Review: β-glucans as Effective Antibiotic Alternatives in Poultry
Abstract
1. The Poultry Immune System
2. Bacterial Infections and Parasitism of the Intestinal Tract in Poultry
3. Antibiotic Use in Poultry Production and Its Effects on Bacterial Resistance
4. Beta-glucans
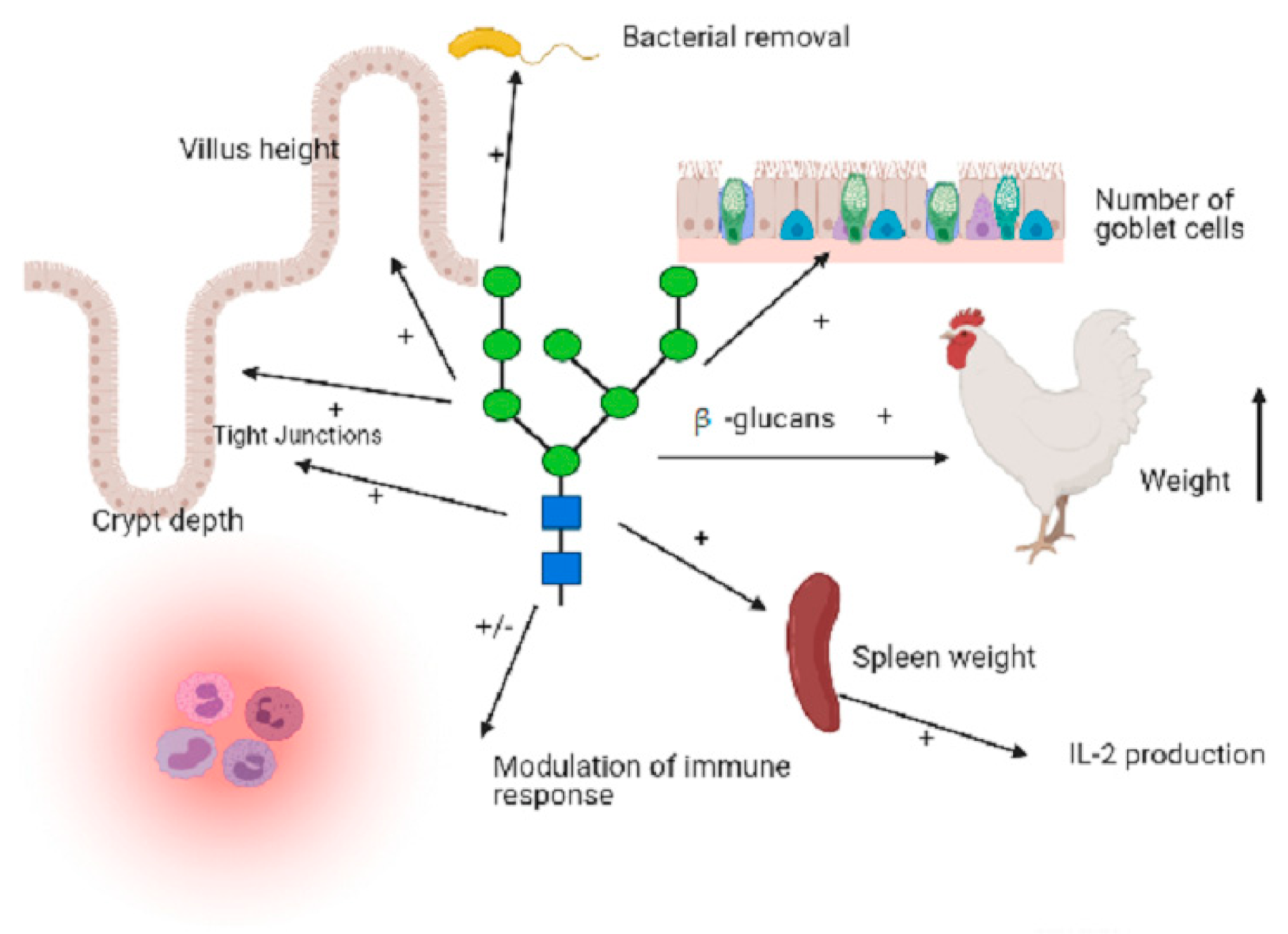
5. Beta-glucans and the Poultry Gastrointestinal and Immune System
6. Glucans as Effective Measure Against Foodborne Disease Infection in Poultry
7. The Putative Mechanisms by which β-glucans may Exert Antibiotic Alternative Effects
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kajiwara, E.; Shigeta, A.; Horiuchi, H.; Matsuda, H.; Furusawa, S. Development of Peyer’s patch and cecal tonsil in gut-associated lymphoid tissues in the chicken embryo. J. Vet. Med. Sci. 2003, 65, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.S.; Trout, J.M. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin. Microbiol. Rev. 1996, 9, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Salmonella infection–prevention and treatment by antibiotics and probiotic yeasts: A review. Microbiology 2018, 164, 1327–1344. [Google Scholar] [CrossRef]
- Ravel, A.; Smolina, E.; Sargeant, J.M.; Cook, A.; Marshall, B.; Fleury, M.D.; Pollari, F. Seasonality in human salmonellosis: Assessment of human activities and chicken contamination as driving factors. Foodborne Pathog. Dis. 2010, 7, 785–794. [Google Scholar] [CrossRef]
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol. 2010, 8, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The population genetics of pathogenic E. coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef]
- Pechine, S.; Collignon, A. Immune responses induced by Clostridium difficile. Anaerobe 2016, 41, 68–78. [Google Scholar] [CrossRef]
- Abt, M.C.; McKenney, P.T.; Pamer, E.G. Clostridium difficile colitis: Pathogenesis and host defence. Nat. Rev. Microbiol. 2016, 14, 609–620. [Google Scholar] [CrossRef]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef] [PubMed]
- López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Overview of Poultry Eimeria Life Cycle and Host-Parasite Interactions. Front. Vet. Sci. 2020, 7, 384. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yan, X.; Lillehoj, H.S.; Oh, S.; Liu, L.; Sun, Z.; Gu, C.; Lee, Y.; Xianyu, Z.; Zhao, H. Eimeria maxima-induced transcriptional changes in the cecal mucosa of broiler chickens. Parasites Vectors 2019, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014; p. 256. Available online: https://www.who.int/drugresistance/documents/surveillancereport/en/ (accessed on 15 April 2021).
- Aarestrup, F.M.; Wegener, H.C.; Collignon, P. Resistance in bacteria of the food chain: Epidemiology and control strategies. Expert Rev. Anti-Infect. Ther. 2008, 6, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Aidara-Kane, A.; Angulo, F.J.; Conly, J.M.; Minato, Y.; Silbergeld, E.K.; McEwen, S.A.; Collignon, P.J. World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrob. Resist. Infect. Control. 2018, 7, 7. [Google Scholar] [CrossRef]
- Heredia, N.; Garcia, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef]
- Andrew Selaledi, L.; Mohammed Hassan, Z.; Manyelo, T.G.; Mabelebele, M. The Current Status of the Alternative Use to Antibiotics in Poultry Production: An African Perspective. Antibiotics 2020, 9, 594. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Natural immunomodulators and their stimulation of immune reaction: True or false? Anticancer. Res. 2014, 34, 2275–2282. [Google Scholar]
- Synytsya, A.; Novak, M. Structural analysis of glucans. Ann. Transl. Med. 2014, 2, 17. [Google Scholar]
- Novak, M.; Vetvicka, V. Glucans as biological response modifiers. Endocr. Metab. Immune. Disord. Drug Targets 2009, 9, 67–75. [Google Scholar] [CrossRef]
- Laroche, C.; Michaud, P. New developments and prospective applications for betaβ (1,3) glucans. Recent Pat. Biotechnol. 2007, 1, 59–73. [Google Scholar] [CrossRef]
- Ohno, N.K.; Yadomae, T. Physicochemical properties and antitumor activities of carboxymethylated derivatives of glucan from Sclerotinia slerotiorum. Chem. Pharm. Bull. 1988, 36, 1118–1125. [Google Scholar] [CrossRef]
- Chae, B.J.; Lohakare, J.D.; Moon, W.K.; Lee, S.L.; Park, Y.H.; Hahn, T.W. Effects of supplementation of betaβ-glucan on the growth performance and immunity in broilers. Res. Vet. Sci. 2006, 80, 291–298. [Google Scholar] [CrossRef]
- Cho, J.H.; Zhang, Z.F.; Kim, I.H. Effects of single or combined dietary supplementation of betaβ-glucan and kefir on growth performance, blood characteristics and meat quality in broilers. Br. Poult. Sci. 2013, 54, 216–221. [Google Scholar] [CrossRef]
- Moon, S.H.; Lee, I.; Feng, X.; Lee, H.Y.; Kim, J.; Ahn, D.U. Effect of Dietary BetaΒ-Glucan on the Performance of Broilers and the Quality of Broiler Breast Meat. Asian -Australas. J. Anim. Sci. 2016, 29, 384–389. [Google Scholar] [CrossRef]
- Langhout, P. New additives for broiler chickens. World Poult. 2000, 16, 22–27. [Google Scholar]
- Rajapakse, J.R.; Buddhika, M.D.; Nagataki, M.; Nomura, H.; Watanabe, Y.; Ikeue, Y.; Agatsuma, T. Effect of Sophy betaβ-glucan on immunity and growth performance in broiler chicken. J. Vet. Med. Sci. 2010, 72, 1629–1632. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.F.; Zhou, T.X.; Ao, X.; Kim, I.H. Effects of β-glucan and Bacillus subtilis on growth performance, blood profiles, relative organ weight and meat quality in broilers fed maize–soybean meal based diets. Livest. Sci. 2012, 150, 419–424. [Google Scholar] [CrossRef]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary Yeast BetaΒ-1,3/1,6-D-glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef]
- Guo, P.; Thomas, J.D.; Bruce, M.P.; Hinton, T.M.; Bean, A.G.; Lowenthal, J.W. The chicken TH1 response: Potential therapeutic applications of ChIFN-gamma. Dev. Comp. Immunol. 2013, 41, 389–396. [Google Scholar] [CrossRef]
- Lowry, V.K.; Farnell, M.B.; Ferro, P.J.; Swaggerty, C.L.; Bahl, A.; Kogut, M.H. Purified betaβ-glucan as an abiotic feed additive up-regulates the innate immune response in immature chickens against Salmonella enterica serovar Enteritidis. Int. J. Food Microbiol. 2005, 98, 309–318. [Google Scholar] [CrossRef]
- Anwar, M.; Muhammad, F.; Awais, M.; Akhtar, M. A review of β-glucans as a growth promoter and antibiotic alternative against enteric pathogens in poultry. World Poult. Sci. J. 2017, 73, 651–661. [Google Scholar] [CrossRef]
- Jacob, J.; Pescatore, A.J. Glucans and the Poultry Immune System. Am. J. Immunol. 2017, 13, 45–49. [Google Scholar] [CrossRef]
- Ding, B.; Zheng, J.; Wang, X.; Zhang, L.; Sun; Xing, Q.; Pirone, A.; Fronte, B. Effects of dietary yeast betaβ-1,3-1,6-glucan on growth performance, intestinal morphology and chosen immunity parameters changes in Haidong chicks. Asian-Australas J. Anim. Sci. 2019, 32, 1558. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Zhang, L.; Yang, R.; Fei, C.; Zhang, K.; Wang, C.; Liu, Y.; Xue, F. Effect of sulfated yeast betaβ-glucan on cyclophosphamide-induced immunosuppression in chickens. Int. Immunopharmacol. 2019, 74, 105690. [Google Scholar] [CrossRef]
- Wang, M.; Yang, R.; Zhang, L.; Meng, X.; Fei, C.; Zhang, K.; Wang, X.; Zheng, W.; Xiao, S.; Zhang, S.; et al. Sulfated glucan can improve the immune efficacy of Newcastle disease vaccine in chicken. Int. J. Biol. Macromol. 2014, 70, 193–198. [Google Scholar] [CrossRef]
- Murphy, P.; Bello, F.D.; O’Doherty, J.V.; Arendt, E.K.; Sweeney, T.; Coffey, A. Effects of cereal betaβ-glucans and enzyme inclusion on the porcine gastrointestinal tract microbiota. Anaerobe 2012, 18, 557–565. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Johnson, I.R.; Madsen, K.L.; Vasanthan, T.; Ball, R.; Field, C.J. Barley-derived betaβ-glucans increases gut permeability, ex vivo epithelial cell binding to E. coli, and naive T-cell proportions in weanling pigs. J. Anim. Sci. 2012, 90, 2652–2662. [Google Scholar] [CrossRef]
- Jacob, J.P.; Pescatore, A.J. Barley betaβ-glucan in poultry diets. Ann. Transl. Med. 2014, 2, 20. [Google Scholar] [PubMed]
- Shao, Y.; Wang, Z.; Tian, X.; Guo, Y.; Zhang, H. Yeast betaβ-d-glucans induced antimicrobial peptide expressions against Salmonella infection in broiler chickens. Int. J. Biol. Macromol. 2016, 85, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, E.E.; Lynch, R.D. The tight junction: A multifunctional complex. Am. J. Physiol. Cell Physiol. 2004, 286, C1213–C1228. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Guttman, J.A.; Samji, F.N.; Li, Y.; Vogl, A.W.; Finlay, B.B. Evidence that tight junctions are disrupted due to intimate bacterial contact and not inflammation during attaching and effacing pathogen infection in vivo. Infect. Immun. 2006, 74, 6075–6084. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Bergstrom, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schutte, A.; van der Post, S.; Svensson, F.; Rodriguez-Pineiro, A.M.; Nystrom, E.E.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef]
- Rubio, L.A. Possibilities of early life programming in broiler chickens via intestinal microbiota modulation. Poult. Sci. 2019, 98, 695–706. [Google Scholar] [CrossRef]
- Swiderska, Z.; Smidova, A.; Buchtova, L.; Bryjova, A.; Fabianova, A.; Munclinger, P.; Vinkler, M. Avian Toll-like receptor allelic diversity far exceeds human polymorphism: An insight from domestic chicken breeds. Sci. Rep. 2018, 8, 17878. [Google Scholar] [CrossRef]
- Guo, Y.; Ali, R.A.; Qureshi, M.A. The influence of betaβ-glucan on immune responses in broiler chicks. Immunopharmacol. Immunotoxicol. 2003, 25, 461–472. [Google Scholar] [CrossRef]
- Muthusamy, N.; Haldar, S.; Ghosh, T.K.; Bedford, M.R. Effects of hydrolysed Saccharomyces cerevisiae yeast and yeast cell wall components on live performance, intestinal histo-morphology and humoral immune response of broilers. Br. Poult. Sci. 2011, 52, 694–703. [Google Scholar] [CrossRef]
- Cox, C.M.; Sumners, L.H.; Kim, S.; McElroy, A.P.; Bedford, M.R.; Dalloul, R.A. Immune responses to dietary β-glucan in broiler chicks during an Eimeria challenge. Poult. Sci. 2010, 89, 2597–2607. [Google Scholar] [CrossRef]
- Karumuthil-Melethil, S.; Gudi, R.; Johnson, B.M.; Perez, N.; Vasu, C. Fungal betaβ-glucan, a Dectin-1 ligand, promotes protection from type 1 diabetes by inducing regulatory innate immune response. J. Immunol. 2014, 193, 3308–3321. [Google Scholar] [CrossRef]
- Zhao, J.; Cheung, P.C. Fermentation of betaβ-glucans derived from different sources by bifidobacteria: Evaluation of their bifidogenic effect. J. Agric. Food Chem. 2011, 59, 5986–5992. [Google Scholar] [CrossRef]
- Le, T.; Le, T.; Doan, T.H.; Quyen, D.; Le, K.X.; Pham, V.; Nagataki, M.; Nomura, H.; Ikeue, Y.; Watanabe, Y.; et al. The adjuvant effect of Sophy betaβ-glucan to the antibody response in poultry immunized by the avian influenza A H5N1 and H5N2 vaccines. J. Microbiol. Biotechnol. 2011, 21, 405–411. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P. The Effects of betaβ–Glucan on Fish Immunity. N. Am. J. Med. Sci. 2013, 5, 580–588. [Google Scholar] [CrossRef]
- de Jesus, R.B.; Petit, J.; Pilarski, F.; Wiegertjes, G.F.; Koch, J.F.A.; de Oliveira, C.A.F.; Zanuzzo, F.S. An early β-glucan bath during embryo development increases larval size of Nile tilapia. Aquac. Res. 2019, 50, 2012–2014. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y.; Wang, Z. The Modulating Effect of β-1, 3/1, 6-glucan Supplementation in the Diet on Performance and Immunological Responses of Broiler Chickens. Asian-Australas. J. Anim. Sci. 2008, 21, 237–244. [Google Scholar] [CrossRef]
- Rathgeber, B.M.; Budgell, K.L.; MacIsaac, J.L.; Mirza, M.A.; Doncaster, K.L. Growth performance and spleen and bursa weight of broilers fed yeast betaβ-glucan. Can. J. Anim. Sci. 2008, 88, 469–473. [Google Scholar] [CrossRef]
- Zhang, S.; Ou, J.; Luo, Z.; Kim, I.H. Effect of dietary β-1,3-glucan supplementation and heat stress on growth performance, nutrient digestibility, meat quality, organ weight, ileum microbiota, and immunity in broilers. Poult. Sci. 2020, 99, 4969–4977. [Google Scholar] [CrossRef]
- Cox, C.M.; Stuard, L.H.; Kim, S.; McElroy, A.P.; Bedford, M.R.; Dalloul, R.A. Performance and immune responses to dietary betaβ-glucan in broiler chicks. Poult. Sci. 2010, 89, 1924–1933. [Google Scholar] [CrossRef]
- Morales-Lopez, R.; Auclair, E.; Garcia, F.; Esteve-Garcia, E.; Brufau, J. Use of yeast cell walls; betaβ-1, 3/1, 6-glucans; and mannoproteins in broiler chicken diets. Poult. Sci. 2009, 88, 601–607. [Google Scholar] [CrossRef]
- Huff, G.R.; Huff, W.E.; Farnell, M.B.; Rath, N.C.; Solis de Los Santos, F.; Donoghue, A.M. Bacterial clearance, heterophil function, and hematological parameters of transport-stressed turkey poults supplemented with dietary yeast extract. Poult. Sci. 2010, 89, 447–456. [Google Scholar] [CrossRef]
- Redmond, S.B.; Tell, R.M.; Coble, D.; Mueller, C.; Palic, D.; Andreasen, C.B.; Lamont, S.J. Differential splenic cytokine responses to dietary immune modulation by diverse chicken lines. Poult. Sci. 2010, 89, 1635–1641. [Google Scholar] [CrossRef]
- Shao, Y.; Guo, Y.; Wang, Z. betaβ-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poult. Sci. 2013, 92, 1764–1773. [Google Scholar] [CrossRef]
- Omara, I.I.; Pender, C.M.; White, M.B.; Dalloul, R.A. The Modulating Effect of Dietary BetaΒ-Glucan Supplementation on Expression of Immune Response Genes of Broilers during a Coccidiosis Challenge. Animals 2021, 11, 159. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Update on mucosal immunoglobulin A in gastrointestinal disease. Curr. Opin. Gastroenterol. 2010, 26, 554–563. [Google Scholar] [CrossRef]
- Revolledo, L.; Ferreira, C.S.; Ferreira, A.J. Prevention of Salmonella Typhimurium colonization and organ invasion by combination treatment in broiler chicks. Poult. Sci. 2009, 88, 734–743. [Google Scholar] [CrossRef]
- Jajere, S.M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World 2019, 12, 504–521. [Google Scholar] [CrossRef]
- de los Santos, F.S.; Donoghue, A.M.; Farnell, M.B.; Huff, G.R.; Huff, W.E.; Donoghue, D.J. Gastrointestinal maturation is accelerated in turkey poults supplemented with a mannan-oligosaccharide yeast extract (Alphamune). Poult. Sci. 2007, 86, 921–930. [Google Scholar] [CrossRef]
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Mackiewicz, A.; Schmidt, M. Secretory IgA in Intestinal Mucosal Secretions as an Adaptive Barrier against Microbial Cells. Int. J. Mol. Sci. 2020, 21, 9254. [Google Scholar] [CrossRef]
- Commins, S.P.; Borish, L.; Steinke, J.W. Immunologic messenger molecules: Cytokines, interferons, and chemokines. J. Allergy Clin. Immunol. 2010, 125 (Suppl. S2), S53–S72. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Weng, B.C.; Chang, M.T.; Liao, Y.H.; Chen, T.T.; Chu, C. Direct enhancement of the phagocytic and bactericidal capability of abdominal macrophage of chicks by betaβ-1,3-1,6-glucan. Poult. Sci. 2008, 87, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. BetaΒ Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Lee, D.-N.; Wen, C.-M.; Weng, C.-F. Effects of β-Glucan Supplementation on Lymphocyte Proliferation, Macrophage Chemotaxis and Specific Immune Responses in Broilers. Asian Aust. J. Anim. Sci. 2004, 17, 1145–1149. [Google Scholar] [CrossRef]
- Zimmermann, C.E.; Cruz, I.B.; Cadona, F.C.; Machado, A.K.; Assmann, C.; Schlemmer, K.B.; Zanette, R.A.; Leal, D.B.; Santurio, J.M. Cytoprotective and genoprotective effects of betaβ-glucans against aflatoxin B(1)-induced DNA damage in broiler chicken lymphocytes. Toxicol. Vitro 2015, 29, 538–543. [Google Scholar] [CrossRef]
- Vetvicka, V.; Oliveira, C. β(1-3)(1-6)-D-Glucan with Strong Effects on Immune Status in Chicken: Potential Importance for Efficiency of Commercial Farming. J. Nutr. Health Sci. 2014, 1, 309–314. [Google Scholar]
- Muthusamy, G.; Joardar, S.N.; Samanta, I.; Roy, D.P.; Kumar, B.T. Dietary administered purified β-glucan of edible mushroom (Pleurotus florida) provides immunostimulation and protection in broiler experimentally challenged with virulent Newcastle disease virus. JoBAZ 2020, 81, 55–65. [Google Scholar] [CrossRef]
| Glucan | Effects | Reference |
|---|---|---|
| Yeast | Increased phagocytosis, microbicidal killing | [34] |
| Yeast | Improved growth parameters | [37] |
| Yeast | Inhibition of immunosuppression | [38] |
| Yeast | Improved efficacy of vaccines | [39] |
| Yeast | Improved GI health | [43] |
| Yeast | Increased phagocytosis | [50] |
| Yeast | Increased phagocytosis, IL-2 production, growth, decreased stress | [77] |
| Yeast | Upregulation of iNOS intestinal expression, downregulation of IL-8 | [52] |
| Yeast | Increased numbers of Treg cells | [53] |
| Yeast | Improved growth | [58] |
| Yeast | Increased anti-infection response | [43] |
| Yeast | Improved intestinal mucosa | [65] |
| Yeast | Increased chemotaxis | [75] |
| Barley | Increased intestinal viscosity | [42] |
| Mushroom | Increased phagocytosis, microbicidal killing | [78] |
| Mushroom | Increased proliferation of splenocytes, IL-2 production | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwartz, B.; Vetvicka, V. Review: β-glucans as Effective Antibiotic Alternatives in Poultry. Molecules 2021, 26, 3560. https://doi.org/10.3390/molecules26123560
Schwartz B, Vetvicka V. Review: β-glucans as Effective Antibiotic Alternatives in Poultry. Molecules. 2021; 26(12):3560. https://doi.org/10.3390/molecules26123560
Chicago/Turabian StyleSchwartz, Betty, and Vaclav Vetvicka. 2021. "Review: β-glucans as Effective Antibiotic Alternatives in Poultry" Molecules 26, no. 12: 3560. https://doi.org/10.3390/molecules26123560
APA StyleSchwartz, B., & Vetvicka, V. (2021). Review: β-glucans as Effective Antibiotic Alternatives in Poultry. Molecules, 26(12), 3560. https://doi.org/10.3390/molecules26123560







