Chimera Spectrum Diagnostics for Peptides Using Two-Dimensional Partial Covariance Mass Spectrometry
Abstract
1. Introduction
1.1. Identification of Chimera Spectra—State of the Art
1.2. Two-Dimensional Partial Covariance Mass Spectrometry
2. Results
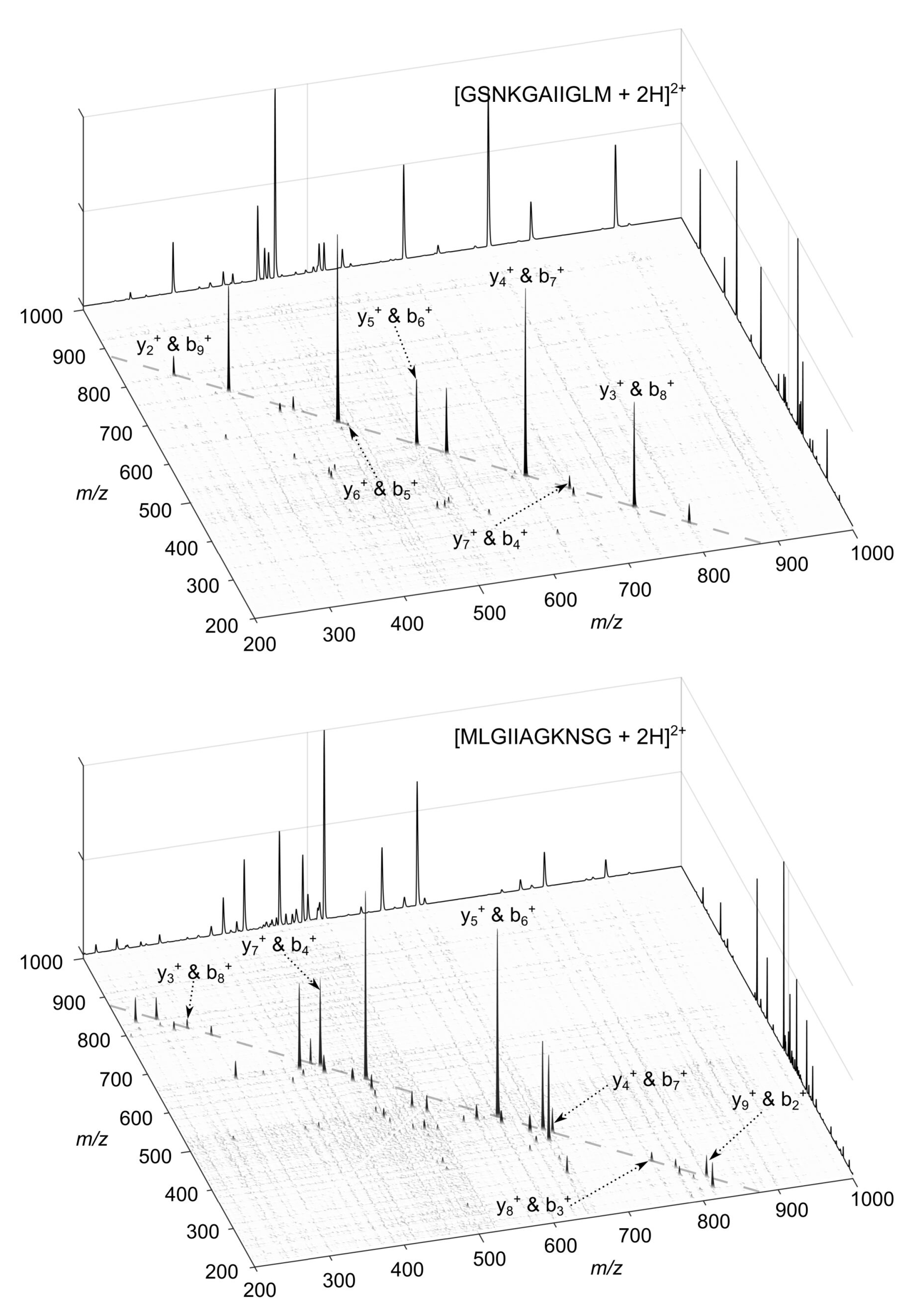
2.1. Principles of Chimera Spectra Detection within 2D-PC-MS
- Scenario 1
- co-fragmented parent ions have different charges, but arbitrarily close ;
- Scenario 2
- co-fragmented parent ions have the same charge, while their masses are close, but distinguishable within the mass resolution of the MS equipment; and
- Scenario 3
- co-fragmented parent ions have the same charge and either indistinguishable (within the mass resolution of the equipment) or exactly the same masses.
2.2. Experimental Demonstration of 3-57 Tags
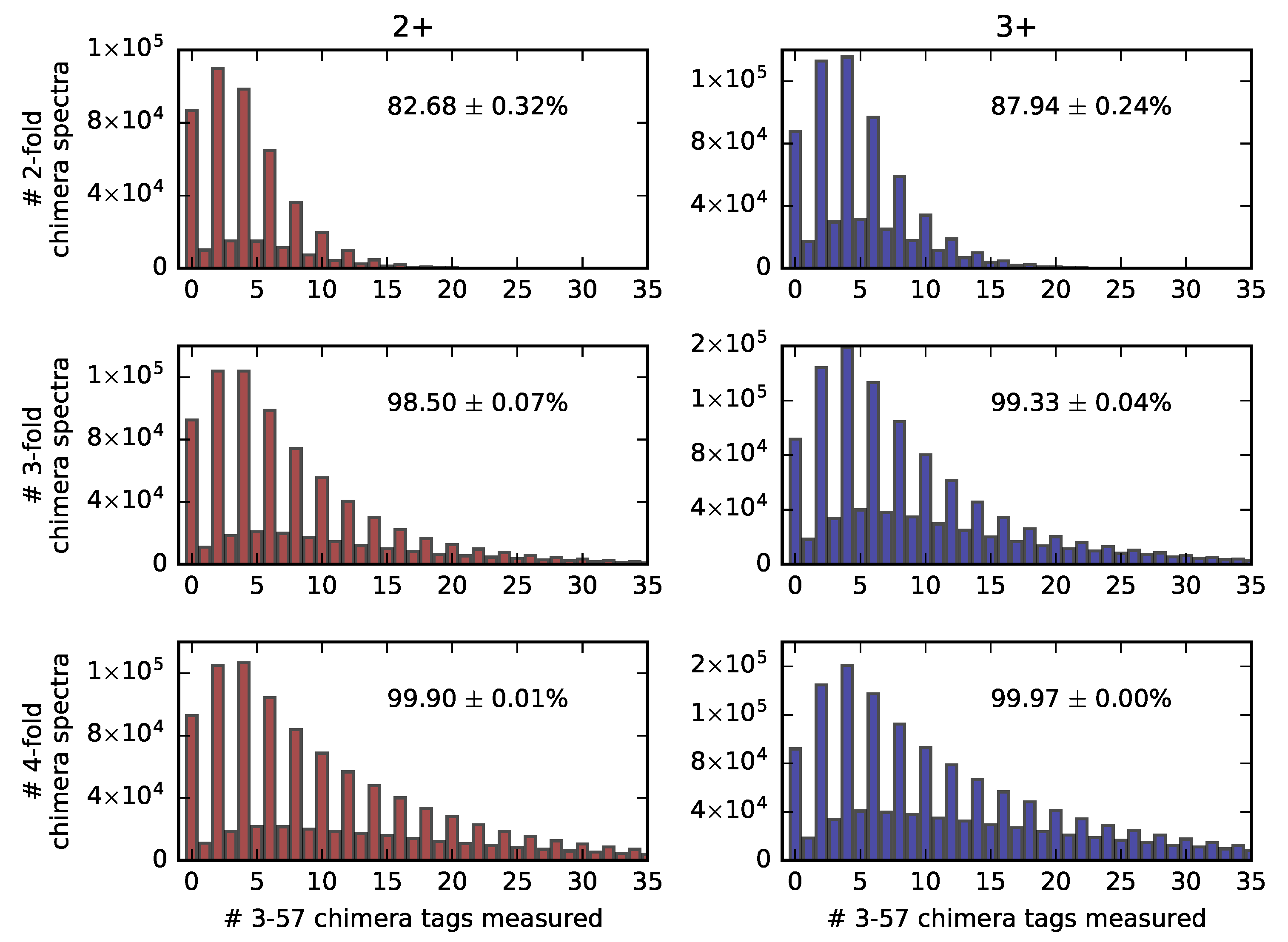
2.3. Estimation of the False-Negative Rate of Chimera Spectra Detection Using 3-57 Tags within 2D-PC-MS
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. MS Analysis and Data Processing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aebersold, R.; Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature 2016, 537, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Houel, S.; Abernathy, R.; Renganathan, K.; Meyer-Arendt, K.; Ahn, N.; Old, W. Quantifying the impact of chimera MS/MS spectra on peptide identification in large scale proteomic studies. J. Proteome Res. 2010, 9, 4152–4160. [Google Scholar] [CrossRef] [PubMed]
- Luethy, R.; Kessner, D.E.; Katz, J.E.; MacLean, B.; Grothe, R.; Kani, K.; Faça, V.; Pitteri, S.; Hanash, S.; Agus, D.B.; et al. Precursor-ion mass re-estimation improves peptide identification on hybrid instruments. J. Proteome Res. 2008, 7, 4031–4039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, X.J.; Ye, M.; Pan, S.; Schwikowski, B.; Aebersold, R. ProbIDtree: An automated software program capable of identifying multiple peptides from a single collision-induced dissociation spectrum collected by a tandem mass spectrometer. Proteomics 2005, 5, 4096–4106. [Google Scholar] [CrossRef] [PubMed]
- Masselon, C.; Paša-Tolić, L.; Lee, S.W.; Li, L.; Anderson, G.A.; Harkewicz, R.; Smith, R.D. Identification of tryptic peptides from large databases using multiplexed tandem mass spectrometry: Simulations and experimental results. Proteomics 2003, 3, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Dorfer, V.; Maltsev, S.; Winkler, S.; Mechtler, K. CharmeRT: Boosting Peptide Identifications by Chimeric Spectra Identification and Retention Time Prediction. J. Proteome Res. 2018, 17, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Kryuchkov, F.; Verano-Braga, T.; Hansen, T.A.; Sprenger, R.R.; Kjeldsen, F. Deconvolution of mixture spectra and increased throughput of peptide identification by utilization of intensified complementary ions formed in tandem mass spectrometry. J. Proteome Res. 2013, 12, 3362–3371. [Google Scholar] [CrossRef] [PubMed]
- Kryuchkov, F.; Verano-Braga, T.; Kjeldsen, F. N-terminal sequence tagging using reliably determined b2 ions: A useful approach to deconvolute tandem mass spectra of co-fragmented peptides in proteomics. J. Proteom. 2014, 103, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Driver, T.; Cooper, B.; Ayers, R.; Pipkorn, R.; Patchkovskii, S.; Averbukh, V.; Klug, D.R.; Marangos, J.P.; Frasinski, L.J.; Edelson-Averbukh, M. Two-Dimensional Partial-Covariance Mass Spectrometry of Large Molecules Based on Fragment Correlations. Phys. Rev. X 2020, 10, 41004. [Google Scholar] [CrossRef]
- Miller, J. Biomolecule mass spectrometry enters a new dimension. Phys. Today 2020, 2020, 1023a. [Google Scholar] [CrossRef]
- Pfändler, P.; Bodenhausen, G.; Rapin, J.; Houriet, R.; Gäumann, T. Two-Dimensional Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Chem. Phys. Lett. 1987, 138, 195–200. [Google Scholar] [CrossRef]
- Driver, T.; Pipkorn, R.; Averbukh, V.; Frasinski, L.J.; Marangos, J.P.; Edelson-Averbukh, M. Breaking the Histone Code with Two-Dimensional Partial Covariance Mass Spectrometry. chemRxiv 2020. chemRxiv:12743669. [Google Scholar]
- Driver, T.; Averbukh, V.; Frasinski, L.J.; Marangos, J.P.; Edelson-Averbukh, M. Two-dimensional partial covariance mass spectrometry for the top-down analysis of intact proteins. arXiv 2020, arXiv:2004.11949. [Google Scholar]
- Driver, T.; Pipkorn, R.; Frasinski, L.J.; Marangos, J.P.; Edelson-Averbukh, M.; Averbukh, V. Proteomic database search engine for two-dimensional partial covariance mass spectrometry. chemRxiv 2020. chemRxiv:13623173. [Google Scholar]
- Wysocki, V.H.; Resing, K.A.; Zhang, Q.; Cheng, G. Mass spectrometry of peptides and proteins. Methods 2005, 35, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Han, S.; Lee, S.; Han, S.Y.; Lee, T.G.; Chung, G.; Lee, D.; Oh, H.B. Observation of pronounced b•,y cleavages electron capture dissociation mass spectrometry of polyamidoamine (PAMAM) dendrimer ions with amide functionalities. J. Am. Soc. Mass Spectrom. 2006, 17, 536–543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zubarev, R.A. Reactions of polypeptide ions with electrons in the gas phase. Mass Spectrom. Rev. 2003, 22, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Edelson-Averbukh, M.; Pipkorn, R.; Lehmann, W.D. Analysis of Protein Phosphorylation in the Regions of Consecutive Serine/Threonine Residues by Negative Ion Electrospray Collision-Induced Dissociation. Approach to Pinpointing of Phosphorylation Sites. Anal. Chem. 2007, 79, 3476–3486. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Martin, M.J.; O’Donovan, C.; Magrane, M.; Alpi, E.; Antunes, R.; Bely, B.; Bingley, M.; Bonilla, C.; Britto, R.; et al. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2017, 45, D158–D169. [Google Scholar] [CrossRef]
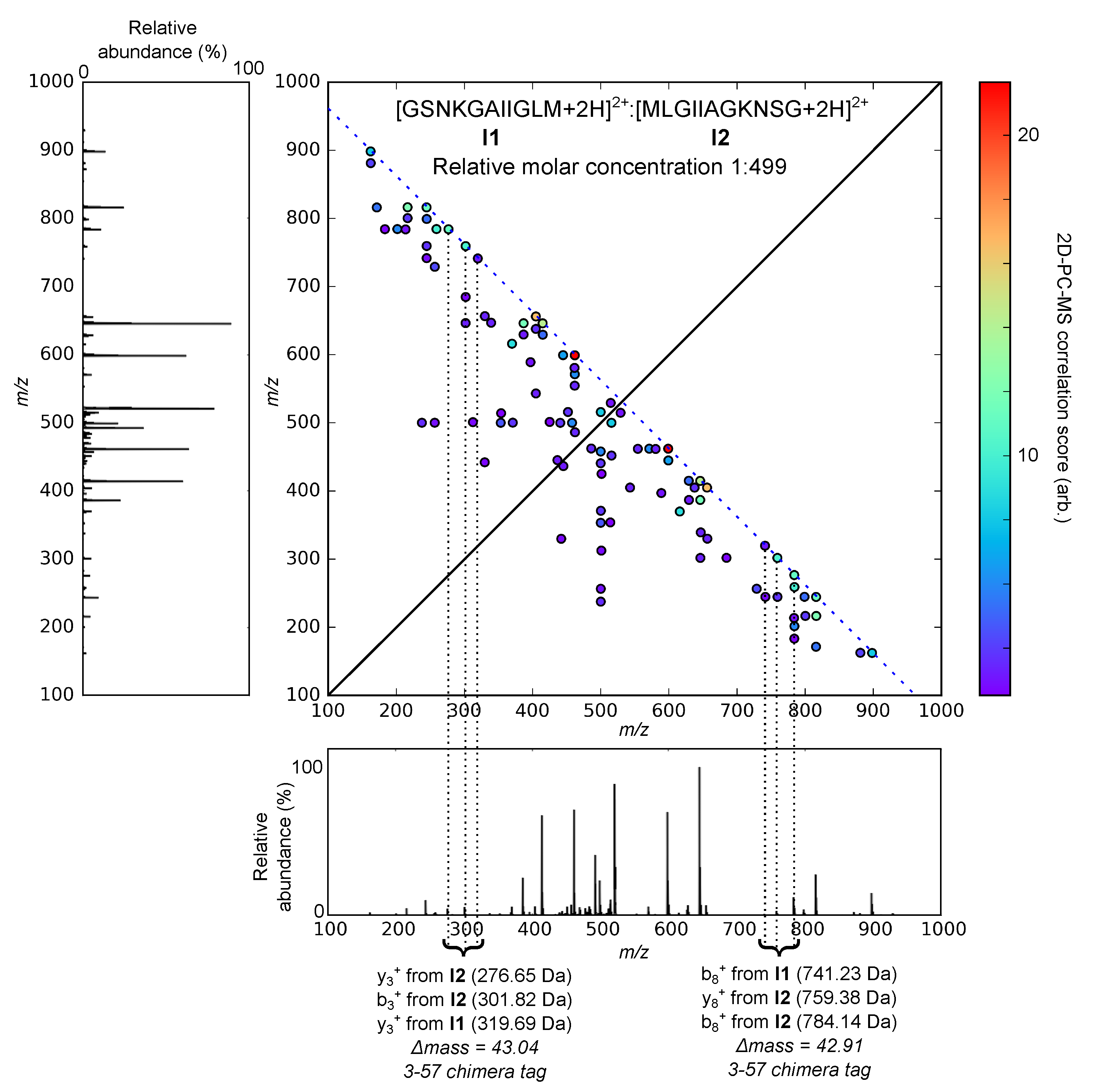
| Analysed Sample | Chimera Spectrum Identified? | # 3-57 Tags |
|---|---|---|
| (1:499) | Y | 2 |
| (1:99) | Y | 6 |
| (1:19) | Y | 10 |
| (1:9) | Y | 10 |
| (1:1) | Y | 14 |
| (I1, pure) | N | 0 |
| (I2, pure) | N | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Driver, T.; Bachhawat, N.; Frasinski, L.J.; Marangos, J.P.; Averbukh, V.; Edelson-Averbukh, M. Chimera Spectrum Diagnostics for Peptides Using Two-Dimensional Partial Covariance Mass Spectrometry. Molecules 2021, 26, 3728. https://doi.org/10.3390/molecules26123728
Driver T, Bachhawat N, Frasinski LJ, Marangos JP, Averbukh V, Edelson-Averbukh M. Chimera Spectrum Diagnostics for Peptides Using Two-Dimensional Partial Covariance Mass Spectrometry. Molecules. 2021; 26(12):3728. https://doi.org/10.3390/molecules26123728
Chicago/Turabian StyleDriver, Taran, Nikhil Bachhawat, Leszek J. Frasinski, Jonathan P. Marangos, Vitali Averbukh, and Marina Edelson-Averbukh. 2021. "Chimera Spectrum Diagnostics for Peptides Using Two-Dimensional Partial Covariance Mass Spectrometry" Molecules 26, no. 12: 3728. https://doi.org/10.3390/molecules26123728
APA StyleDriver, T., Bachhawat, N., Frasinski, L. J., Marangos, J. P., Averbukh, V., & Edelson-Averbukh, M. (2021). Chimera Spectrum Diagnostics for Peptides Using Two-Dimensional Partial Covariance Mass Spectrometry. Molecules, 26(12), 3728. https://doi.org/10.3390/molecules26123728






