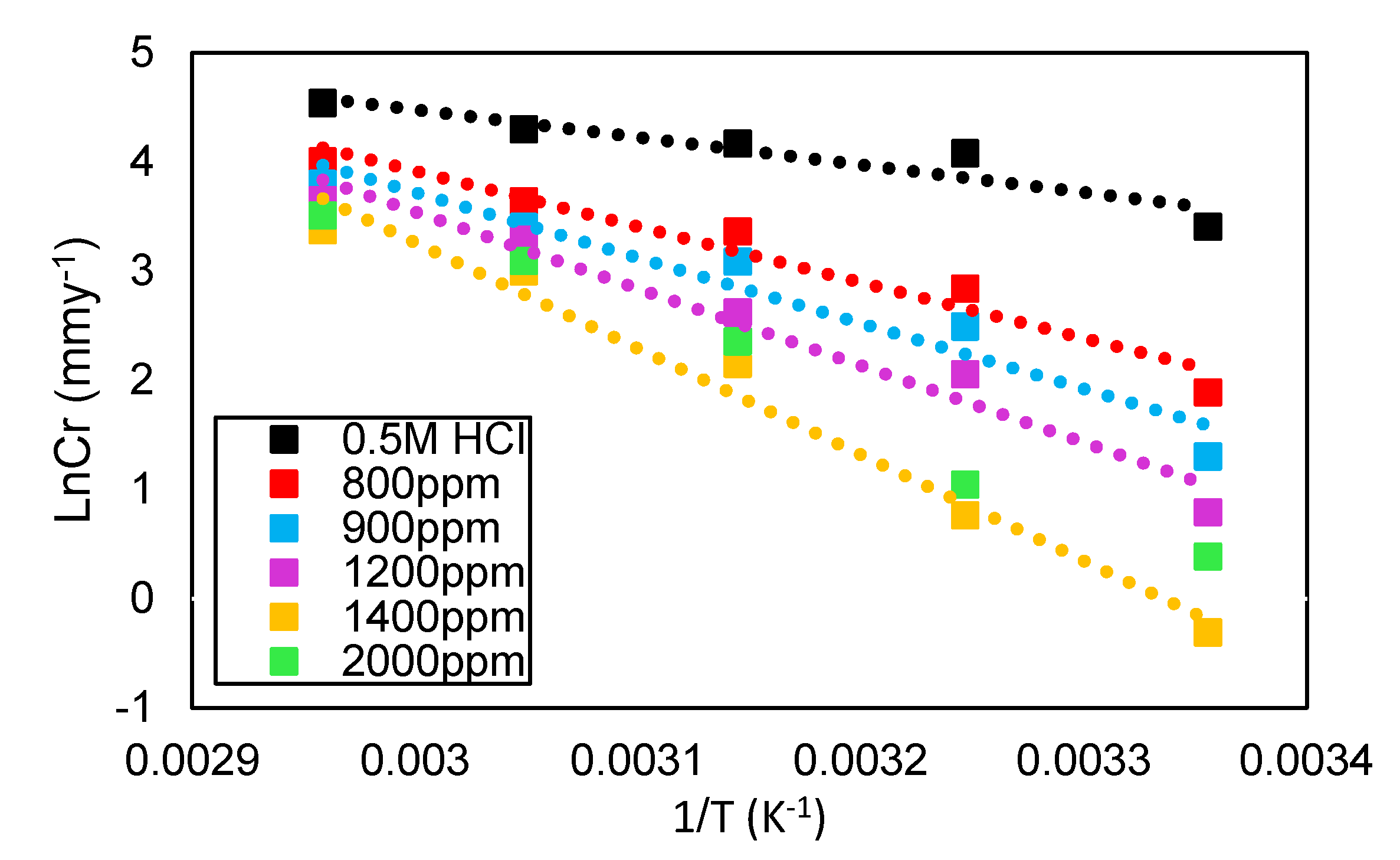
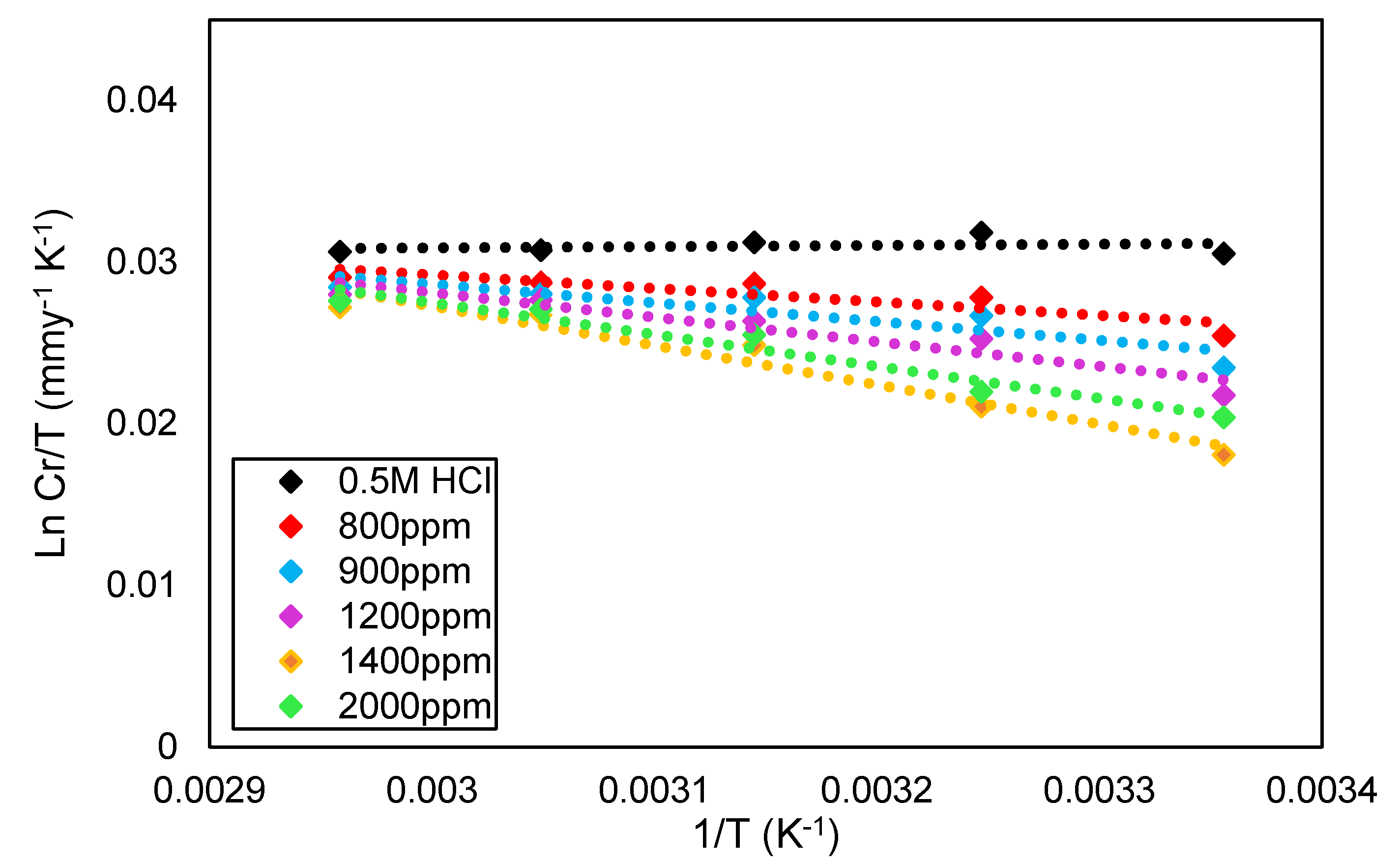
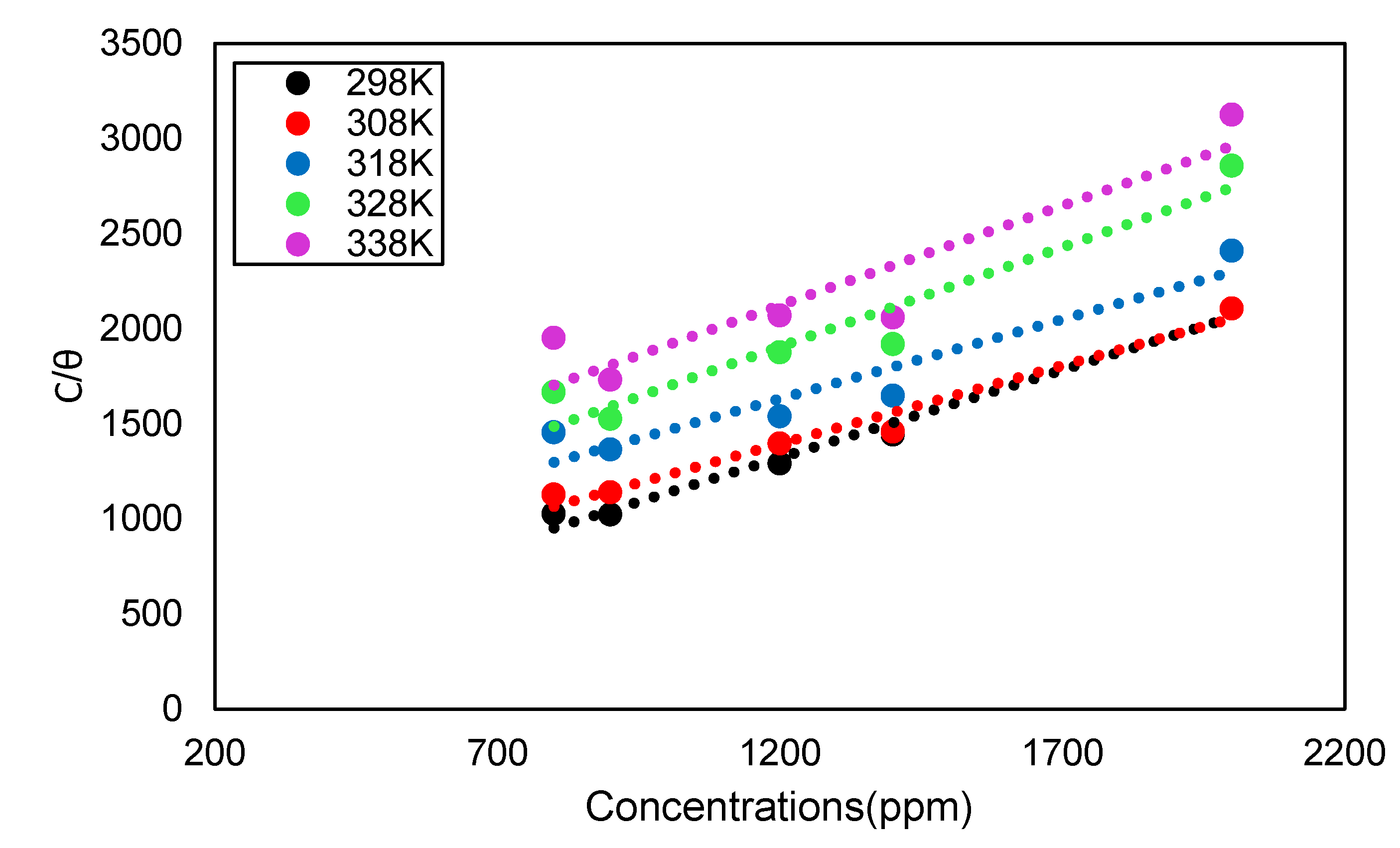
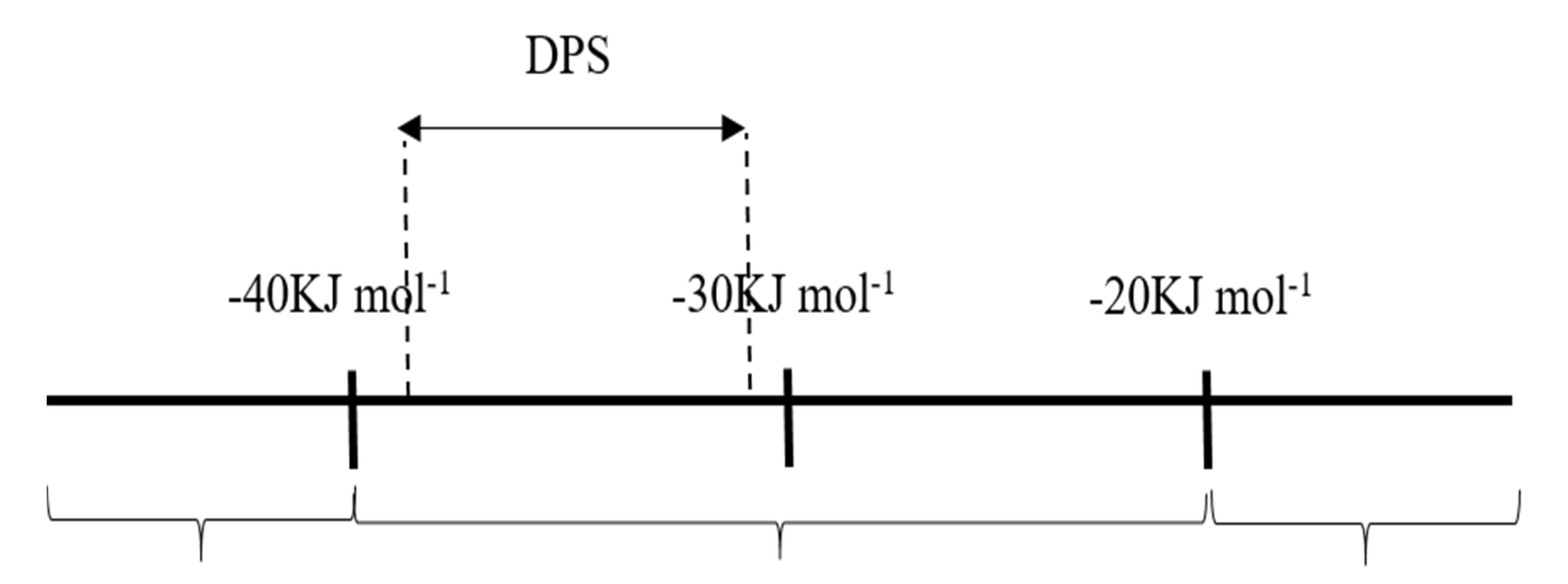
3.4. Corrosion Inhibitor in HCl Solution Operational Mechanism
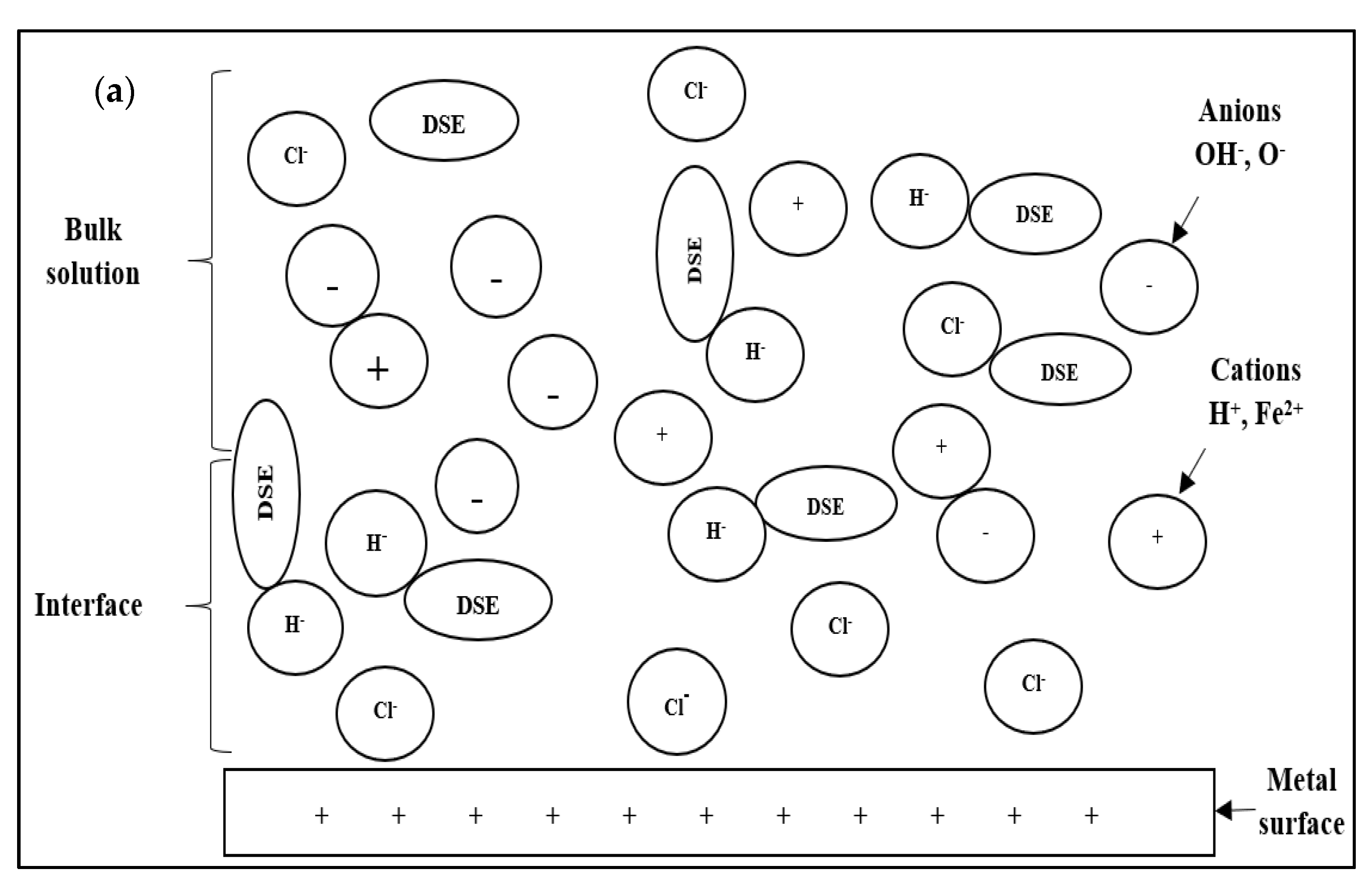
Figure 10 shows the proposed corrosion inhibition mechanism by DPS inhibitor. Corrosion inhibitors will generally be ionised in acidic solutions and acquire a positive charge due to the ionisation process. The ionisation reaction of the DSE inhibitor solution occurs where the water molecule (H
2O) is separated into a hydrogen cation (H
+) and the hydroxyl anion (OH
−) [
40].
In this study, date seed extract with various functional groups, such as OH, C=O, and OCH
3, which are nucleophilic, will be protonated or bonded to H
+ in an acidic aqueous solution (
Figure 10a). Cl
− and OH
− anions tend to move to more electrophilic cations (Fe
+2). Thus, the steel surface will be negatively charged by the anions CI
− and OH
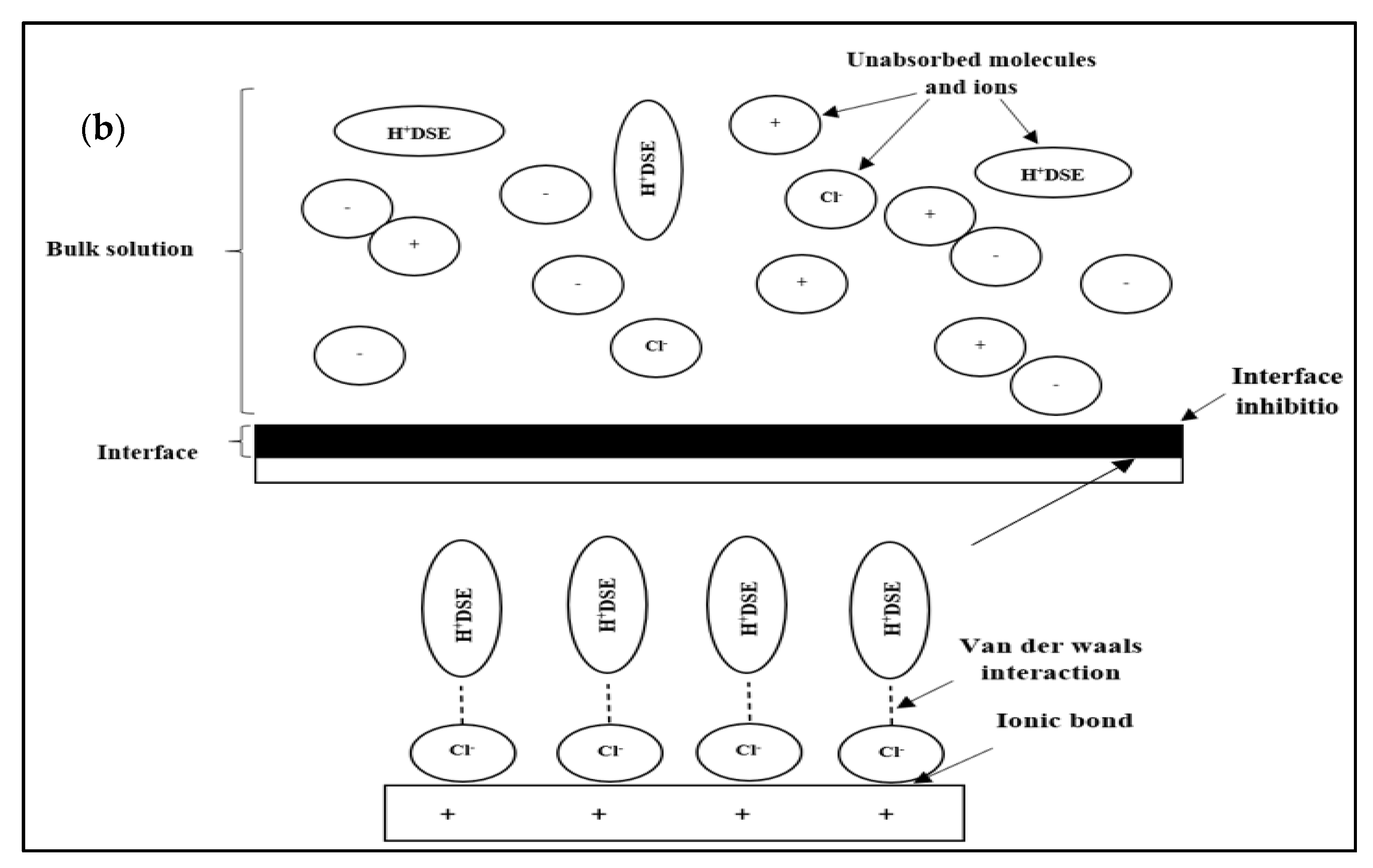
− and undergo corrosion reactions and oxide formation. Next, the proton corrosion inhibitor interacts with the negatively charged steel surface (
Figure 10b). The positive charge on the active site of the DPS corrosion inhibitor ties to the negative charge on the steel. These interactions cause the inhibition of the corrosion process. Anion Cl
− was found adsorbed by coupling with the inhibitor molecule, where it lies between the steel surface (Fe
2+) and the corrosion inhibitor (H
+ inhibitor) through the interaction of ionic bonds and Van der Waals forces. Coupling adsorption usually involves interactions between anions and active sites of the corrosion inhibitor [
42].
3.5. Quantum Chemical Calculation
The quantum parameters of chemicals listed in
Table 6 were calculated to understand the mechanism of inhibition offered by lauric, myristic, oleic, phthalic, caprylic, and palmitic acids on the Fe surface. The performance of an inhibitor can be predicted using its E
HOMO, E
LUMO, and ΔE values. The tendency of the inhibitor molecule to contribute electrons to the metal surface is correlated with E
HOMO, while E
LUMO is used to explain the ability of inhibitor molecules to obtain electrons from the metal surface [
43]. Inhibitors with higher E
HOMO values show a high propensity to donate electrons to the metal surface of the unoccupied d-orbital [
44]. The E
HOMO increasing values facilitate adsorption on the metal surface, thereby improving the effectiveness of inhibition.
On the other hand, the inhibitors with lower E
LUMO values show a high capability to accept electrons. In this research, oleic acid has a high E
HOMO value, as shown in
Table 6, which indicates a greater tendency to give electrons to the Fe surface. In comparison, the lower E
LUMO value of phthalic acid exhibits a higher tendency to allow electrons, followed by the Fe surface.
The energy gap (ΔE), which is defined as the difference between E
LUMO and E
HOMO, is another quantum chemical parameter that can be correlated with inhibition efficiency. The ΔE can elucidate the adsorption reactiveness of the inhibitor molecules with the Fe surface. A lower ∆E value of an inhibitor molecule shows that the molecule has higher reactivity and can have better IE compared to the molecules with higher ΔE. Phthalic acid possesses a low ΔE value, which suggests greater reactivity and strong inhibition performance on the Fe surface. The ΔE of oleic acid is lower than lauric acid, as also found by [
44]. Other work reports by [
45] on lauric, myristic, oleic and caprylic acid show the ΔE of 7.60 eV for lauric and myristic while oleic, caprylic acid have ΔE of 6.16 eV and 7.62 eV, respectively. The deviation between the HOMO-LUMO energy gap value obtained in this work and the other work is due to the difference in the software used and other calculations parameters such as basis sets and exchange-correlation functional. Due to the lower inhibitor electronegativity (χ) values as compared to the work function of Fe, given as 4.82 eV in Fe, the electrons flow from the inhibitor to the Fe surface. The electronegativity value of oleic acid is the lowest amongst other DPS molecules, although the difference in electronegativity value is too small as compared to those of lauric, myristic, and palmitic acids.
Global hardness (η) and softness (σ) are terms used to describe the resistance of atoms to the deformation of their electron cloud [
46]. These are important characteristics for calculating molecular stability and reactivity. A large ΔE exists in hard molecules, while the ΔE is low in soft molecules. A hard molecule is less reactive than a soft molecule because it cannot easily give electrons to the acceptor molecule. Thus, a soft-molecule inhibitor is supposed to be more reactive and has more inhibition efficiency than a hard-molecule inhibitor because soft molecules easily provide electrons to the metal surface. In this research, the values of η and σ of lauric, myristic, caprylic, and palmitic acids show small differences, which suggest that the resistance of the species is similar to the disfigurement of its electron structure. As hard molecules are less interactive than soft molecules, the alikeness of the η and σ values of lauric, myristic, caprylic, and palmitic acids suggests that these acids will have the same reactivity towards the Fe surface as inhibitors. This can also be explained by the small differences in their ΔE values. However, the values of η and σ for oleic acid and phthalic acid are not quite as close to those of other inhibitors, which indicates that they would have different reactivity towards the Fe surface. As shown in
Table 6, the lowest hardness value for phthalic acid is 1.740 eV, and the lowest softness value is 0.3783 eV
−1, which is assumed to be the most effective for a corrosion inhibitor.
The IE% through the electrons transferred (ΔN) was higher for oleic acid, indicating a higher tendency to contribute electrons to the Fe surface. All ∆N values are also shown to be lower than 3.6, and according to Lukovits [
46], ∆N value of less than 3.6 indicates higher inhibition efficiency on the metal surface due to higher electron-donating power. If ∆N exceeds 0, electrons are transferred from the inhibitor molecules to the Fe surface. On the other hand, if ∆N is less than 0, the opposite process occurs, and electrons are transferred from the Fe surface to the inhibitor molecules [
47]. The results show that the inhibitors examined in this study were electron donors and the Fe surface was the electron acceptor.
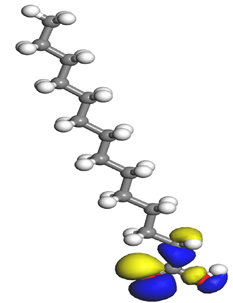
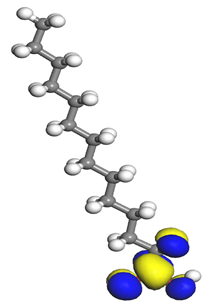
The electrophilic attack sites representing the regions where the inhibitor molecule and metal surface show that the highest bonding ability is carried by the HOMO orbital. Nucleophilic attack sites are carried by LUMO orbital; they exhibit an antibonding orbital occurring between inhibitor molecules and metal surface to create a bond of feedback that strengthens the interaction between the inhibitor and the surface of Fe.
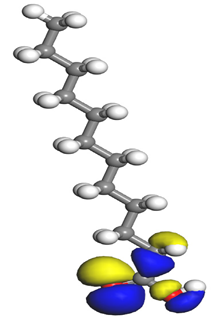
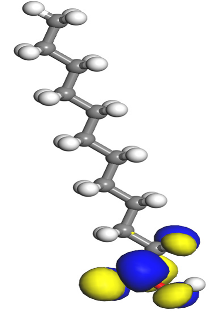
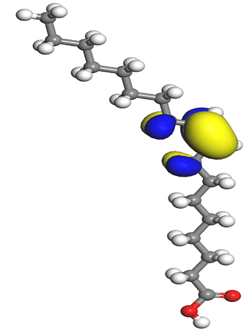
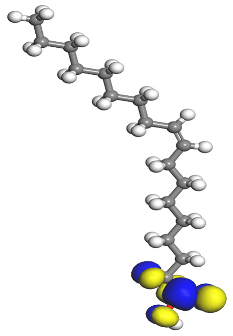
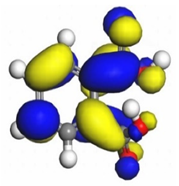
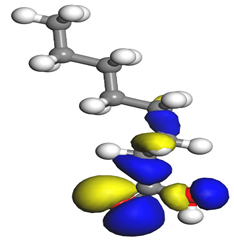
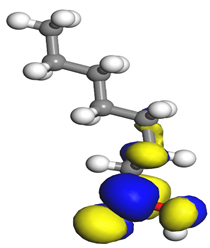
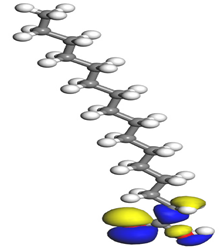
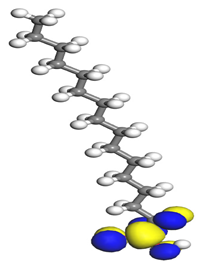
Table 7 presents the distribution of HOMO electron density over the inhibitor means that the inhibitor molecule is likely to be active in the donation of electrons to the empty Fe orbital, resulting in efficient corrosion inhibition. The distribution of LUMO density over the molecule, on the other hand, confirms that electrons from the occupied Fe orbitals can be effectively accepted, which is a major factor for interactions between the donor and the acceptor to occur. It can be observed that the HOMO and LUMO of lauric, myristic, phthalic, caprylic, and palmitic acid molecules are mainly localised around the carboxyl (COOH) group. However, in the case of oleic acid, the HOMO was mainly localised on the C=C bond, and this finding is consistent with another report [
48]. This can be due to the middle of the oleic acid contain a double bond, so the HOMO was saturated around the area. This is supported by a finding from [
49], which found that the major contribution to the HOMO comes from the carbon atoms adjacent to the C=C in the oleic acid side.
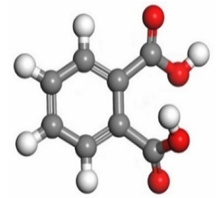
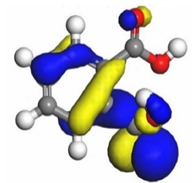
Therefore, these sites are the most active for the electron donation-acceptance type of interaction and most likely to facilitate adsorption over the Fe surface. Also, the distribution of HOMO-LUMO indicates that all double bonds in simple molecules serve as active HOMO sites, suggesting their tendency to share electronic charge with the atoms of surface metal. In the case of phthalic acid, which has two groups of carboxyl, the distribution of HOMO and LUMO around its benzene ring is observed over the entire molecule.