Novel Amphiphilic Block Copolymers for the Formation of Stimuli-Responsive Non-Lamellar Lipid Nanoparticles
Abstract
1. Introduction
2. Results
2.1. Synthesis of Stimuli-Responsive ABCs
2.2. Formulation and Characterization of MO Nanoparticles Stabilized by the Synthetic ABCs
2.3. H2O2-Responsiveness of the Formulated Nanoparticles
2.4. pH-Responsiveness of the Formulated Nanoparticles
2.5. Temperature-Responsiveness of the Formulated Nanoparticles
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization of ABC1 (PEG114-RAFT)
4.3. Synthesis and Characterization of ABC2 (PEG114-PTBA5)
4.4. Synthesis and Characterization of ABC3 (PEG114-PTBA9)
4.5. Synthesis and Characterization of ABC4 (PEG114-PDMAEMA17-RAFT)
4.6. Synthesis and Characterization of ABC5 (PEG114-PDMAEMA17-PTBA5-RAFT)
4.7. Synthesis and Characterization of ABC6 (PEG114-PDMAEMA17-PTBA9-RAFT)
4.8. Formulation of ABC-Stabilized Nanoparticles
4.9. High Throughput Synchrotron SAXS Characterization
4.10. SAXS Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Barauskas, J.; Johnsson, M.; Joabsson, F.; Tiberg, F. Cubic phase nanoparticles (cubosome): Principles for controlling size, structure, and stability. Langmuir 2005, 21, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-D.; Larson, I.; Hanley, T.; Boyd, B.J. Bulk and Dispersed Aqueous Phase Behavior of Phytantriol: Effect of Vitamin E Acetate and F127 Polymer on Liquid Crystal Nanostructure. Langmuir 2006, 22, 9512–9518. [Google Scholar] [CrossRef] [PubMed]
- Tilley, A.J.; Drummond, C.J.; Boyd, B.J. Disposition and association of the steric stabilizer Pluronic® F127 in lyotropic liquid crystalline nanostructured particle dispersions. J. Colloid Interface Sci. 2013, 392, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Mulet, X.; Boyd, B.J.; Drummond, C.J. Advances in drug delivery and medical imaging using colloidal lyotropic liquid crystalline dispersions. J. Colloid Interface Sci. 2013, 393, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The Next Generation of Smart Lipid Nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef]
- Zhai, J.; Fong, C.; Tran, N.; Drummond, C.J. Non-Lamellar Lyotropic Liquid Crystalline Lipid Nanoparticles for the Next Generation of Nanomedicine. ACS Nano 2019, 13, 6178–6206. [Google Scholar] [CrossRef]
- Sagalowicz, L.; Michel, M.; Adrian, M.; Frossard, P.; Rouvet, M.; Watzke, H.J.; Yaghmur, A.; De Campo, L.; Glatter, O.; Leser, M.E. Crystallography of dispersed liquid crystalline phases studied by cryo-transmission electron microscopy. J. Microsc. 2006, 221, 110–121. [Google Scholar] [CrossRef]
- Seddon, J.M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim. Biophys. Acta Rev. Biomembr. 1990, 1031, 1–69. [Google Scholar] [CrossRef]
- Demurtas, D.; Guichard, P.; Martiel, I.; Mezzenga, R.; Hebert, C.; Sagalowicz, L. Direct visualization of dispersed lipid bicontinuous cubic phases by cryo-electron tomography. Nat. Commun. 2015, 6, 8915. [Google Scholar] [CrossRef] [PubMed]
- Fong, W.-K.; Sanchez-Ferrer, A.; Ortelli, F.G.; Sun, W.; Boyd, B.J.; Mezzenga, R. Dynamic formation of nanostructured particles from vesicles via invertase hydrolysis for on-demand delivery. RSC Adv. 2017, 7, 4368–4377. [Google Scholar] [CrossRef]
- Zhai, J.; Luwor, R.B.; Ahmed, N.; Escalona, R.; Tan, F.H.; Fong, C.; Ratcliffe, J.; Scoble, J.A.; Drummond, C.J.; Tran, N. Paclitaxel-Loaded Self-Assembled Lipid Nanoparticles as Targeted Drug Delivery Systems for the Treatment of Aggressive Ovarian Cancer. ACS Appl. Mater. Interfaces 2018, 10, 25174–25185. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Swarnakar, N.K.; Mishra, P.R.; Verma, A.; Kaul, A.; Mishra, A.K.; Jain, N.K. Paclitaxel loaded PEGylated gleceryl monooleate based nanoparticulate carriers in chemotherapy. Biomaterials 2012, 33, 7206–7220. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Mu, H. Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharm. Sin. B 2021, 11, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.B.; Ferreira, D.A.; de Paula, D.; Garcia, M.T.J.; Thomazini, J.A.; Fantini, M.C.; Bentley, M.V.L. Reverse hexagonal phase nanodispersion of monoolein and oleic acid for topical delivery of peptides: In vitro and in vivo skin penetration of cyclosporin A. Pharm. Res. 2006, 23, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Leal, C. Cuboplexes: Topologically Active siRNA Delivery. ACS Nano 2015, 9, 10214–10226. [Google Scholar] [CrossRef]
- Jenni, S.; Picci, G.; Fornasier, M.; Mamusa, M.; Schmidt, J.; Talmon, Y.; Sour, A.; Heitz, V.; Murgia, S.; Caltagirone, C. Multifunctional cubic liquid crystalline nanoparticles for chemo- and photodynamic synergistic cancer therapy. Photochem. Photobiol. Sci. 2020, 19, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cui, Z.; Huo, Y.; Sun, Y.; Zhang, X.; Guan, J.; Mao, S. Influence of drug-carrier compatibility and preparation method on the properties of paclitaxel-loaded lipid liquid crystalline nanoparticles. J. Pharm. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Tian, D.; Sun, L.; Wang, X.; Tian, M. Theranostic combinatorial drug-loaded coated cubosomes for enhanced targeting and efficacy against cancer cells. Cell Death Dis. 2020, 11, 1. [Google Scholar] [CrossRef]
- Tran, N.; Bye, N.; Moffat, B.A.; Wright, D.K.; Cuddihy, A.; Hinton, T.M.; Hawley, A.M.; Reynolds, N.P.; Waddington, L.J.; Mulet, X.; et al. Dual-modality NIRF-MRI cubosomes and hexosomes: High throughput formulation and in vivo biodistribution. Mater. Sci. Eng. C 2017, 71, 584–593. [Google Scholar] [CrossRef]
- Bye, N.; Hutt, O.E.; Hinton, T.M.; Acharya, D.P.; Waddington, L.J.; Moffat, B.A.; Wright, D.K.; Wang, H.X.; Mulet, X.; Muir, B.W. Nitroxide-Loaded Hexosomes Provide MRI Contrast in Vivo. Langmuir 2014, 30, 8898–8906. [Google Scholar] [CrossRef]
- Muir, B.W.; Acharya, D.P.; Kennedy, D.F.; Mulet, X.; Evans, R.A.; Pereira, S.M.; Wark, K.L.; Boyd, B.J.; Nguyen, T.-H.; Hinton, T.M.; et al. Metal-free and MRI visible theranostic lyotropic liquid crystal nitroxide-based nanoparticles. Biomaterials 2012, 33, 2723–2733. [Google Scholar] [CrossRef]
- Fong, W.-K.; Negrini, R.; Vallooran, J.J.; Mezzenga, R.; Boyd, B.J. Responsive self-assembled nanostructured lipid systems for drug delivery and diagnostics. J. Colloid Interface Sci. 2016, 484, 320–339. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Glatter, O. Characterization and potential applications of nanostructured aqueous dispersions. Adv. Colloid Interface Sci. 2009, 147–148, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Rizwan, S.B.; Dong, Y.-D.; Hook, S.; Rades, T. Self-assembled geometric liquid-crystalline nanoparticles imaged in three dimensions: Hexosomes are not necessarily flat hexagonal prisms. Langmuir 2007, 23, 12461–12464. [Google Scholar] [CrossRef]
- Mulet, X.; Kennedy, D.F.; Conn, C.E.; Hawley, A.; Drummond, C.J. High throughput preparation and characterisation of amphiphilic nanostructured nanoparticulate drug delivery vehicles. Int. J. Pharm. 2010, 395, 290–297. [Google Scholar] [CrossRef]
- Sarkar, S.; Tran, N.; Soni, S.K.; Nasa, Z.; Drummond, C.J.; Conn, C.E. Cuboplex-Mediated Nonviral Delivery of Functional siRNA to Chinese Hamster Ovary (CHO) Cells. ACS Appl. Mater. Interfaces 2021, 13, 2336–2345. [Google Scholar] [CrossRef]
- Boge, L.; Bysell, H.; Ringstad, L.; Wennman, D.; Umerska, A.; Cassisa, V.; Eriksson, J.; Joly-Guillou, M.-L.; Edwards, K.; Andersson, M. Lipid-Based Liquid Crystals As Carriers for Antimicrobial Peptides: Phase Behavior and Antimicrobial Effect. Langmuir 2016, 32, 4217–4228. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Mutafchieva, R.; Lesieur, S.; Couvreur, P. Self-Assembled Multicompartment Liquid Crystalline Lipid Carriers for Protein, Peptide, and Nucleic Acid Drug Delivery. Acc. Chem. Res. 2010, 44, 147–156. [Google Scholar] [CrossRef]
- Géral, C.; Angelova, A.; Lesieur, S. From Molecular to Nanotechnology Strategies for Delivery of Neurotrophins: Emphasis on Brain-Derived Neurotrophic Factor (BDNF). Pharmaceutics 2013, 5, 127–167. [Google Scholar] [CrossRef] [PubMed]
- Negrini, R.; Fong, W.-K.; Boyd, B.J.; Mezzenga, R. pH-responsive lyotropic liquid crystals and their potential therapeutic role in cancer treatment. Chem. Commun. 2015, 51, 6671–6674. [Google Scholar] [CrossRef] [PubMed]
- Vallooran, J.J.; Handschin, S.; Bolisetty, S.; Mezzenga, R. Twofold Light and Magnetic Responsive Behavior in Nanoparticle–Lyotropic Liquid Crystal Systems. Langmuir 2012, 28, 5589–5595. [Google Scholar] [CrossRef] [PubMed]
- Szlezak, M.; Nieciecka, D.; Joniec, A.; Pękała, M.; Gorecka, E.; Emo, M.; Stébé, M.J.; Krysiński, P.; Bilewicz, R. Monoolein Cubic Phase Gels and Cubosomes Doped with Magnetic Nanoparticles–Hybrid Materials for Controlled Drug Release. ACS Appl. Mater. Interfaces 2017, 9, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Angelova, A.; Hu, F.; Garamus, V.M.; Peng, C.; Li, N.; Liu, J.; Liu, D.; Zou, A. pH Responsiveness of Hexosomes and Cubosomes for Combined Delivery of Brucea javanica Oil and Doxorubicin. Langmuir 2019, 35, 14532–14542. [Google Scholar] [CrossRef]
- Zhai, J.; Tan, F.H.; Luwor, R.B.; Srinivasa Reddy, T.; Ahmed, N.; Drummond, C.J.; Tran, N. In Vitro and In Vivo Toxicity and Biodistribution of Paclitaxel-Loaded Cubosomes as a Drug Delivery Nanocarrier: A Case Study Using an A431 Skin Cancer Xenograft Model. ACS Appl. Bio Mater. 2020, 3, 4198–4207. [Google Scholar] [CrossRef]
- Abdelrahman, F.E.; Elsayed, I.; Gad, M.K.; Badr, A.; Mohamed, M.I. Investigating the cubosomal ability for transnasal brain targeting: In vitro optimization, ex vivo permeation and in vivo biodistribution. Int. J. Pharm. 2015, 490, 281–291. [Google Scholar] [CrossRef]
- Nasr, M.; Ghorab, M.K.; Abdelazem, A. In vitro and in vivo evaluation of cubosomes containing 5-fluorouracil for liver targeting. Acta Pharm. Sin. B 2015, 5, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Stefania, B.; Laura, A.; Claudia, C.; Chiara, G.; Angela, M.F.; Vito, L.; Andrea, L.; Paolo, M.; Valeria, M.; Maura, M.; et al. Cubosomes for in vivo fluorescence lifetime imaging. Nanotechnology 2017, 28, 055102. [Google Scholar]
- Fong, W.K.; Hanley, T.; Boyd, B.J. Stimuli responsive liquid crystals provide ‘on-demand’ drug delivery in vitro and in vivo. J. Control Release 2009, 135, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Negrini, R.; Mezzenga, R. pH-Responsive Lyotropic Liquid Crystals for Controlled Drug Delivery. Langmuir 2011, 27, 5296–5303. [Google Scholar] [CrossRef]
- Rahanyan-Kagi, N.; Aleandri, S.; Speziale, C.; Mezzenga, R.; Landau, E.M. Stimuli-responsive lipidic cubic phase: Triggered release and sequestration of guest molecules. Chemistry 2015, 21, 1873–1877. [Google Scholar] [CrossRef]
- Rarokar, N.R.; Saoji, S.D.; Raut, N.A.; Taksande, J.B.; Khedekar, P.B.; Dave, V.S. Nanostructured Cubosomes in a Thermoresponsive Depot System: An Alternative Approach for the Controlled Delivery of Docetaxel. AAPS PharmSciTech 2016, 17, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.Y.; Nguyen, T.-H.; Hanley, T.; Boyd, B.J. Nanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugs. Int. J. Pharm. 2009, 365, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Mulet, X.; Hawley, A.M.; Hinton, T.M.; Mudie, S.T.; Muir, B.W.; Giakoumatos, E.C.; Waddington, L.J.; Kirby, N.M.; Drummond, C.J. Nanostructure and cytotoxicity of self-assembled monoolein-capric acid lyotropic liquid crystalline nanoparticles. RSC Adv. 2015, 5, 26785–26795. [Google Scholar] [CrossRef]
- Mertins, O.; Mathews, P.D.; Angelova, A. Advances in the Design of pH-Sensitive Cubosome Liquid Crystalline Nanocarriers for Drug Delivery Applications. Nanomaterials 2020, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Hawley, A.M.; Zhai, J.; Muir, B.W.; Fong, C.; Drummond, C.J.; Mulet, X. High-throughput screening of saturated fatty acid influence on nanostructure of lyotropic liquid crystalline lipid nanoparticles. Langmuir 2016, 32, 4509–4520. [Google Scholar] [CrossRef]
- Fong, C.; Zhai, J.; Drummond, C.J.; Tran, N. Micellar Fd3m cubosomes from monoolein—long chain unsaturated fatty acid mixtures: Stability on temperature and pH response. J. Colloid Interface Sci. 2020, 566, 98–106. [Google Scholar] [CrossRef]
- Rajesh, S.; Zhai, J.; Drummond, C.J.; Tran, N. Synthetic ionizable aminolipids induce a pH dependent inverse hexagonal to bicontinuous cubic lyotropic liquid crystalline phase transition in monoolein nanoparticles. J. Colloid Interface Sci. 2021, 589, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Chong, J.Y.T.; Mulet, X.; Boyd, B.J.; Drummond, C.J. Chapter Five—Steric Stabilizers for Cubic Phase Lyotropic Liquid Crystal Nanodispersions (Cubosomes). In Advances in Planar Lipid Bilayers and Liposomes; Iglič, A., Kulkarni, C.V., Rappolt, M., Eds.; Academic Press: Waltham, MA, USA, 2015; pp. 131–187. [Google Scholar]
- Zhai, J.; Suryadinata, R.; Luan, B.; Tran, N.; Hinton, T.M.; Ratcliffe, J.; Hao, X.; Drummond, C.J. Amphiphilic brush polymers produced using the RAFT polymerisation method stabilise and reduce the cell cytotoxicity of lipid lyotropic liquid crystalline nanoparticles. Faraday Discuss. 2016, 191, 545–563. [Google Scholar] [CrossRef]
- Chong, J.Y.T.; Mulet, X.; Keddie, D.J.; Waddington, L.; Mudie, S.T.; Boyd, B.J.; Drummond, C.J. Novel Steric Stabilizers for Lyotropic Liquid Crystalline Nanoparticles: PEGylated-Phytanyl Copolymers. Langmuir 2015, 31, 2615–2629. [Google Scholar] [CrossRef]
- Yu Helvig, S.; Woythe, L.; Pham, S.; Bor, G.; Andersen, H.; Moein Moghimi, S.; Yaghmur, A. A structurally diverse library of glycerol monooleate/oleic acid non-lamellar liquid crystalline nanodispersions stabilized with nonionic methoxypoly(ethylene glycol) (mPEG)-lipids showing variable complement activation properties. J. Colloid Interface Sci. 2021, 582, 906–917. [Google Scholar] [CrossRef]
- Angelov, B.; Angelova, A.; Drechsler, M.; Garamus, V.M.; Mutafchieva, R.; Lesieur, S. Identification of large channels in cationic PEGylated cubosome nanoparticles by synchrotron radiation SAXS and Cryo-TEM imaging. Soft Matter 2015, 11, 3686–3692. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Waddington, L.; Wooster, T.J.; Aguilar, M.I.; Boyd, B.J. Revisiting β-casein as a stabilizer for lipid liquid crystalline nanostructured particles. Langmuir 2011, 27, 14757–14766. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Hinton, T.M.; Waddington, L.J.; Fong, C.; Tran, N.; Mulet, X.; Drummond, C.J.; Muir, B.W. Lipid–PEG Conjugates Sterically Stabilize and Reduce the Toxicity of Phytantriol-Based Lyotropic Liquid Crystalline Nanoparticles. Langmuir 2015, 31, 10871–10880. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Sugihara, K.; Gillilland, M.G.; Jon, S.; Kamada, N.; Moon, J.J. Hyaluronic acid–bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 2020, 19, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Fornasier, M.; Biffi, S.; Bortot, B.; Macor, P.; Manhart, A.; Wurm, F.R.; Murgia, S. Cubosomes stabilized by a polyphosphoester-analog of Pluronic F127 with reduced cytotoxicity. J. Colloid Interface Sci. 2020, 580, 286–297. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Perinelli, D.R.; Pippa, N.; Chrysostomou, V.; Forys, A.; Otulakowski, L.; Bonacucina, G.; Trzebicka, B.; Pispas, S.; Demetzos, C. Physicochemical, morphological and thermal evaluation of lyotropic lipidic liquid crystalline nanoparticles: The effect of stimuli-responsive polymeric stabilizer. Colloids Surf. A 2020, 595, 124678. [Google Scholar] [CrossRef]
- Fan, B.; Wan, J.; Zhai, J.; Chen, X.; Thang, S.H. Triggered Degradable Colloidal Particles with Ordered Inverse Bicontinuous Cubic and Hexagonal Mesophases. ACS Nano 2021, 15, 4688–4698. [Google Scholar] [CrossRef]
- Stubbs, E.; Laskowski, E.; Conor, P.; Heinze, D.A.; Karis, D.; Glogowski, E.M. Control of pH- and temperature-responsive behavior of mPEG-b-PDMAEMA copolymers through polymer composition. J. Macromol. Sci. Part A 2017, 54, 228–235. [Google Scholar] [CrossRef]
- Mezzenga, R.; Seddon, J.M.; Drummond, C.J.; Boyd, B.J.; Schröder-Turk, G.E.; Sagalowicz, L. Nature-Inspired Design and Application of Lipidic Lyotropic Liquid Crystals. Adv. Mater. 2019, 31, 1900818. [Google Scholar] [CrossRef]
- Mulet, X.; Conn, C.E.; Fong, C.; Kennedy, D.F.; Moghaddam, M.J.; Drummond, C.J. High-throughput development of amphiphile self-assembly materials: Fast-tracking synthesis, characterization, formulation, application, and understanding. Acc. Chem. Res. 2013, 46, 1497–1505. [Google Scholar] [CrossRef]
- Wan, J.; Fan, B.; Liu, Y.; Hsia, T.; Qin, K.; Junkers, T.; Teo, B.M.; Thang, S.H. Room temperature synthesis of block copolymer nano-objects with different morphologies via ultrasound initiated RAFT polymerization-induced self-assembly (sono-RAFT-PISA). Polym. Chem. 2020, 11, 3564–3572. [Google Scholar] [CrossRef]
- Fan, B.; Wan, J.; McKay, A.; Qu, Z.; Thang, S.H. Facile synthesis of well-controlled poly(1-vinyl imidazole) by the RAFT process. Polym. Chem. 2020, 11, 5649–5658. [Google Scholar] [CrossRef]
- Fan, B.; Wan, J.; Liu, Y.; Tian, W.W.; Thang, S.H. Functionalization of liquid metal nanoparticles via the RAFT process. Polym. Chem. 2021, 12, 3015–3025. [Google Scholar] [CrossRef]
- Fan, B.; Liu, Y.; Wan, J.; Crawford, S.; Thang, S.H. Polymerization-Induced Self-Assembly (PISA) and “Host–Guest” Complexation-Directed Polymer/Gold Nanocomposites. ACS Mater. Lett. 2020, 2, 492–498. [Google Scholar] [CrossRef]
- Tran, N.; Mulet, X.; Hawley, A.M.; Fong, C.; Zhai, J.; Le, T.C.; Ratcliffe, J.; Drummond, C.J. Manipulating the Ordered Nanostructure of Self-Assembled Monoolein and Phytantriol Nanoparticles with Unsaturated Fatty Acids. Langmuir 2018, 34, 2764–2773. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, V.; Pispas, S. Stimuli-responsive amphiphilic PDMAEMA-b-PLMA copolymers and their cationic and zwitterionic analogs. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 598–610. [Google Scholar] [CrossRef]
- Clogston, J.; Rathman, J.; Tomasko, D.; Walker, H.; Caffrey, M. Phase behavior of a monoacylglycerol: (Myverol 18-99K)/water system. Chem. Phys. Lipids 2000, 107, 191–220. [Google Scholar] [CrossRef]
- Van’t Hag, L.; Gras, S.L.; Conn, C.E.; Drummond, C.J. Lyotropic liquid crystal engineering moving beyond binary compositional space—ordered nanostructured amphiphile self-assembly materials by design. Chem. Soc. Rev. 2017, 46, 2705–2731. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Xiao, W.; Song, X.; Wang, W.; Dong, X. Recent Advances in Tumor Microenvironment Hydrogen Peroxide-Responsive Materials for Cancer Photodynamic Therapy. Nano-Micro Lett. 2020, 12, 15. [Google Scholar] [CrossRef]
- Loh, X.J.; Ong, S.J.; Tung, Y.T.; Choo, H.T. Dual responsive micelles based on poly[(R)-3-hydroxybutyrate] and poly(2-(di-methylamino)ethyl methacrylate) for effective doxorubicin delivery. Polym. Chem. 2013, 4, 2564–2574. [Google Scholar] [CrossRef]
- Drummond, C.J.; Grieser, F.; Healy, T.W. Acid-base equilibria in aqueous micellar solutions. Part 1.—‘Simple’ weak acids and bases. J. Chem. Soc. Faraday Trans. I 1989, 85, 521–535. [Google Scholar] [CrossRef]
- Tannock, I.F.; Rotin, D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989, 49, 4373–4384. [Google Scholar] [PubMed]
- El-Kenawi, A.; Gatenbee, C.; Robertson-Tessi, M.; Bravo, R.; Dhillon, J.; Balagurunathan, Y.; Berglund, A.; Vishvakarma, N.; Ibrahim-Hashim, A.; Choi, J.; et al. Acidity promotes tumour progression by altering macrophage phenotype in prostate cancer. Br. J. Cancer 2019, 121, 556–566. [Google Scholar] [CrossRef]
- Seddon, J.M.; Squires, A.M.; Conn, C.E.; Ces, O.; Heron, A.J.; Mulet, X.; Shearman, G.C.; Templer, R.H. Pressure-jump X-ray studies of liquid crystal transitions in lipids. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2006, 364, 2635–2655. [Google Scholar] [CrossRef]


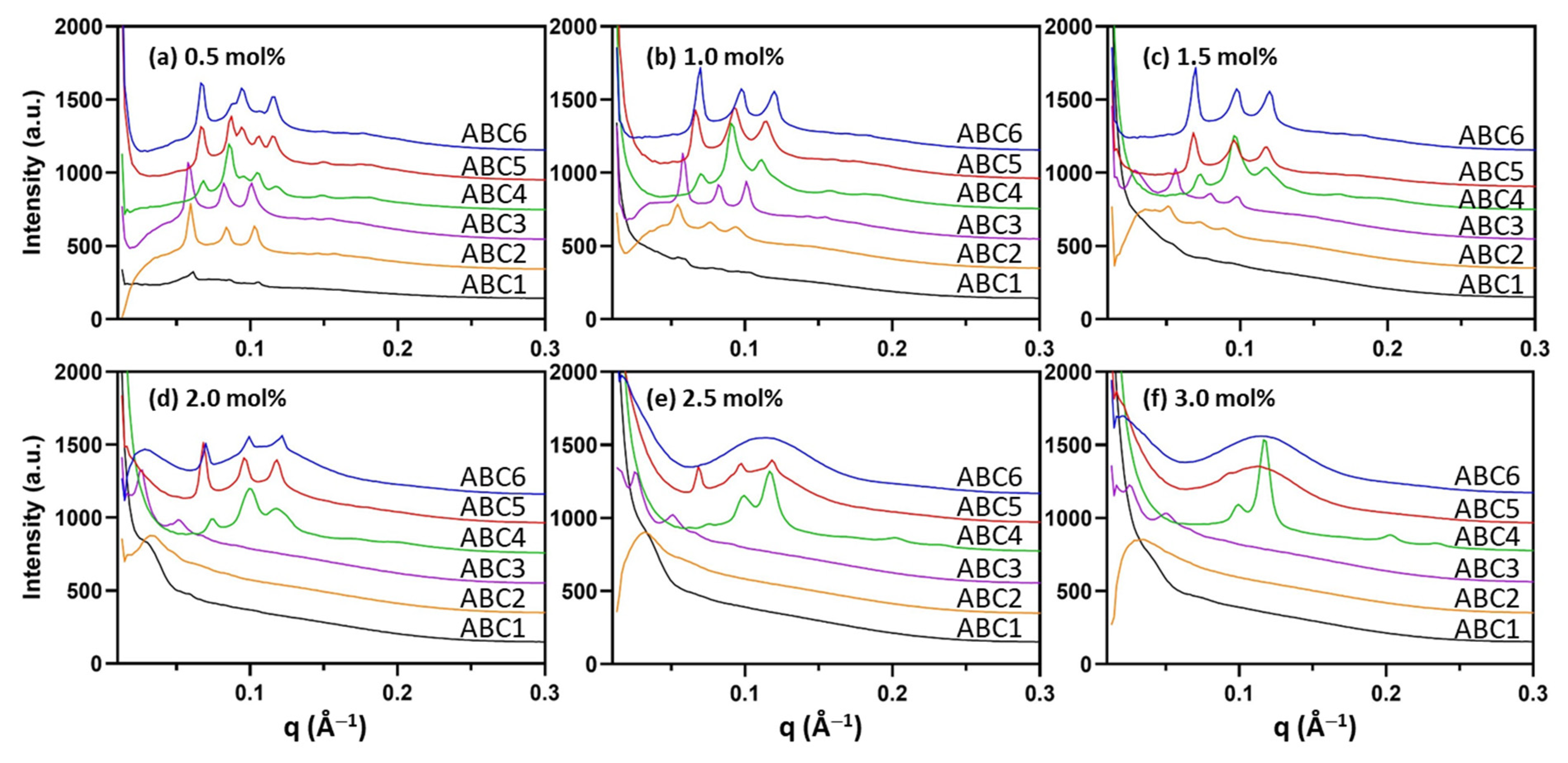
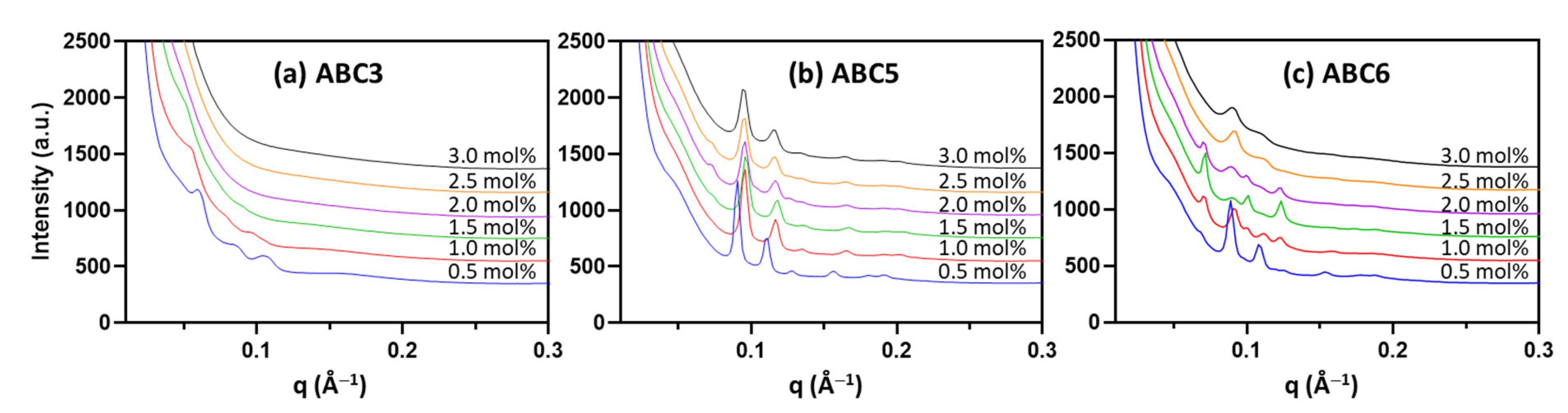
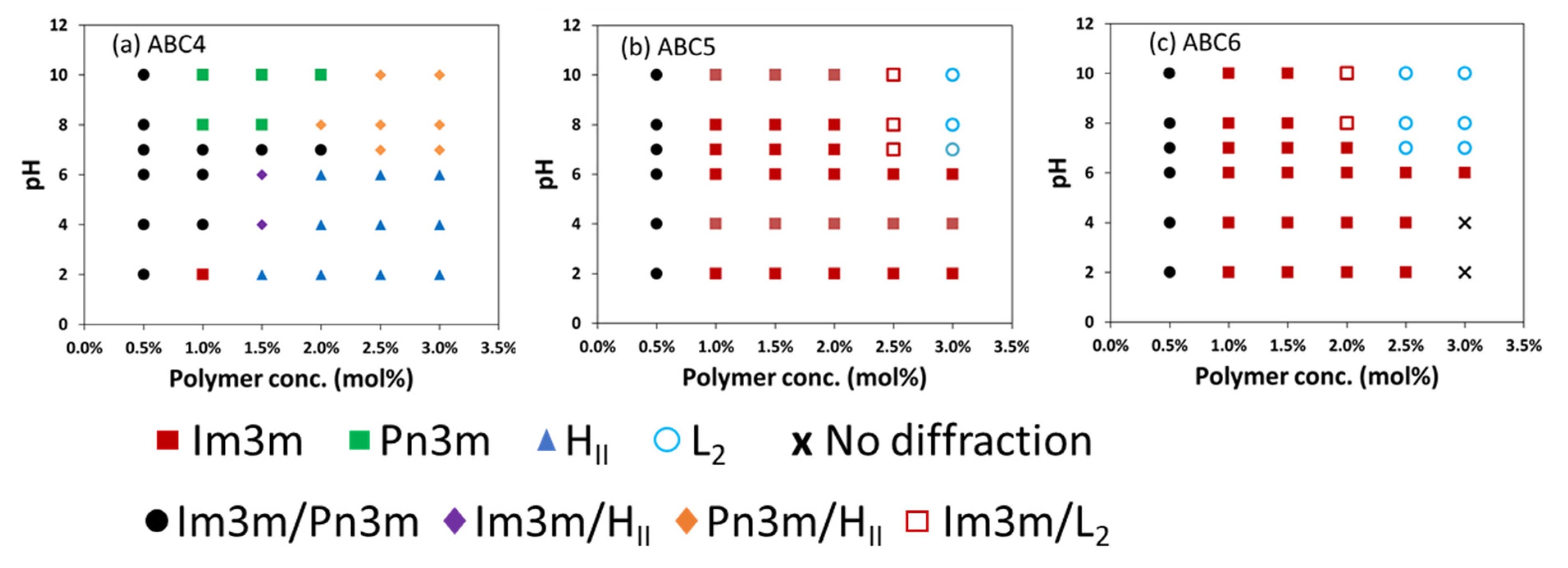
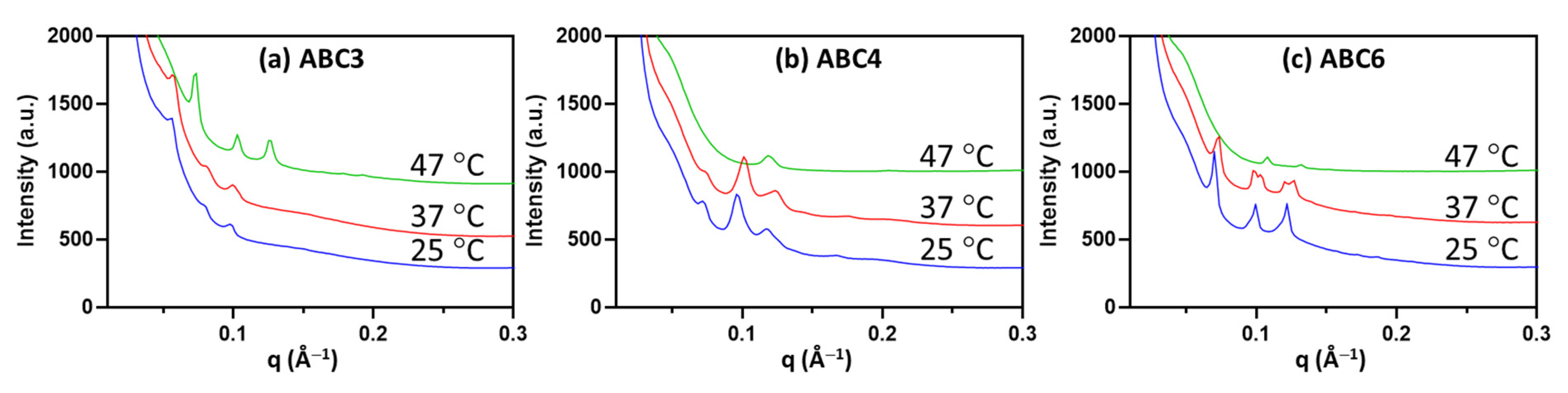
| mol% | ABC1 | ABC2 | ABC3 | ABC4 | ABC5 | ABC6 |
|---|---|---|---|---|---|---|
| 0.5 | Weak Im3m signal | Im3m (145) | Im3m (153) | Pn3m (101); Im3m (127) | Im3m (131); Pn3m (103) | Im3m (130) Pn3m (103) |
| 1.0 | Weak Im3m signal | Im3m (162) | Im3m (153) | Pn3m (98); Im3m (115) | Im3m (132) | Im3m (126) |
| 1.5 | ND | Im3m (174) | Im3m (157) | Pn3m (92); Im3m (112) | Im3m (130) | Im3m (126) |
| 2.0 | ND | ND | Im3m (176) | Pn3m (89); Im3m (112) | Im3m (129) | Im3m (125) |
| 2.5 | ND | ND | Im3m (176) | Pn3m (90); H2 (61) | Im3m (130) L2 | L2 |
| 3.0 | ND | ND | Im3m (178) | Pn3m (90); H2 (62) | L2 | L2 |
| mol% | ABC3 | ABC5 | ABC6 |
|---|---|---|---|
| 0.5 | Im3m (148) | Pn3m (98) | Pn3m (100) |
| 1.0 | Im3m (161) | Pn3m (92) | Im3m (122) Pn3m (97) |
| 1.5 | ND | Pn3m (91) | Im3m (125) Pn3m (100) |
| 2.0 | ND | Pn3m (93) Im3m (weak) | Im3m (120) Pn3m (100) |
| 2.5 | ND | Pn3m (94) (Im3m weak) | Pn3m (100) |
| 3.0 | ND | Pn3m (94) | Pn3m (101) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, J.; Fan, B.; Thang, S.H.; Drummond, C.J. Novel Amphiphilic Block Copolymers for the Formation of Stimuli-Responsive Non-Lamellar Lipid Nanoparticles. Molecules 2021, 26, 3648. https://doi.org/10.3390/molecules26123648
Zhai J, Fan B, Thang SH, Drummond CJ. Novel Amphiphilic Block Copolymers for the Formation of Stimuli-Responsive Non-Lamellar Lipid Nanoparticles. Molecules. 2021; 26(12):3648. https://doi.org/10.3390/molecules26123648
Chicago/Turabian StyleZhai, Jiali, Bo Fan, San H. Thang, and Calum J. Drummond. 2021. "Novel Amphiphilic Block Copolymers for the Formation of Stimuli-Responsive Non-Lamellar Lipid Nanoparticles" Molecules 26, no. 12: 3648. https://doi.org/10.3390/molecules26123648
APA StyleZhai, J., Fan, B., Thang, S. H., & Drummond, C. J. (2021). Novel Amphiphilic Block Copolymers for the Formation of Stimuli-Responsive Non-Lamellar Lipid Nanoparticles. Molecules, 26(12), 3648. https://doi.org/10.3390/molecules26123648






