Biological Effects of β-Glucans on Osteoclastogenesis
Abstract
1. Introduction
2. Immunoreceptor-Mediated Regulation of Ostetoclastogenesis
3. β-Glucan Receptors in Osteoclasts
4. Biological Effect of β-Glucans on Bone
4.1. Inhibitory Effects of β-Glucans on Osteoclast Differentiation In Vitro
| β-Glucan | Cell | Receptor | Effect | Molecular Mechanisms | References |
|---|---|---|---|---|---|
| Curdlan | BMCs RAW264.7 | Dectin-1 | Direct | Suppression of NFATc1 activation by down-regulation of Syk signaling | [45] |
| Curdlan | BMCs | Dectin-1 | Direct | Suppression of NFATc1 activation by stimulation of MafB induced by IL-33 | [69] |
| β-glucan from baker’s yeast | BMCs RAW264.7 | Dectin-1 | Direct | Suppression of NFATc1 activation by down-regulation of NF-κB and c-fos, stimulation of Irf-8, and induction of autophagy and ubiquitin/proteasome-mediated Syk protein degradation | [70] |
| Zymosan | BMCs | TLRs | Direct | Unknown | [71,72] |
| Curdlan (low MW) | BMCs cultured with osteoblasts | TLR2 TLR6 | Indirect | Suppression of RANKL expression on osteoblasts | [74] |
4.2. Inhibitory Effects of β-Glucans on Bone Resorption In Vivo
4.3. Effects of β-Glucans on Bone Regeneration and Bone Metabolism
4.4. Catabolic Effects of β-Glucans on Bone and Cartilage Tissue
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, G.D.; Gordon, S. Fungal beta-glucans and mammalian immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Du, B.; Bian, Z.; Xu, B. Skin health promotion effects of natural beta-glucan derived from cereals and microorganisms: A review. Phytother. Res. 2014, 28, 159–166. [Google Scholar] [CrossRef]
- Zhang, M.; Kim, J.A.; Huang, A.Y. Optimizing Tumor Microenvironment for Cancer Immunotherapy: β-Glucan-Based Nanoparticles. Front. Immunol. 2018, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Camilli, G.; Tabouret, G.; Quintin, J. The Complexity of Fungal β-Glucan in Health and Disease: Effects on the Mononuclear Phagocyte System. Front. Immunol. 2018, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Jin, Y.; Li, P.; Wang, F. β-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef]
- Yuan, H.; Lan, P.; He, Y.; Li, C.; Ma, X. Effect of the Modifications on the Physicochemical and Biological Properties of β-Glucan-A Critical Review. Molecules 2019, 25, 57. [Google Scholar] [CrossRef]
- Karsenty, G.; Kronenberg, H.M.; Settembre, C. Genetic control of bone formation. Annu. Rev. Cell Dev. Biol. 2009, 25, 629–648. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Osteoclasts: What do they do and how do they do it? Am. J. Pathol. 2007, 170, 427–435. [Google Scholar] [CrossRef]
- Bonewald, L.F.; Johnson, M.L. Osteocytes, mechanosensing and Wnt signaling. Bone 2008, 42, 606–615. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Y.K.; Harris, S.; Ahuja, S.S.; Bonewald, L.F. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J. Bone Miner. Res. 2002, 17, 2068–2079. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L.; Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003, 4, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Long, F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 2011, 13, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.C.; Kim, G.K.; Maurizio, P.L.; Molnar, E.E.; Choi, Y. TRAF6 autoubiquitination-independent activation of the NFkappaB and MAPK pathways in response to IL-1 and RANKL. PLoS ONE 2008, 3, e4064. [Google Scholar] [CrossRef] [PubMed]
- Darnay, B.G.; Ni, J.; Moore, P.A.; Aggarwal, B.B. Activation of NF-kappaB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-kappaB-inducing kinase. Identification of a novel TRAF6 interaction motif. J. Biol. Chem. 1999, 274, 7724–7731. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Humphrey, M.B.; Nakamura, M.C. A Comprehensive Review of Immunoreceptor Regulation of Osteoclasts. Clin. Rev. Allergy Immunol. 2016, 51, 48–58. [Google Scholar] [CrossRef]
- Lanier, L.L.; Bakker, A.B. The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function. Immunol. Today 2000, 21, 611–614. [Google Scholar] [CrossRef]
- Kubagawa, H.; Burrows, P.D.; Cooper, M.D. A novel pair of immunoglobulin-like receptors expressed by B cells and myeloid cells. Proc. Natl. Acad. Sci. USA 1997, 94, 5261–5266. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, M.B.; Lanier, L.L.; Nakamura, M.C. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol. Rev. 2005, 208, 50–65. [Google Scholar] [CrossRef]
- Miyazaki, T.; Tanaka, S.; Sanjay, A.; Baron, R. The role of c-Src kinase in the regulation of osteoclast function. Mod. Rheumatol. 2006, 16, 68–74. [Google Scholar] [CrossRef]
- Horne, W.C.; Sanjay, A.; Bruzzaniti, A.; Baron, R. The role(s) of Src kinase and Cbl proteins in the regulation of osteoclast differentiation and function. Immunol. Rev. 2005, 208, 106–125. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Kitaura, H.; Reeve, J.; Long, F.; Tybulewicz, V.L.; Shattil, S.J.; Ginsberg, M.H.; Ross, F.P.; Teitelbaum, S.L. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J. Cell Biol. 2007, 176, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Lee, Y.; Kim, H.J.; Lee, Z.H.; Hyung, S.W.; Lee, S.W.; Kim, H.H. Lyn inhibits osteoclast differentiation by interfering with PLCgamma1-mediated Ca2+ signaling. FEBS Lett. 2009, 583, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Ozkirimli, E.; Shah, K.; Harrison, M.L.; Geahlen, R.L. Generation of an analog-sensitive Syk tyrosine kinase for the study of signaling dynamics from the B cell antigen receptor. J. Biol. Chem. 2007, 282, 33760–33768. [Google Scholar] [CrossRef]
- Hasegawa, H.; Kido, S.; Tomomura, M.; Fujimoto, K.; Ohi, M.; Kiyomura, M.; Kanegae, H.; Inaba, A.; Sakagami, H.; Tomomura, A. Serum calcium-decreasing factor, caldecrin, inhibits osteoclast differentiation by suppression of NFATc1 activity. J. Biol. Chem. 2010, 285, 25448–25457. [Google Scholar] [CrossRef]
- Tomomura, M.; Hasegawa, H.; Suda, N.; Sakagami, H.; Tomomura, A. Serum calcium-decreasing factor, caldecrin, inhibits receptor activator of NF-κB ligand (RANKL)-mediated Ca2+ signaling and actin ring formation in mature osteoclasts via suppression of Src signaling pathway. J. Biol. Chem. 2012, 287, 17963–17974. [Google Scholar] [CrossRef]
- Okamoto, K.; Nakashima, T. Osteoimmunology. Cold Spring Harb. Perspect. Med. 2019, 9, a031245. [Google Scholar] [CrossRef]
- Faccio, R.; Zou, W.; Colaianni, G.; Teitelbaum, S.L.; Ross, F.P. High dose M-CSF partially rescues the Dap12-/- osteoclast phenotype. J. Cell Biochem. 2003, 90, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Mócsai, A.; Humphrey, M.B.; Van Ziffle, J.A.; Hu, Y.; Burghardt, A.; Spusta, S.C.; Majumdar, S.; Lanier, L.L.; Lowell, C.A.; Nakamura, M.C. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc. Natl. Acad. Sci. USA 2004, 101, 6158–6163. [Google Scholar] [CrossRef] [PubMed]
- Kittaka, M.; Yoshimoto, T.; Schlosser, C.; Rottapel, R.; Kajiya, M.; Kurihara, H.; Reichenberger, E.J.; Ueki, Y. Alveolar Bone Protection by Targeting the SH3BP2-SYK Axis in Osteoclasts. J. Bone Miner. Res. 2020, 35, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Konda, V.R.; Desai, A.; Darland, G.; Bland, J.S.; Tripp, M.L. META060 inhibits osteoclastogenesis and matrix metalloproteinases in vitro and reduces bone and cartilage degradation in a mouse model of rheumatoid arthritis. Arthritis Rheum. 2010, 62, 1683–1692. [Google Scholar] [CrossRef]
- Liao, C.; Hsu, J.; Kim, Y.; Hu, D.Q.; Xu, D.; Zhang, J.; Pashine, A.; Menke, J.; Whittard, T.; Romero, N.; et al. Selective inhibition of spleen tyrosine kinase (SYK) with a novel orally bioavailable small molecule inhibitor, RO9021, impinges on various innate and adaptive immune responses: Implications for SYK inhibitors in autoimmune disease therapy. Arthritis Res. Ther. 2013, 15, R146. [Google Scholar] [CrossRef]
- Jia, Y.; Miao, Y.; Yue, M.; Shu, M.; Wei, Z.; Dai, Y. Tetrandrine attenuates the bone erosion in collagen-induced arthritis rats by inhibiting osteoclastogenesis via spleen tyrosine kinase. FASEB J. 2018, 32, 3398–3410. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.H.; Baek, J.M.; Erkhembaatar, M.; Kim, M.S.; Yoon, K.H.; Oh, J.; Lee, M.S. Harpagoside Inhibits RANKL-Induced Osteoclastogenesis via Syk-Btk-PLCγ2-Ca(2+) Signaling Pathway and Prevents Inflammation-Mediated Bone Loss. J. Nat. Prod. 2015, 78, 2167–2174. [Google Scholar] [CrossRef]
- Joung, Y.H.; Darvin, P.; Kang, D.Y.; Sp, N.; Byun, H.J.; Lee, C.H.; Lee, H.K.; Yang, Y.M. Methylsulfonylmethane Inhibits RANKL-Induced Osteoclastogenesis in BMMs by Suppressing NF-κB and STAT3 Activities. PLoS ONE 2016, 11, e0159891. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Tao, Y.; Lv, C.; Xia, Y.; Wei, Z.; Dai, Y. Tetrandrine enhances the ubiquitination and degradation of Syk through an AhR-c-src-c-Cbl pathway and consequently inhibits osteoclastogenesis and bone destruction in arthritis. Cell Death Dis. 2019, 10, 38. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Immune recognition. A new receptor for beta-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef]
- Ariizumi, K.; Shen, G.L.; Shikano, S.; Xu, S.; Ritter, R., 3rd; Kumamoto, T.; Edelbaum, D.; Morita, A.; Bergstresser, P.R.; Takashima, A. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J. Biol. Chem. 2000, 275, 20157–20167. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 2006, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.R.; Brown, G.D.; Reid, D.M.; Willment, J.A.; Martinez-Pomares, L.; Gordon, S.; Wong, S.Y. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 2002, 169, 3876–3882. [Google Scholar] [CrossRef]
- Charles, J.F.; Hsu, L.Y.; Niemi, E.C.; Weiss, A.; Aliprantis, A.O.; Nakamura, M.C. Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function. J. Clin. Investig. 2012, 122, 4592–4605. [Google Scholar] [CrossRef]
- Yamasaki, T.; Ariyoshi, W.; Okinaga, T.; Adachi, Y.; Hosokawa, R.; Mochizuki, S.; Sakurai, K.; Nishihara, T. The dectin 1 agonist curdlan regulates osteoclastogenesis by inhibiting nuclear factor of activated T cells cytoplasmic 1 (NFATc1) through Syk kinase. J. Biol. Chem. 2014, 289, 19191–191203. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Rossnagle, E.; Lowell, C.A.; Simmons, R.M. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 2005, 106, 2543–2550. [Google Scholar] [CrossRef]
- Thwe, P.M.; Fritz, D.I.; Snyder, J.P.; Smith, P.R.; Curtis, K.D.; O’Donnell, A.; Galasso, N.A.; Sepaniac, L.A.; Adamik, B.J.; Hoyt, L.R.; et al. Syk-dependent glycolytic reprogramming in dendritic cells regulates IL-1β production to β-glucan ligands in a TLR-independent manner. J. Leukoc Biol. 2019, 106, 1325–1335. [Google Scholar] [CrossRef]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. Beta-glucan recognition by the innate immune system. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef]
- Ross, G.D.; Vetvicka, V.; Yan, J.; Xia, Y.; Vetvicková, J. Therapeutic intervention with complement and beta-glucan in cancer. Immunopharmacology 1999, 42, 61–74. [Google Scholar] [CrossRef]
- Vetvicka, V.; Thornton, B.P.; Ross, G.D. Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J. Clin. Investig. 1996, 98, 50–61. [Google Scholar] [CrossRef]
- Yan, J.; Vetvicka, V.; Xia, Y.; Coxon, A.; Carroll, M.C.; Mayadas, T.N.; Ross, G.D. Beta-glucan, a “specific” biologic response modifier that uses antibodies to target tumors for cytotoxic recognition by leukocyte complement receptor type 3 (CD11b/CD18). J. Immunol. 1999, 163, 3045–3052. [Google Scholar]
- Li, D.; Bai, C.; Zhang, Q.; Li, Z.; Shao, D.; Li, X. β-1,3-Glucan/CR3/SYK pathway-dependent LC3B-II accumulation enhanced the fungicidal activity in human neutrophils. J. Microbiol. 2019, 57, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Clark, H.L.; Abbondante, S.; Minns, M.S.; Greenberg, E.N.; Sun, Y.; Pearlman, E. Protein Deiminase 4 and CR3 Regulate Aspergillus fumigatus and β-Glucan-Induced Neutrophil Extracellular Trap Formation, but Hyphal Killing Is Dependent only on CR3. Front. Immunol. 2018, 9, 1182. [Google Scholar] [CrossRef] [PubMed]
- Mangham, D.C.; Scoones, D.J.; Drayson, M.T. Complement and the recruitment of mononuclear osteoclasts. J. Clin. Pathol. 1993, 46, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Akiyama, M.; Nakahama, K.; Koshiishi, T.; Takeda, S.; Morita, I. Role of intercellular adhesion molecule-2 in osteoclastogenesis. Genes Cells 2012, 17, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Nakahama, K.; Sato, T.; Tuchiya, T.; Asakawa, Y.; Maemura, T.; Tanaka, M.; Morita, M.; Morita, I. The role of Mac-1 (CD11b/CD18) in osteoclast differentiation induced by receptor activator of nuclear factor-kappaB ligand. FEBS Lett. 2008, 582, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chen, X.; Yan, Z.; Zhu, Q.; Yang, C. CD11b promotes the differentiation of osteoclasts induced by RANKL through the spleen tyrosine kinase signalling pathway. J. Cell Mol. Med. 2017, 21, 3445–3452. [Google Scholar] [CrossRef]
- Park-Min, K.H.; Lee, E.Y.; Moskowitz, N.K.; Lim, E.; Lee, S.K.; Lorenzo, J.A.; Huang, C.; Melnick, A.M.; Purdue, P.E.; Goldring, S.R.; et al. Negative regulation of osteoclast precursor differentiation by CD11b and β2 integrin-B-cell lymphoma 6 signaling. J. Bone Miner. Res. 2013, 28, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrières, V. Molecular Interactions of β-(1→3)-Glucans with Their Receptors. Molecules 2015, 20, 9745–9766. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhen, H.; Wang, N.; Yan, Q.; Jing, H.; Jiang, Z. Curdlan oligosaccharides having higher immunostimulatory activity than curdlan in mice treated with cyclophosphamide. Carbohydr. Polym. 2019, 207, 131–142. [Google Scholar] [CrossRef]
- Liu, J.; Tang, J.; Li, X.; Yan, Q.; Ma, J.; Jiang, Z. Curdlan (Alcaligenes faecalis) (1→3)-β-d-Glucan Oligosaccharides Drive M1 Phenotype Polarization in Murine Bone Marrow-Derived Macrophages via Activation of MAPKs and NF-κB Pathways. Molecules 2019, 24, 4251. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zou, S.; Xu, H.; Liu, Q.; Song, J.; Xu, M.; Xu, X.; Zhang, L. The linear structure of β-glucan from baker’s yeast and its activation of macrophage-like RAW264.7 cells. Carbohydr. Polym. 2016, 148, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Lu, M.K.; Lu, T.J.; Lai, M.N.; Ng, L.T. A (1→6)-Branched (1→4)-β-d-Glucan from Grifola frondosa Inhibits Lipopolysaccharide-Induced Cytokine Production in RAW264.7 Macrophages by Binding to TLR2 Rather than Dectin-1 or CR3 Receptors. J. Nat. Prod. 2020, 83, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.P.C.; Lerner, U.H. Finding a Toll on the Route: The Fate of Osteoclast Progenitors after Toll-Like Receptor Activation. Front. Immunol. 2019, 10, 1663. [Google Scholar] [CrossRef]
- Kamohara, A.; Hirata, H.; Xu, X.; Shiraki, M.; Yamada, S.; Zhang, J.Q.; Kukita, T.; Toyonaga, K.; Hara, H.; Urano, Y.; et al. IgG immune complexes with Staphylococcus aureus protein A enhance osteoclast differentiation and bone resorption by stimulating Fc receptors and TLR2. Int. Immunol. 2020, 32, 89–104. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Xu, Q.; Harber, G.; Feng, X.; Michalek, S.M.; Katz, J. TLR2-dependent modulation of osteoclastogenesis by Porphyromonas gingivalis through differential induction of NFATc1 and NF-kappaB. J. Biol. Chem. 2011, 286, 24159–24169. [Google Scholar] [CrossRef] [PubMed]
- Ohgi, K.; Kajiya, H.; Goto, T.K.; Okamoto, F.; Yoshinaga, Y.; Okabe, K.; Sakagami, R. Toll-like receptor 2 activation primes and upregulates osteoclastogenesis via lox-1. Lipids Health Dis. 2018, 17, 132. [Google Scholar] [CrossRef]
- Yang, J.; Ryu, Y.H.; Yun, C.H.; Han, S.H. Impaired osteoclastogenesis by staphylococcal lipoteichoic acid through Toll-like receptor 2 with partial involvement of MyD88. J. Leukoc Biol. 2009, 86, 823–831. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, Y.; Jiang, Y.; Qin, T.; Chen, J.; Chu, X.; Yi, Q.; Gao, S.; Wang, S. Dectin-1 signaling inhibits osteoclastogenesis via IL-33-induced inhibition of NFATc1. Oncotarget 2017, 8, 53366–53374. [Google Scholar] [CrossRef]
- Hara, S.; Nagai-Yoshioka, Y.; Yamasaki, R.; Adachi, Y.; Fujita, Y.; Watanabe, K.; Maki, K.; Nishihara, T.; Ariyoshi, W. Dectin-1-mediated suppression of RANKL-induced osteoclastogenesis by glucan from baker’s yeast. J. Cell Physiol. 2021. ahead of print. [Google Scholar]
- Takami, M.; Kim, N.; Rho, J.; Choi, Y. Stimulation by toll-like receptors inhibits osteoclast differentiation. J. Immunol. 2002, 169, 1516–1523. [Google Scholar] [CrossRef]
- Mochizuki, A.; Takami, M.; Kawawa, T.; Suzumoto, R.; Sasaki, T.; Shiba, A.; Tsukasaki, H.; Zhao, B.; Yasuhara, R.; Suzawa, T.; et al. Identification and characterization of the precursors committed to osteoclasts induced by TNF-related activation-induced cytokine/receptor activator of NF-kappa B ligand. J. Immunol. 2006, 177, 4360–4368. [Google Scholar] [CrossRef]
- Yamanaka, D.; Kurita, S.; Hanayama, Y.; Adachi, Y. Split Enzyme-Based Biosensors for Structural Characterization of Soluble and Insoluble β-Glucans. Int. J. Mol. Sci. 2021, 22, 1576. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, M.; Watanabe, K.; Tominari, T.; Matsumoto, C.; Hirata, M.; Grundler, F.M.W.; Inada, M.; Miyaura, C. Low Molecular-Weight Curdlan, (1→3)-β-Glucan Suppresses TLR2-Induced RANKL-Dependent Bone Resorption. Biol. Pharm. Bull. 2018, 41, 1282–1285. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kang, S.J.; Kim, J.W.; Cho, H.R.; Moon, S.B.; Kim, K.Y.; Lee, H.S.; Han, C.H.; Ku, S.K.; Lee, Y.J. Effects of Polycan, a β-glucan, on experimental periodontitis and alveolar bone loss in Sprague-Dawley rats. J. Periodontal. Res. 2012, 47, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Kang, S.J.; Han, C.H.; Kim, J.W.; Song, C.H.; Lee, S.N.; Ku, S.K.; Lee, Y.J. The Effects of Topical Application of Polycal (a 2:98 (g/g) Mixture of Polycan and Calcium Gluconate) on Experimental Periodontitis and Alveolar Bone Loss in Rats. Molecules 2016, 21, 527. [Google Scholar] [CrossRef]
- Jung, M.Y.; Kim, J.W.; Kim, K.Y.; Choi, S.H.; Ku, S.K. Polycan, a β-glucan from Aureobasidium pullulans SM-2001, mitigates ovariectomy-induced osteoporosis in rats. Exp. Ther. Med. 2016, 12, 1251–1262. [Google Scholar] [CrossRef]
- Cho, C.S.; Jeong, H.S.; Kim, I.Y.; Jung, G.W.; Ku, B.H.; Park, D.C.; Moon, S.B.; Cho, H.R.; Bashir, K.M.I.; Ku, S.K.; et al. Anti-osteoporotic effects of mixed compositions of extracellular polymers isolated from Aureobasidium pullulans and Textoria morbifera in ovariectomized mice. BMC Complement. Altern. Med. 2018, 18, 295. [Google Scholar] [CrossRef]
- De, O.S.V.; Lobato, R.V.; Andrade, E.F.; Orlando, D.R.; Borges, B.D.B.; Zangeronimo, M.G.; de Sousa, R.V.; Pereira, L.J. Effects of β-Glucans Ingestion on Alveolar Bone Loss, Intestinal Morphology, Systemic Inflammatory Profile, and Pancreatic β-Cell Function in Rats with Periodontitis and Diabetes. Nutrients 2017, 9, 1016. [Google Scholar] [CrossRef]
- Silva Vde, O.; Lobato, R.V.; Andrade, E.F.; de Macedo, C.G.; Napimoga, J.T.; Napimoga, M.H.; Messora, M.R.; Murata, R.M.; Pereira, L.J. β-Glucans (Saccharomyces cereviseae) Reduce Glucose Levels and Attenuate Alveolar Bone Loss in Diabetic Rats with Periodontal Disease. PLoS ONE 2015, 10, e0134742. [Google Scholar] [CrossRef]
- Breivik, T.; Opstad, P.K.; Engstad, R.; Gundersen, G.; Gjermo, P.; Preus, H. Soluble beta-1,3/1,6-glucan from yeast inhibits experimental periodontal disease in Wistar rats. J. Clin. Periodontol. 2005, 32, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A.; Ginalska, G. Addition of 1,3-β-d-glucan to chitosan-based composites enhances osteoblast adhesion, growth, and proliferation. Int. J. Biol. Macromol. 2014, 70, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A.; Ginalska, G. Enhanced differentiation of osteoblastic cells on novel chitosan/β-1,3-glucan/bioceramic scaffolds for bone tissue regeneration. Biomed. Mater. 2015, 10, 015009. [Google Scholar] [CrossRef] [PubMed]
- Klimek, K.; Przekora, A.; Pałka, K.; Ginalska, G. New method for the fabrication of highly osteoconductive β-1,3-glucan/HA scaffold for bone tissue engineering: Structural, mechanical, and biological characterization. J. Biomed. Mater. Res. A 2016, 104, 2528–2536. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A.; Benko, A.; Blazewicz, M.; Ginalska, G. Hybrid chitosan/β-1,3-glucan matrix of bone scaffold enhances osteoblast adhesion, spreading and proliferation via promotion of serum protein adsorption. Biomed. Mater. 2016, 11, 045001. [Google Scholar] [CrossRef]
- Przekora, A.; Ginalska, G. In vitro evaluation of the risk of inflammatory response after chitosan/HA and chitosan/β-1,3-glucan/HA bone scaffold implantation. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 355–361. [Google Scholar] [CrossRef]
- Przekora, A.; Ginalska, G. Chitosan/β-1,3-glucan/hydroxyapatite bone scaffold enhances osteogenic differentiation through TNF-α-mediated mechanism. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 225–233. [Google Scholar] [CrossRef]
- Borkowski, L.; Lübek, T.; Jojczuk, M.; Nogalski, A.; Belcarz, A.; Palka, K.; Hajnos, M.; Ginalska, G. Behavior of new hydroxyapatite/glucan composite in human serum. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2653–2664. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Park, M.Y.; Kim, J.D.; Cho, H.R.; Choi, I.S.; Kim, J.W. Safety and efficacy of polycalcium for improving biomarkers of bone metabolism: A 4-week open-label clinical study. J. Med. Food 2013, 16, 263–267. [Google Scholar] [CrossRef]
- Ohlin, A.; Sjögren, U.; Lerner, U.H. Bone resorbing activity released from zymosan-activated mouse peritoneal macrophages--the role of prostanoids and interleukin-1. Inflamm. Res. 1999, 48, 181–192. [Google Scholar] [CrossRef]
- Maruyama, K.; Takayama, Y.; Kondo, T.; Ishibashi, K.I.; Sahoo, B.R.; Kanemaru, H.; Kumagai, Y.; Martino, M.M.; Tanaka, H.; Ohno, N.; et al. Nociceptors Boost the Resolution of Fungal Osteoinflammation via the TRP Channel-CGRP-Jdp2 Axis. Cell Rep. 2017, 19, 2730–2742. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Muneta, T.; Tsuji, K.; Sekiya, I. BMP-7 inhibits cartilage degeneration through suppression of inflammation in rat zymosan-induced arthritis. Cell Tissue Res. 2011, 344, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Ganova, P.; Gyurkovska, V.; Belenska-Todorova, L.; Ivanovska, N. Functional complement activity is decisive for the development of chronic synovitis, osteophyte formation and processes of cell senescence in zymosan-induced arthritis. Immunol. Lett. 2017, 190, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, P.; Ivanovska, N.; Schwaeble, W.; Gyurkovska, V.; Stover, C. The role of properdin in murine zymosan-induced arthritis. Mol. Immunol. 2010, 47, 1458–1466. [Google Scholar] [CrossRef]
- Pakozdi, A.; Amin, M.A.; Haas, C.S.; Martinez, R.J.; Haines, G.K., 3rd; Santos, L.L.; Morand, E.F.; David, J.R.; Koch, A.E. Macrophage migration inhibitory factor: A mediator of matrix metalloproteinase-2 production in rheumatoid arthritis. Arthritis Res. Ther. 2006, 8, R132. [Google Scholar] [CrossRef]
- Van de Loo, F.A.; Bennink, M.B.; Arntz, O.J.; Smeets, R.L.; Lubberts, E.; Joosten, L.A.; van Lent, P.L.; Coenen-de Roo, C.J.; Cuzzocrea, S.; Segal, B.H.; et al. Deficiency of NADPH oxidase components p47phox and gp91phox caused granulomatous synovitis and increased connective tissue destruction in experimental arthritis models. Am. J. Pathol. 2003, 163, 1525–1537. [Google Scholar] [CrossRef]
- Weinberger, A.; Halpern, M.; Zahalka, M.A.; Quintana, F.; Traub, L.; Moroz, C. Placental immunomodulator ferritin, a novel immunoregulator, suppresses experimental arthritis. Arthritis Rheum. 2003, 48, 846–853. [Google Scholar] [CrossRef]
- Schalkwijk, J.; van den Berg, W.B.; van de Putte, L.B.; Joosten, L.A.; van der Sluis, M. Effects of experimental joint inflammation on bone marrow and periarticular bone. A study of two types of arthritis, using variable degrees of inflammation. Br. J. Exp. Pathol. 1985, 66, 435–444. [Google Scholar] [CrossRef]
- Min, H.K.; Kim, J.K.; Lee, S.Y.; Kim, E.K.; Lee, S.H.; Lee, J.; Kwok, S.K.; Cho, M.L.; Park, S.H. Rebamipide prevents peripheral arthritis and intestinal inflammation by reciprocally regulating Th17/Treg cell imbalance in mice with curdlan-induced spondyloarthritis. J. Transl. Med. 2016, 14, 190. [Google Scholar] [CrossRef]
- Jeong, H.; Bae, E.K.; Kim, H.; Lim, D.H.; Chung, T.Y.; Lee, J.; Jeon, C.H.; Koh, E.M.; Cha, H.S. Spondyloarthritis features in zymosan-induced SKG mice. Joint. Bone Spine 2018, 85, 583–591. [Google Scholar] [CrossRef]
- Duygulu, F.; Yakan, B.; Karaoglu, S.; Kutlubay, R.; Karahan, O.I.; Ozturk, A. The effect of zymosan and the protective effect of various antioxidants on fracture healing in rats. Arch. Orthop. Trauma Surg. 2007, 127, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by beta-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.C.; Chan, W.K.; Sze, D.M. The effects of beta-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Seviour, R. Medicinal importance of fungal beta-(1-->3), (1-->6)-glucans. Mycol. Res. 2007, 111, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Raymond, K. Beta-glucans in the treatment of diabetes and associated cardiovascular risks. VASC Health Risk Manag. 2008, 4, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Nakashima, T.; Shinohara, M.; Negishi-Koga, T.; Komatsu, N.; Terashima, A.; Sawa, S.; Nitta, T.; Takayanagi, H. Osteoimmunology: The Conceptual Framework Unifying the Immune and Skeletal Systems. Physiol. Rev. 2017, 97, 1295–1349. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Zhang, Q.; Tong, L.; Liu, L.; Zhou, X.; Zhou, S. Molecular weight degradation and rheological properties of schizophyllan under ultrasonic treatment. Ultrason. Sonochem. 2015, 23, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef]
- Chen, J.; Chen, L.; Lin, S.; Liu, C.; Cheung, P.C.K. Preparation and structural characterization of a partially depolymerized beta-glucan obtained from Poria cocos sclerotium by ultrasonic treatment. Food Hydrocoll. 2015, 46, 1–9. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, J.; Liu, D.; Ye, X.; Ke, F. Impact of ultrasonic treatment on properties of starch film-forming dispersion and the resulting films. Carbohydr. Polym. 2010, 81, 707–711. [Google Scholar] [CrossRef]
- Ishimoto, Y.; Ishibashi, K.I.; Yamanaka, D.; Adachi, Y.; Kanzaki, K.; Okita, K.; Iwakura, Y.; Ohno, N. Modulation of an innate immune response by soluble yeast β-glucan prepared by a heat degradation method. Int. J. Biol. Macromol. 2017, 104, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.-H.; Kim, J.-H.; Sung, N.-Y.; Choi, J.-I.; Lim, S.-T.; Kim, K.-H.; Yook, H.-S.; Byun, M.-W.; Lee, J.-W. Effects of gamma irradiation on the physical and structural properties of β-glucan. Radiat. Phys. Chem. 2008, 77, 781–786. [Google Scholar] [CrossRef]
- Chang, Y.J.; Lee, S.; Yoo, M.A.; Lee, H.G. Structural and biological characterization of sulfated-derivatized oat beta-glucan. J. Agric. Food Chem. 2006, 54, 3815–3818. [Google Scholar] [CrossRef] [PubMed]
- Han, M.D.; Han, Y.S.; Hyun, S.H.; Shin, H.W. Solubilization of water-insoluble beta-glucan isolated from Ganoderma lucidum. J. Environ. Biol. 2008, 29, 237–242. [Google Scholar] [PubMed]
- Williams, D.L.; McNamee, R.B.; Jones, E.L.; Pretus, H.A.; Ensley, H.E.; Browder, I.W.; Di Luzio, N.R. A method for the solubilization of a (1→3)-β-d-glucan isolated from Saccharomyces cerevisiae. Carbohydr. Res. 1991, 219, 203–213. [Google Scholar] [CrossRef]
- Chen, X.; Xu, X.; Zhang, L.; Zeng, F. Chain conformation and anti-tumor activities of phosphorylated (1→3)-β-d-glucan from Poria cocos. Carbohydr. Polym. 2009, 78, 581–587. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, L. Preparation, chain conformation and anti-tumor activities of water-soluble phosphated (1→3)-α-d-glucan from Poria cocos mycelia. Carbohydr. Polym. 2011, 83, 1363–1369. [Google Scholar] [CrossRef]
- Park, S.Y.; Bae, I.Y.; Lee, S.; Lee, H.G. Physicochemical and hypocholesterolemic characterization of oxidized oat beta-glucan. J. Agric. Food Chem. 2009, 57, 439–443. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Yang, Z.; Zhu, Y.; Wu, Y.; Huang, J.; Mao, J. Oxidation of β-glucan extracted from Poria cocos and its physiological activities. Carbohydr. Polym. 2011, 85, 798–802. [Google Scholar] [CrossRef]

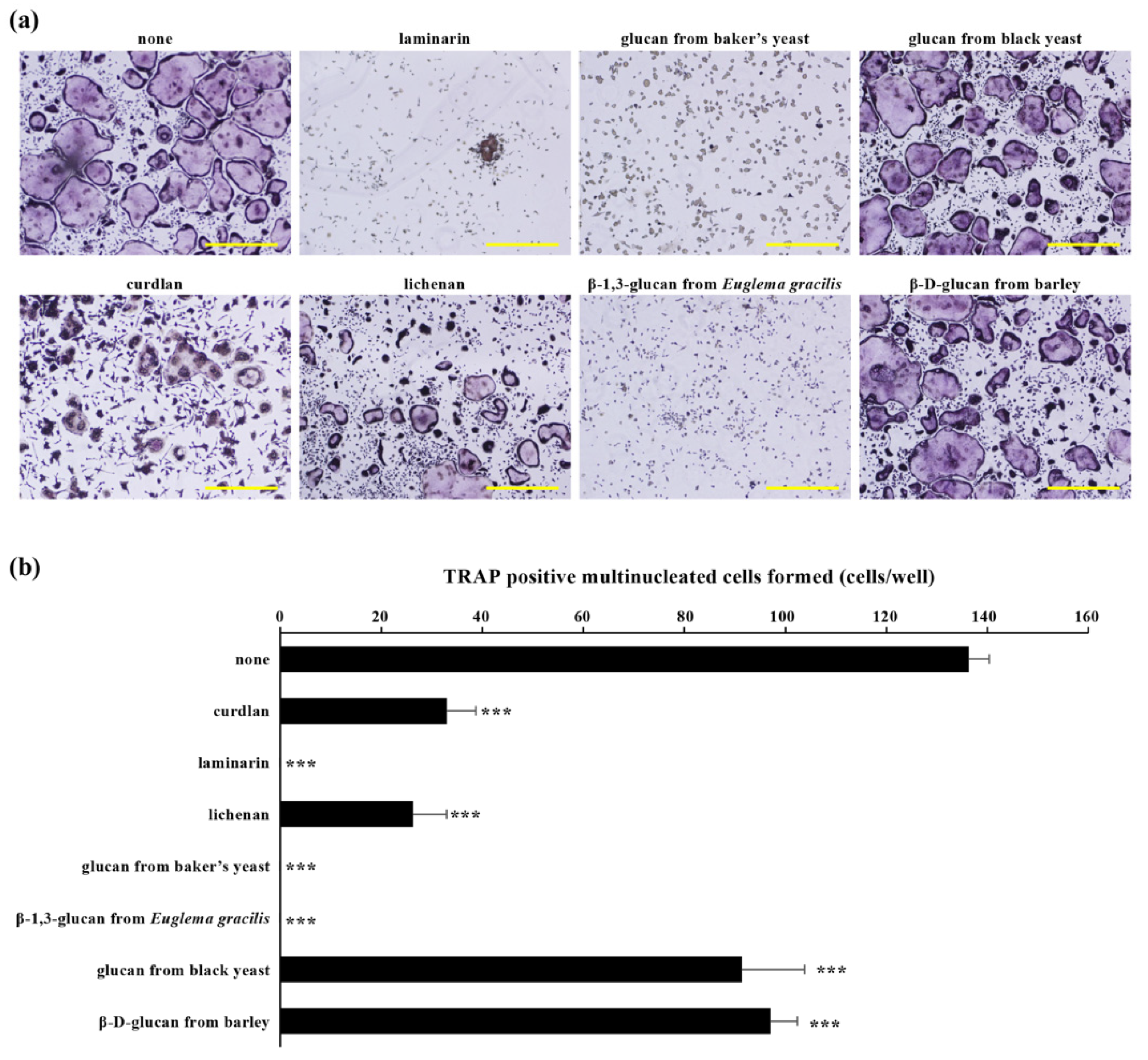
| β-Glucan | Cell | Structure |
|---|---|---|
| Curdlan | Alcaligenes faecalis var. myxogenes | Linear chain of β-d-(1-3)-glucopyranosyl units |
| Laminarin | Laminaria sp. | Linear chain of β-d-(1-3)-glucopyranosyl units with some 6-O-branching in the main chain and some β-(1,6)-intrachain links |
| Lichenan | Cetraria islandica | Linear chains of β-d-glucopyranosyl units linked via (1,3) and (1,4) linkage |
| Glucan from baker’s yeast | Saccharomyces cerevisiae | Linear chain of β-d-(1-3)-glucopyranosyl units |
| β-1,3-glucan from Euglena gracilis | Euglena gracilis | Linear chain of β-d-(1-3)-glucopyranosyl units |
| Glucan from black yeast | Aureobasidium pullulans | Backbone of β-d-(1-3)-glucopyranosyl units with one β-d-(1-6)-branching unit every three residues |
| β-d-glucan from barley | Hordeum vulgare L. | Linear chains of β-d-glucopyranosyl units linked via (1,3) and (1,4) linkage |
| β-Glucan | Organism | Analysis | Results | References |
|---|---|---|---|---|
| Polycan | Male Sprague-Dawley rats | Methylene blue assay Detection of IL-1β and TNF-α Measurement of MPO activity MDA measurement iNos activity measurement Histopathology and histomorphology | Inhibited ligature-induced periodontitis and related alveolar bone loss via an antioxidant effect. | [75] |
| Polycan | Male SD (Crl:CD1) rats | Measurement of alveolar bone loss Microbiological analysis Measurement of MPO activity Detection of IL-1β and TNF-α MDA measurement iNos activity measurement Histopathology | Inhibited ligature-induced experimental periodontitis and related alveolar bone loss mediated by antibacterial, anti-inflammatory, and anti-oxidative activities. | [76] |
| Polycan | Female Sprague-Dawley rats | Detection of serum levels of osteocalcin, bALP, calcium and phosphorus Detection of urinary levels of deoxypyridinoline and creatinine Measurement of BMC, BMD and FL Histology and histomorphometry | Preserved bone mass and strength, and increased the rate of bone formation in ovariectomy-induced osteoporosis model. | [77] |
| β-glucan from Aureobasidium pullulans | Female ICR mice | Measurement of BMD, bone weight, and FL Detection of serum levels of osteocalcin and bALP Measurement of femur mineral contents Histopathology | Mixture of extracellular polymeric substances isolated from A pullulans and Textoria morbifera Nakai inhibited the ovariectomy-induced osteoporotic symptoms. | [78] |
| β-glucan from Saccharomyces cerevisiae | Male Wistar rats | Detection of β-cell function Detection of serum levels of TNF-α and IL-10 Measurement of alveolar bone loss Histometric analysis | Inhibited the systemic inflammatory profile, prevented alveolar bone loss, and improved β-cell function in streptozotocin-induced diabetic model with periodontitis. | [79] |
| β-glucan from Saccharomyces cerevisiae | Male Wistar rats | Measurement of blood glucose RT-PCR for COX-2, RANKL and OPG Morphometric analysis for alveolar bone loss | Reduced blood glucose levels and attenuated alveolar bone loss in streptozotocin-induced diabetes model with periodontitis. | [80] |
| Soluble β-1,3/1,6-glucan from Saccharomyces cerevisiae | Male Wistar rats | Radiographic examination Measurement of corticosterone Detection of serum levels of IL-10, TGF-β1 and TNF-α | Inhibited ligature-induced periodontal bone loss. | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariyoshi, W.; Hara, S.; Koga, A.; Nagai-Yoshioka, Y.; Yamasaki, R. Biological Effects of β-Glucans on Osteoclastogenesis. Molecules 2021, 26, 1982. https://doi.org/10.3390/molecules26071982
Ariyoshi W, Hara S, Koga A, Nagai-Yoshioka Y, Yamasaki R. Biological Effects of β-Glucans on Osteoclastogenesis. Molecules. 2021; 26(7):1982. https://doi.org/10.3390/molecules26071982
Chicago/Turabian StyleAriyoshi, Wataru, Shiika Hara, Ayaka Koga, Yoshie Nagai-Yoshioka, and Ryota Yamasaki. 2021. "Biological Effects of β-Glucans on Osteoclastogenesis" Molecules 26, no. 7: 1982. https://doi.org/10.3390/molecules26071982
APA StyleAriyoshi, W., Hara, S., Koga, A., Nagai-Yoshioka, Y., & Yamasaki, R. (2021). Biological Effects of β-Glucans on Osteoclastogenesis. Molecules, 26(7), 1982. https://doi.org/10.3390/molecules26071982







