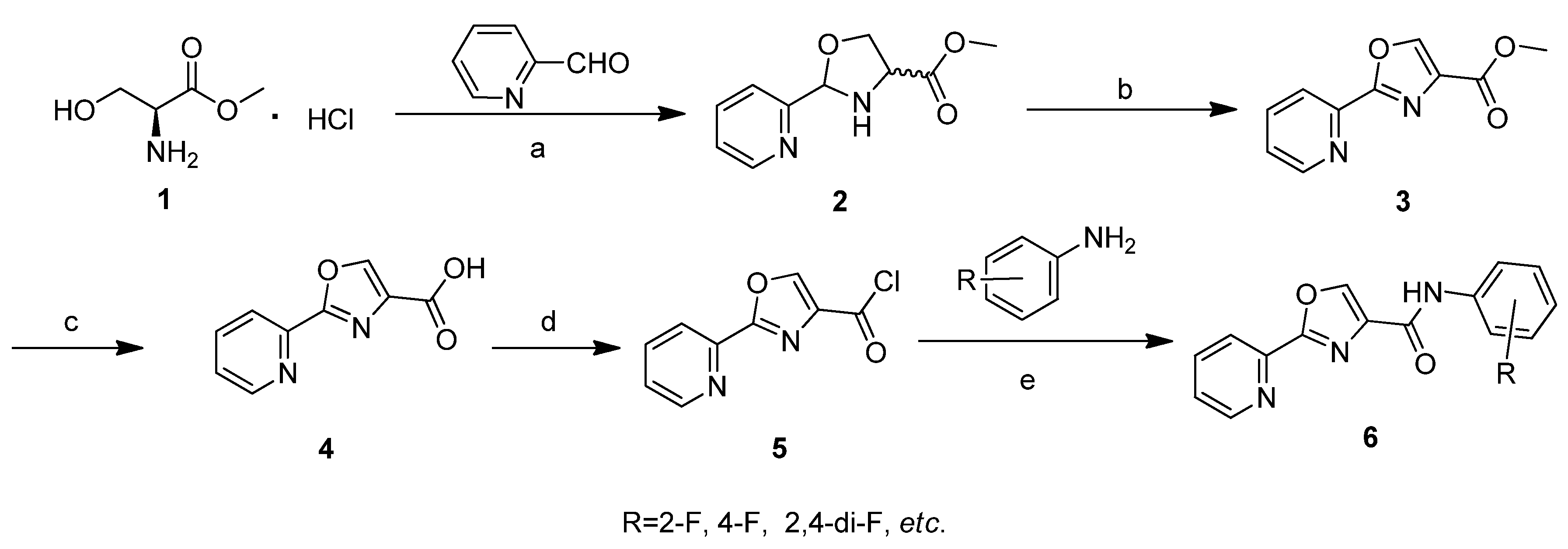
2. Results and Discussion
2.1. Biological Activities of Target Compounds
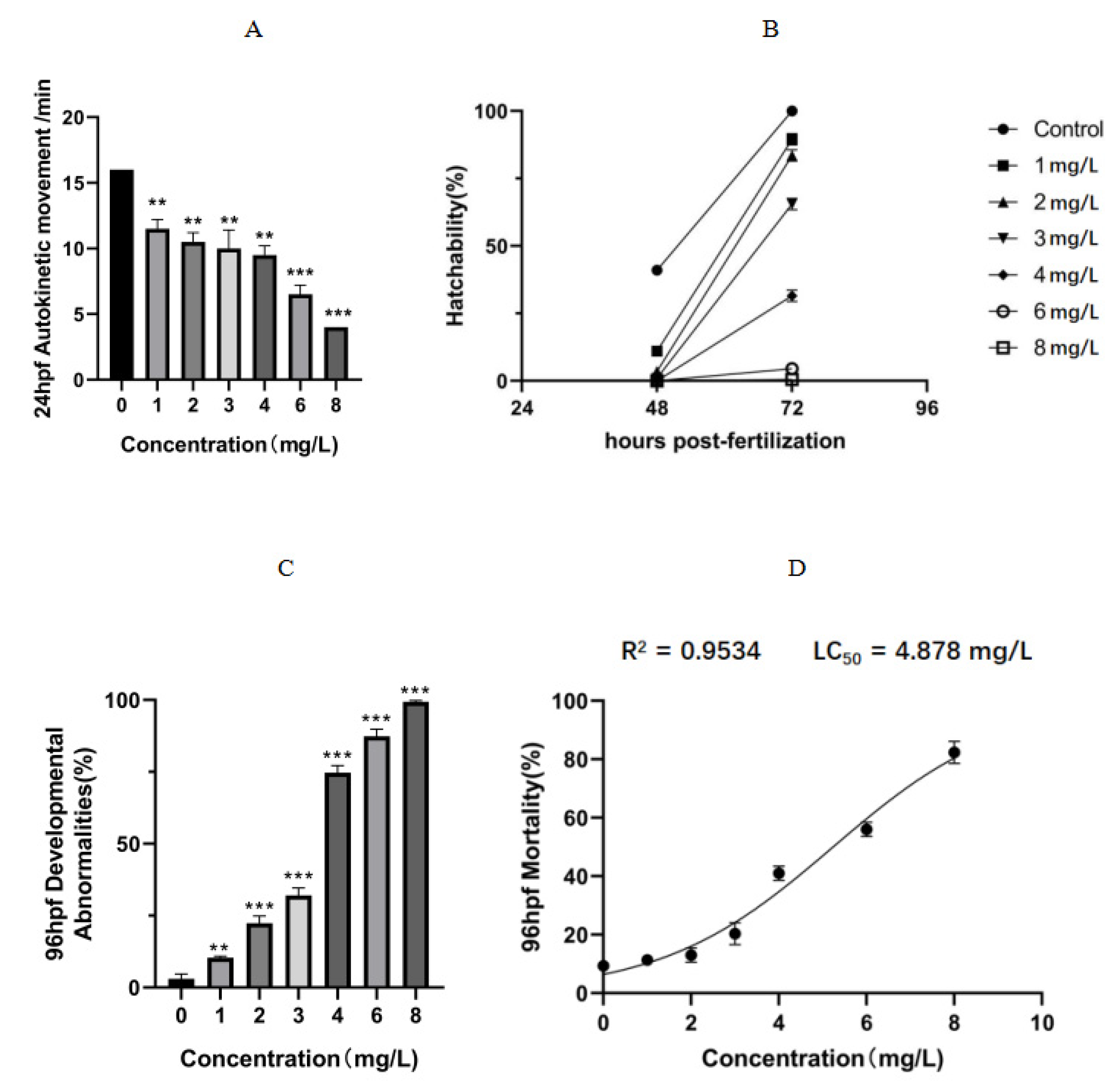
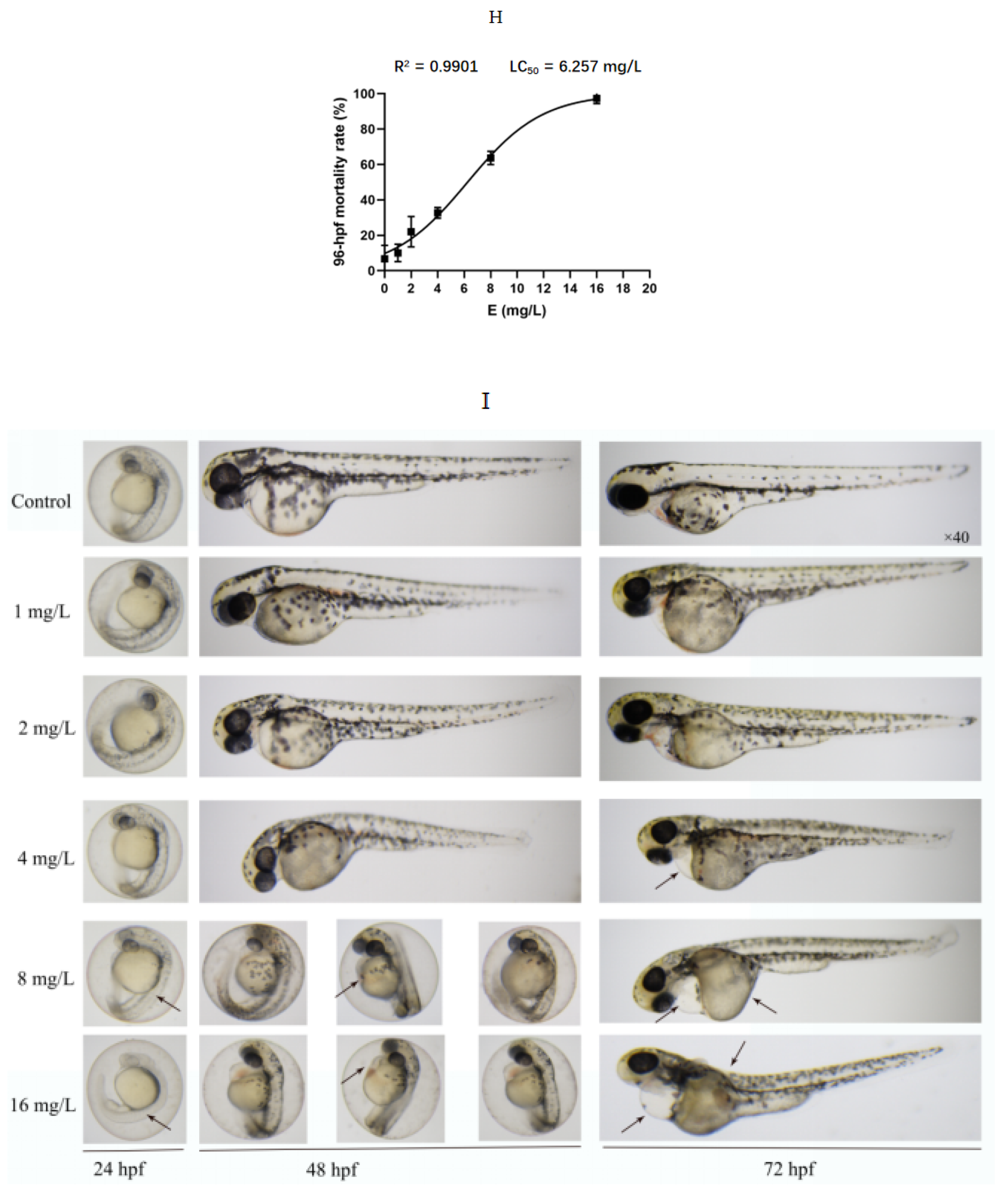
2.2. Toxicity to Zebrafish Embryos
3. Materials and Methods
3.1. Materials
3.2. Fish Husbandry and Embryo Collection
3.3. Ethics Statement
3.4. Fungicidal Activity and Toxicity Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.J.; Xu, Y.Q.; He, J.H.; Yu, H.P.; Huang, C.J.; Gao, J.M.; Dong, Q.X.; Xuan, Y.X.; Li, C.Q. Human cardiotoxic drugs delivered by soaking and microinjection induce cardiovascular toxicity in zebrafifish. J. Appl. Toxicol. 2014, 34, 139–148. [Google Scholar] [CrossRef]
- Fan, C.Y.; Cowden, J.; Simmons, S.O.; Padilla, S.; Ramabhadran, R. Gene expression changes in developing zebrafish as potential markers for rapid developmental neurotoxicity screening. Neurotoxicol. Teratol. 2010, 32, 91–98. [Google Scholar] [CrossRef]
- Ou, H.; Simon, J.A.; Rubel, E.W.; Raible, D.W. Screening for chemicals that affect hair cell death and survival in the zebrafish lateral line. Hear. Res. 2012, 288, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.C.; Guan, A.Y.; Yang, F.; Liu, C.L. Progress of Fungicides in China and Abroad. Modern Agrochem. 2015, 14, 1–9. [Google Scholar]
- Wu, Z.B.; Kuang, J.Q.; Yin, J.; Wu, S.X.; Cai, H. Synthesis and Antifungal Activity of Picolinamide Compounds. Guizhou Agric. Sci. 2013, 41, 93–94. [Google Scholar]
- Kaspady, M.; Narayanaswamy, V.K.; Raju, M.; Rao, G.K. Synthesis, Antibacterial Activity of 2,4-Disubstituted Oxazoles and Thiazoles as Bioisosteres. Lett. Drug Des. Discov. 2009, 6, 21–28. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.Q.; Liu, J.; Yang, X.P.; Liu, Z.J. Stereoselective Synthesis and Antifungal Activities of (E)-α-(Methoxyimino)benzeneacetate Derivatives Containing 1,3,5-Substituted Pyrazole Ring. J. Agric. Food Chem. 2006, 54, 3636–3640. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhuang, H.Y.; Shi, L.; Li, G.; Zhang, H.J.; Fang, Y.; Dai, B.J. Synthesis and Biological Activities of Novel Substituted Pyrazole Oximes Containing Substituted Pyridine Group. Chin. J. Org. Chem. 2015, 35, 2399–2404. [Google Scholar] [CrossRef] [Green Version]
- Dai, H.; Li, Y.Q.; Du, D.; Qin, X.; Zhang, X.; Yu, H.B.; Fang, J.X. Synthesis and biological activities of novel pyrazole oxime derivatives containing a 2-chloro-5-thiazolyl moiety. J. Agric. Food Chem. 2008, 56, 10805–10810. [Google Scholar] [CrossRef]
- Dai, H.; Li, H.; Jin, Z.C.; Liu, W.Y.; Xiao, Y.; He, H.B.; Wang, Q.M.; Shi, Y.J. Synthesis and Bioactivity of Novel 1-Methyl-3-trifluoromethyl-5-subsituent-1H-pyrazole-4-carbaldehydeO-(4-trifluoromethylbenzoyl)oximes. Chin. J. Org. Chem. 2016, 36, 185–190. [Google Scholar] [CrossRef]
- Yang, Y.G.; Meng, S.Q.; Qi, Z.Q.; Ji, M.S.; Li, X.H. Synthesis and fungicidal activity evaluation of 2-thiazolyl amide cyclohexane-sulfonamide. Chin. J. Pestic. Sci. 2018, 20, 287–293. [Google Scholar]
- Wu, J.; Song, B.A.; Kang, S.D.; Yang, S.; Hu, D.Y. The Heterocyclic Amide Derivatives with Herbicidal Activity. Agrochemicals 2012, 51, 625–631. [Google Scholar]
- Liu, X.H.; Zhao, W.; Shen, Z.H.; Xing, J.H.; Yuan, J.; Yang, G.; Xu, T.M.; Peng, W.L. Synthesis, nematocidal activity and docking study of novel chiral 1-(3-chloropyridin-2-yl)-3-(trifluoromethyl)-1H-pyrazole-4-carboxamide derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 3626. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhao, W.; Shen, Z.H.; Xing, J.H.; Xu, T.M.; Peng, W.L. Eur. Synthesis, nematocidal activity and SAR study of novel difluoromethylpyrazole carboxamide derivatives containing flexible alkyl chain moieties. J. Med. Chem. 2017, 125, 881–889. [Google Scholar] [CrossRef]
- Lahm, G.P.; Cordova, D.; Barry, J.D. New and selective ryanodine receptor activators for insect control. Bioorg. Med. Chem. 2009, 17, 4127–4133. [Google Scholar] [CrossRef]
- Ji, W.J.; Xu, T.M.; Zheng, Z.W.; Zhu, B.C.; Li, J.; Hu, W.Q.; Kong, X.L. Synthesis and fungicidal activity of 1-(3-chloropyridin-2-yl)-5-difluoromethyl-1H-pyrazole-4-carboxamide derivatives. Chin. J. Pestic. Sci. 2013, 15, 393–397. [Google Scholar]
- Eryılmaz, S.; Çelikoğlu, E.T.; İdil, Ö.; İnkaya, E.; Kozak, Z.; Mısır, E.; Gül, M. Derivatives of Pyridine and Thiazole Hybrid: Synthesis, DFT, Biological Evaluation via Antimicrobial and DNA Cleavage Activity. Bioorg. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Liu, C.L.; Zhao, W.G.; Li, Z.M. Synthesis and biological activity of multiple substituted pyridines and derivatives. Chin. J. Org. Chem. 2004, 24, 204. [Google Scholar]
- Ouyang, G.P.; Cai, X.J.; Chen, Z.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y.; Zeng, S. Synthesis and antiviral activities of pyrazole derivatives containing an oxime moiety. J. Agric. Food Chem. 2008, 56, 10160–10167. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.Q.; Fu, C.R.; Zou, X.M. Synthesis and Herbicidal Activity of 2-Cyano-3-(2-substituted phenyoxypyridin-5-yl) Amino Acrylates Containing Substituted Phenoxyl Group. Chin. J. Org. Chem. 2015, 35, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.M.; Zhang, Q.Q.; Yue, K.; Li, Q.S.; Xu, F.B. Synthesis and Insecticidal Activity of o-Carboxamidobenzamide Compounds Containing 2-(Substituted phenyl)oxazole Group. Chin. J. Org. Chem. 2017, 37, 1774–1780. [Google Scholar] [CrossRef]
- Gideens, A.C.; Boshoff, H.I.M.; Franzblau, S.G.; Barry, C.E., III; Coppa, B.R. Antimycobacterial natural products: Synthesis and preliminary biological evaluation of the oxazole-containing alkaloid texaline. Tetrahedron Lett. 2005, 46, 7355–7357. [Google Scholar] [CrossRef]
- Prakash, T.B.; Reddy, G.D.; Padmaja, A.; Padmavathi, V. Synthesis and antimicrobial activity of amine linked bis- and tris-heterocycles. Eur. J. Med. Chem. 2014, 82, 347–354. [Google Scholar] [CrossRef]
- Lin, J.; Chen, J.W.; Cai, X.Y.; Qiao, X.L.; Huang, L.P.; Wang, D.G.; Wang, Z. Evolution of Toxicity upon Hydrolysis of Fenoxaprop-p-ethyl. J. Agric. Food Chem. 2007, 55, 7626–7629. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.J.; Du, X.C.; Wang, X.L.; Chen, Q.W.; Li, L.; Dai, H.; Xu, C.Q.; Zhang, J.Y.; Ling, Y. Synthesis and Herbicidal Activity of Novel Cyanoacrylate Derivatives Containing Substituted Oxazole Moiety. Chin. J. Org. Chem. 2018, 38, 1772–1778. [Google Scholar] [CrossRef]
- Liu, T.T.; Ni, Y.; Zhong, L.K.; Huang, H.Y.; Hu, W.Q.; Xu, T.M.; Tan, C.X. Synthesis and Fungicidal Activity of Difluoromethy Substituted Carboxamide Derivatives. Chin. J. Org. Chem. 2015, 35, 422–427. [Google Scholar] [CrossRef]
- Graham, T.H. A Direct Synthesis of Oxazoles from Aldehydes. Org. Lett. 2010, 12, 3614–3617. [Google Scholar] [CrossRef] [PubMed]
- Credico, B.D.; Reginato, G.; Gonsalvi, L.; Peruzzini, M.; Rossin, A. Selective synthesis of 2-substituted 4-carboxy oxazoles, thiazoles and thiazolidines from serine or cysteine amino acids. Tetrahedron 2011, 67, 267–274. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Liu, M.; Liu, J.F.; Wang, X.D.; Wang, C.H.; Ai, W.M.; Chen, S.B.; Wang, H.L. Combined toxicity of triclosan, 2,4-dichlorophenol and 2,4,6-trichlorophenol to zebrafish ( Danio rerio ). Environ. Toxicol. Pharmacol. 2018, 57, 9–18. [Google Scholar] [CrossRef]
- Liu, J.F.; Sun, L.M.; Zhang, H.Q.; Shi, M.R.; Dahlgren, R.A.; Wang, X.D.; Wang, H.L. Response mechanisms to joint exposure of triclosan and its chlorinated derivatives on zebrafish ( Danio rerio ) behavior. Chemosphere 2018, 193, 820–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colwill, R.M.; Creton, R. Locomotor behaviors in zebrafish (Danio rerio) larvae. Behav. Process. 2011, 86, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhu, H.W.; Shang, J.F.; Wang, B.L.; Li, Z.M. Synthesis and Biological Activities of Novel 3-(((3-Bromo1-(3-chloropyridin-2-yl)-1H-pyrazol-5-yl) methylene) amino)-substituted-benzo [d] [1,2,3] triazin-4(3H)-ones. Chin. J. Org. Chem. 2019, 39, 861–866. [Google Scholar] [CrossRef]
- Chen, W.T.; Wang, Q.; Min, L.J.; Wu, H.K.; Weng, J.Q.; Tan, C.X.; Zhang, Y.G.; Hu, B.Z.; Liu, X.H. Synthesis, Crystal Structure, Fungicidal Activity, Molecular Docking, and Density Functional Theory Study of 2-Chloro-N-(p-tolylcarbamoyl) nicotinamide. Indian J. Heterocycl. Chem. 2019, 29, 429–435. [Google Scholar]
- Zhang, Y.; Wang, X.; Yin, X.; Wang, H. Toxicity assessment of combined fluoroquinolone and tetracycline exposure in zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2016, 31, 736–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Compd. | R | Botrytis cinereal | |||
|---|---|---|---|---|---|
| 100 mg/L | 50 mg/L | 25 mg/L | 12.5 mg/L | ||
| 6a | 2-F | 100 | 45.88 | 12.36 | nt |
| 6b | 4-F | 100 | 52.06 | 41.18 | 30.08 |
| 6c | 2,4-di-F | 100 | 63.83 | 40.30 | 29.71 |
| 6d | 2,6-di-F | 100 | 62.06 | 49.41 | 32.94 |
| 6e | 3-Cl | 100 | 66.47 | 14.71 | nt |
| 6f | 3-Cl-2-CH3 | 90 | 65.00 | 44.71 | 17.36 |
| 6g | 4-CH3 | 100 | 41.76 | 7.65 | 3.83 |
| 6h | 2,4-di-CH3 | 100 | 64.41 | 28.83 | 7.06 |
| Azoxystrobin | 100 | 40 | 20 | 0 | |
| Compd. | R | Rhizoctonia solani | |||
|---|---|---|---|---|---|
| 100 mg/L | 50 mg/L | 25 mg/L | 12.5 mg/L | ||
| 6a | 2-F | 90 | 47.06 | 29.31 | 17.69 |
| 6b | 4-F | 100 | 51.77 | 41.71 | 31.77 |
| 6c | 2,4-di-F | 80 | 79.12 | 18.24 | 16.77 |
| 6d | 2,6-di-F | 80 | 50.00 | 42.06 | 20.06 |
| 6e | 3-Cl | 90 | 57.90 | 18.83 | 11.77 |
| 6f | 3-Cl-2-CH3 | 80 | 66.77 | 36.77 | 6.18 |
| 6g | 4-CH3 | 70 | nt | nt | nt |
| 6h | 2,4-di-CH3 | 90 | 55.30 | 34.41 | 4.41 |
| Azoxystrobin | 100 | 60 | 20 | 0 | |
| Compd. | R | 1H-NMR | 13C-NMR | HRMS |
|---|---|---|---|---|
| 6a | 2-F | 1H-NMR (600 MHz, DMSO-d6) δ:9.92 (s, 1H, NH), 8.98 (s, 1H, oxazole-H), 8.77 (d, J = 4.8 Hz, 1H, Py-H), 8.22 (d, J = 7.8 Hz, 1H, Py-H), 8.07 (m, 1H, Py-H), 7.81–7.82 (m, 1H, Ph-H), 7.62–7.63 (m, 1H, Py-H), 7.34–7.35 (m, 1H, Ph-H), 7.27–7.28 (m, 2H, Ph-H) | 13C-NMR (151 MHz, DMSO-d6) δ: 160.4, 158.9, 156.3, 154.6, 150.6, 145.1, 144.5, 138.2, 137.3, 127.2 (d, J = 7.6 Hz), 126.3 (d, J = 26.0 Hz), 125.5 (d, J = 11.9 Hz), 125.0 (d, J = 3.3 Hz), 123.1, 116.2 (d, J = 19.6 Hz) | calcd for C15H11FN3O2 ([M + H]+) 284.0830, found 284.0822 |
| 6b | 4-F | 1H-NMR (600 MHz, DMSO-d6) δ: 10.32 (s, 1H, NH), 8.95 (s, 1H, oxazole-H), 8.77 (d, J = 4.8 Hz, 1H, Py-H), 8.23 (d, J = 8.0 Hz, 1H, Py-H), 8.07 (td, J = 8.0, 1.5 Hz, 1H, Py-H), 7.84–7.87 (m, 2H, Ph-H), 7.61–7.62 (m, 1H, Py-H), 7.21 (t, J = 9.0 Hz, 2H, Ph-H) | 13C-NMR (151 MHz, DMSO-d6) δ: 160.28, 159.79, 158.97, 158.19, 150.58, 145.25, 144.44, 138.18, 137.87, 135.10, 126.28, 123.01, 123.0, 122.9, 115.70 (d, J = 22.1 Hz) | calcd for C15H11FN3O2 ([M + H]+) 284.0830 found 284.0820 |
| 6c | 2,4-di-F | 1H-NMR (500 MHz, DMSO-d6) δ: 10.06 (s, 1H, NH), 8.97 (s, 1H, oxazole-H), 8.77 (d, J = 5.0, 1H, Py-H), 8.21 (d, J = 8.0 Hz, 1H, Py-H), 8.06–8.07 (m, 1H, Py-H), 7.71–7.72 (m, 1H, Ph-H), 7.61 (q, J = 3.0 Hz, 1H, Py-H), 7.39–7.40 (m, 1H, Ph-H), 7.14 (t, J = 4.5 Hz, 1H, Ph-H) | 13C-NMR (126 MHz, DMSO-d6) δ: 159.87, 158.57, 150.09, 144.68, 144.02, 137.70, 136.73, 127.60 (d, J = 11.0 Hz), 125.81, 122.55, 111.33 (d, J = 3.4 Hz), 111.15 (d, J = 3.4 Hz), 104.54, 104.33 (d, J = 2.8 Hz), 104.13 | calcd for C15H10F2N3O2 ([M + H]+) 302.0736 found 302.0731 |
| 6d | 2,6-di-F | 1H-NMR (600 MHz, DMSO-d6) δ: 10.03 (s, 1H, NH), 8.97 (s, 1H, oxazole-H), 8.77–8.79 (m, 1H, Py-H), 8.21 (d, J = 7.8 Hz, 1H, Py-H), 8.06–8.07 (m, 1H, Py-H), 7.72–7.73 (m, 1H, Ph-H), 7.61–7.63 (m, 1H, Py-H), 7.39–7.41 (m, 1H, Ph-H), 7.15–7.16 (m, 1H, Ph-H) | 13C-NMR (151 MHz, DMSO-d6) δ: 160.4, 159.1, 150.6, 145.2, 144.5, 138.2, 137.2, 128.2 (d, J = 9.5 Hz), 126.3, 123.1, 111.7 (dd, J = 22.1, 3.5 Hz), 104.8 (dd, J = 26.6, 24.2 Hz) | calcd for C15H10F2N3O2 ([M + H]+) 302.0736, found 302.0726 |
| 6e | 3-Cl | 1H-NMR (600 MHz, DMSO-d6) δ: 10.44 (s, 1H, NH), 8.98 (s, 1H, oxazole-H), 8.77 (d, J = 4.8 Hz, 1H, Py-H), 8.24 (d, J = 7.8 Hz, 1H, Py-H), 8.07–8.08 (m, 1H, Py-H), 8.03–8.04 (m, 1H, Ph-H), 7.80 (d, J = 8.2, 2.4 Hz, 1H, Ph-H), 7.62–7.63 (m, 1H, Py-H), 7.40 (t, J = 8.2 Hz, 1H, Ph-H), 7.19–7.20 (m, 1H, Ph-H) | 13C-NMR (126 MHz, DMSO) δ: 159.8, 158.8, 150.1, 144.7, 144.2, 139.8, 137.7, 137.2, 132.9, 130.3, 125.8, 123.0, 122.6, 120.0, 118.9 | calcd for C15H11ClN3O2 ([M + H]+) 300.0534, found 300.0522 |
| 6f | 3-Cl-2-CH3 | 1H-NMR (500 MHz, DMSO-d6) δ: 10.15 (s, 1H, NH), 8.95 (s, 1H, oxazole-H), 8.77 (d, J = 5.0 Hz, 1H, Py-H), 8.22 (d, J = 7.8 Hz, 1H, Py-H), 8.07–8.08 (m, 1H, Py-H), 7.62–7.63 (m, 1H, Py-H), 7.43 (dd, J = 7.8, 1.2 Hz, 1H, Ph-H), 7.38–7.39 (m, 1H, Ph-H), 7.26 (t, J = 7.8 Hz, 1H, Ph-H), 2.28 (s, 3H, CH3) | 13C-NMR (126 MHz, DMSO) δ: 160.3, 159.1, 150.6, 145.2, 144.4, 138.2, 137.6, 137.6, 134.2, 132.0, 127.4, 127.2, 126.3, 125.7, 123.0, 15.7 | calcd for C16H13ClN3O2 ([M + H]+) 314.0691, found 314.0690 |
| 6g | 4-CH3 | 1H-NMR (600 MHz, DMSO-d6) δ: 10.12 (s, 1H, NH), 8.92 (s, 1H, oxazole-H), 8.76 (d, J = 4.2 Hz, 1H, Py-H), 8.24 (d, J = 7.8 Hz, 1H, Py-H), 8.06–8.07 (m, 1H, Py-H), 7.70 (d, J = 8.4 Hz, 2H, Ph-H), 7.60–7.61 (m, 1H, Py-H), 7.17 (d, J = 17.2 Hz, 2H, Ph-H), 2.29 (s, 3H, CH3) | 13C-NMR (151 MHz, DMSO-d6) δ: 160.2, 158.8, 150.6, 145.3, 144.2, 138.2, 138.0, 136.2, 133.5, 129.5, 126.3, 123.1, 121.0, 21.0 | calcd for C16H14N3O2 ([M + H]+) 280.1081, found 280.1065 |
| 6h | 2. 4-di-CH3 | 1H-NMR (500 MHz, DMSO-d6) δ: 9.73 (s, 1H, NH), 8.91 (s, 1H, oxazole-H), 8.76–8.78 (m, 1H, Py-H), 8.22 (d, J = 8.0 Hz, 1H, Py-H), 8.06–8.08 (m, 1H, Py-H), 7.61–7.63 (m, 1H, Py-H), 7.36–7.37 (m, 1H, Ph-H), 7.10 (d, J = 2.0 Hz, 1H, Ph-H), 7.03 (d, J = 8.0 Hz, 1H, Ph-H), 2.29 (s, 3H, CH3), 2.23 (s, 3H, CH3) | 13C-NMR (126 MHz, CDCl3) δ: 159.8, 158.3, 150.1, 144.8, 143.4, 137.6, 137.4, 134.9, 132.9, 132.2, 130.9, 126.6, 125.7, 125.3, 20.5, 17.6 | calcd for C17H16N3O2 ([M + H]+) 294.1237, found 294.1226 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zhang, D.-L.; Ren, C.-L.; Zou, W.-Q.; Tian, X.-Y.; Du, X.-H.; Tan, C.-X. Novel Pyridyl–Oxazole Carboxamides: Toxicity Assay Determination in Fungi and Zebrafish Embryos. Molecules 2021, 26, 3883. https://doi.org/10.3390/molecules26133883
Chen S, Zhang D-L, Ren C-L, Zou W-Q, Tian X-Y, Du X-H, Tan C-X. Novel Pyridyl–Oxazole Carboxamides: Toxicity Assay Determination in Fungi and Zebrafish Embryos. Molecules. 2021; 26(13):3883. https://doi.org/10.3390/molecules26133883
Chicago/Turabian StyleChen, Shu, Dong-Lin Zhang, Chao-Li Ren, Wen-Qian Zou, Xiao-Yu Tian, Xiao-Hua Du, and Cheng-Xia Tan. 2021. "Novel Pyridyl–Oxazole Carboxamides: Toxicity Assay Determination in Fungi and Zebrafish Embryos" Molecules 26, no. 13: 3883. https://doi.org/10.3390/molecules26133883
APA StyleChen, S., Zhang, D.-L., Ren, C.-L., Zou, W.-Q., Tian, X.-Y., Du, X.-H., & Tan, C.-X. (2021). Novel Pyridyl–Oxazole Carboxamides: Toxicity Assay Determination in Fungi and Zebrafish Embryos. Molecules, 26(13), 3883. https://doi.org/10.3390/molecules26133883






