Active Barrier Coating for Packaging Paper with Controlled Release of Sunflower Oils
Abstract
1. Introduction
2. Experimental Details
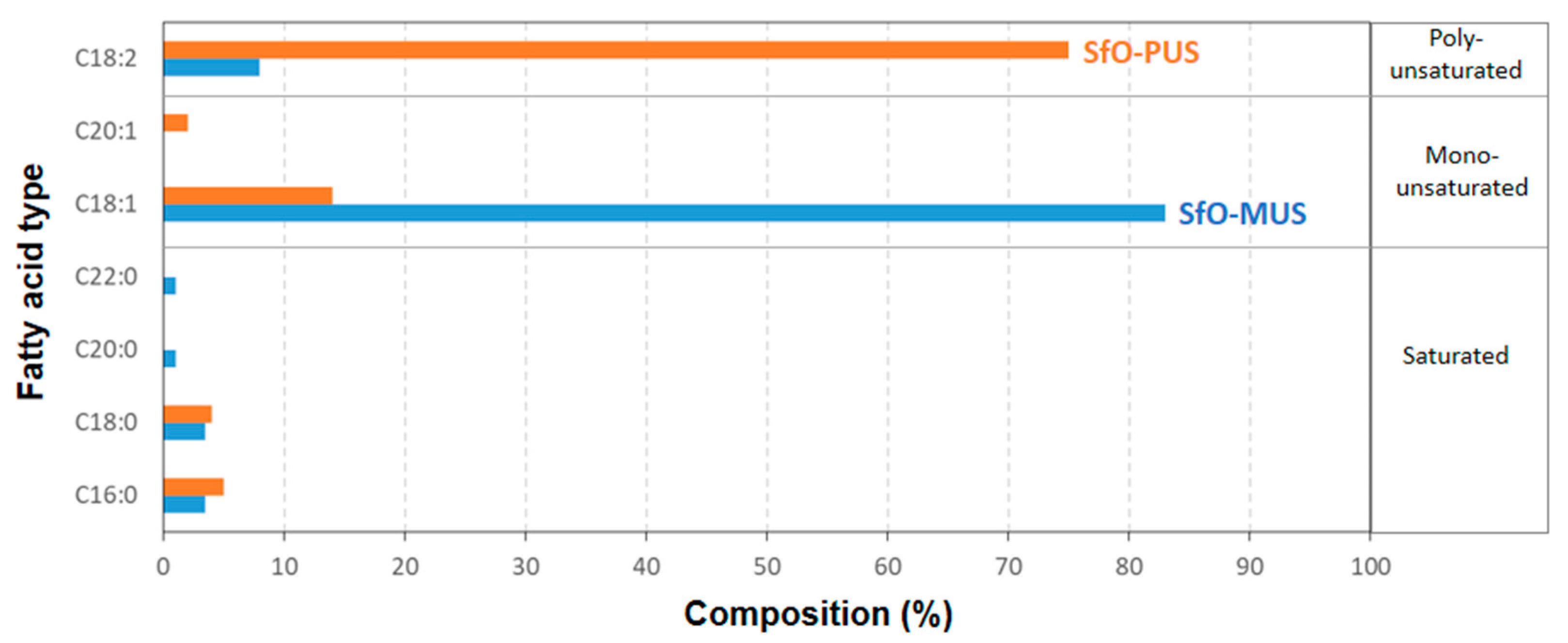
2.1. Materials
2.2. Coating Application
2.3. Characterization Methods
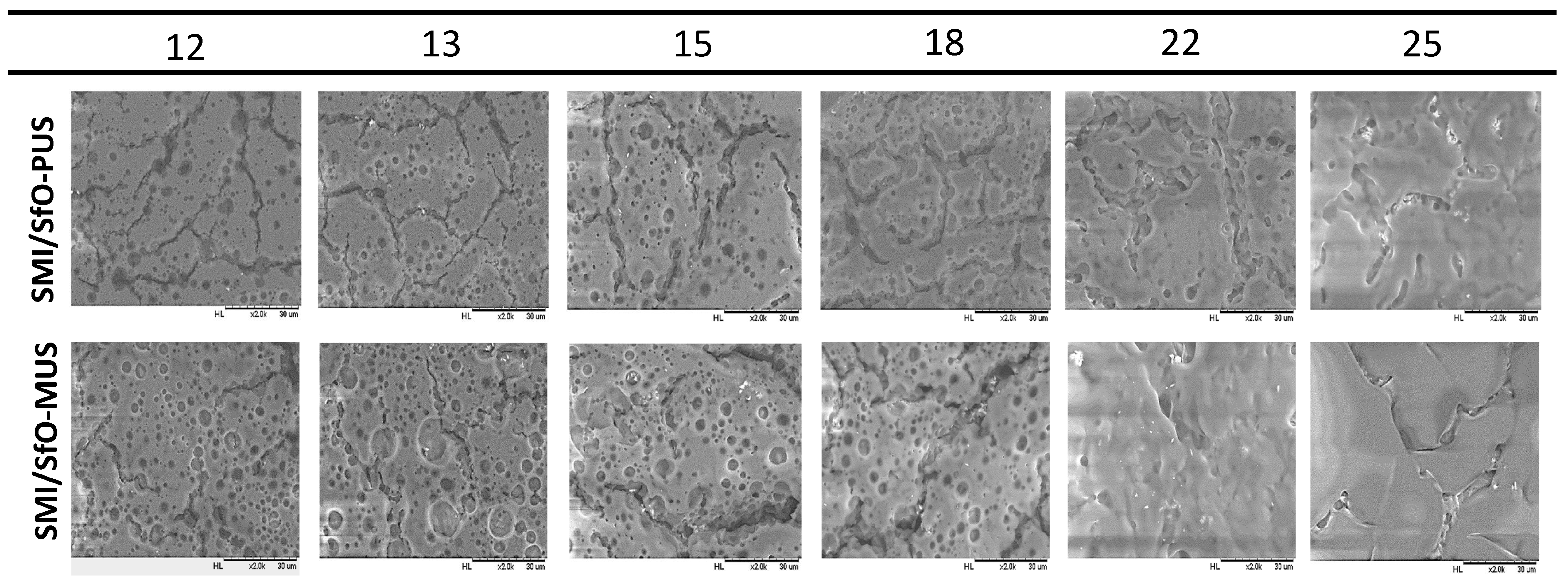
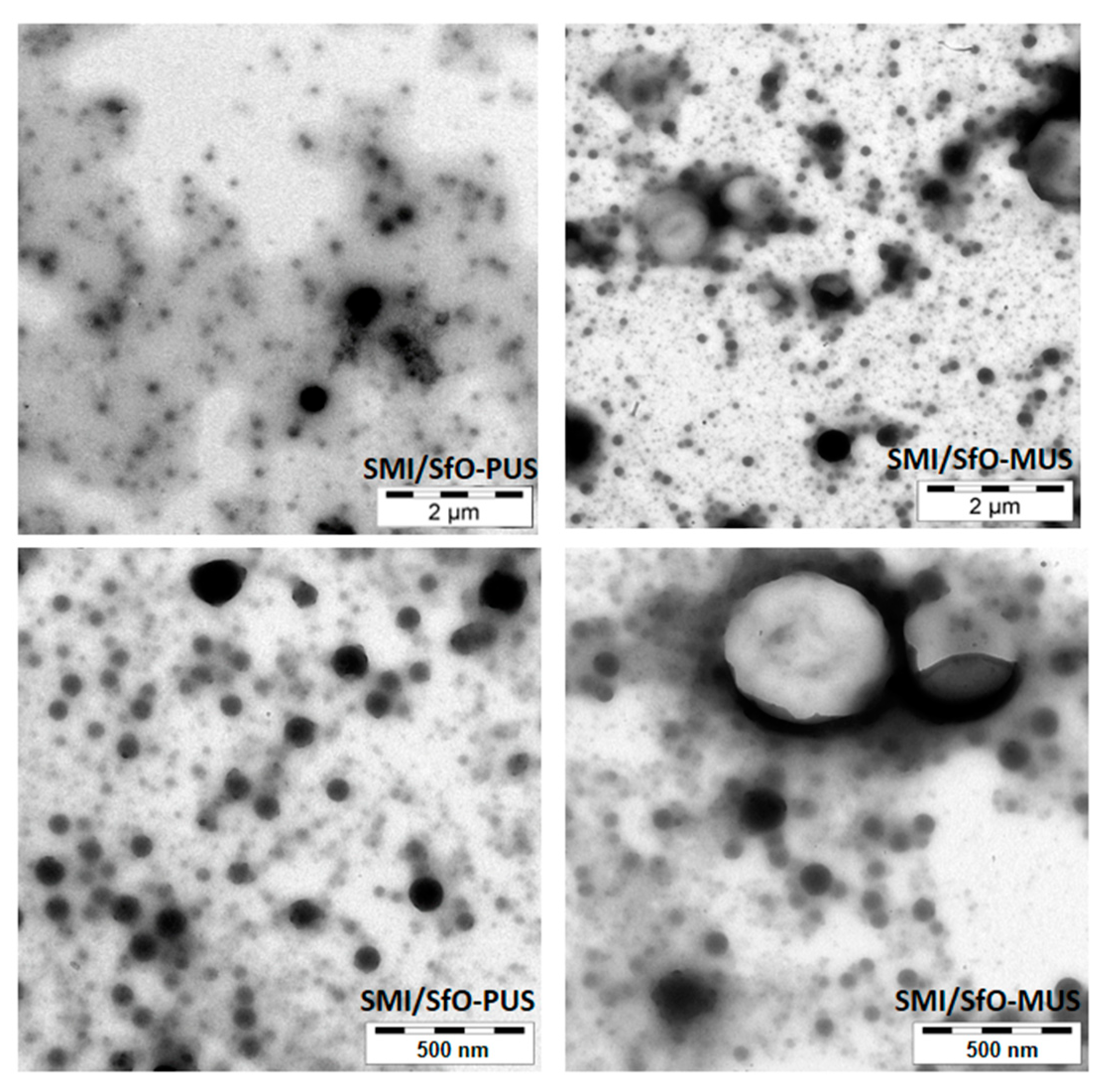
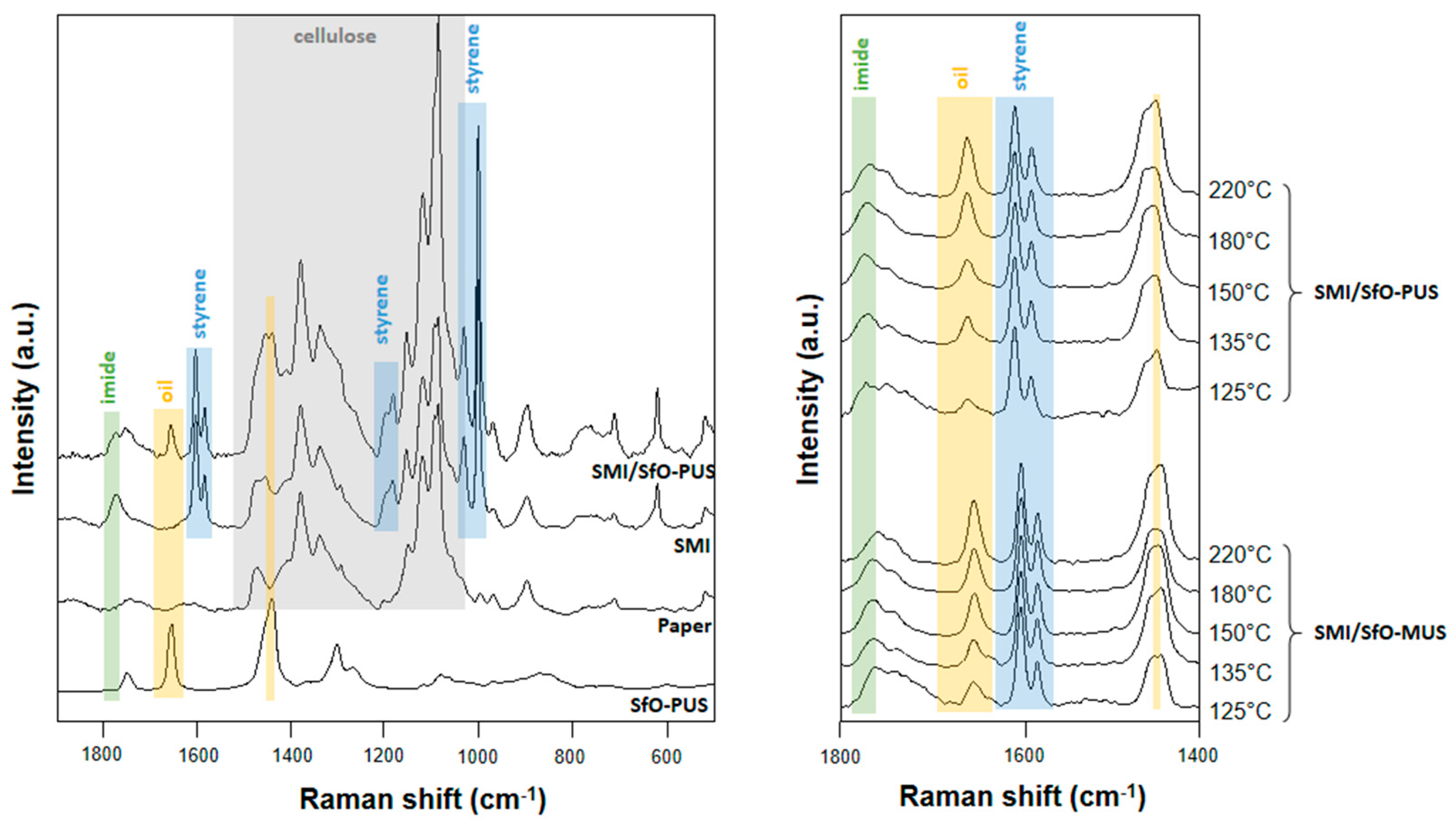
3. Test Results
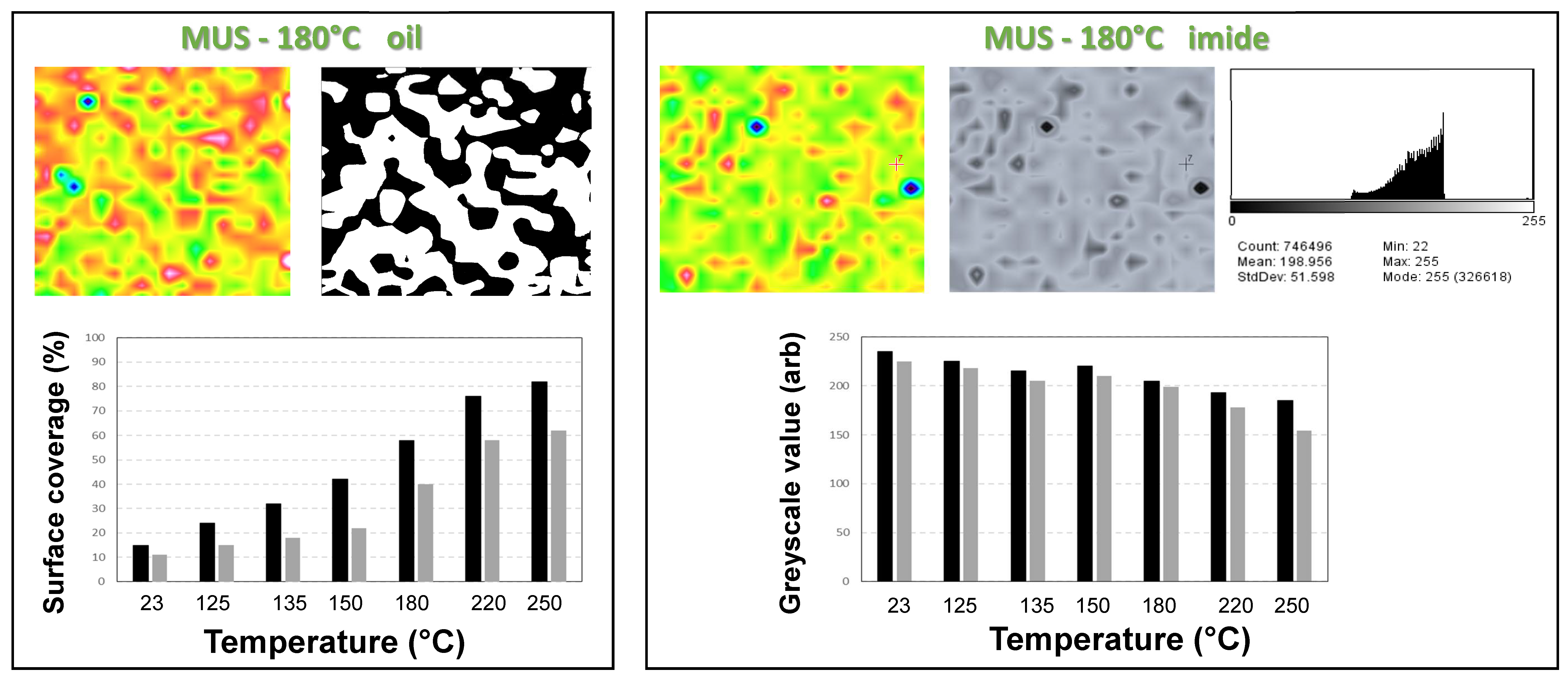
3.1. Microscopic Morphology of Paper Coatings
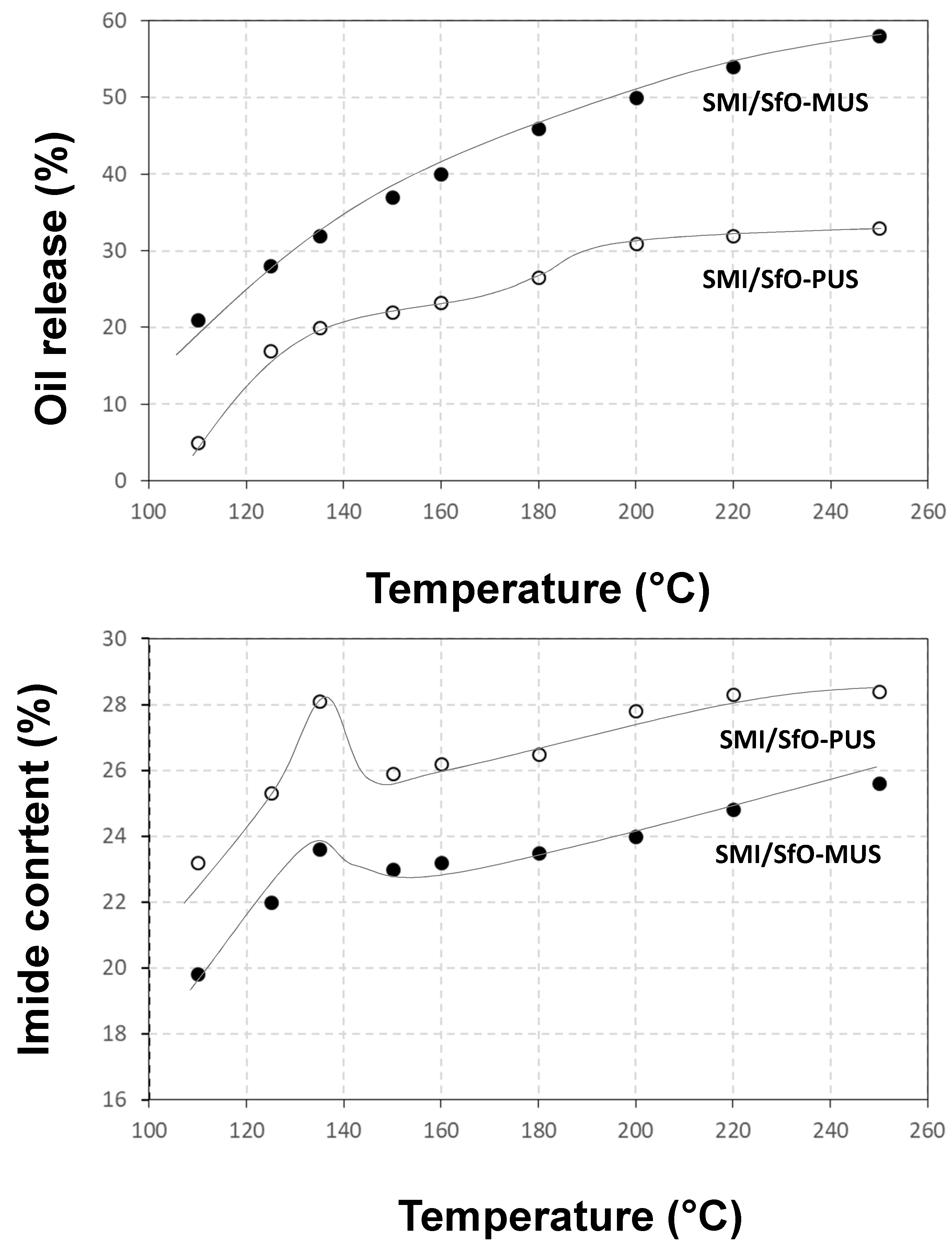
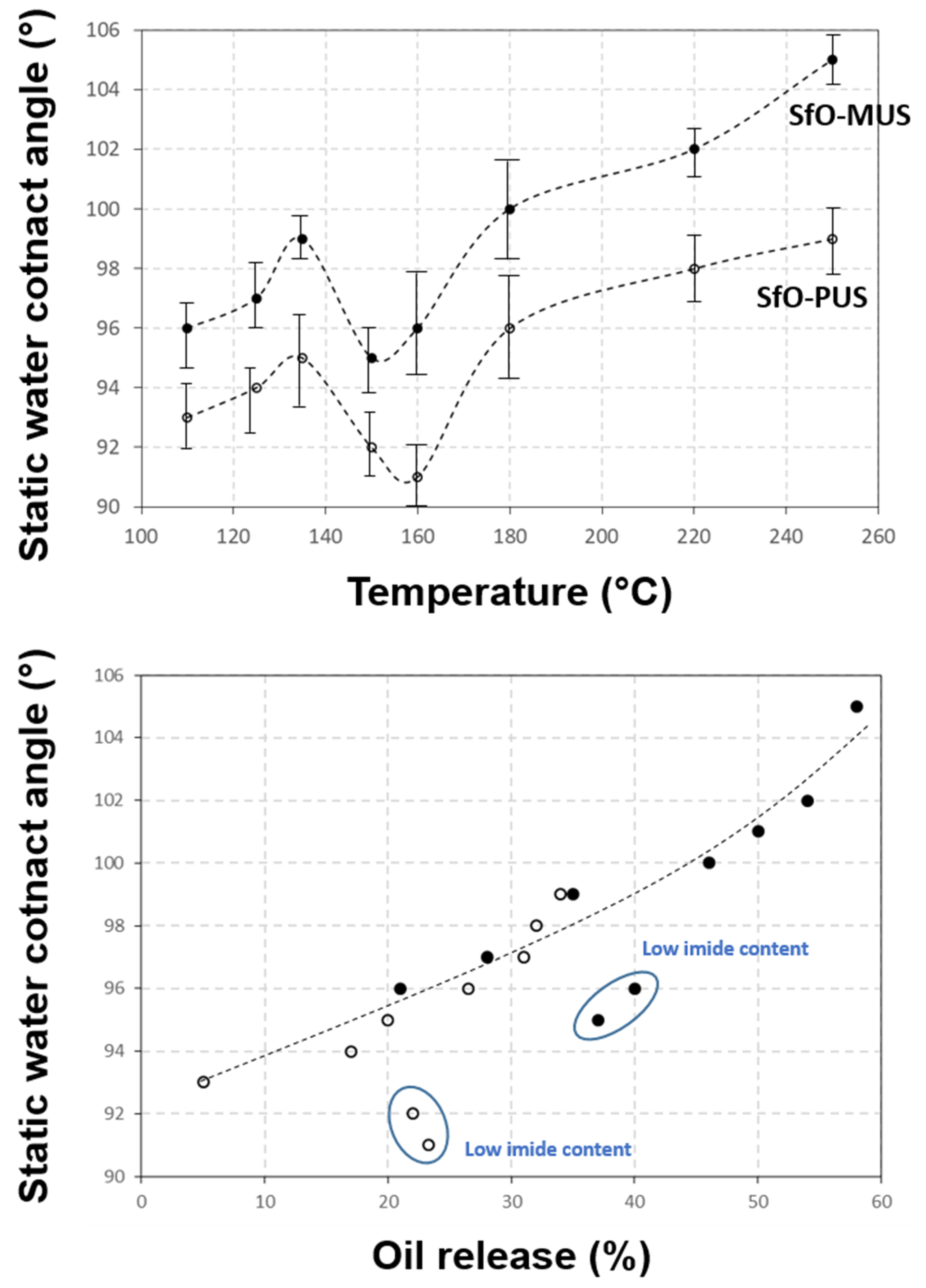
3.2. Quantification of Release Profiles from Single-Point Micro-Raman Spectroscopy
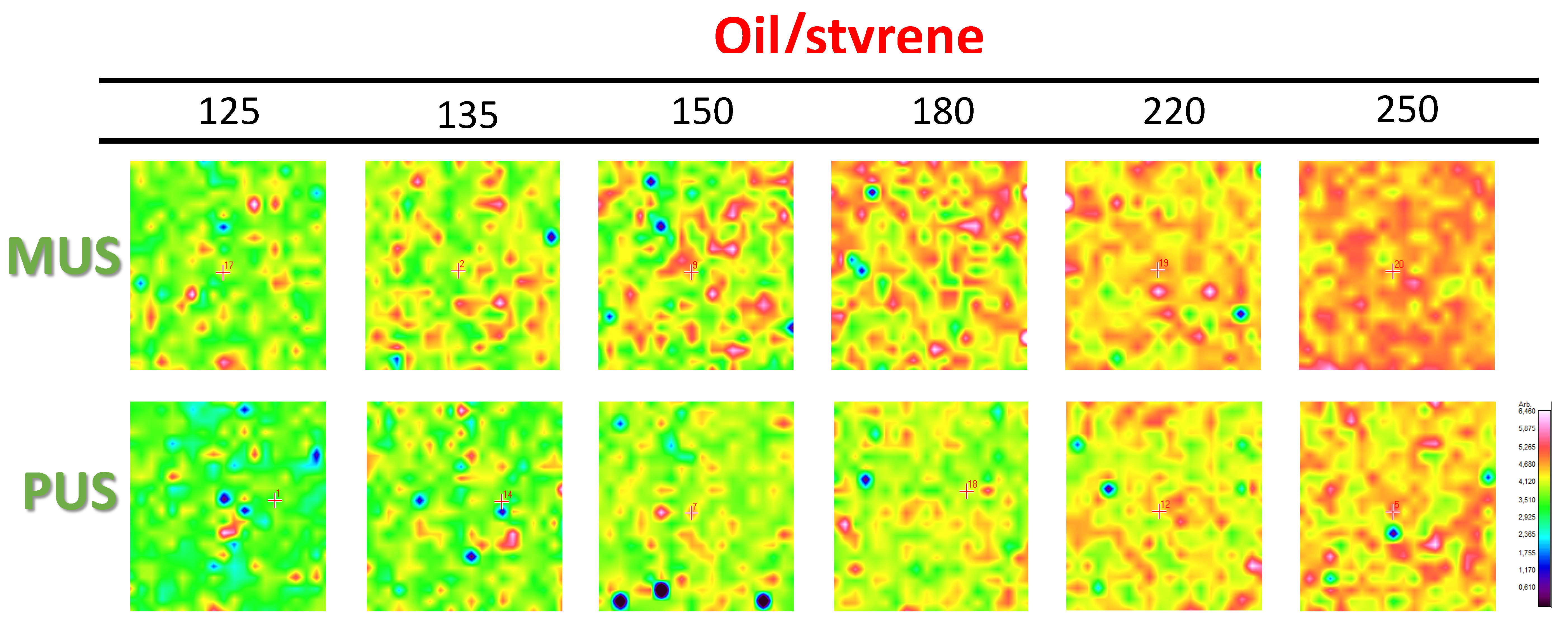
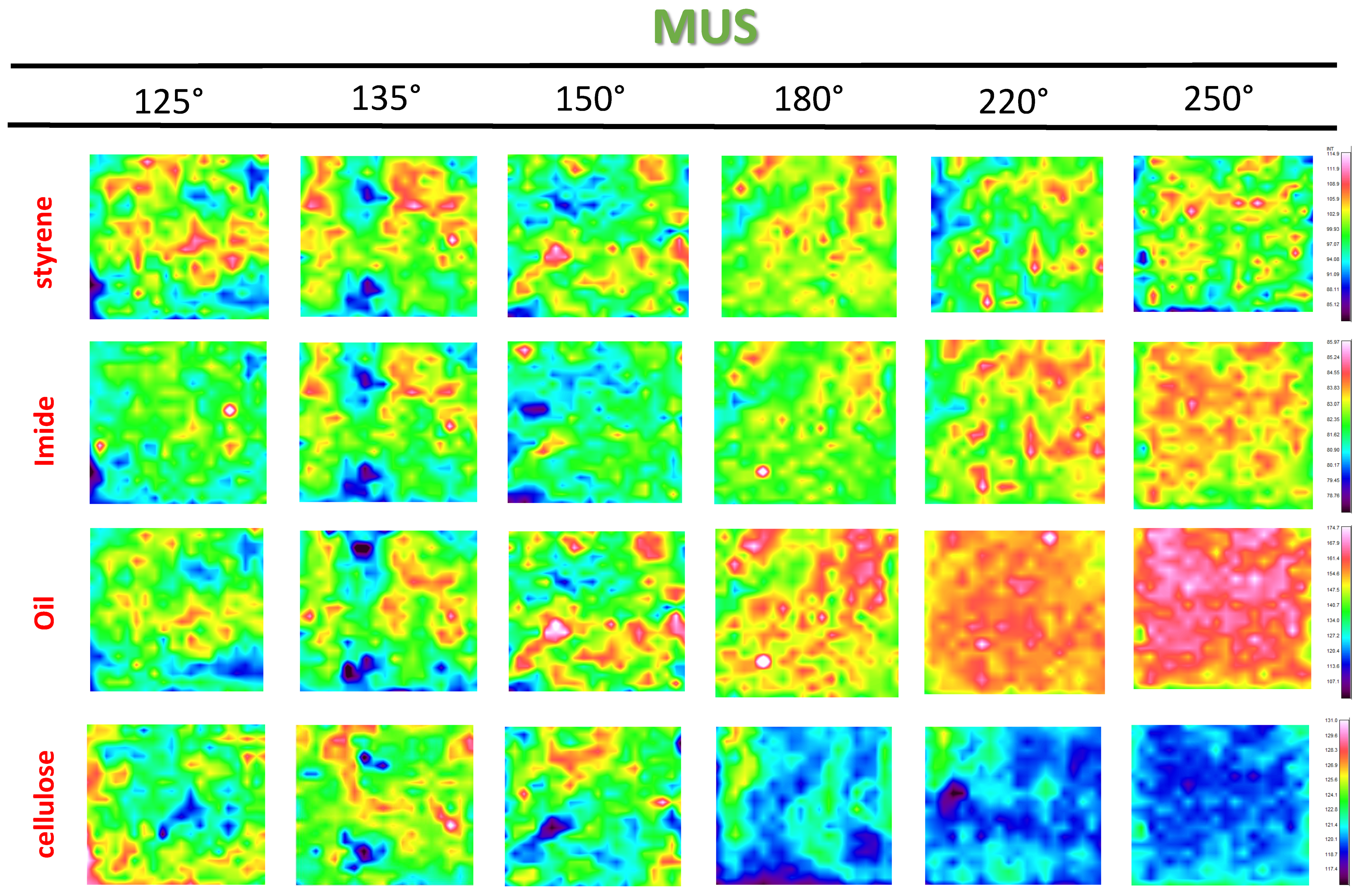
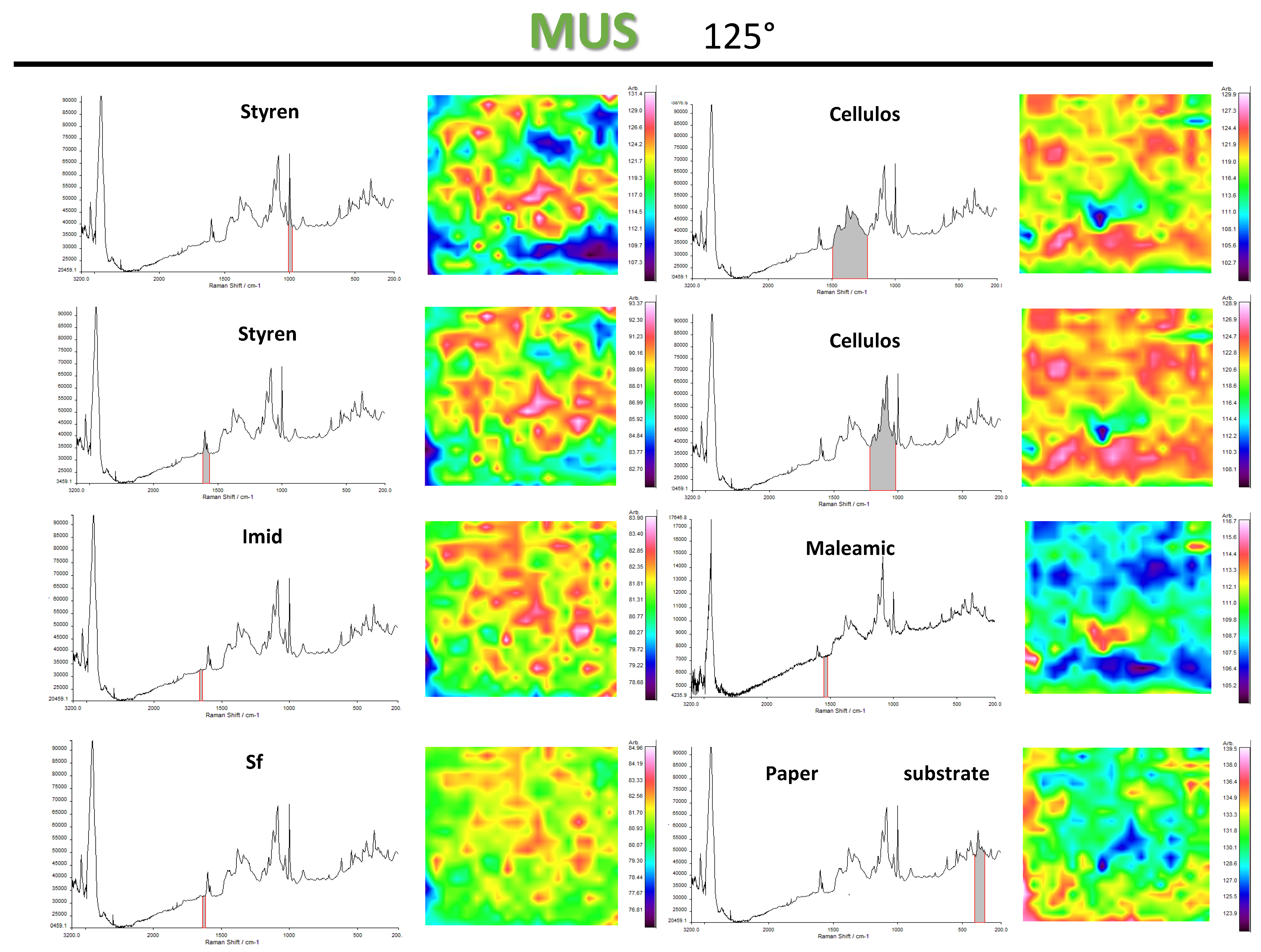
3.3. Chemical Surface Mapping by Micro-Raman Spectroscopy
3.4. Quantification of Release Profiles from Micro-Raman Mapping
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Otto, S.; Strenger, M.; Maier-Nörth, A.; Schmid, M. Food packaging and sustainability—consumer perception vs. correlated scientific facts: A review. J. Clean Prod. 2021, 298, 126733. [Google Scholar] [CrossRef]
- Vasile, C.; Baican, M. Progresses in food packaging, food quality, and safety—controlled-release antioxidant and/or antimicrobial packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, K.; Singh, S.; Ajji, A. Moisture absorbers for food packaging applications. Environ. Chem. Lett. 2019, 17, 609–628. [Google Scholar] [CrossRef]
- Hirvikorpi, T.; Vaha-Nissi, M.; Harlin, A.; Karppinen, M. Comparison of some coating techniques to fabricate barrier layers on packaging materials. Thin Solid Films 2010, 518, 5463–5466. [Google Scholar] [CrossRef]
- Bastarrachea, L.J.; Wong, D.E.; Roman, M.J.; Lin, Z.; Goddard, J.M. Active packaging coatings. Coatings 2015, 5, 771–791. [Google Scholar] [CrossRef]
- Conte, A.; Gammariello, D.; Di Guilio, S.; Attanasio, M.; Del Nobile, M.A. Active coating and modified-atmosphere packaging to extend the shelf life of Fior di Latte cheese. J. Diary Sci. 2009, 92, 887–894. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Advances in controlled release devices for food packaging applications. Trends Food Sci. Technol. 2010, 21, 591–598. [Google Scholar] [CrossRef]
- Chen, X.; Chen, M.; Xu, C.; Yam, K.L. Critical review of controlled release packaging to improve food safety and quality. Crit. Rev. Food Sci. Nutr. 2019, 59, 2386–2399. [Google Scholar] [CrossRef]
- Almasi, H.; Oskouie, M.J.; Saleh, A. A review on techniques utilized for design of controlled release food active packaging. Crit. Rev. Food Sci. Nutr. 2020, 1–21. [Google Scholar] [CrossRef]
- Oloyede, O.O.; Lignou, S. Sustainable paper-based packaging: A consumer’s perspective. Foods 2021, 10, 1035. [Google Scholar] [CrossRef]
- Jasmani, L.; Ainun, Z.M.A.; Adnan, S.; Ibrahim, R.; Sapuan, S.M.; Ilyas, R.A. Sustainable Paper-Based Packaging. In Bio-Based Packaging: Material, Environmental and Economic Aspects; Sapuan, S.M., Ilyas, R.A., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 225–244. [Google Scholar]
- Samyn, P. Wetting and hydrophobic modification of cellulose surfaces for paper applications. J. Mater. Sci. 2013, 48, 6455–6498. [Google Scholar] [CrossRef]
- Nemli, G.; Colakoglu, G. The influence of lamination technique on the properties of particleboard. Build. Environ. 2005, 40, 83–87. [Google Scholar] [CrossRef]
- Andersson, C.; Ernstsson, M.; Järnström, L. Barrier properties and heat sealability/failure mechanisms of dispersion-coated paperboard. Pack. Technol. Sci. 2003, 15, 209–241. [Google Scholar] [CrossRef]
- Qin, C.; Wang, W.; Li, W.; Zhang, S. Reactive water vapor barrier coatings derived from cellulose undecenoyl esters for paper packaging. Coatings 2020, 10, 1032. [Google Scholar] [CrossRef]
- Hua, Q.; Liu, L.Y.; Karaaslan, M.A.; Rennecker, S. Aqueous dispersions of esterified lignin particles for hydrophobic coatings. Front. Chem. 2019, 7, 515. [Google Scholar] [CrossRef] [PubMed]
- Samain, X.; Langlois, V.; Renard, E.; Lorang, G. Grafting biodegradable polyesters onto cellulose. J. Appl. Polym. Sci. 2011, 121, 1183–1192. [Google Scholar] [CrossRef]
- Liu, R.; Li, Q.; Liu, J.; Duan, Y.; Gao, T. Graft copolymerization of MA/(TFEA or TFPM) onto cellulosic fibers for surface hydrophobicity. Cellulose 2021, 28, 3981–3995. [Google Scholar] [CrossRef]
- Tambe, C.; Graiver, D.; Narayan, R. Moisture resistance coating of packaging paper from biobased silylated soybean oil. Prog. Org. Coat. 2016, 101, 270–278. [Google Scholar] [CrossRef]
- Tucekova, Z.K.; Galmiz, O.; Kelar, J.; Kovacik, D.; Stupavska, M.; Sramkova, P.; Zemalk, M.; Vallade, J.; Cernak, M. Adhesive properties of silicone-coated release liner paper enhanced by atmospheric pressure plasma preand post-treatment. Coatings 2020, 10, 1102. [Google Scholar] [CrossRef]
- Dankovich, T.A.; Hsieh, Y.L. Surface modification of cellulose with plant triglycerides for hydrophobicity. Cellulose 2007, 14, 469–480. [Google Scholar] [CrossRef]
- Cordt, C.; Geissler, A.; Biesalski, M. Regenerative superhydrophobic paper coatings by in situ formation of waxy nanostructures. Adv. Mater. Interfac. 2020, 8, 2001265. [Google Scholar] [CrossRef]
- Gras, S.L.; Mahmud, T.; Rosengarten, G.; Mitchell, A.; Kalantar-Zadeh, K. Intelligent control of surface hydrophobicity. Chem. Phys. Chem. 2007, 8, 2036–2050. [Google Scholar] [CrossRef]
- Nau, M.; Seelinger, D.; Biesalski, M. Independent two way switching of the wetting behavior of cellulose-derived nanoparticle surface coatings by light and by temperature. Adv. Mater. Interfac. 2019, 6, 1900378. [Google Scholar] [CrossRef]
- Madhan, S.; Espirito Santo, C.; Andrade, L.P.; Da Silva, P.; Gaspar, P.D. Active and intelligent packaging with phase change materials to promote the shelf life extension of food products. KnE Eng. 2020, 5, 232–241. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, Y.S. Phase change materials for advanced cooling packaging. Environ. Chem. Lett. 2018, 16, 845–859. [Google Scholar] [CrossRef]
- Firouz, M.S.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.A.; Azizi-Malabadi, M.; Tavassoli, M.; Mohammadi, K.; McClements, D.J. Recent advances in the development of smart and active biodegradable packaging materials. Nanomaterials 2021, 11, 1331. [Google Scholar] [CrossRef]
- Nogueira, G.F.; de Oliveira, R.A.; Velasco, J.I.; Fakhouri, F.M. Methods of incorporating plant-derived bioactive compounds into films made with agro-based polymers for application as food packaging: A brief review. Polymers 2020, 12, 2518. [Google Scholar] [CrossRef]
- Zanetti, M.; Carniel, T.K.; Dalcanton, F.; dos Anjos, R.S.; Riella, H.G.; de Araujo, P.H.H.; de Oliveira, D.; Fiori, M.A. Use of encapsulated natural compounds as antimicrobial additives in food packaging: A brief review. Trends Food Sci. Technol. 2018, 81, 51–60. [Google Scholar] [CrossRef]
- Gemili, S.; Yemenicioglu, A.; Altinkaya, S.A. Development of antioxidant food packaging materials with controlled release properties. J. Food Eng. 2010, 96, 325–332. [Google Scholar] [CrossRef]
- Tian, F.; Decker, E.A.; Goddard, J.M. Controlling lipid oxidation of food by active packaging technologies. Food Funct. 2013, 4, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Chen, X.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of essential oil as a sustained release preparation in food packaging. Trends Food Sci. Technol. 2019, 92, 22–32. [Google Scholar] [CrossRef]
- Sumiga, B.; Sumiga, B.; Ravnjak, D.; Podgornik, B.B. Antimicrobial paper coatings containing microencapsulated cymbopogon citratus oil. Coatings 2019, 9, 470. [Google Scholar] [CrossRef]
- Martin, A.; Varona, S.; Navarette, A.; Cocero, M.J. Encapsulation and co-precipitation processes with supercritical fluids: Applications with essential oils. Open Chem. Eng. J. 2010, 4, 31–41. [Google Scholar] [CrossRef]
- Becerril, R.; Nerin, C.; Silva, F. Encapsulation systems for antimicrobial food packaging components: An update. Molecules 2020, 25, 1134. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Nunes, I.L. Oil nanoencapsulation: Development, application, and incorporation into the food market. Nanoscale Res. Lett. 2019, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- You, Y.Z.; Kalebaila, K.K.; Brock, S.L.; Oupicky, D. Temperature-controlled uptake and release in PNIPAM-modified porous silica nanoparticles. Chem. Mater. 2008, 20, 3354–3359. [Google Scholar] [CrossRef]
- Giroux, H.J.; Britten, M. Encapsulation of hydrophobic aroma in whey protein nanoparticles. J. Microencapsul. 2011, 28, 337–343. [Google Scholar] [CrossRef]
- Soliman, O.; Ibrahim, S. Encapsulation of carbon black through miniemulsion polymerization as hydrophobic coating for packaging materials. Der Pharma Chem. 2016, 8, 245–252. [Google Scholar]
- Razaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocol. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Saffarionpour, S. Nanoencapsulation of hydrophobic food flavor ingredients and their cyclodextrin inclusion complexes. Food Bioprocess Technol. 2019, 12, 1157–1173. [Google Scholar] [CrossRef]
- Lopez-Gomez, A.; Ros-Chumillas, M.; Buendia-Moreno, L.; Martinez-Hernandez, G.M. Active cardboard packaging with encapsulated essential oils for enhancing the shelf life of fruit and vegetables. Front. Nutr. 2020, 7, 559978. [Google Scholar] [CrossRef]
- Buendia-Moreno, L.; Soto-Jover, S.; Ros-Chumillas, M.; Antolinos, V.; Navarro-Segura, L.; Sanchez-Martinez, J.; Martinez-Hernandez, G.B.; Lopez-Gomez, A. Innovative cardboard active packaging with a coating including encapsulated essential oils to extend cherry tomato shelf life. LWT 2019, 116, 108584. [Google Scholar] [CrossRef]
- Rungwasantisuk, A.; Raibhu, S. Application of encapsulating lavender essential oil in gelatin/gum-arabic complex coacervate and varnish screen-printing in making fragrant gift-wrapping paper. Prog. Org. Coat. 2020, 149, 105924. [Google Scholar] [CrossRef]
- Ozdemir, M.; Kemerli, T. Innovative applications of micro and nanoencapsulation in food packaging. In Encapsulation and Controlled Release Technologies in Food Systems, 2nd ed.; Lakkis, J.M., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 333–378. [Google Scholar]
- Chaudhari, A.K.; Singh, V.P.; Das, S.; Dubey, N.K. Nanoencapsulation of essential oils and their bioactive constituents: A novel strategy to control mycotoxin contamination in food system. Food Chem. Toxicol. 2021, 149, 112019. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Gu, K.; Qiang, S.; Wang, C. Environmental stimuli-responsive self-repairing waterbased superhydrophobic coatings. RSC Adv. 2017, 7, 543–550. [Google Scholar] [CrossRef]
- Wang, B.; Ye, Z.; Xu, Q.; Liu, H.; Lin, Q.; Chen, H.; Nan, K. Construction of a temperature-responsive terpolymer coating with recyclable bactericidal and self-cleaning antimicrobial properties. Biomater. Sci. 2016, 4, 1731–1741. [Google Scholar] [CrossRef]
- Samyn, P.; Schoukens, G.; Stanssens, D.; Vonck, L.; Van den Abbeele, H. Incorporating different vegetable oils into an aqueous dispersion of hybrid organic nanoparticles. J. Nanopart. Res. 2012, 14, 1075–1085. [Google Scholar] [CrossRef]
- Roberts, C.C.; Francis, L.F. Drying and cracking of soft latex coatings. J. Coat. Technol. Res. 2013, 10, 441–451. [Google Scholar] [CrossRef]
- Samyn, P.; Deconinck, M.; Schoukens, G.; Stanssens, D.; Vonck, L.; Van den Abbeele, H. Synthesis and characterization of imidized poly(styrene-maleic anhydride) organic nanoparticles in stable aqueous dispersion. Polym. Adv. Technol. 2012, 23, 311–325. [Google Scholar] [CrossRef]
- Seo, Y.; Lee, S.M.; Kim, D.Y.; Kim, K.U. Kinetic study of the imidization of a poly(ester amic acid) by ft-raman spectroscopy. Macromolecules 1997, 30, 3747–3753. [Google Scholar] [CrossRef]
- Wang, A.; Freema, J.J.; Jolliff, B.L. Understanding the Raman spectral features of phyllosilicates. J. Raman Spectrosc. 2014, 46, 829–845. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samyn, P. Active Barrier Coating for Packaging Paper with Controlled Release of Sunflower Oils. Molecules 2021, 26, 3561. https://doi.org/10.3390/molecules26123561
Samyn P. Active Barrier Coating for Packaging Paper with Controlled Release of Sunflower Oils. Molecules. 2021; 26(12):3561. https://doi.org/10.3390/molecules26123561
Chicago/Turabian StyleSamyn, Pieter. 2021. "Active Barrier Coating for Packaging Paper with Controlled Release of Sunflower Oils" Molecules 26, no. 12: 3561. https://doi.org/10.3390/molecules26123561
APA StyleSamyn, P. (2021). Active Barrier Coating for Packaging Paper with Controlled Release of Sunflower Oils. Molecules, 26(12), 3561. https://doi.org/10.3390/molecules26123561





