Biochemical Studies in Perfundates and Homogenates of Isolated Porcine Kidneys after Flushing with Zinc or Zinc–Prolactin Modified Preservation Solution Using a Static Cold Storage Technique
Abstract
1. Introduction
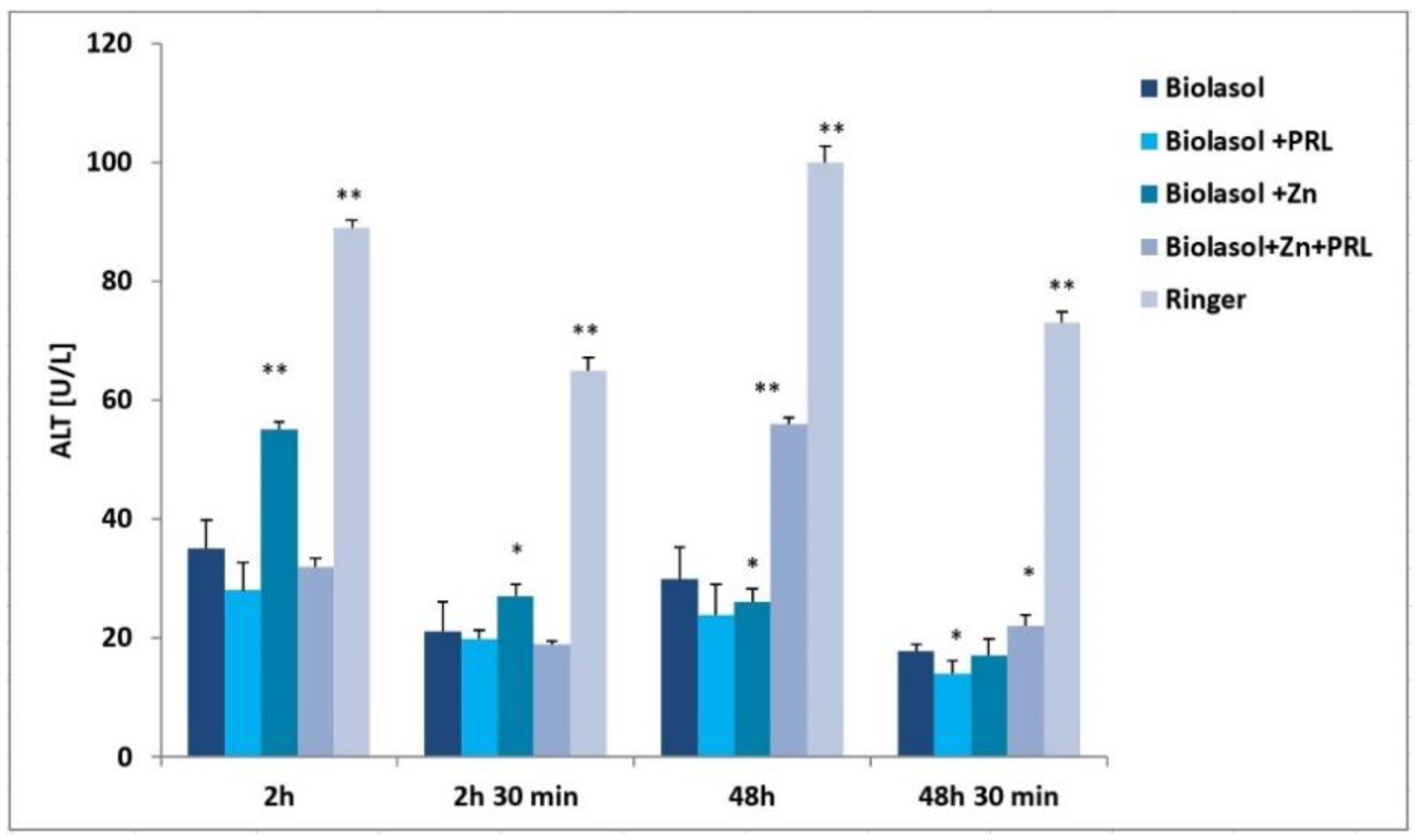
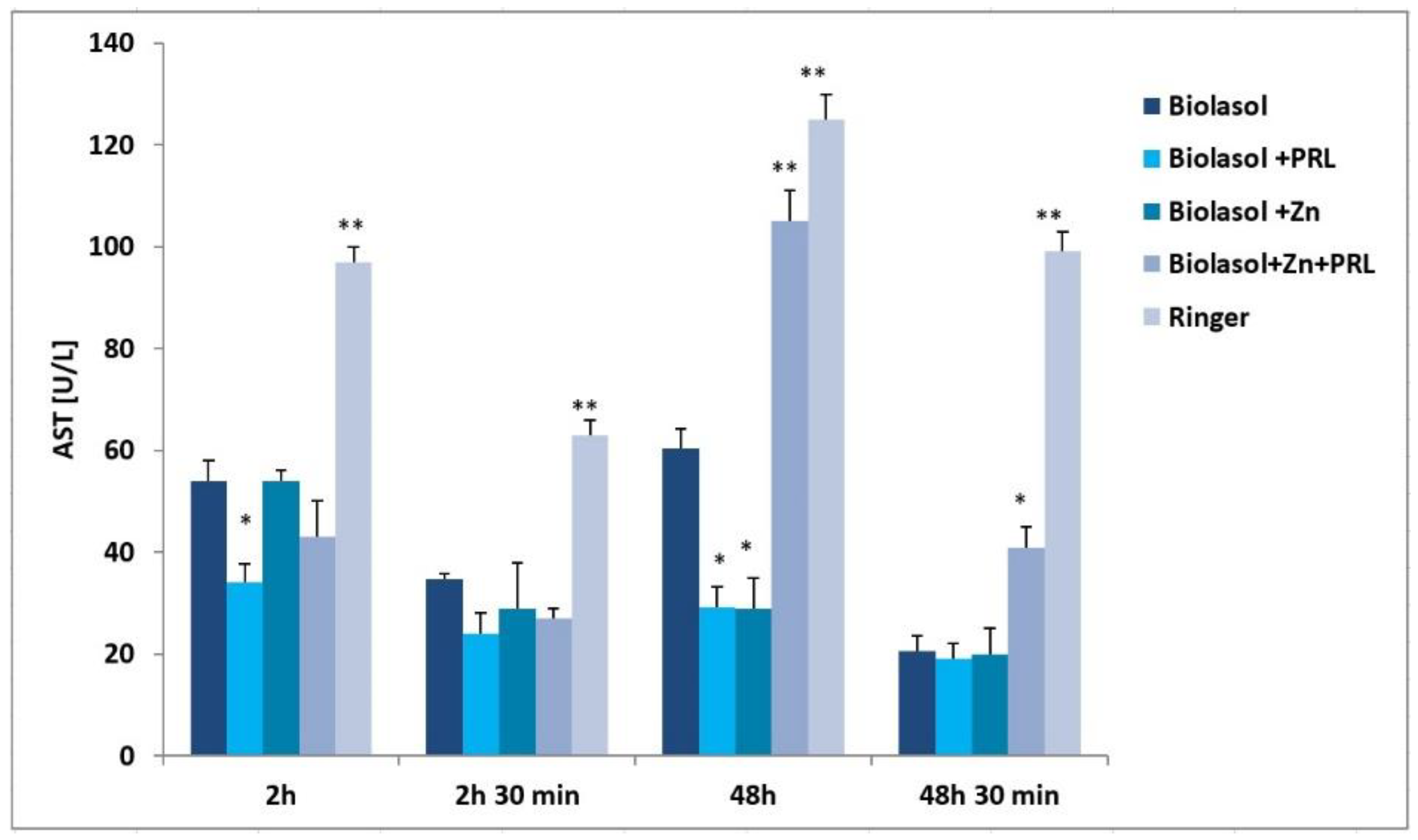
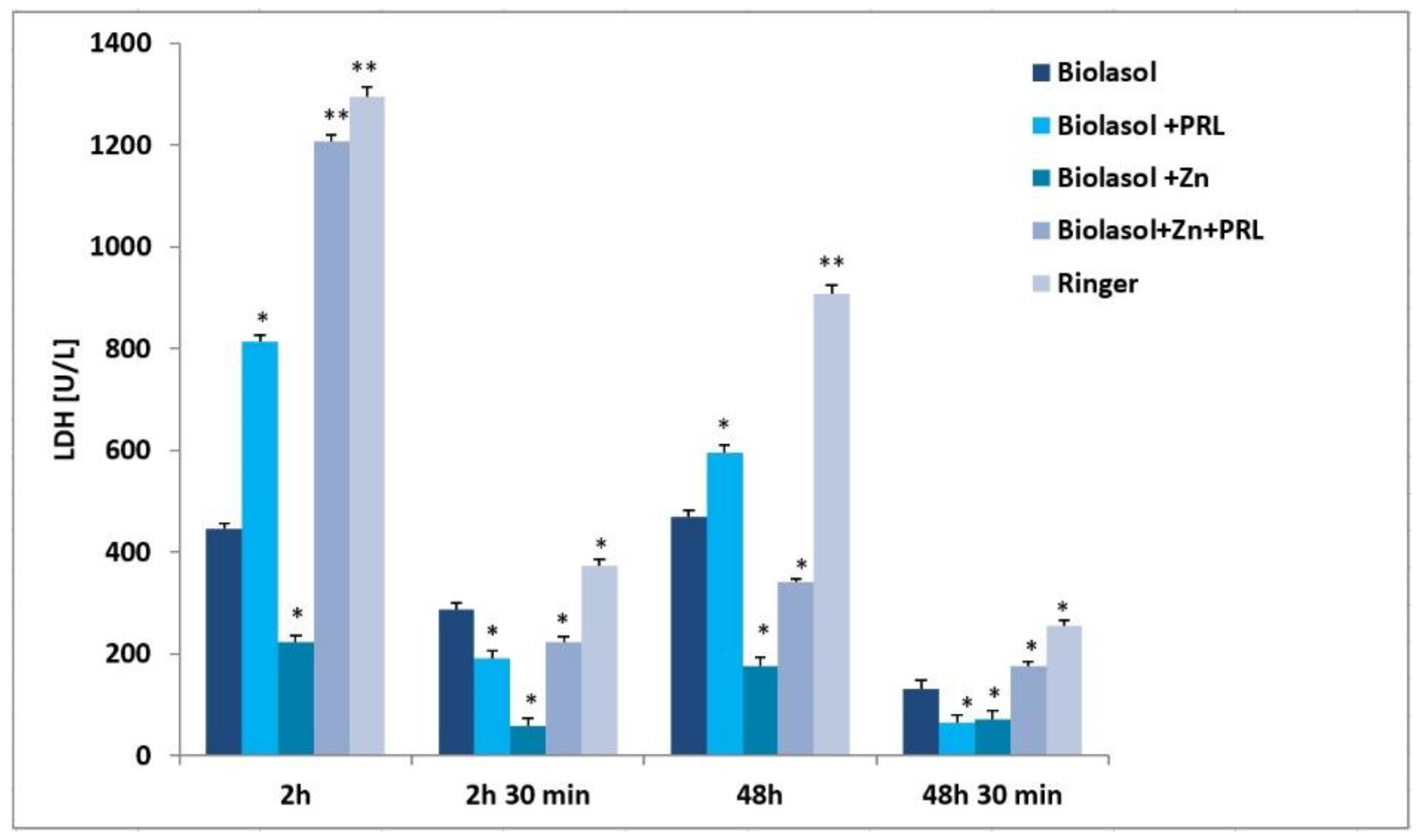
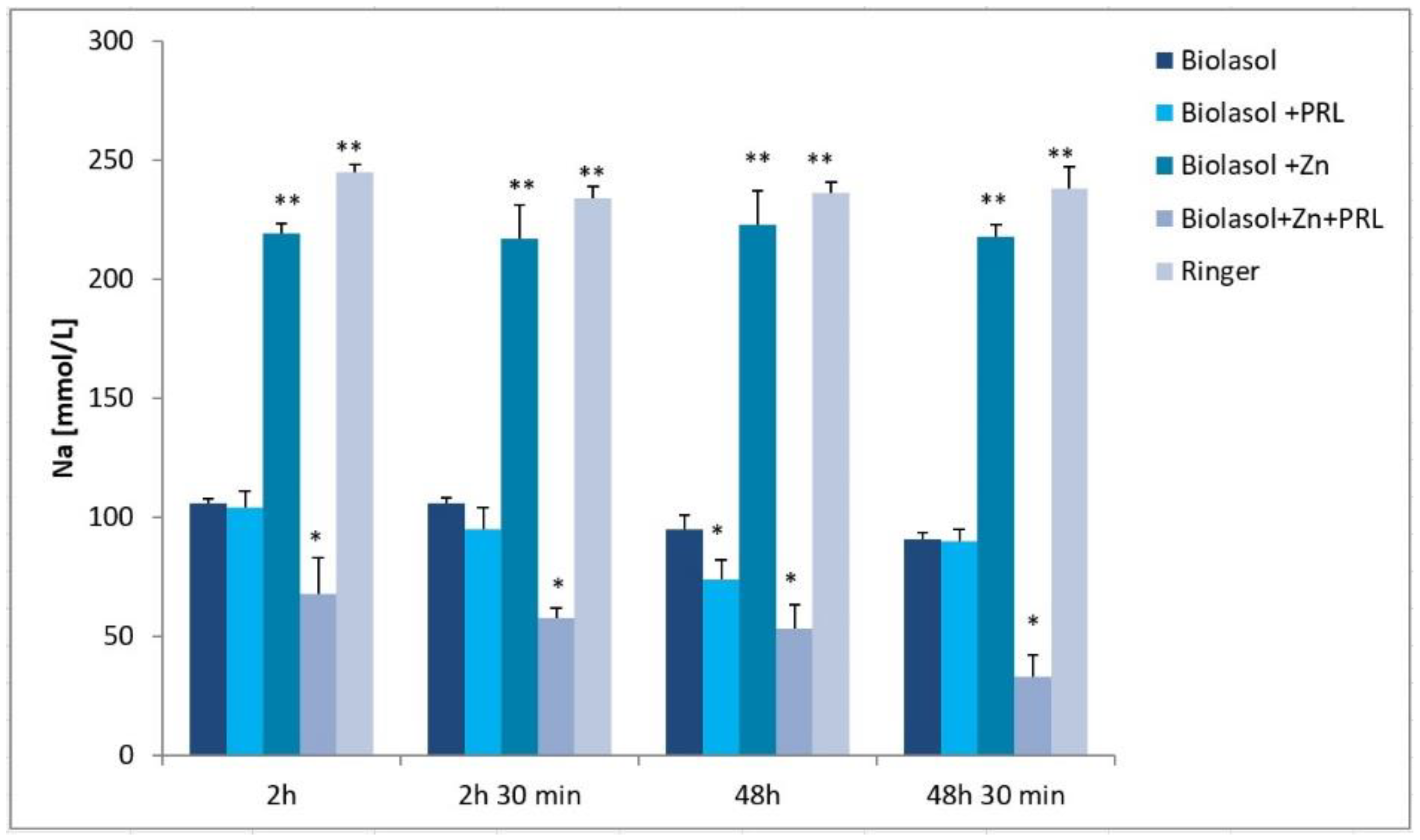
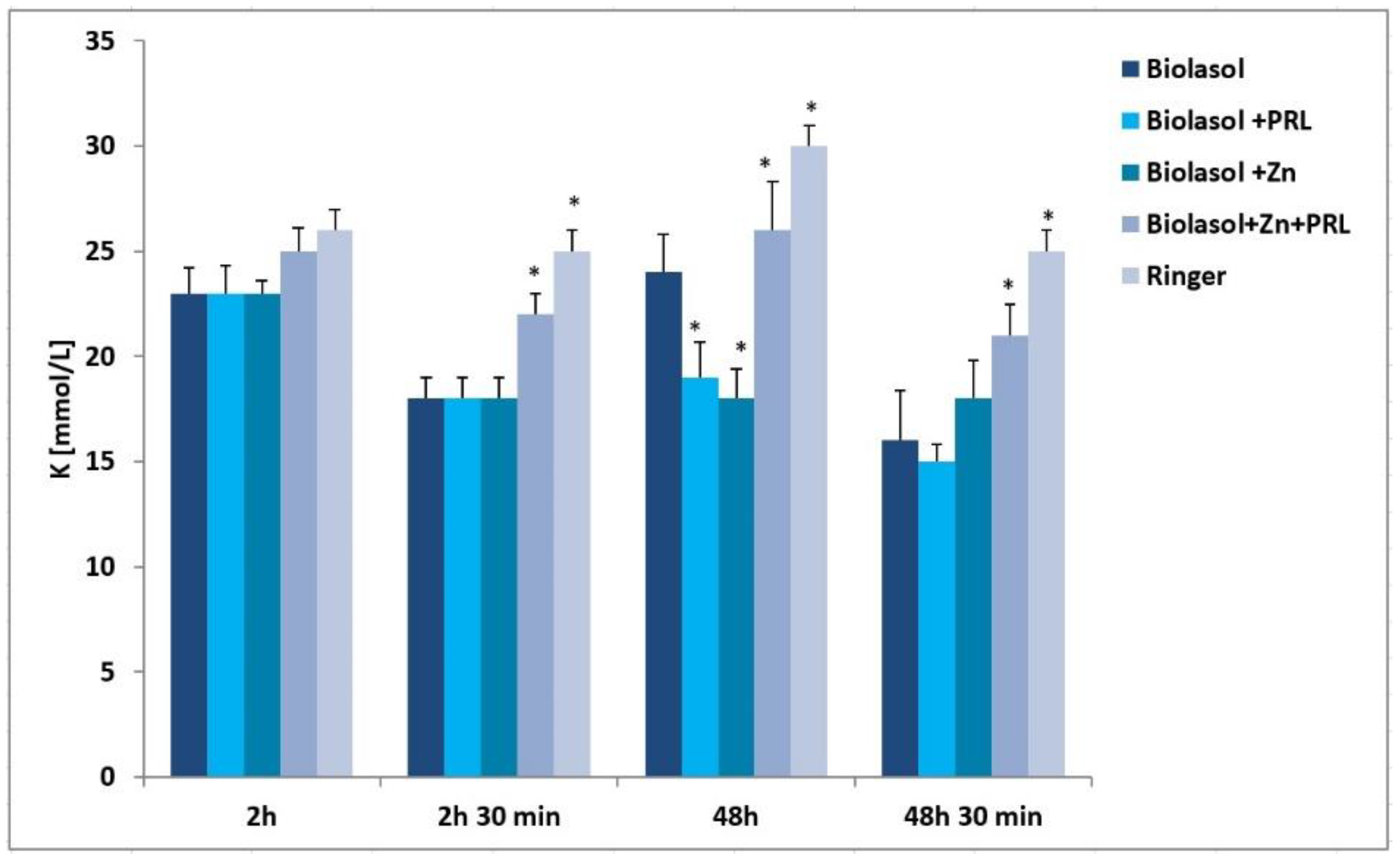
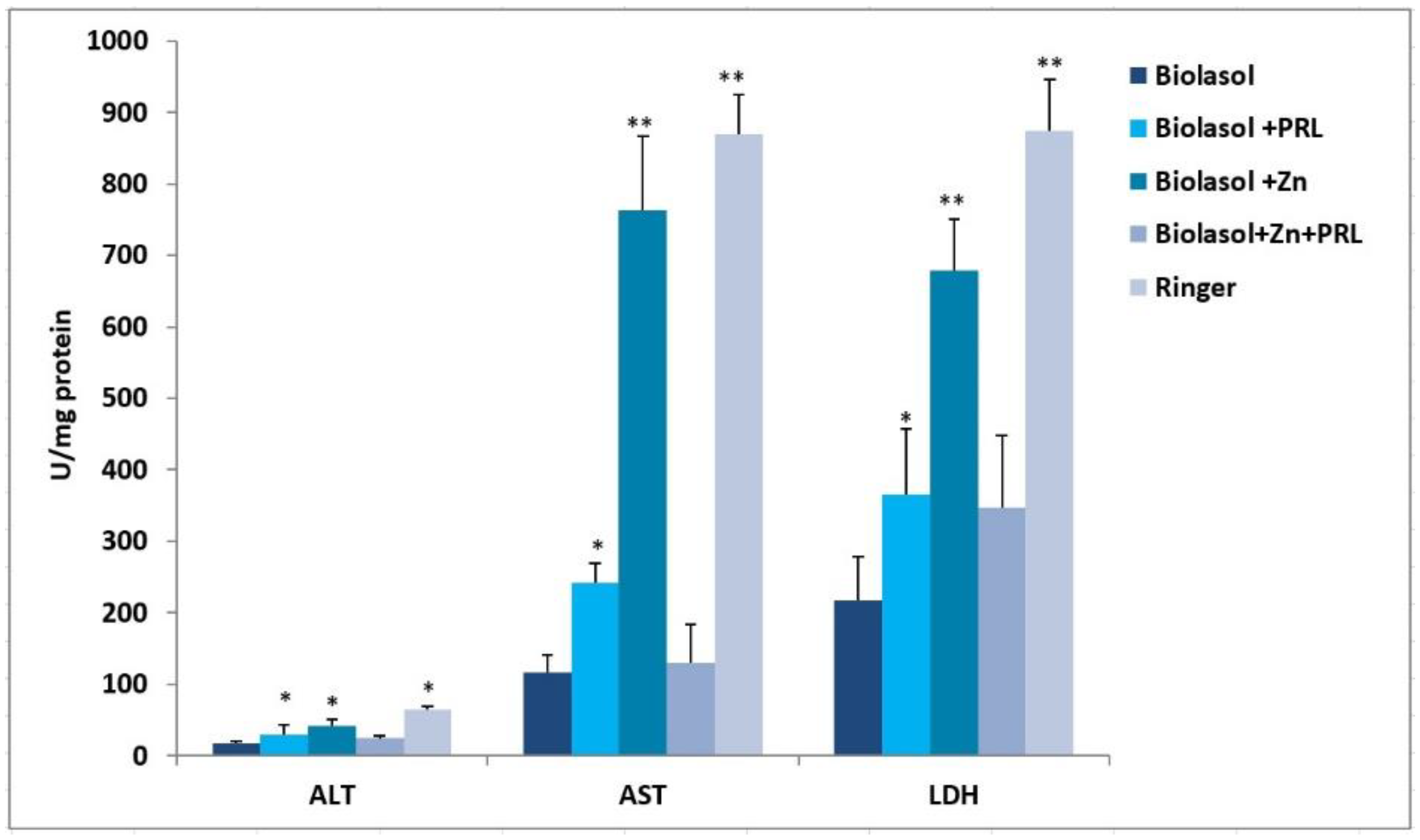
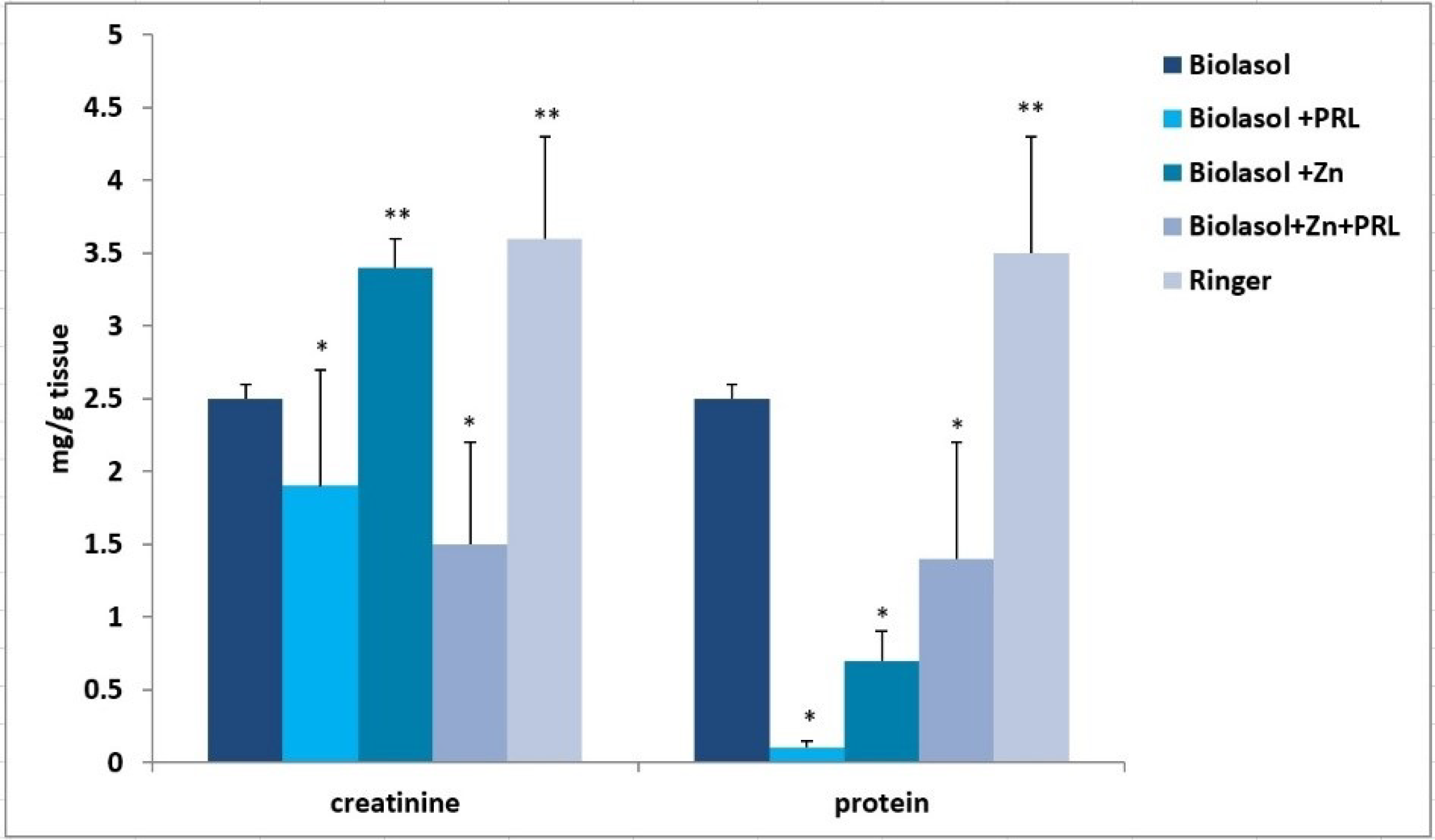
2. Results
3. Discussion
4. Materials and Methods
4.1. Preservation Solution
4.2. Animals
4.3. Ethical Issues
4.4. Kidney Procurement and Experimental Groups
4.4.1. Group A, Control
4.4.2. Group B1
4.4.3. Group B2
4.4.4. Group B3
4.4.5. Group C
4.5. Experimental Protocol
4.6. Determination of Alanine Aminotransferase
4.7. Determination of Aspartate Aminotransferase
4.8. Determination of Lactate Dehydrogenase Activity
4.9. Determination of Sodium Concentration
4.10. Determination of Potassium Concentration
4.11. Determination of Creatinine Concentration
4.12. Determination of Total Protein Concentration
4.13. Apparatus
4.14. Biochemical Analysis in Kidney Homogenates
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gapys, B.; Specht, A.; Bielarczyk, H. Role of zinc in physiological and pathological processes of the body. J. Lab. Diagn. 2014, 50, 45–52. [Google Scholar]
- Powell, S.R. The antioxidant properties of zinc. J. Nutr. 2000, 130, 1447S–1454S. [Google Scholar] [CrossRef]
- Grüngreiff, K.; Gottstein, T.; Reinhold, D. Zinc Deficiency—An Independent Risk Factor in the Pathogenesis of Haemorrhagic Stroke? Nutrients 2020, 12, 3548. [Google Scholar] [CrossRef]
- Maret, W.; Li, Y. Coordination dynamics of zinc in proteins. Chem. Rev. 2009, 109, 4682–4707. [Google Scholar] [CrossRef]
- Stork, C.J.; Li, Y.V. Intracellular zinc elevation measured with a “calcium-specific” indicator during ischemia and reperfusion in rat hippocampus: A question on calcium overload. J. Neurosci. 2006, 26, 10430–10437. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.K.; Alhosin, M. The role of zinc deficiency in endothelial dysfunction. Eur. J. Cell. Sci. 2019, 1, 22–25. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Shaikh, A.A.; Braakhuis, A.J.; Bishop, K.S. The Mediterranean Diet and Breast Cancer: A Personalised Approach. Healthcare 2019, 7, 104. [Google Scholar] [CrossRef]
- Ryszka, F.; Dolińska, B.; Czyż, K.; Jelińska, M.; Strabel, A.; Bocheńska, J. Effect of Recombinant Human Prolactin Addition to Biolasol Solution on Biochemical Indicators in Perfundates of Porcine Kidneys. Transplant. Proc. 2016, 48, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- Ostróżka-Cieślik, A.; Dolińska, B.; Ryszka, F. The Effect of Modified Biolasol Solution on the Efficacy of Storing Isolated Porcine Kidneys. BioMed Res. Int. 2018, 2018, 7465435. [Google Scholar] [CrossRef]
- Cierpka, L.; Ryszka, F.; Dolińska, B.; Smorąg, Z.; Słomski, R.; Wiaderkiewicz, R.; Caban, A.; Budziński, G.; Oczkowicz, G.; Wieczorek, J. Biolasol: Novel perfusion and preservation solution for kidneys. Transplant. Proc. 2014, 46, 2539–2541. [Google Scholar] [CrossRef]
- Permyakov, E.A.; Veprintsev, D.B.; Deikus, G.Y.; Permyakov, S.E.; Kalinichenko, L.P.; Grishchenko, V.M.; Brooks, C.L. pH-induced transition and Zn2+-binding properties of bovine prolactin. FEBS Lett. 1997, 405, 273–276. [Google Scholar] [CrossRef]
- Dolińska, B.; Ostróżka-Cieślik, A.; Caban, A.; Cierpka, L.; Ryszka, F. Comparing the effect of Biolasol® and HTK solutions on maintaining proper homeostasis, indicating the kidney storage efficiency prior to transplantation. Ann. Transplant. 2012, 17, 74–78. [Google Scholar]
- Ostróżka-Cieślik, A.; Dolińska, B.; Ryszka, F. Tips for optimizing organ preservation solutions. Acta Biochim. Pol. 2018, 65, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Ryszka, F.; Cierpka, L.; Dolińska, B. Fluid for Perfusion, Preservation and Reperfusion of Organs and Tissues. Patent PL 205012 B1, 31 March 2010. [Google Scholar]
- Budziński, G.; Suszka-Świtek, A.; Roman, P.; Caban, A.; Oczkowicz, G.; Wiaderkiewicz, A.; Mały, A.; Wiaderkiewicz, R.; Smorąg, Z.; Ryszka, F.; et al. Interleukin-6 concentration in the transgenic pig’s liver preserved for 24 hours in Biolasol solution. Transplant Proc. 2014, 46, 2552–2554. [Google Scholar] [CrossRef]
- Jozwik, A.; Domagala, P.; Kieszek, R.; Wszola, M.; Bieniasz, M.; Serwanska-Swietek, M.; Durlik, M.; Ryszka, F.; Chmura, A.; Kwiatkowski, A. Storage Kidneys Prior to Transplantation Using First Polish Preservation Solution “Biolasol”—Preliminary Report. Am. J. Transplant. 2016, 16 (Suppl. 3), 739. [Google Scholar]
- Kimura, T.; Kambe, T. The Functions of Metallothionein and ZIP and ZnT Transporters: An Overview and Perspective. Int. J. Mol. Sci. 2016, 17, 336. [Google Scholar] [CrossRef]
- Ranaldi, G.; Perozzi, G.; Truong-Tran, A.; Zalewski, P.; Murgia, C. Intracellular distribution of labile Zn(II) and zinc transporter expression in kidney and MDCK cells. Am. J. Physiol. Renal Physiol. 2002, 283, F1365–F1375. [Google Scholar] [CrossRef]
- Moslemi, F.; Talebi, A.; Nematbakhsh, M. Protective Effect of Zinc Supplementation on Renal Ischemia/Reperfusion Injury in Rat: Gender-related Difference. Int. J. Prev. Med. 2019, 10, 68. [Google Scholar] [PubMed]
- Abdallah, N.H.; Baulies, A.; Bouhlel, A.; Bejaoui, M.; Zaouali, M.A.; Ben Mimouna, S.; Messaoudi, I.; Fernandez-Checa, J.C.; García, R.C.; Ben Abdennebi, H. Zinc mitigates renal ischemiareperfusion injury in rats by modulating oxidative stress, endoplasmic reticulum stress, and autophagy. J. Cell Physiol. 2018, 233, 8677–8690. [Google Scholar] [CrossRef] [PubMed]
- Barekat, F.; Talebi, A.; Nematbakhsh, M. The protective roles of zinc and estradiol in renal ischemia/reperfusion injury in ovariectomized rats. J. Nephropathol. 2018, 7, 88–92. [Google Scholar] [CrossRef]
- O’Kane, D.; Gibson, L.; May, C.N.; du Plessis, J.; Shulkes, A.; Baldwin, G.S.; Bolton, D.; Ischia, J.; Patel, O. Zinc preconditioning protects against renal ischaemia reperfusion injury in a preclinical sheep large animal model. Biometals 2018, 31, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, B.; Emami, F.; Moslemi, F.; Talebi, A.; Nematbakhsh, M. Zinc Supplementation and Ischemia Pre-conditioning in Renal Ischemia/Reperfusion Injury. Malays J. Med. Sci. 2019, 26, 39–46. [Google Scholar] [CrossRef]
- Hegenauer, J.; Saltman, P.; Fairchild, R.; Halasz, N.A. Improved function of reperfused rabbit kidney following administration of zinc histidine. J. Trace Elem. Exp. Med. 1991, 4, 103–108. [Google Scholar]
- Rao, K.; Sethi, K.; Ischia, J.; Gibson, L.; Galea, L.; Xiao, L.; Yim, M.; Chang, M.; Papa, N.; Bolton, D.; et al. Protective effect of zinc preconditioning against renal ischemia reperfusion injury is dose dependent. PLoS ONE. 2017, 7, e0180028. [Google Scholar] [CrossRef]
- Karmazyn, M.; Gan, X.T.; Humphreys, R.A.; Yoshida, H.; Kusumoto, K. The myocardial Na+/H+ exchan-ge: Structure, regulation and its role its heart disease. Circ. Res. 1999, 85, 777–786. [Google Scholar] [CrossRef]
- Gachot, B.; Tauc, M.; Morat, L.; Poujeol, P. Zinc uptake by proximal cells isolated from rabbit kidney: Effects of cysteine and histidine. Pflug. Arch. 1991, 419, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.E.; Kim, K.J.; Goodman, B.E.; Wells, J.R.; Crandall, E.D. Sodium-amino acid cotransport by type II alveolar epithelial cells. J. Appl. Physiol. 1985, 59, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.G.; Farrell, N.P. The dynamics of zinc sites in proteins: Electronic basis for coordination sphere expansion at structural sites. Metallomics 2014, 6, 2230–2241. [Google Scholar] [CrossRef]
- Tettamanzi, M.C.; Keeler, C.; Meshack, S.; Hodsdon, M.E. Analysis of site-specific histidine protonation in human prolactin. Biochemistry 2008, 47, 8638–8647. [Google Scholar] [CrossRef][Green Version]
- Ducoudret, O.; Barbier, O.; Tauc, M.; Fuchs, M.; Poujeol, P. Characterization of Zn(2+) transport in Madin-Darby canine kidney cells. Biochim. Biophys. Acta. 2003, 1611, 171–179. [Google Scholar] [CrossRef]
- Dünkelberg, S.; Maywald, M.; Schmitt, A.K.; Schwerdtle, T.; Meyer, S.; Rink, L. The Interaction of Sodium and Zinc in the Priming of T Cell Subpopulations Regarding Th17 and Treg Cells. Mol. Nutr. Food. Res. 2020, 64, e1900245. [Google Scholar] [CrossRef]
- Prasad, A.S. Sodium and Zinc. In Interactions of Zinc with Other Micronutrient; Prasad, A.S., Ed.; Plenum Press: New York, NY, USA, 1993; pp. 170–171. [Google Scholar]
- Ibarra, F.; Crambert, S.; Eklöf, A.C.; Lundquist, A.; Hansell, P.; Holtbäck, U. Prolactin, a natriuretic hormone, interacting with the renal dopamine system. Kidney Int. 2005, 68, 1700–1707. [Google Scholar] [CrossRef][Green Version]
- Caban, A.; Dolińska, B.; Oczkowicz, G.; Budziński, G.; Ostróżka-Cieślik, A.; Krzysztofik, M.; Cierpka, L.; Ryszka, F. The effect of HTK solution modification by addition of prolactin on biochemical indices reflecting ischaemic damage to porcine kidney. Transpl. Proc. 2010, 42, 3981–3983. [Google Scholar] [CrossRef]
- Iran-Nejad, A.; Nematbakhsh, M.; Eshraghi-Jazi, F.; Talebi, A. Preventive role of estradiol on kidney injury induced by renal ischemia-reperfusion in male and female rats. Int. J. Prev. Med. 2015, 6, 22. [Google Scholar]
- Kang, K.P.; Lee, J.E.; Lee, A.S.; Jung, Y.J.; Kim, D.; Lee, S.; Hwang, H.P.; Kim, W.; Park, S.K. Effect of gender differences on the regulation of renal ischemia-reperfusion-induced inflammation in mice. Mol. Med. Rep. 2014, 9, 2061–2068. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Dolińska, B. The Role of Hormones and Trophic Factors as Components of Preservation Solutions in Protection of Renal Function before Transplantation: A Review of the Literature. Molecules 2020, 25, 2185. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Dolińska, B.; Ryszka, F. Effect of Lutropin Concentration on the Efficiency of Isolated Porcine Kidney Storage in Modified Biolasol Solution. Transpl. Proc. 2020, 52, 2055–2058. [Google Scholar] [CrossRef]
- Brandão, N.J.; de Mendonça, B.B.; Shuhama, T.; Marchini, J.S.; Madureira, G.; Pimenta, W.P.; Tornero, M.T. Zinc: An inhibitor of prolactin (PRL) secretion in humans. Horm. Metab. Res. 1989, 21, 203–206. [Google Scholar]
- Prasad, A.S. Zinc and Prolactin. In Interactions of Zinc with Other Micronutrient; Prasad, A.S., Ed.; Plenum Press: New York, NY, USA, 1993; pp. 110–111. [Google Scholar]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, Y.; Liang, J. Zinc is a potent heat shock protein inducer during liver cold preservation in rats. Chin. Med. J. 2002, 115, 1777–1779. [Google Scholar] [PubMed]
- Weng, Z.; Liu, Z.; Zhang, S.; Tao, H.; Ji, X. Zinc protection in fetal rats for maternal mercury exposure-induced growth retardation is probably associated with S100B expression. J. Obstet. Gynaecol. Res. 2017, 43, 73–77. [Google Scholar] [CrossRef]
- Moser, M.A.J.; Sawicka, K.; Sawicka, J.; Franczak, A.; Cohen, A.; Bil-Lula, I.; Sawicki, G. Protection of the transplant kidney during cold perfusion with doxycycline: Proteomic analysis in a rat model. Proteome Sci. 2020, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Mimura, Y. Antioxidant effect of zinc on acute renal failure induced by ischemia-reperfusion injury in rats. Am. J. Nephrol. 1999, 19, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Odeniyi, D.T.; Apostolov, E.O.; Savenka, A.; Fite, T.; Wangila, G.W.; Walker, R.B.; Basnakian, A.G. Protective effect of zinc-N-acetylcysteine on the rat kidney during cold storage. Am. J. Physiol. Renal Physiol. 2013, 305, F1022–F1030. [Google Scholar] [CrossRef]
- Karagulova, G.; Yue, Y.; Moreyra, A.; Boutjdir, M.; Korichneva, I. Protective role of intracellular zinc in myocardial ischemia/reperfusion is associated with preservation of protein kinase C isoforms. J.; Pharmacol. Exp. Ther. 2007, 321, 517–525. [Google Scholar] [CrossRef]
- Guo, L.; Li, P.; Meng, C.; Lu, R.; Yang, Y.; Zhou, Y.; Zhang, Y.; Chen, H.; Yin, F. Protective effect of zinc on mouse renal ischemia-reperfusion injury by anti-apoptosis and antioxidation. Curr. Pharm. Biotechnol. 2014, 15, 577–582. [Google Scholar] [CrossRef]
- Ischia, J.; Bolton, D.M.; Patel, O. Why is it worth testing the ability of zinc to protect against ischaemia reperfusion injury for human application. Metallomics 2019, 11, 1330–1343. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A.; Dolińska, B.; Ryszka, F. Therapeutic Potential of Selenium as a Component of Preservation Solutions for Kidney Transplantation. Molecules 2020, 25, 3592. [Google Scholar] [CrossRef]
- Hadj, A.N.; Baulies, A.; Bouhlel, A.; Bejaoui, M.; Zaouali, M.A.; Ben, M.S.; Messaoudi, I.; Fernandez-Checa, J.C.; García, R.C.; Ben, A.H. The effect of zinc acexamate on oxidative stress, inflammation and mitochondria induced apoptosis in rat model of renal warm ischemia. Biomed. Pharmacother. 2018, 105, 573–581. [Google Scholar] [CrossRef]
- Lulat, S.I.; Yadav, Y.C.; Balaraman, R.; Maheshwari, R. Antiurolithiatic effect of lithocare against ethylene glycol-induced urolithiasis in Wistar rats. Indian. J. Pharmacol. 2016, 48, 78–82. [Google Scholar] [PubMed]
- McHugh, M.L. Multiple comparison analysis testing in ANOVA. Biochem. Med. 2011, 21, 203–209. [Google Scholar] [CrossRef]
| Component | Effect | |
|---|---|---|
| K+ | 10 mmol/L | Electrolytes |
| Na+ | 105 mmol/L | Electrolytes |
| Ca2+ | 0.5 mmol/L | Electrolytes |
| Mg2+ | 5 mmol/L | Electrolytes |
| Cl− | 10.5 mmol/L | Electrolytes |
| Dextran 70 | 0.7 g/L | Colloids |
| HCO3− | 5 mmol/L | Buffers |
| Citrate Glucose | 30 mmol/L 167 mmol/L | Impermeant Impermeant Energy substrates |
| Di-sodium edetate Fumarate | 5 mmol/L 5 mmol/L | Additives Additives |
| Ascorbic acid | 0.5 mmol/L | Antioxidant |
| pH | 7.4 | |
| Viscosity (cP) | 2.90 | |
| Osmolality (mOsm/kg H2O) | 330 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostróżka-Cieślik, A.; Dolińska, B.; Ryszka, F. Biochemical Studies in Perfundates and Homogenates of Isolated Porcine Kidneys after Flushing with Zinc or Zinc–Prolactin Modified Preservation Solution Using a Static Cold Storage Technique. Molecules 2021, 26, 3465. https://doi.org/10.3390/molecules26113465
Ostróżka-Cieślik A, Dolińska B, Ryszka F. Biochemical Studies in Perfundates and Homogenates of Isolated Porcine Kidneys after Flushing with Zinc or Zinc–Prolactin Modified Preservation Solution Using a Static Cold Storage Technique. Molecules. 2021; 26(11):3465. https://doi.org/10.3390/molecules26113465
Chicago/Turabian StyleOstróżka-Cieślik, Aneta, Barbara Dolińska, and Florian Ryszka. 2021. "Biochemical Studies in Perfundates and Homogenates of Isolated Porcine Kidneys after Flushing with Zinc or Zinc–Prolactin Modified Preservation Solution Using a Static Cold Storage Technique" Molecules 26, no. 11: 3465. https://doi.org/10.3390/molecules26113465
APA StyleOstróżka-Cieślik, A., Dolińska, B., & Ryszka, F. (2021). Biochemical Studies in Perfundates and Homogenates of Isolated Porcine Kidneys after Flushing with Zinc or Zinc–Prolactin Modified Preservation Solution Using a Static Cold Storage Technique. Molecules, 26(11), 3465. https://doi.org/10.3390/molecules26113465








