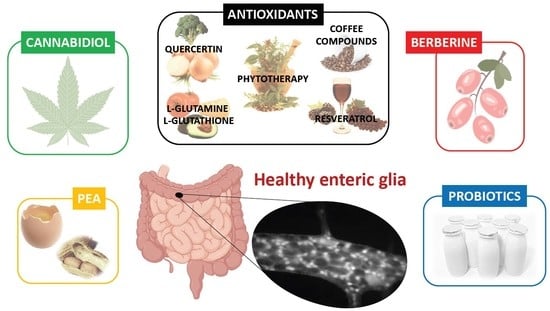
Nutraceuticals and Enteric Glial Cells
Abstract
1. Introduction
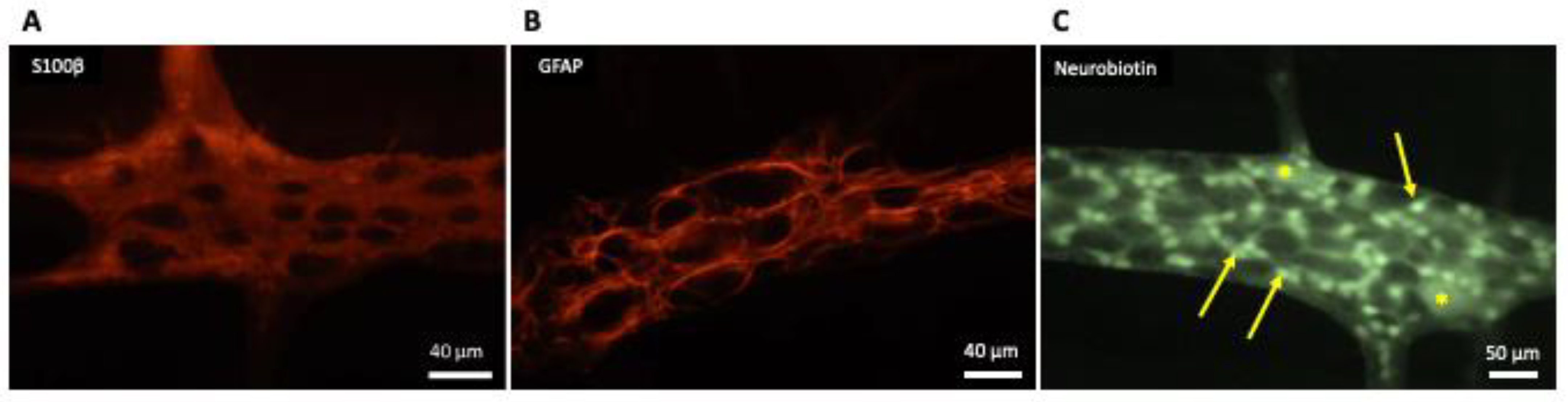
2. Enteric Glial Cells
2.1. Cellular and Tissular Roles of EGCs
2.2. Physiological Changes in the Population of EGCs
2.3. Role of EGCs in GI Pathophysiology
2.3.1. Intestinal Inflammation
2.3.2. Chronic Constipation
2.3.3. Postoperative Ileus
2.3.4. Irritable Bowel Syndrome
2.3.5. EGC and Pathophysiology Outer the GI Tract
2.4. Effects of Nutraceuticals on Enteric Glial Cells
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 15d-PGJ2 | 15-deoxy-Δ12,14-prostaglandin J2 | |
| ATP | adenosin triphosphate | |
| B.b. | Bifidobacterium bifidum | |
| BDNF | brain derived neurotrophic factor | |
| B.f. | Bacteroides fragilis | |
| BR1 | bradykinin receptor 1 | |
| Ca2+ | calcium | |
| CA | caffeic acid | |
| CBD | cannabidiol | |
| CGA | chlorogenic acid | |
| CD | Crohn’s disease | |
| CD44 | membrane glycoprotein | |
| CNS | central nervous system | |
| COX-2 | cyclo-oxygenase-2 | |
| Cx43 | connexin 43 | |
| DRG | dorsal root ganglion, dorsal root ganglia | |
| DSS | dextran sodium sulfate | |
| EGC | enteric glial cell | |
| ELISA | enzyme linked immunosorbent assay | |
| ENS | enteric nervous system | |
| ET-B | endothelin-1 receptor B | |
| GABA | gamma amino butyric acid | |
| GAT2 | GABA transporter | |
| GDNF | glial cell-derived neurotrophic factor | |
| GFAP | glial fibrillary acidic protein | |
| GI | gastrointestinal | |
| GSH | glutathione | |
| HA | haemagglutinin | |
| HIV | human immunodeficiency virus | |
| IBD | inflammatory bowel disease | |
| IBS-C | constipation-predominant irritable bowel syndrome | |
| IBS-D | diarrhea-irritable bowel syndrome | |
| IBS-M | mixed irritable bowel syndrome | |
| IBS | irritable bowel syndrome | |
| IFN | interferon | |
| IL | interleukin | |
| IL-1R | receptor for interleukin 1 | |
| iNOS | inducible isoform of nitric oxide synthase | |
| I/R | ischemia-reperfusion | |
| IR | immunoreactive | |
| LPS | lipopolysaccharide | |
| M-CSF | macrophage colony-stimulating factor | |
| MCP1 | monocyte chemotactic protein 1 | |
| MHC | major histocompatibility complex | |
| mRNA | messenger ribonucleic acid | |
| MT | metallothionein | |
| NF-κB | nuclear factor kappa B | |
| NGF | nerve growth factor | |
| NLRP | nod-like receptor family, pyrin domain-containing | |
| NO | nitric oxide | |
| NrF2 | nuclear factor erythroid-derived 2-like 2 | |
| NT-3 | neurotrophin-3 | |
| P2 | purinergic receptor 2 | |
| PCR | polymerase-chain reaction | |
| PEA | palmitoylethanolamide | |
| PD | Parkinson’s disease | |
| PNS | peripheral nervous system | |
| PI-IBS | post-infectious irritable bowel syndrome | |
| PKC | protein kinase C | |
| POI | postoperative ileus | |
| PPAR | peroxisome proliferator-activated receptor | |
| Pro-EGF | pro-epidermal growth factor | |
| Ran-2 | rat neural antigen-2 | |
| ROS | reactive oxygen species | |
| SP | substance P | |
| STZ | streptozotocin-induced | |
| TACE | TNF-α converting enzyme | |
| TGF | transforming growth factor | |
| TLR | Toll-like receptor | |
| TNF | tumor necrosis factor | |
| Trk | tropomyosin receptor kinase | |
| UC | ulcerative colitis |
References
- Seguella, L.; Gulbransen, B.D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol. 2021. Available online: https://www.nature.com/articles/s41575-021-00423-7 (accessed on 15 March 2021). [CrossRef]
- Furness, J. The Enteric Nervous System, 1st ed.; Blackwell Publishing: Hoboken, NJ, USA, 2007. [Google Scholar]
- Hanani, M.; Reichenbach, A. Morphology of horseradish peroxidase (HRP)-injected glial cells in the myenteric plexus of the guinea-pig. Cell Tissue Res. 1994, 278, 153–160. [Google Scholar] [CrossRef]
- Ferri, G.L.; Probert, L.; Cocchia, D.; Michetti, F.; Marangos, P.J.; Polak, J.M. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature 1982, 297, 409–410. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature 1980, 286, 736–737. [Google Scholar] [CrossRef]
- Grundmann, D.; Loris, E.; Maas-Omlor, S.; Huang, W.; Scheller, A.; Kirchhoff, F.; Schafer, K.H. Enteric Glia: S100, GFAP, and Beyond. Anat Rec. 2019, 302, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2017, 18, 942–952. [Google Scholar] [CrossRef]
- Eddleston, M.; Mucke, L. Molecular profile of reactive astrocytes-implications for their role in neurologic disease. Neuroscience 1993, 54, 15–36. [Google Scholar] [CrossRef]
- Hanani, M.; Zamir, O.; Baluk, P. Glial cells in the guinea pig myenteric plexus are dye coupled. Brain Res. 1989, 497, 245–249. [Google Scholar] [CrossRef]
- Ochoa-Cortes, F.; Turco, F.; Linan-Rico, A.; Soghomonyan, S.; Whitaker, E.; Wehner, S.; Cuomo, R.; Christofi, F.L. Enteric Glial Cells: A New Frontier in Neurogastroenterology and Clinical Target for Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2016, 22, 433–449. [Google Scholar] [CrossRef] [PubMed]
- McClain, J.L.; Grubišić, V.; Fried, D.; Gomez-Suarez, R.A.; Leinninger, G.M.; Sévigny, J.; Parpura, V.; Gulbransen, B.D. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 2014, 146, 497–507.e1. [Google Scholar] [CrossRef]
- Bornstein, J.C. Purinergic mechanisms in the control of gastrointestinal motility. Purinergic Signal. 2008, 4, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Vanderwinden, J.M.; Timmermans, J.P.; Schiffmann, S.N. Glial cells, but not interstitial cells, express P2X7, an ionotropic purinergic receptor, in rat gastrointestinal musculature. Cell Tissue Res. 2003, 312, 149–154. [Google Scholar] [CrossRef]
- Kimball, B.C.; Mulholland, M.W. Enteric Glia Exhibit P2U Receptors that Increase Cytosolic Calcium by a Phospholipase C-Dependent Mechanism. J. Neurochem. 2002, 66, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Van Nassauw, L.; Costagliola, A.; den Bosch, J.V.O.; Cecio, A.; Vanderwinden, J.M.; Burnstock, G.; Timmermans, J.P. Region-specific distribution of the P2Y4 receptor in enteric glial cells and interstitial cells of Cajal within the guinea-pig gastrointestinal tract. Auton. Neurosci. Basic Clin. 2006, 126–127, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Nasser, Y.; Keenan, C.M.; Ma, A.C.; McCafferty, D.M.; Sharkey, K.A. Expression of a functional metabotropic glutamate receptor 5 on enteric glia is altered in states of inflammation. Glia 2007, 55, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Giaroni, C.; Zanetti, E.; Chiaravalli, A.M.; Albarello, L.; Dominioni, L.; Capella, C.; Lecchini, S.; Frigo, G. Evidence for a glutamatergic modulation of the cholinergic function in the human enteric nervous system via NMDA receptors. Eur. J. Pharmacol. 2003, 476, 63–69. [Google Scholar] [CrossRef]
- Fletcher, E.L.; Clark, M.J.; Furness, J.B. Neuronal and glial localization of GABA transporter immunoreactivity in the myenteric plexus. Cell Tissue Res. 2002, 308, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Galligan, J.J.; Lepard, K.J.; Schneider, D.A.; Zhou, X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J. Auton. Nerv. Syst. 2000, 81, 97–103. [Google Scholar] [CrossRef]
- Eiko, A.; Semba, R.; Kashiwamata, S. Evidence for the presence of l-arginine in the glial components of the peripheral nervous system. Brain Res. 1991, 559, 159–162. [Google Scholar] [CrossRef]
- Nagahama, M.; Semba, R.; Tsuzuki, M.; Aoki, E. L-arginine immunoreactive enteric glial cells in the enteric nervous system of rat ileum. Neuro Signals 2001, 10, 336–340. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. Astrocyte-like glia in the peripheral nervous system: An immunohistochemical study of enteric glia. J. Neurosci. 1983, 3, 2206–2218. [Google Scholar] [CrossRef]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Turco, F.; Steardo, L.; Cuomo, R. S100B protein in the gut: The evidence for enteroglial sustained intestinal inflammation. World J. Gastroenterol. 2011, 17, 1261–1266. [Google Scholar] [CrossRef]
- Bradley, J.S.; Parr, E.J.; Sharkey, K.A. Effects of inflammation on cell proliferation in the myenteric plexus of the guinea-pig ileum. Cell Tissue Res. 1997, 289, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Pochard, C.; Coquenlorge, S.; Freyssinet, M.; Naveilhan, P.; Bourreille, A.; Neunlist, M.; Rolli-Derkinderen, M. The multiple faces of inflammatory enteric glial cells: Is Crohn’s disease a gliopathy? Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G1–G11. [Google Scholar] [CrossRef]
- Von Boyen, G.B.T.; Steinkamp, M.; Reinshagen, M.; Schäfer, K.-H.; Adler, G.; Kirsch, J. Nerve Growth Factor Secretion in Cultured Enteric Glia Cells is Modulated by Proinflammatory Cytokines. J. Neuroendocrinol. 2006, 18, 820–825. [Google Scholar] [CrossRef] [PubMed]
- von Georg, B.T.; Nadine, D.; Christoph, H.; Guido Adler, M.S. The endothelin axis influences enteric glia cell functions. Med. Sci Monit. 2010, 16, 161–167. [Google Scholar]
- Esposito, G.; Capoccia, E.; Turco, F.; Palumbo, I.; Lu, J.; Steardo, A.; Cuomo, R.; Sarnelli, G.; Steardo, L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut 2014, 63, 1300–1312. [Google Scholar] [CrossRef]
- Murakami, M.; Ohta, T.; Ito, S. Interleukin-1β enhances the action of bradykinin in rat myenteric neurons through up-regulation of glial B1 receptor expression. Neuroscience 2008, 151, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Grosso, M.; Petruzzelli, R.; Izzo, P.; Cali, G.; D’Armiento, F.P.; Rocco, A.; Nardone, G.; et al. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol. Motil. 2009, 21, 1209-e112. [Google Scholar] [CrossRef]
- Von Boyen, G.B.T.; Steinkamp, M.; Reinshagen, M.; Schäfer, K.H.; Adler, G.; Kirsch, J. Proinflammatory cytokines increase glial fibrillary acidic protein expression in enteric glia. Gut 2004, 53, 222–228. [Google Scholar] [CrossRef]
- Cirillo, C.; Sarnelli, G.; Turco, F.; Mango, A.; Grosso, M.; Aprea, G.; Masone, S.; Cuomo, R. Proinflammatory stimuli activates human-derived enteroglial cells and induces autocrine nitric oxide production. Neurogastroenterol. Motil. 2011, 23, e372–e382. [Google Scholar] [CrossRef]
- Rosenbaum, C.; Schick, M.A.; Wollborn, J.; Heider, A.; Scholz, C.J.; Cecil, A.; Niesler, B.; Hirrlinger, J.; Walles, H.; Metzger, M. Activation of myenteric glia during acute inflammation in vitro and in vivo. PLoS ONE 2016, 11, e0151335. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.J.; Pachnis, V. Development of the enteric nervous system: Bringing together cells, signals and genes. Neurogastroenterol. Motil. 2009, 21, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Steinkamp, M.; Geerling, I.; Reinshagen, M.; Schäfer, K.; Adler, G.; Kirsch, J. Proinflammatory cytokines induce neurotrophic factor expression in enteric glia: A key to the regulation of epithelial apoptosis in Crohn’s disease. Inflamm. Bowel Dis. 2006, 12, 346–354. [Google Scholar]
- Sharkey, K.A. Emerging roles for enteric glia in gastrointestinal disorders. J. Clin. Investig. 2015, 125, 918–925. [Google Scholar] [CrossRef]
- Liu, Y.A.; Chung, Y.C.; Pan, S.T.; Shen, M.Y.; Hou, Y.C.; Peng, S.J.; Pasricha, P.J.; Tang, S.C. 3-D imaging, illustration, and quantitation of enteric glial network in transparent human colon mucosa. Neurogastroenterol. Motil. 2013, 25, 324–338. [Google Scholar] [CrossRef]
- Steinkamp, M.; Geerling, I.; Seufferlein, T.; von Boyen, G.; Egger, B.; Grossmann, J.; Ludwig, L.; Adler, G.; Reinshagen, M. Glial-derived neurotrophic factor regulates apoptosis in colonic epithelial cells. Gastroenterology 2003, 124, 1748–1757. [Google Scholar] [CrossRef]
- Tanaka, F.; Tominaga, K.; Fujikawa, Y.; Nagami, Y.; Komata, N.; Yamagami, H.; Tanigawa, T.; Shiba, M.; Watanabe, T.; Fujiwara, Y.; et al. Concentration of Glial Cell Line-Derived neurotrophic Factor Positively Correlates with Symptoms in Functional Dyspepsia. Dig. Dis Sci. 2016, 61, 3478–3485. [Google Scholar] [CrossRef]
- Van Landeghem, L.; Chevalier, J.; Mahé, M.M.; Wedel, T.; Urvil, P.; Derkinderen, P.; Savidge, T.; Neunlist, M. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G976. [Google Scholar] [CrossRef]
- Savidge, T.C.; Newman, P.; Pothoulakis, C.; Ruhl, A.; Neunlinst, M.; Bourreille, A.; Hurst, R.; Sofroniew, M.V. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 2007, 132, 1344–1358. [Google Scholar] [CrossRef]
- Bach-Ngohou, K.; Mahé, M.M.; Aubert, P.; Abdo, H.; Boni, S.; Bourreille, A.; Denis, M.G.; Lardeux, B.; Neunlist, M.; Masson, D. Enteric glia modulate epithelial cell proliferation and differentiation through 15-deoxy-Δ12,14-prostaglandin J2. J. Physiol. 2010, 588, 2533–2544. [Google Scholar] [CrossRef]
- Flamant, M.; Aubert, P.; Rolli-Derkinderen, M.; Bourreille, A.; Neunlist, M.R.; Mahé, M.M.; Meurette, G.; Marteyn, B.; Savidge, T.; Galmiche, J.P.; et al. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: A role for S-nitrosoglutathione. Gut 2011, 60, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Rico, A.L.; Grants, I.; Needleman, B.J.; Williams, K.C.; Soghomonyan, S.; Turco, F.; Cuomo, R.; Grider, J.R.; Kendig, D.M.; Murthy, K.S.; et al. Gliomodulation of Neuronal and Motor Behavior in the Human GI Tract. Gastroenterology 2015, 148, S-18. [Google Scholar] [CrossRef]
- Aubé, A.C.; Cabarrocas, J.; Bauer, J.; Philippe, D.; Aubert, P.; Doulay, F.; Liblau, R.; Galmiche, J.P.; Neunlist, M. Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption. Gut 2006, 55, 630–637. [Google Scholar] [CrossRef]
- Nasser, Y.; Fernandez, E.; Keenan, C.M.; Ho, W.; Oland, L.D.; Tibbles, L.A.; Schemann, M.; MacNaughton, W.K.; Ruhl, A.; Sharkey, K.A. Role of enteric glia in intestinal physiology: Effects of the gliotoxin fluorocitrate on motor and secretory function. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G912–G927. [Google Scholar] [CrossRef]
- Morales-Soto, W.; Gulbransen, B.D. Enteric Glia: A New Player in Abdominal Pain. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Grubišić, V.; McClain, J.L.; Fried, D.E.; Grants, I.; Rajasekhar, P.; Csizmadia, E.; Ajijola, O.; Watson, R.E.; Poole, D.P.; Robson, S.C.; et al. Enteric Glia Modulate Macrophage Phenotype and Visceral Sensitivity following Inflammation. Cell Rep. 2020, 32, 108100. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.J.; Kieffer, E.J.; Powley, T.L. Loss of glia and neurons in the myenteric plexus of the aged Fischer 344 rat. Anat Embryol. 2004, 209, 19–30. [Google Scholar] [CrossRef]
- Stenkamp-Strahm, C.; Patterson, S.; Boren, J.; Gericke, M.; Balemba, O. High-fat diet and age-dependent effects on enteric glial cell populations of mouse small intestine. Auton. Neurosci. Basic Clin. 2013, 177, 199–210. [Google Scholar] [CrossRef]
- Baudry, C.; Reichardt, F.; Marchix, J.; Bado, A.; Schemann, M.; des Varannes, S.B.; Neunlist, M.; Moriez, R. Diet-induced obesity has neuroprotective effects in murine gastric enteric nervous system: Involvement of leptin and glial cell line-derived neurotrophic factor. J. Physiol. 2012, 590, 533–544. [Google Scholar] [CrossRef]
- Voss, U.; Sand, E.; Olde, B.; Ekblad, E. Enteric neuropathy can be induced by high fat diet in vivo and palmitic acid exposure in vitro. PLoS ONE 2013, 8, e81413. [Google Scholar] [CrossRef]
- Schoffen, J.P.F.; Santi Rampazzo, A.P.; Cirilo, C.P.; Zapater, M.C.U.; Vicentini, F.A.; Comar, J.F.; Bracht, A.; Marcal Natali, M.R. Food restriction enhances oxidative status in aging rats with neuroprotective effects on myenteric neuron populations in the proximal colon. Exp. Gerontol. 2014, 51, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Bush, T.G.; Savidge, T.C.; Freeman, T.C.; Cox, H.J.; Campbell, E.A.; Mucke, L.; Johnson, M.H.; Sofroniew, M.V. Fulminant jejuno-ileitis following ablation of enteric gila in adult transgenic mice. Cell 1998, 93, 189–201. [Google Scholar] [CrossRef]
- Cornet, A.; Savidge, T.C.; Cabarrocas, J.; Deng, W.L.; Colombel, J.F.; Lassmann, H.; Desreumaux, P.; Liblau, R.S. Enterocolitis induced by autoimmune targeting of enteric glial cells: A possible mechanism in Crohn’s disease? Proc. Natl. Acad. Sci. USA 2001, 98, 13306–13311. [Google Scholar] [CrossRef]
- Von Boyen, G.B.T.; Schulte, N.; Pflüger, C.; Spaniol, U.; Hartmann, C.; Steinkamp, M. Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol. 2011, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Villanacci, V.; Bassotti, G.; Nascimbeni, R.; Antonelli, E.; Cadei, M.; Fisogni, S.; Salerni, B.; Geboes, K. Enteric nervous system abnormalities in inflammatory bowel diseases. Neurogastroenterol. Motil. 2008, 20, 1009–1016. [Google Scholar] [CrossRef]
- Zhang, D.K.; He, F.Q.; Li, T.K.; Pang, X.H.; Cui, D.J.; Xie, Q.; Huang, L.H.; Gan, H.T. Glial-derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis. J. Pathol. 2010, 222, 213–222. [Google Scholar] [CrossRef]
- Hoff, S.; Zeller, F.; von Weyhern, C.W.H.; Wegner, M.; Schemann, M.; Klaus, M.; Ruhl, A. Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. J. Comp. Neurol. 2008, 509, 356–371. [Google Scholar] [CrossRef]
- Coquenlorge, S.; Van Landeghem, L.; Jaulin, J.; Cenac, N.; Vergnolle, N.; Duchalais, E.; Neunlist, M.; Rolli-Derkinderen, M. The arachidonic acid metabolite 11β-ProstaglandinF2α controls intestinal epithelial healing: Deficiency in patients with Crohn’s disease. Sci. Rep. 2016, 6, 25203. [Google Scholar] [CrossRef] [PubMed]
- Geboes, K.; Rutgeerts, P.; Ectors, N.; Mebis, J.; Penninckx, F.; Vantrappen, G.; Desmet, V.J. Major histocompatibility class II expression on the small intestinal nervous system in Crohn’s disease. Gastroenterology 1992, 103, 439–447. [Google Scholar] [CrossRef]
- Koretz, K.; Momburg, F.; Otto, H.F.; Möller, P. Sequential induction of MHC antigens on autochthonous cells of ileum affected by Crohn’s disease. Am. J. Pathol. 1987, 129, 493–502. [Google Scholar]
- Turco, F.; Sarnelli, G.; Cirillo, C.; Palumbo, I.; De Giorgi, F.; D’Alessandro, A.; Cammarota, M.; Giuliano, M.; Cuomo, R. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut 2014, 63, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Villanacci, V.; Cathomas, G.; Maurer, C.A.; Fisogni, S.; Cadei, M.; Baron, L.; Morelli, A.; Valloncini, E.; Salerni, B. Enteric neuropathology of the terminal ileum in patients with intractable slow-transit constipation. Hum. Pathol. 2006, 37, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Villanacci, V.; Maurer, C.A.; Fisogni, S.; Di Fabio, F.; Cadei, M.; Morelli, A.; Panagiotis, T.; Cathomas, G.; Salerni, B. The role of glial cells and apoptosis of enteric neurones in the neuropathology of intractable slow transit constipation. Gut 2006, 55, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Villanacci, V.; Nascimbeni, R.; Asteria, C.R.; Fisogni, S.; Nesi, G.; Legrenzi, L.; Mariano, M.; Tonelli, F.; Morelli, A.; et al. Colonic neuropathological aspects in patients with intractable constipation due to obstructed defecation. Mod. Pathol. 2007, 20, 367–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iantorno, G.; Bassotti, G.; Kogan, Z.; Lumi, C.M.; Cabanne, A.M.; Fisogni, S.; Varrica, L.M.; Bilder, C.R.; Munoz, J.P.; Liserre, B.; et al. The Enteric Nervous System in Chagasic and Idiopathic Megacolon. Am. J. Surg. Pathol. 2007, 31, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, B.; Hupa, K.J.; Snoek, S.A.; Van Bree, S.; Stein, K.; Schwandt, T.; Vilz, T.O.; Lysson, M.; Van’t Veer, C.; Kummer, M.P.; et al. Postoperative ileus involves interleukin-1 receptor signaling in enteric glia. Gastroenterology 2014, 146, 176–187.e1. [Google Scholar] [CrossRef]
- Lilli, N.L.; Quénéhervé, L.; Haddara, S.; Brochard, C.; Aubert, P.; Rolli-Derkinderen, M.; Durand, T.; Naveilhan, P.; Hardouin, J.B.; De Giorgio, R.; et al. Glioplasticity in irritable bowel syndrome. Neurogastroenterol. Motil. 2018, 30, e13232. [Google Scholar] [CrossRef]
- Jackson, M.; Olefson, S.; MacHan, J.T.; Kelly, C.R. A high rate of alternative diagnoses in patients referred for presumed clostridium difficile infection. J. Clin. Gastroenterol. 2016, 50, 742–746. [Google Scholar] [CrossRef]
- Klem, F.; Wadhwa, A.; Prokop, L.J.; Sundt, W.J.; Farrugia, G.; Camilleri, M.; Singh, S.; Grover, M. Prevalence, Risk Factors, and Outcomes of Irritable Bowel Syndrome After Infectious Enteritis: A Systematic Review and Meta-analysis. Gastroenterology 2017, 152, 1042–1054.e1. [Google Scholar] [CrossRef] [PubMed]
- Swan, C.; Duroudier, N.P.; Campbell, E.; Zaitoun, A.; Hastings, M.; Dukes, G.E.; Cox, J.; Kelly, F.M.; Wilde, J.; Lennon, M.G.; et al. Identifying and testing candidate genetic polymorphisms in the irritable bowel syndrome (IBS): Association with TNFSF15 and TNFα. Gut 2013, 62, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R.C.; Jenkins, D.; Thornley, J.P.; Hebden, J.M.; Wright, T.; Skinner, M.; Neal, K. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000, 47, 804–811. [Google Scholar] [CrossRef]
- Fettucciari, K.; Ponsini, P.; Gioè, D.; Macchioni, L.; Palumbo, C.; Antonelli, E.; Coaccioli, S.; Villanacci, V.; Corazzi, L.; Marconi, P.; et al. Enteric glial cells are susceptible to Clostridium difficile toxin B. Cell Mol. Life Sci. 2017, 74, 1527–1551. [Google Scholar] [CrossRef] [PubMed]
- Bassotti, G.; Macchioni, L.; Corazzi, L.; Marconi, P.; Fettucciari, K. Clostridium difficile-related postinfectious IBS: A case of enteroglial microbiological stalking and/or the solution of a conundrum? Cell Mol. Life Sci. 2018, 75, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Zuo, X.L.; Zhao, Q.J.; Chen, F.X.; Yang, J.; Dong, Y.Y.; Wang, P.; Li, Y.Q. Brain-derived neurotrophic factor contributes to abdominal pain in irritable bowel syndrome. Gut 2012, 61, 685–694. [Google Scholar] [CrossRef]
- Hoehner, J.C.; Wester, T.; Pahlman, S.; Olsen, L. Localization of neurotrophins and their high-affinity receptors during human enteric nervous system development. Gastroenterology 1996, 110, 756–767. [Google Scholar] [CrossRef]
- Wang, P.; Du, C.; Chen, F.X.; Li, C.Q.; Yu, Y.B.; Han, T.; Akhtar, S.; Zuo, Z.L.; Di Tan, X.; Li, Y.-Q. BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci. Rep. 2016, 6, 20320. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; Biagi, E.; Heilig, H.G.H.J.; Kajander, K.; Kekkonen, R.A.; Tims, S.; de Vos, V.M. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1792–1801. [Google Scholar] [CrossRef]
- Jeffery, I.B.; O’Toole, P.W.; Ohman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M.M.; Simren, M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012, 61, 997–1006. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.E.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable Carbohydrate Restriction Reduces Luminal Bifidobacteria and Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef]
- Long, X.; Li, M.; Li, L.-X.; Sun, Y.-Y.; Zhang, W.-X.; Zhao, D.-Y.; Li, Y.Q. Butyrate promotes visceral hypersensitivity in an IBS-like model via enteric glial cell-derived nerve growth factor. Neurogastroenterol. Motil. 2018, 30, e13227. [Google Scholar] [CrossRef] [PubMed]
- Devos, D.; Lebouvier, T.; Lardeux, B.; Biraud, M.; Rouaud, T.; Pouclet, H.; Coron, E.; des Varannes, S.B.; Naveilhan, P.; Nguyen, J.-M.; et al. Colonic inflammation in Parkinson’s disease. Neurobiol Dis. 2013, 50, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Clairembault, T.; Kamphuis, W.; Leclair-Visonneau, L.; Rolli-Derkinderen, M.; Coron, E.; Neunlist, M.; Hol, E.M.; Derkinderen, P. Enteric GFAP expression and phosphorylation in Parkinson’s disease. J. Neurochem. 2014, 130, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.R.S.; Arantes, C.P.; Muras, A.G.; Nomizo, R.; Brentani, R.R.; Martins, V.R. Cellular prion protein expression in astrocytes modulates neuronal survival and differentiation. J. Neurochem. 2007, 103, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Ferrucci, M.; Lazzeri, G.; Paparelli, A.; Fornai, F. Transmission of prions within the gut and toward the central nervous system. Prion 2011, 5, 142–149. [Google Scholar] [CrossRef]
- Kujala, P.; Raymond, C.R.; Romeijn, M.; Godsave, S.F.; van Kasteren, S.I.; Wille, H.; Prusiner, S.B.; Mabbott, N.A.; Peters, P.J. Prion uptake in the gut: Identification of the first uptake and replication sites. PLoS Pathog. 2011, 7, 1002449. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Liu, D.; Li, C.; He, W.-X.; Zhang, C.-L.; Chang, M.-J. Enteric glial cell activation protects enteric neurons from damage due to diabetes in part via the promotion of neurotrophic factor release. Neurogastroenterol. Motil. 2018, 30, e13368. [Google Scholar] [CrossRef]
- Piovezana Bossolani, G.D.; Silva, B.T.; Colombo Martins Perles, J.V.; Lima, M.M.; Vieira Frez, F.C.; Garcia de Souza, S.R.; Sehaber-Sierakowski, C.C.; Bersani-Amado, C.A.; Zanoni, J.N. Rheumatoid arthritis induces enteric neurodegeneration and jejunal inflammation, and quercetin promotes neuroprotective and anti-inflammatory actions. Life Sci. 2019, 238, 116956. [Google Scholar] [CrossRef]
- Panizzon, C.P. do N.B.; Zanoni, J.N.; Hermes-Uliana, C.; Trevizan, A.R.; Sehaber, C.C.; Pereira, R.V.F.; Linden, D.R.; Hubner, M.; Neto, M. Desired and side effects of the supplementation with L-glutamine and L-glutathione in enteric glia of diabetic rats. Acta Histochem. 2016, 118, 625–631. [Google Scholar] [CrossRef]
- Pereira, R.V.F.; Tronchini, E.A.; Tashima, C.M.; Alves, E.P.B.; Lima, M.M.H.; Zanoni, J.N. L-glutamine supplementation prevents myenteric neuron loss and has gliatrophic effects in the ileum of diabetic rats. Dig. Dis Sci. 2011, 56, 3507–3516. [Google Scholar] [CrossRef]
- Do Nascimento Bonato Panizzon, C.P.; De Miranda Neto, M.H.; Ramalho, F.V.; Longhini, R.; De Mello, J.C.P.; Zanoni, J.N. Ethyl acetate fraction from Trichilia catigua confers partial neuroprotection in components of the enteric innervation of the jejunum in diabetic rats. Cell Physiol. Biochem. 2019, 53, 76–86. [Google Scholar]
- De Santi-Rampazzo, A.P.; Schoffen, J.P.F.; Cirilo, C.P.; Zapater, M.C.V.U.; Vicentini, F.A.; Soares, A.A.; Peralta, R.M.; Bracht, A.; Buttow, N.C.; Natali, M.R.M. Aqueous extract of Agaricus blazei Murrill prevents age-related changes in the myenteric plexus of the jejunum in rats. Evid. Based Complement. Alternat Med. 2015, 2015, 287153. [Google Scholar]
- Almeida, P.P.D.; Thomasi, B.B.D.M.; Costa, N.D.S.; Valdetaro, L.; Pereira, A.D.A.; Gomes, A.L.T.; Stockler-Pinto, M.B. Brazil Nut (Bertholletia excelsa H.B.K) Retards Gastric Emptying and Modulates Enteric Glial Cells in a Dose-Dependent Manner. J. Am. Coll Nutr. 2020, 1–9. [Google Scholar] [CrossRef]
- Abdo, H.; Mahé, M.M.; Derkinderen, P.; Bach-Ngohou, K.; Neunlist, M.; Lardeux, B. The omega-6 fatty acid derivative 15-deoxy-Δ 12,14 -prostaglandin J2 is involved in neuroprotection by enteric glial cells against oxidative stress. J. Physiol. 2012, 590, 2739–2750. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, I.; Isooka, N.; Wada, K.; Kikuoka, R.; Kitamura, Y.; Asanuma, M. Effects of Enteric Environmental Modification by Coffee Components on Neurodegeneration in Rotenone-Treated Mice. Cells 2019, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.E.B.; Lopes, C.R.P.; Alves, A.M.P.; Alves, É.P.B.; Linden, D.R.; Zanoni, J.N.; Buttow, N.C. Diabetic neuropathy: An evaluation of the use of quercetin in the cecum of rats. World J. Gastroenterol. 2013, 19, 6416–6426. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.R.P.; Ferreira, P.E.B.; Zanoni, J.N.; Alves, A.M.P.; Alves, É.P.B.; Buttow, N.C. Neuroprotective effect of quercetin on the duodenum enteric nervous system of streptozotocin-induced diabetic rats. Dig. Dis. Sci. 2012, 57, 3106–3115. [Google Scholar] [CrossRef]
- De Souza, S.R.G.; de Neto, M.H.; Perles, J.V.C.M.; Frez, F.C.V.; Zignani, I.; Ramalho, F.V.; Hermes-Uliana, C.; Piovezana Bossolani, G.D.; Zanoni, J.N. Antioxidant effects of the quercetin in the jejunal myenteric innervation of diabetic rats. Front. Med. 2017, 4, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferreira, P.E.B.; Beraldi, E.J.; Borges, S.C.; Natali, M.R.M.; Buttow, N.C. Resveratrol promotes neuroprotection and attenuates oxidative and nitrosative stress in the small intestine in diabetic rats. Biomed. Pharmacother. 2018, 105, 724–733. [Google Scholar] [CrossRef]
- Borges, S.C.; da Silva de Souza, A.C.; Beraldi, E.J.; Schneider, L.C.L.; Buttow, N.C. Resveratrol promotes myenteric neuroprotection in the ileum of rats after ischemia-reperfusion injury. Life Sci. 2016, 166, 54–59. [Google Scholar] [CrossRef]
- Borges, S.C.; Ferreira, P.E.B.; da Silva, L.M.; de Paula Werner, M.F.; Irache, J.M.; Cavalcanti, O.A.; Buttow, N.C. Evaluation of the treatment with resveratrol-loaded nanoparticles in intestinal injury model caused by ischemia and reperfusion. Toxicology 2018, 396–397, 13–22. [Google Scholar] [CrossRef]
- Sarnelli, G.; Seguella, L.; Pesce, M.; Lu, J.; Gigli, S.; Bruzzese, E.; Lattanzi, R.; D’Alessandro, A.; Cuomo, R.; Steardo, L.; et al. HIV-1 Tat-induced diarrhea is improved by the PPARalpha agonist, palmitoylethanolamide, by suppressing the activation of enteric glia. J. Neuroinflammation. 2018, 15, 94. [Google Scholar] [CrossRef]
- De Filippis, D.; Esposito, G.; Cirillo, C.; Cipriano, M.; De Winter, B.Y.; Scuderi, C.; Sarnelli, G.; Cuomo, R.; Steardo, L.; De Man, J.G.; et al. Cannabidiol Reduces Intestinal Inflammation through the Control of Neuroimmune Axis. Gaetani S, editor. PLoS ONE 2011, 6, e28159. [Google Scholar] [CrossRef]
- Li, H.; Fan, C.; Lu, H.; Feng, C.; He, P.; Yang, X.; Xiang, C.; Zuo, J.; Tang, W. Protective role of berberine on ulcerative colitis through modulating enteric glial cells–intestinal epithelial cells–immune cells interactions. Acta Pharm Sin. B 2020, 10, 447–461. [Google Scholar] [CrossRef]
- Hosseinifard, E.S.; Morshedi, M.; Bavafa-Valenlia, K.; Saghafi-Asl, M. The novel insight into anti-inflammatory and anxiolytic effects of psychobiotics in diabetic rats: Possible link between gut microbiota and brain regions. Eur J. Nutr. 2019, 58, 3361–3375. [Google Scholar] [CrossRef] [PubMed]
- Di Giancamillo, A.; Vitari, F.; Bosi, G.; Savoini, G.; Domeneghini, C. The chemical code of porcine enteric neurons and the number of enteric glial cells are altered by dietary probiotics. Neurogastroenterol. Motil. 2010, 22, e271-8. [Google Scholar] [CrossRef]
- Yang, P.C.; Li, X.J.; Yang, Y.H.; Qian, W.; Li, S.Y.; Yan, C.H.; Wang, J.; Wang, Q.; Hou, X.H.; Dai, C.B. The Influence of Bifidobacterium bifidum and Bacteroides fragilis on Enteric Glial Cell–Derived Neurotrophic Factors and Inflammasome. Inflammation 2020, 43, 2166–2177. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Almeida, L.M.; Dinis, T.C.P. Dietary polyphenols: A novel strategy to modulate microbiota-gut-brain axis. Trends Food Sci. Technol. 2018, 78, 224–233. [Google Scholar] [CrossRef]
- Kessler, M.; Ubeaud, G.; Jung, L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J. Pharm Pharmacol. 2003, 55, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, G.H. Quercetin accumulation by chronic administration causes the caspase-3 activation in liver and brain of mice. Biofactors 2010, 36, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Sibaev, A.; Yüce, B.; Kemmer, M.; Van Nassauw, L.; Broedl, U.; Allescher, H.D.; Goke, B.; Timmermans, J.P.; Storr, M. Cannabinoid-1 (CB1) receptors regulate colonic propulsion by acting at motor neurons within the ascending motor pathways in mouse colon. Am. J. Physiol. Gastrointest Liver Physiol. 2009, 296, G119–G128. [Google Scholar] [CrossRef]
- Stanzani, A.; Galiazzo, G.; Giancola, F.; Tagliavia, C.; De Silva, M.; Pietra, M.; Fracassi, F.; Chiocchetti, R. Localization of cannabinoid and cannabinoid related receptors in the cat gastrointestinal tract. Histochem Cell Biol. 2020, 153, 339–356. [Google Scholar] [CrossRef]
- Galiazzo, G.; Giancola, F.; Stanzani, A.; Fracassi, F.; Bernardini, C.; Forni, M.; Pietra, M.; Chiocchetti, R. Localization of cannabinoid receptors CB1, CB2, GPR55, and PPARα in the canine gastrointestinal tract. Histochem Cell Biol. 2018, 150, 187–205. [Google Scholar] [CrossRef]
- Maselli, D.B.; Camilleri, M. Pharmacology, Clinical Effects, and Therapeutic Potential of Cannabinoids for Gastrointestinal and Liver Diseases. Clin. Gastroenterol. Hepatol. 2020, S1542–S3565, 30504–30508. [Google Scholar] [CrossRef] [PubMed]
- Inglet, S.; Winter, B.; Yost, S.E.; Entringer, S.; Lian, A.; Biksacky, M.; Pitt, R.D.; Mortensen, W. Clinical Data for the Use of Cannabis-Based Treatments: A Comprehensive Review of the Literature. Ann. Pharmacother. 2020, 54, 1109–1143. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; Del Castillo, M.D.; Abalo, R. Cannabidiol and Other Non-Psychoactive Cannabinoids for Prevention and Treatment of Gastrointestinal Disorders: Useful Nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
| Type | Morphology | Location | |
|---|---|---|---|
| Type I Protoplasmic EGCs | Star-shaped cells with short, irregularly branched processes |  | Intraganglionic (enteric ganglia) |
| Type II Fibrous EGCs | Elongated glia with branches |  | Within interganglionic fiber tracts |
| Type III Mucosal EGCs | Long branched processes |  | Extraganglionic: subepithelial glia |
| Type IV Intermuscular EGCs | Elongated glia |  | Extraganglionic: accompanying the nerve fibers and encircling the smooth muscles |
| Feature | Enteric Glial Cells | Astrocytes |
|---|---|---|
| Morphology | Irregularly branched processes | In vivo: numerous processes forming well-delineated bushy territories In culture: few processes, polygonal fibroblast-like shape Astrocytes show structural plasticity: their morphology differs between brain areas, and it may be changed (stellation or process growth) by different stimuli |
| Subtypes | Protoplasmic Fibrous Mucosal Intermuscular | Protoplasmic Fibrous |
| Location | Enteric nervous system (submucosal and myenteric plexus) | Central nervous system |
| Identification | GFAP Calcium-binding protein S100β Transcription factors (SOX8, SOX9, SOX10) | GFAP Calcium-binding protein S100β Glutamine synthase CD44 Vimentin Ran-2 Astrocytes from different brain regions can exhibit pronounced molecular differences |
| Adjacent cell coupling | Gap junction coupling | Gap junction coupling |
| Activation | Release of pro-inflammatory cytokines (i.e., IL-1β, TNF-α) Increased expression of c-fos, TrkA, ET-B, TLR-4, BR1 Enhanced expression of glial cell markers | Release of pro-inflammatory cytokines (i.e., IL-1β, IL-6, TNF-α, TGF-β) Increased expression of adhesion-related molecules (CD44) Increased expression of receptors for EGF, TNF-α Enhanced expression of glial cell markers: GFAP, vimentin, nestin |
| Involved in | Physiopathological modulation of GI functions | Development and plasticity of dendritic spines and synapses Elimination of dendritic spines, synapse formation Regulation of neurotransmission and plasticity |
| Nutraceutical (Pathology) | Characteristics | Source | Effects | System | References |
|---|---|---|---|---|---|
| L-glutamine (Diabetes) | Amino acid | Protein-rich foods Available as a dietary supplement | Antioxidant | Wistar rats | [90,91] |
| L-glutathione (Diabetes) | Tripeptide | Supply of the raw nutritional materials used to generate GSH, such as cysteine and glycine | Antioxidant | Wistar rats | [90] |
| Phytotherapy (Aging, diabetes) | Procyanidin B2, epicatechin Glutamate, polyphenols Selenium, ellagic acid | Trichilia catigua Agaricus blazei Murrill, Bertholletia excelsa H.B., (plants/fungi extracts and foods) | Antioxidant | Wistar rats | [92,93,94] |
| 15d-PGJ2 | Omega-6 fatty acid metabolite | Many sources (including B. excelsa H.B.) | PPARγ, Nrf2 activation | Non-transformed or transformed EGCs cultures | [42,95] |
| Coffee compounds (Parkinson’s disease) | Caffeic acid and chlorogenic acid | Coffee | Antioxidants Prevent MT-1,2 downregulation | C57BL/6 mice, EGC cultures | [96] |
| Quercetin (Diabetes, rheumatoid arthritis) | Flavonoid polyphenol | Fruits and vegetables Available as a dietary supplement. | Antioxidant, anti-inflammatory, Nrf 2 activation | Wistar rats, Holtzman rats | [89,97,98,99] |
| Resveratrol (Diabetes, intestinal ischemia-reperfusion) | Non-flavonoid polyphenol | Concentrated mostly in the skins and seeds of grapes and berries. Available as a dietary supplement. | Antioxidant, regulation of EGC proliferation | Wistar rats | [100,101,102] |
| PEA (IBD, HIV) | Endogenous or exogenous fatty acid amide | Soy lecithin, soybeans, egg yolk, peanuts, alfalfa. Available as a food-supplement named PeaPure. | PPARα activation, anti-inflammatory effects | Wistar rats, CD-1 mice, human intestinal biopsies. | [28,103] |
| Cannabidiol (UC) | Phytocannabinoid | Cannabis sativa | PPARγ receptor activation, anti-inflammatory effects | Mice, human biopsies | [104] |
| Berberine (UC) | Isoquinoline alkaloid | Plants from Berberidaceae family Available as a dietary supplement. | Modulation of interactions between EGCs, epithelial cells and immune cells. | C57BL/6 mice, Rat EGC cell line, CRL-2690 cultures | [105] |
| Probiotics (Diabetes, IBS) | Lactobacillus plantarum Bifidobacterium bifidum Bacteroides fragilis Pediococcus acidilactici | Fermented food and dairy products | Modulation of inflammation | Wistar rats, EGC cultures, pigs | [106,107,108] |
| Inulin (Diabetes) | Polysaccharide | Plants, also available in supplement form or as an ingredient | Prebiotic fiber | Wistar rats | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Gómez, L.; Szymaszkiewicz, A.; Zielińska, M.; Abalo, R. Nutraceuticals and Enteric Glial Cells. Molecules 2021, 26, 3762. https://doi.org/10.3390/molecules26123762
López-Gómez L, Szymaszkiewicz A, Zielińska M, Abalo R. Nutraceuticals and Enteric Glial Cells. Molecules. 2021; 26(12):3762. https://doi.org/10.3390/molecules26123762
Chicago/Turabian StyleLópez-Gómez, Laura, Agata Szymaszkiewicz, Marta Zielińska, and Raquel Abalo. 2021. "Nutraceuticals and Enteric Glial Cells" Molecules 26, no. 12: 3762. https://doi.org/10.3390/molecules26123762
APA StyleLópez-Gómez, L., Szymaszkiewicz, A., Zielińska, M., & Abalo, R. (2021). Nutraceuticals and Enteric Glial Cells. Molecules, 26(12), 3762. https://doi.org/10.3390/molecules26123762