Activity and Mechanism of Action of Antifungal Peptides from Microorganisms: A Review
Abstract
1. Introduction
2. Microorganisms Producing Antifungal Peptides
| Microbial Species | Source | Name of Antifungal Peptide | Molecular Weight/Da | Fungal Species | References |
|---|---|---|---|---|---|
| Bacillus BH072 | Bacillus | Flagellin | 35 615 | Aspergillus niger, Pythium, Botrytis cinerea, Fusarium oxysporum | [23] |
| Bacillus AH-E-1 | Bacillus | Not named | 500-1000 | A variety of plant and human pathogenic fungi | [24] |
| Bacillus B9987 | Bacillus | Metabolites BMME-1 | Not mentioned | Alternaria solani | [25] |
| Bacillus subtilis CCTCCM207209 | Bacillus | Iturin A | 1095.5 | Candida, Hyphomyces cerevisiae, Fusarium and Aspergillus | [26,27] |
| Bacillus subtilis B25 | Bacillus | Not named | 38708.67 | Fusarium oxysporum, Alternaria solani, Corynespora, Botrytis cinerea, Colletotrichum gloeosporioide | [28] |
| Bacillus amyloliquefaciens SWB16 | Bacillus | Subtilin, Iturin | 1042.6-1505.9 | Beauveria bassiana | [29] |
| Bacillus cereus YQ 308 | Bacillus | Chitinase, chitosanase, protease | 48,000 | Fusarium oxysporum, Fusarium solani, Pythium ultimum | [30,31] |
| Bacillus thuringiensis S4 | Bacillus | Chitin-binding protein CBP24 | 21,000 | Fusarium, Rhizoctonia subtilis | [32] |
| Bacillus licheniformis W10 | Bacillus | Serine protease | 48,794.16 | Botrytis cinerea | [33] |
| Bacillus pumilus HN-10 | Bacillus | P-1 | 1149.14 | Trichothecium roseum | [22] |
| Bacillus bereis DTU001 | Bacillus | Not named | Not mentioned | Candida, Penicillium, Aspergillus, etc. | [34] |
| Paenibacillus polymyxa KT-8 | Paenibacillus | Fusaricidin A | About 883 | Fusarium oxysporum, Aspergillus niger, Saccharomyces cerevisiae, Magnaporthe grisea, etc. | [35,36] |
| Paenibacillus ehimensis MA2012 | Paenibacillus | Not named | 1115 | A variety of plant pathogenic fungi, Colletotrichum | [37] |
| Pseudomonas syringae | Pseudomonas | Syringostatin A, syringostatin E | About 1179.7, About 1161.3 | Yeasts, filamentous fungi | [21] |
| Helicobacter pylori | Spirillum | HP 2-20 | About 2320.8 | Candida albicans, Hyphomyces burnetii | [38] |
| Enterococcus faecalis | Enterococcus | EntV | 3000-10,000 | C. albicans, C. tropicalis, C. paraplanatus, etc. | [39] |
| Aspergillus nidulans | Aspergillus | Echinocandin B | About 1 060.2 | Candida | [15] |
| Aspergillus clavatus | Aspergillus | AcAFP | 5773 | Fusarium oxysporum, Aspergillus niger, Botrytis cinerea, etc. | [40] |
| Penicillium citrinum W1 | Penicillium | PcPAF | About 10,000 | Trichoderma viride, Fusarium oxysporum, Paecilomyces variotii, and Alternaria longipes | [41] |
| Aureobasidium pullulans | Aureobasidium | Aureobasidin A(AbA) | 1070-1148 | Candida, Cryptococcus neoformans, Blastomyces dermatitis, etc. | [42,43] |
| Acremonium persicinum | Acremonium | VL-2397 | About 914.9 | Aspergillus, Cryptococcus neoformans, Candida glabrata, etc. | [44] |
| Marine streptomyces DA11 | Streptomyces | Chitinase | About 34000 | Aspergillus niger, Candida albicans | [45] |
| Marine Actinomycetes M045 | cladothrix actinomyces | Chandrananimycin A | About 270.24 | M. miehei | [46] |
| Actinomycete Streptomyces cacaoi | Streptomyces | Polyoxin D | About 521.4 | Candida albicans, Cryptococcus neoformans, etc. | [47] |
| Streptomyces tendae | Streptomyces | Nikkomycin Z | About 495.4 | Glomus, Aspergillus fumigatus, etc. | [48,49] |
3. Stability of Antifungal Peptides
4. Toxicity of Antifungal Peptides
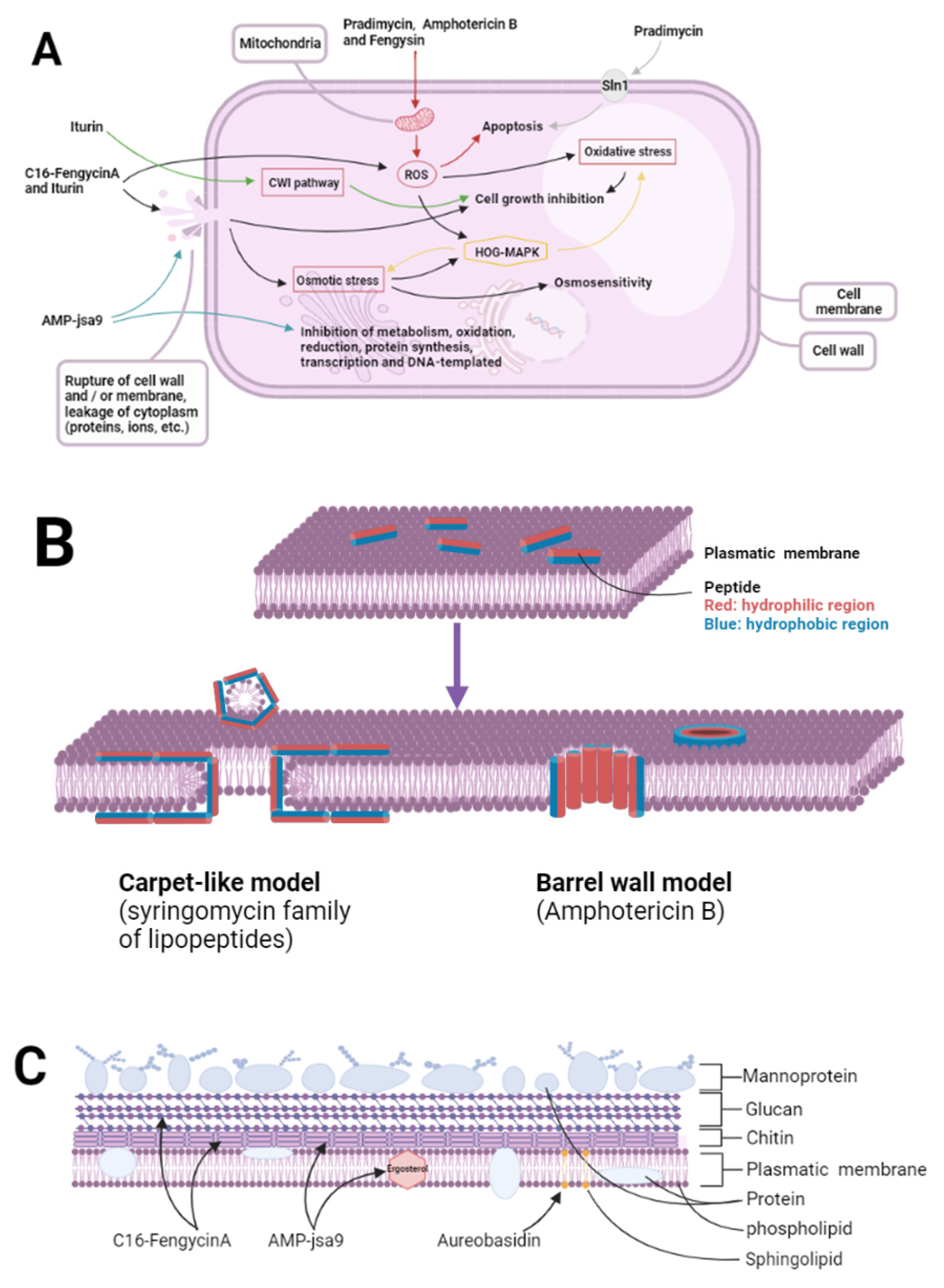
5. Mechanism of Action of Antifungal Peptides
5.1. Effect of Antifungal Peptides on Pathogenic Bacteria
5.1.1. Targeting of Cell Walls
5.1.2. Targeting Cell Membranes
5.1.3. Targeting Nucleic Acids, Organelles, and Intracellular Macromolecules
5.2. Effects of Antifungal Peptides on Their Own Strains
5.3. Competitive Effects of Antifungal Peptides on Host Targets and Nutrients
5.4. Brief Summary
6. Expectations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shentsova, E.S.; Lytkina, L.I.; Shevtsov, A.A. Reduction of the content of aflatoxin-forming fungi in contaminated grains by methods of hydrothermal treatment. Gig. I Sanit. 2015, 94, 64–67. [Google Scholar]
- Lestrade, P.P.; Bentvelsen, R.G.; Schauwvlieghe, A.; Schalekamp, S.; van der Velden, W.; Kuiper, E.J.; van Paassen, J.; van der Hoven, B.; van der Lee, H.A.; Melchers, W.J.G.; et al. Voriconazole Resistance and Mortality in Invasive Aspergillosis: A Multicenter Retrospective Cohort Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 68, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Martel, C.M.; Parker, J.E.; Bader, O.; Weig, M.; Gross, U.; Warrilow, A.G.; Kelly, D.E.; Kelly, S.L. A clinical isolate of Candida albicans with mutations in ERG11 (encoding sterol 14alpha-demethylase) and ERG5 (encoding C22 desaturase) is cross resistant to azoles and amphotericin B. Antimicrob. Agents Chemother. 2010, 54, 3578–3583. [Google Scholar] [CrossRef] [PubMed]
- Farahyar, S.; Zaini, F.; Kordbacheh, P.; Rezaie, S.; Safara, M.; Raoofian, R.; Heidari, M. Overexpression of aldo-keto-reductase in azole-resistant clinical isolates of Candida glabrata determined by cDNA-AFLP. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2013, 21, 1. [Google Scholar] [CrossRef]
- Fan, X.; Xiao, M.; Liao, K.; Kudinha, T.; Wang, H.; Zhang, L.; Hou, X.; Kong, F.; Xu, Y.C. Notable Increasing Trend in Azole Non-susceptible Candida tropicalis Causing Invasive Candidiasis in China (August 2009 to July 2014): Molecular Epidemiology and Clinical Azole Consumption. Front. Microbiol. 2017, 8, 464. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Steiner, H.; Hultmark, D.; Engström, A.; Bennich, H.; Boman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 1981, 292, 246–248. [Google Scholar] [CrossRef]
- Fry, D.E. Antimicrobial Peptides. Surg. Infect. 2018, 19, 804–811. [Google Scholar] [CrossRef]
- Landy, M.; Warren, G.H.; RosenmanM, S.B.; Colio, L.G. Bacillomycin: An antibiotic from Bacillus subtilis active against pathogenic fungi. Proceedings of the Society for Experimental Biology and Medicine. Soc. Exp. Biol. Med. 1948, 67, 539–541. [Google Scholar] [CrossRef]
- Banerjee, N.; Bose, S.K. Mode of action of mycobacillin, a new antifungal antibiotic. J. Bacteriol. 1963, 86, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.B.; Bose, S.K. Biosynthesis of mycobacillin, a new antifungal peptide. i. role of nucleic acid. J. Bacteriol. 1964, 87, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G. Antibacterial peptides: Key components needed in immunity. Cell 1991, 65, 205–207. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Wei, Z.; Guan, Z.; Cai, Y.; Liao, X. Antifungal Activity of Isolated Bacillus amyloliquefaciens SYBC H47 for the Biocontrol of Peach Gummosis. PLoS ONE 2016, 11, e0162125. [Google Scholar] [CrossRef] [PubMed]
- Nyfeler, R.; Keller-Schierlein, W. Metabolites of microorganisms. 143. Echinocandin B, a novel polypeptide-antibiotic from Aspergillus nidulans var. echinulatus: Isolation and structural components. Helv. Chim. Acta 1974, 57, 2459–2477. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014, 169, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Xue, Y.; Gao, W.; Li, J.; Zu, X.; Fu, D.; Feng, S.; Bai, X.; Zuo, Y.; Li, P. Actinobacteria-Derived peptide antibiotics since 2000. Peptides 2018, 103, 48–59. [Google Scholar] [CrossRef]
- Martín Mazuelos, E.; Rodríguez-Tudela, J.L. In vitro activity of anidulafungin. Comparison with the activity of other echinocandins. Enferm. Infecc. y Microbiol. Clin. 2008, 26, 7–13. [Google Scholar] [CrossRef]
- Yao, J.; Liu, H.; Zhou, T.; Chen, H.; Miao, Z.; Sheng, C.; Zhang, W. Total synthesis and structure-activity relationships of new echinocandin-like antifungal cyclolipohexapeptides. Eur. J. Med. Chem. 2012, 50, 196–208. [Google Scholar] [CrossRef]
- Zhao, Y.; Perlin, D.S. Review of the Novel Echinocandin Antifungal Rezafungin: Animal Studies and Clinical Data. J. Fungi 2020, 6, 192. [Google Scholar] [CrossRef]
- Sorensen, K.N.; Kim, K.H.; Takemoto, J.Y. In vitro antifungal and fungicidal activities and erythrocyte toxicities of cyclic lipodepsinonapeptides produced by Pseudomonas syringae pv. syringae. Antimicrob. Agents Chemother. 1996, 40, 2710–2713. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Yun, J.; Ai, D.; Zhang, W.; Bai, J.; Guo, J. Two novel cationic antifungal peptides isolated from Bacillus pumilus HN-10 and their inhibitory activity against Trichothecium roseum. World J. Microbiol. Biotechnol. 2018, 34, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, Z.J.; Han, Y.; Wang, Z.Z.; Fan, J.; Xiao, H.Z. Isolation and identification of antifungal peptides from Bacillus BH072, a novel bacterium isolated from honey. Microbiol. Res. 2013, 168, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Jin, M.; Qu, H.M.; Chen, Z.Q.; Chen, Z.L.; Qiu, Z.G.; Wang, X.W.; Li, J.W. Isolation and characterization of Bacillus sp. producing broad-spectrum antibiotics against human and plant pathogenic fungi. J. Microbiol. Biotechnol. 2012, 22, 256–263. [Google Scholar] [CrossRef]
- Gao, W.; Tian, L.; Zhou, J.; Shi, Z.; Zheng, L.; Cui, Z.; Li, Y. Antifungal mechanism of Bacillus marinus B-9987. Wei Sheng Wu Xue Bao = Acta Microbiol. Sin. 2009, 49, 1494–1501. [Google Scholar]
- Lei, S.; Zhao, H.; Pang, B.; Qu, R.; Lian, Z.; Jiang, C.; Shao, D.; Huang, Q.; Jin, M.; Shi, J. Capability of iturin from Bacillus subtilis to inhibit Candida albicans in vitro and in vivo. Appl. Microbiol. Biotechnol. 2019, 103, 4377–4392. [Google Scholar] [CrossRef]
- Klich, M.A.; Lax, A.R.; Bland, J.M. Inhibition of some mycotoxigenic fungi by iturin A, a peptidolipid produced by Bacillus subtilis. Mycopathologia 1991, 116, 77–80. [Google Scholar] [CrossRef]
- Tan, Z.; Lin, B.; Zhang, R. A novel antifungal protein of Bacillus subtilis B25. SpringerPlus 2013, 2, 543. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, D.; Liu, Y.; Ao, X.; Fan, R.; Duan, Z.; Liu, Y.; Chen, Q.; Jin, Z.; Wan, Y. Antagonism against Beauveria bassiana by lipopeptide metabolites produced by entophyte Bacillus amyloliquefaciens strain SWB16. Wei Sheng Wu Xue Bao = Acta Microbiol. Sin. 2014, 54, 778–785. [Google Scholar]
- Chang, W.T.; Chen, C.S.; Wang, S.L. An antifungal chitinase produced by Bacillus cereus with shrimp and crab shell powder as a carbon source. Curr. Microbiol. 2003, 47, 102–108. [Google Scholar] [CrossRef]
- Chang, W.T.; Chen, Y.C.; Jao, C.L. Antifungal activity and enhancement of plant growth by Bacillus cereus grown on shellfish chitin wastes. Bioresour. Technol. 2007, 98, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, M.A.; Latif, M.; Hussain, K.; Gull, M.; Latif, F.; Rajoka, M.I. Heterologous expression of the antifungal β-chitin binding protein CBP24 from bacillus thuringiensis and its synergistic action with bacterial chitinases. Protein Pept. Lett. 2015, 22, 39–44. [Google Scholar] [CrossRef]
- Ji, Z.L.; Peng, S.; Chen, L.L.; Liu, Y.; Yan, C.; Zhu, F. Identification and characterization of a serine protease from Bacillus licheniformis W10: A potential antifungal agent. Int. J. Biol. Macromol. 2020, 145, 594–603. [Google Scholar] [CrossRef]
- Devi, S.; Kiesewalter, H.T.; Kovács, R.; Frisvad, J.C.; Weber, T.; Larsen, T.O.; Kovács, Á.T.; Ding, L. Depiction of secondary metabolites and antifungal activity of Bacillus velezensis DTU001. Synth. Syst. Biotechnol. 2019, 4, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, Y.; Kaneda, M. Fusaricidin A, a new depsipeptide antibiotic produced by Bacillus polymyxa KT-8. Taxonomy, fermentation, isolation, structure elucidation and biological activity. J. Antibiot. 1996, 49, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Feng, H.; Han, J.; Qiao, Z.; Wang, X.; Zhang, Q.; Liu, Z.; Wu, Z. Isolation and Characterization of a New Fusaricidin-Type Antibiotic Produced by Paenibacillus bovis sp. nov BD3526. Curr. Microbiol. 2020, 77, 3990–3999. [Google Scholar] [CrossRef]
- Naing, K.W.; Lee, Y.S.; Nguyen, X.H.; Jeong, M.H.; Anees, M.; Oh, B.S.; Cho, J.Y.; Moon, J.H.; Kim, K.Y. Isolation and characterization of an antimicrobial lipopeptide produced by Paenibacillus ehimensis MA2012. J. Basic Microbiol. 2015, 55, 857–868. [Google Scholar] [CrossRef]
- Ribeiro, P.D.; Medina-Acosta, E. Prevention of lethal murine candidiasis using HP (2-20), an antimicrobial peptide derived from the N-terminus of Helicobacter pylori ribosomal protein L1. Peptides 2003, 24, 1807–1814. [Google Scholar] [CrossRef]
- Graham, C.E.; Cruz, M.R.; Garsin, D.A.; Lorenz, M.C. Enterococcus faecalis bacteriocin EntV inhibits hyphal morphogenesis, biofilm formation, and virulence of Candida albicans. Proc. Natl. Acad. Sci. USA 2017, 114, 4507–4512. [Google Scholar] [CrossRef]
- Skouri-Gargouri, H.; Gargouri, A. First isolation of a novel thermostable antifungal peptide secreted by Aspergillus clavatus. Peptides 2008, 29, 1871–1877. [Google Scholar] [CrossRef]
- Wen, C.; Guo, W.; Chen, X. Purification and identification of a novel antifungal protein secreted by Penicillium citrinum from the Southwest Indian Ocean. J. Microbiol. Biotechnol. 2014, 24, 1337–1345. [Google Scholar] [CrossRef]
- Takesako, K.; Kuroda, H.; Inoue, T.; Haruna, F.; Yoshikawa, Y.; Kato, I.; Uchida, K.; Hiratani, T.; Yamaguchi, H. Biological properties of aureobasidin A, a cyclic depsipeptide antifungal antibiotic. J. Antibiot. 1993, 46, 1414–1420. [Google Scholar] [CrossRef]
- Takesako, K.; Ikai, K.; Haruna, F.; Endo, M.; Shimanaka, K.; Sono, E.; Nakamura, T.; Kato, I.; Yamaguchi, H. Aureobasidins, new antifungal antibiotics. Taxonomy, fermentation, isolation, and properties. J. Antibiot. 1991, 44, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Yoshimura, S.; Masaki, T.; Takase, S.; Ohsumi, K.; Hashimoto, M.; Furukawa, S.; Fujie, A. ASP2397: A novel antifungal agent produced by Acremonium persicinum MF-347833. J. Antibiot. 2017, 70, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, B.; Zhang, F.; Miao, X.; Li, Z. Characterization of antifungal chitinase from marine Streptomyces sp. DA11 associated with South China Sea sponge Craniella australiensis. Mar. Biotechnol. 2009, 11, 132–140. [Google Scholar] [CrossRef]
- Maskey, R.P.; Li, F.; Qin, S.; Fiebig, H.H.; Laatsch, H. Chandrananimycins A approximately C: Production of novel anticancer antibiotics from a marine Actinomadura sp. isolate M048 by variation of medium composition and growth conditions. J. Antibiot. 2003, 56, 622–629. [Google Scholar] [CrossRef]
- Becker, J.M.; Covert, N.L.; Shenbagamurthi, P.; Steinfeld, A.S.; Naider, F. Polyoxin D inhibits growth of zoopathogenic fungi. Antimicrob. Agents Chemother. 1983, 23, 926–929. [Google Scholar] [CrossRef]
- Hector, R.F.; Zimmer, B.L.; Pappagianis, D. Evaluation of nikkomycins X and Z in murine models of coccidioidomycosis, histoplasmosis, and blastomycosis. Antimicrob. Agents Chemother. 1990, 34, 587–593. [Google Scholar] [CrossRef]
- Ganesan, L.T.; Manavathu, E.K.; Cutright, J.L.; Alangaden, G.J.; Chandrasekar, P.H. In-vitro activity of nikkomycin Z alone and in combination with polyenes, triazoles or echinocandins against Aspergillus fumigatus. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2004, 10, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Xu, H.; Li, L.; Chen, R.; Gao, X.; Xu, Z. Antifungal activity of endophytic Bacillus safensis B21 and its potential application as a biopesticide to control rice blast. Pestic. Biochem. Physiol. 2020, 162, 69–77. [Google Scholar] [CrossRef]
- Seyedjavadi, S.S.; Khani, S.; Zare-Zardini, H.; Halabian, R.; Goudarzi, M.; Khatami, S.; Imani Fooladi, A.A.; Amani, J.; Razzaghi-Abyaneh, M. Isolation, functional characterization, and biological properties of MCh-AMP1, a novel antifungal peptide from Matricaria chamomilla L. Chem. Biol. Drug Des. 2019, 93, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.X.; Zhang, Y.; Shan, H.H.; Tong, Y.H.; Chen, X.J.; Liu, F.Q. Isolation and identification of antifungal peptides from Bacillus amyloliquefaciens W10. Environ. Sci. Pollut. Res. Int. 2017, 24, 25000–25009. [Google Scholar] [CrossRef]
- Wang, N.N.; Yan, X.; Gao, X.N.; Niu, H.J.; Kang, Z.S.; Huang, L.L. Purification and characterization of a potential antifungal protein from Bacillus subtilis E1R-J against Valsa mali. World J. Microbiol. Biotechnol. 2016, 32, 63. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Q.; Wang, K.; Brian, K.; Liu, C.; Gu, Y. Study of the antifungal activity of Bacillus vallismortis ZZ185 in vitro and identification of its antifungal components. Bioresour. Technol. 2010, 101, 292–297. [Google Scholar] [CrossRef]
- Ramachandran, R.; Chalasani, A.G.; Lal, R.; Roy, U. A broad-spectrum antimicrobial activity of Bacillus subtilis RLID 12.1. Sci. World J. 2014, 2014, 968487. [Google Scholar] [CrossRef]
- Schmidt, G.; Krings, U.; Nimtz, M.; Berger, R.G. A surfactant tolerant laccase of Meripilus giganteus. World J. Microbiol. Biotechnol. 2012, 28, 1623–1632. [Google Scholar] [CrossRef]
- Shokri, D.; Zaghian, S.; Khodabakhsh, F.; Fazeli, H.; Mobasherizadeh, S.; Ataei, B. Antimicrobial activity of a UV-stable bacteriocin-like inhibitory substance (BLIS) produced by Enterococcus faecium strain DSH20 against vancomycin-resistant Enterococcus (VRE) strains. J. Microbiol. Immunol. Infect. Wei Mian Yu Gan Ran Za Zhi 2014, 47, 371–376. [Google Scholar] [CrossRef]
- Wu, S.; Jia, S.; Sun, D.; Chen, M.; Chen, X.; Zhong, J.; Huan, L. Purification and characterization of two novel antimicrobial peptides Subpeptin JM4-A and Subpeptin JM4-B produced by Bacillus subtilis JM4. Curr. Microbiol. 2005, 51, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Arendt, E.K.; Thery, T.L.C. Isolation and characterisation of the antifungal activity of the cowpea defensin Cp-thionin II. Food Microbiol. 2019, 82, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Tverdek, F.P.; Kofteridis, D.; Kontoyiannis, D.P. Antifungal agents and liver toxicity: A complex interaction. Expert Rev. Anti-Infect. Ther. 2016, 14, 765–776. [Google Scholar] [CrossRef]
- Hamill, R.J. Amphotericin B formulations: A comparative review of efficacy and toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, P.H.; Manavathu, E.K. Caspofungin. Drugs Today 2002, 38, 829–846. [Google Scholar] [CrossRef]
- Denning, D.W. Echinocandin antifungal drugs. Lancet 2003, 362, 1142–1151. [Google Scholar] [CrossRef]
- Hector, R.F.; Bierer, D.E. New β-glucan inhibitors as antifungal drugs. Expert Opin. Ther. Pat. 2011, 21, 1597–1610. [Google Scholar] [CrossRef]
- Debono, M.; Gordee, R.S. Antibiotics that inhibit fungal cell wall development. Annu. Rev. Microbiol. 1994, 48, 471–497. [Google Scholar] [CrossRef]
- Aranda, F.J.; Teruel, J.A.; Ortiz, A. Further aspects on the hemolytic activity of the antibiotic lipopeptide iturin A. Biochim. Et Biophys. Acta 2005, 1713, 51–56. [Google Scholar] [CrossRef] [PubMed]
- López-Abarrategui, C.; McBeth, C.; Mandal, S.M.; Sun, Z.J.; Heffron, G.; Alba-Menéndez, A.; Migliolo, L.; Reyes-Acosta, O.; García-Villarino, M.; Nolasco, D.O.; et al. Cm-p5: An antifungal hydrophilic peptide derived from the coastal mollusk Cenchritis muricatus (Gastropoda: Littorinidae). FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 3315–3325. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, M.H.; Hossain, M.A.; Shin, S.Y.; Kim, Y.; Stella, L.; Wade, J.D.; Park, Y.; Hahm, K.S. Amphipathic alpha-helical peptide, HP (2-20), and its analogues derived from Helicobacter pylori: Pore formation mechanism in various lipid compositions. Biochim. Biophys. Acta 2008, 1778, 229–241. [Google Scholar] [CrossRef]
- Heidler, S.A.; Radding, J.A. Inositol phosphoryl transferases from human pathogenic fungi. Biochim. Biophys. Acta 2000, 1500, 147–152. [Google Scholar] [CrossRef]
- Yamamoto, K.N.; Hirota, K.; Kono, K.; Takeda, S.; Sakamuru, S.; Xia, M.; Huang, R.; Austin, C.P.; Witt, K.L.; Tice, R.R. Characterization of environmental chemicals with potential for DNA damage using isogenic DNA repair-deficient chicken DT40 cell lines. Environ. Mol. Mutagenesis 2011, 52, 547–561. [Google Scholar] [CrossRef]
- Zeng, H.; Feng, P.X.; Wan, C.X. Antifungal effects of actinomycin D on Verticillium dahliae via a membrane-splitting mechanism. Nat. Prod. Res. 2019, 33, 1751–1755. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Chandna, S. Inhibition of microRNA-14 contributes to actinomycin-D-induced apoptosis in the Sf9 insect cell line. Cell Biol. Int. 2010, 34, 851–857. [Google Scholar] [CrossRef]
- Debono, M.; Abbott, B.J.; Turner, J.R.; Howard, L.C.; Gordee, R.S.; Hunt, A.S.; Barnhart, M.; Molloy, R.M.; Willard, K.E.; Fukuda, D.; et al. Synthesis and evaluation of LY121019, a member of a series of semisynthetic analogues of the antifungal lipopeptide echinocandin B. Ann. N. Y. Acad. Sci. 1988, 544, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2019, 10, 2993. [Google Scholar] [CrossRef]
- Akramiene, D.; Kondrotas, A.; Didziapetriene, J.; Kevelaitis, E. Effects of beta-glucans on the immune system. Medicina 2007, 43, 597–606. [Google Scholar] [CrossRef]
- Wasmann, R.E.; Muilwijk, E.W.; Burger, D.M.; Verweij, P.E.; Knibbe, C.A.; Brüggemann, R.J. Clinical Pharmacokinetics and Pharmacodynamics of Micafungin. Clin. Pharm. 2018, 57, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lan, N.; Xu, L.; Yue, Q. Biosynthesis of pneumocandin lipopeptides and perspectives for its production and related echinocandins. Appl. Microbiol. Biotechnol. 2018, 102, 9881–9891. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E. Chitin synthesis and inhibition: A revisit. Pest. Manag. Sci. 2001, 57, 946–950. [Google Scholar] [CrossRef]
- Lenardon, M.D.; Munro, C.A.; Gow, N.A. Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 2010, 13, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Gow, N.A.; Munro, C.A. Elevated chitin content reduces the susceptibility of Candida species to caspofungin. Antimicrob. Agents Chemother. 2013, 57, 146–154. [Google Scholar] [CrossRef]
- Plaza, V.; Silva-Moreno, E.; Castillo, L. Breakpoint: Cell Wall and Glycoproteins and their Crucial Role in the Phytopathogenic Fungi Infection. Curr. Protein Pept. Sci. 2020, 21, 227–244. [Google Scholar] [CrossRef]
- Mizuhara, N.; Kuroda, M.; Ogita, A.; Tanaka, T.; Usuki, Y.; Fujita, K. Antifungal thiopeptide cyclothiazomycin B1 exhibits growth inhibition accompanying morphological changes via binding to fungal cell wall chitin. Bioorganic Med. Chem. 2011, 19, 5300–5310. [Google Scholar] [CrossRef]
- Larwood, D.J. Nikkomycin Z-Ready to Meet the Promise? J. Fungi 2020, 6, 261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Miller, M.J. Polyoxins and nikkomycins: Progress in synthetic and biological studies. Curr. Pharm. Des. 1999, 5, 73–99. [Google Scholar]
- Fernandes, C.; Anjos, J.; Walker, L.A.; Silva, B.M.; Cortes, L.; Mota, M.; Munro, C.A.; Gow, N.A.; Gonçalves, T. Modulation of Alternaria infectoria cell wall chitin and glucan synthesis by cell wall synthase inhibitors. Antimicrob. Agents Chemother. 2014, 58, 2894–2904. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.A.; Lee, K.K.; Munro, C.A.; Gow, N.A. Caspofungin Treatment of Aspergillus fumigatus Results in ChsG-Dependent Upregulation of Chitin Synthesis and the Formation of Chitin-Rich Microcolonies. Antimicrob. Agents Chemother. 2015, 59, 5932–5941. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, H.S.; Yang, S.Y.; Kim, I.S.; Yamaguchi, T.; Sohng, J.K.; Park, S.K.; Kim, J.C.; Lee, C.H.; Gardener, B.M.; et al. Both extracellular chitinase and a new cyclic lipopeptide, chromobactomycin, contribute to the biocontrol activity of Chromobacterium sp. C61. Mol. Plant Pathol. 2014, 15, 122–132. [Google Scholar] [CrossRef]
- Fukazawa, Y.; Kagaya, K. Molecular bases of adhesion of Candida albicans. J. Med. Vet. Mycol. 1997, 35, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.A.; Bates, S.; Lenardon, M.D.; Maccallum, D.M.; Wagener, J.; Lowman, D.W.; Kruppa, M.D.; Williams, D.L.; Odds, F.C.; Brown, A.J.; et al. The Mnn2 mannosyltransferase family modulates mannoprotein fibril length, immune recognition and virulence of Candida albicans. PLoS Pathog. 2013, 9, e1003276. [Google Scholar] [CrossRef]
- Walsh, T.J.; Giri, N. Pradimicins: A novel class of broad-spectrum antifungal compounds. Eur. J. Clin. Microbiol. Infect. Dis. 1997, 16, 93–97. [Google Scholar] [CrossRef]
- Hiramoto, F.; Nomura, N.; Furumai, T.; Oki, T.; Igarashi, Y. Apoptosis-like cell death of Saccharomyces cerevisiae induced by a mannose-binding antifungal antibiotic, pradimicin. J. Antibiot. 2003, 56, 768–772. [Google Scholar] [CrossRef]
- Hiramoto, F.; Nomura, N.; Furumai, T.; Igarashi, Y.; Oki, T. Pradimicin resistance of yeast is caused by a mutation of the putative N-glycosylation sites of osmosensor protein Sln1. Biosci. Biotechnol. Biochem. 2005, 69, 238–241. [Google Scholar] [CrossRef]
- Yasuoka, A.; Oka, S.; Komuro, K.; Shimizu, H.; Kitada, K.; Nakamura, Y.; Shibahara, S.; Takeuchi, T.; Kondo, S.; Shimada, K.; et al. Successful treatment of Pneumocystis carinii Pneumonia in mice with benanomicin A (ME1451). Antimicrob. Agents Chemother. 1995, 39, 720–724. [Google Scholar] [CrossRef]
- Złoch, M.; Rogowska, A.; Pomastowski, P.; Railean-Plugaru, V.; Walczak-Skierska, J.; Rudnicka, J.; Buszewski, B. Use of Lactobacillus paracasei strain for zearalenone binding and metabolization. Toxicon Off. J. Int. Soc. Toxinology 2020, 181, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, H.; Mathieu, F.; Taillandier, P.; Lebrihi, A. Ochratoxin A removal in synthetic and natural grape juices by selected oenological Saccharomyces strains. J. Appl. Microbiol. 2004, 97, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Hammer, J.; Pate, M.; Zhang, Y.; Zhu, F.; Zmuda, E.; Blazyk, J. Antimicrobial activities and structures of two linear cationic peptide families with various amphipathic beta-sheet and alpha-helical potentials. Antimicrob. Agents Chemother. 2005, 49, 4957–4964. [Google Scholar] [CrossRef]
- Blazyk, J.; Wiegand, R.; Klein, J.; Hammer, J.; Epand, R.M.; Epand, R.F.; Maloy, W.L.; Kari, U.P. A novel linear amphipathic beta-sheet cationic antimicrobial peptide with enhanced selectivity for bacterial lipids. J. Biol. Chem. 2001, 276, 27899–27906. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.M.; Sforça, M.L.; Amino, R.; Juliano, M.A.; Oyama, S., Jr.; Juliano, L.; Pertinhez, T.A.; Spisni, A.; Schenkman, S. Lytic activity and structural differences of amphipathic peptides derived from trialysin. Biochemistry 2006, 45, 1765–1774. [Google Scholar] [CrossRef]
- Cabiaux, V.; Agerberth, B.; Johansson, J.; Homblé, F.; Goormaghtigh, E.; Ruysschaert, J.M. Secondary structure and membrane interaction of PR-39, a Pro+Arg-rich antibacterial peptide. Eur. J. Biochem. 1994, 224, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Izadpanah, A.; Gallo, R.L. Antimicrobial peptides. J. Am. Acad. Dermatol. 2005, 52, 381–390. [Google Scholar] [CrossRef]
- Aoki, W.; Ueda, M. Characterization of Antimicrobial Peptides toward the Development of Novel Antibiotics. Pharmaceuticals 2013, 6, 1055–1081. [Google Scholar] [CrossRef]
- Wang, G. Post-translational Modifications of Natural Antimicrobial Peptides and Strategies for Peptide Engineering. Curr. Biotechnol. 2012, 1, 72–79. [Google Scholar] [CrossRef]
- Lee, M.T.; Chen, F.Y.; Huang, H.W. Energetics of pore formation induced by membrane active peptides. Biochemistry 2004, 43, 3590–3599. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, D.G. Antimicrobial Peptides (AMPs) with Dual Mechanisms: Membrane Disruption and Apoptosis. J. Microbiol. Biotechnol. 2015, 25, 759–764. [Google Scholar] [CrossRef]
- Matsuzaki, K. Membrane Permeabilization Mechanisms. Adv. Exp. Med. Biol. 2019, 1117, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Umegawa, Y.; Yamagami, M.; Suzuki, T.; Tsuchikawa, H.; Hanashima, S.; Matsumori, N.; Murata, M. The Perpendicular Orientation of Amphotericin B Methyl Ester in Hydrated Lipid Bilayers Supports the Barrel-Stave Model. Biochemistry 2019, 58, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, N.; Tahara, K.; Yamamoto, H.; Morooka, A.; Doi, M.; Oishi, T.; Murata, M. Direct interaction between amphotericin B and ergosterol in lipid bilayers as revealed by 2H NMR spectroscopy. J. Am. Chem. Soc. 2009, 131, 11855–11860. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Román, E.; Sánchez-Fresneda, R.; Casas, C.; Herrero, E.; Argüelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The production of reactive oxygen species is a universal action mechanism of Amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob. Agents Chemother. 2014, 58, 6627–6638. [Google Scholar] [CrossRef]
- Czub, J.; Baginski, M. Modulation of amphotericin B membrane interaction by cholesterol and ergosterol---A molecular dynamics study. J. Phys. Chem. B 2006, 110, 16743–16753. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, Y.; Hou, S.; Miao, Z.; Ma, Q. Interaction of amphotericin B and saturated or unsaturated phospholipid monolayers containing cholesterol or ergosterol at the air-water interface. Biophys. Chem. 2020, 258, 106317. [Google Scholar] [CrossRef] [PubMed]
- Umegawa, Y.; Nakagawa, Y.; Tahara, K.; Tsuchikawa, H.; Matsumori, N.; Oishi, T.; Murata, M. Head-to-tail interaction between amphotericin B and ergosterol occurs in hydrated phospholipid membrane. Biochemistry 2012, 51, 83–89. [Google Scholar] [CrossRef]
- Santos, J.R.; Gouveia, L.F.; Taylor, E.L.; Resende-Stoianoff, M.A.; Pianetti, G.A.; César, I.C.; Santos, D.A. Dynamic interaction between fluconazole and amphotericin B against Cryptococcus gattii. Antimicrob. Agents Chemother. 2012, 56, 2553–2558. [Google Scholar] [CrossRef]
- Junior, E.F.C.; Guimarães, C.; Franco, L.L.; Alves, R.J.; Kato, K.C.; Martins, H.R.; de Souza Filho, J.D.; Bemquerer, M.P.; Munhoz, V.H.O.; Resende, J.M.; et al. Glycotriazole-peptides derived from the peptide HSP1: Synergistic effect of triazole and saccharide rings on the antifungal activity. Amino Acids 2017, 49, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Hutchison, M.L.; Tester, M.A.; Gross, D.C. Role of biosurfactant and ion channel-forming activities of syringomycin in transmembrane ion flux: A model for the mechanism of action in the plant-pathogen interaction. Mol. Plant-Microbe Interact. MPMI 1995, 8, 610–620. [Google Scholar] [CrossRef]
- Abraham, T.; Lewis, R.N.; Hodges, R.S.; McElhaney, R.N. Isothermal titration calorimetry studies of the binding of the antimicrobial peptide gramicidin S to phospholipid bilayer membranes. Biochemistry 2005, 44, 11279–11285. [Google Scholar] [CrossRef]
- Verly, R.M.; Rodrigues, M.A.; Daghastanli, K.R.; Denadai, A.M.; Cuccovia, I.M.; Bloch, C., Jr.; Frézard, F.; Santoro, M.M.; Piló-Veloso, D.; Bemquerer, M.P. Effect of cholesterol on the interaction of the amphibian antimicrobial peptide DD K with liposomes. Peptides 2008, 29, 15–24. [Google Scholar] [CrossRef]
- Christoffersen, H.F.; Hansen, S.K.; Vad, B.S.; Nielsen, E.H.; Nielsen, J.T.; Vosegaard, T.; Skrydstrup, T.; Otzen, D.E. The natural, peptaibolic peptide SPF-5506-A4 adopts a β-bend spiral structure, shows low hemolytic activity and targets membranes through formation of large pores. Biochim. Biophys. Acta 2015, 1854, 882–889. [Google Scholar] [CrossRef]
- Yang, L.; Harroun, T.A.; Weiss, T.M.; Ding, L.; Huang, H.W. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 2001, 81, 1475–1485. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Sugishita, K.; Harada, M.; Fujii, N.; Miyajima, K. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of Gram-negative bacteria. Biochim. Biophys. Acta 1997, 1327, 119–130. [Google Scholar] [CrossRef]
- Maget-Dana, R.; Peypoux, F. Iturins, a special class of pore-forming lipopeptides: Biological and physicochemical properties. Toxicology 1994, 87, 151–174. [Google Scholar] [CrossRef]
- Iessi, E.; Marconi, M.; Manganelli, V.; Sorice, M.; Malorni, W.; Garofalo, T.; Matarrese, P. On the role of sphingolipids in cell survival and death. Int. Rev. Cell Mol. Biol. 2020, 351, 149–195. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Sakoh, H.; Yamada, K. IPC synthase as a useful target for antifungal drugs. Curr. Drug Targets. Infect. Disord. 2004, 4, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Nagiec, M.M.; Nagiec, E.E.; Baltisberger, J.A.; Wells, G.B.; Lester, R.L.; Dickson, R.C. Sphingolipid synthesis as a target for antifungal drugs. Complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. J. Biol. Chem. 1997, 272, 9809–9817. [Google Scholar] [CrossRef] [PubMed]
- Katragkou, A.; Williams, M.; Sternberg, S.; Pantazatos, D.; Roilides, E.; Walsh, T.J. Micafungin alters the amino acid, nucleic acid and central carbon metabolism of Candida albicans at subinhibitory concentrations: Novel insights into mechanisms of action. J. Antimicrob. Chemother. 2017, 72, 712–716. [Google Scholar] [CrossRef]
- Lee, M.R.; Raman, N.; Ortiz-Bermúdez, P.; Lynn, D.M.; Palecek, S.P. 14-Helical β-Peptides Elicit Toxicity against C. albicans by Forming Pores in the Cell Membrane and Subsequently Disrupting Intracellular Organelles. Cell Chem. Biol. 2019, 26, 289–299.e284. [Google Scholar] [CrossRef]
- Han, J.; Wang, F.; Gao, P.; Ma, Z.; Zhao, S.; Lu, Z.; Lv, F.; Bie, X. Mechanism of action of AMP-jsa9, a LI-F-type antimicrobial peptide produced by Paenibacillus polymyxa JSa-9, against Fusarium moniliforme. Fungal Genet. Biol. FG B 2017, 104, 45–55. [Google Scholar] [CrossRef]
- Dietl, A.M.; Misslinger, M.; Aguiar, M.M.; Ivashov, V.; Teis, D.; Pfister, J.; Decristoforo, C.; Hermann, M.; Sullivan, S.M.; Smith, L.R.; et al. The Siderophore Transporter Sit1 Determines Susceptibility to the Antifungal VL-2397. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Nakamura, I.; Ohsumi, K.; Takeda, S.; Katsumata, K.; Matsumoto, S.; Akamatsu, S.; Mitori, H.; Nakai, T. ASP2397 Is a Novel Natural Compound That Exhibits Rapid and Potent Fungicidal Activity against Aspergillus Species through a Specific Transporter. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shao, J.; Li, B.; Yan, X.; Shen, Q.; Zhang, R. Contribution of bacillomycin D in Bacillus amyloliquefaciens SQR9 to antifungal activity and biofilm formation. Appl. Environ. Microbiol. 2013, 79, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.G.; Hancock, R.E. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit. Rev. Immunol. 2000, 20, 407–431. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef]
- Ramírez-Valdespino, C.A.; Casas-Flores, S.; Olmedo-Monfil, V. Trichoderma as a Model to Study Effector-Like Molecules. Front. Microbiol. 2019, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K. Beneficial effects of Trichoderma secondary metabolites on crops. Phytother. Res. PTR 2020, 34, 2835–2842. [Google Scholar] [CrossRef]
- Marra, R.; Coppola, M.; Pironti, A.; Grasso, F.; Lombardi, N.; d’Errico, G.; Sicari, A.; Bolletti Censi, S.; Woo, S.L.; Rao, R.; et al. The Application of Trichoderma Strains or Metabolites Alters the Olive Leaf Metabolome and the Expression of Defense-Related Genes. J. Fungi 2020, 6, 369. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.J.; Kravchenko, L.V.; Simons, M. Tomato seed and root exudate sugars: Composition, utilization by Pseudomonas biocontrol strains and role in rhizosphere colonization. Environ. Microbiol. 1999, 1, 439–446. [Google Scholar] [CrossRef]
- Wang, S.; Ruan, C.; Yi, L.; Deng, L.; Yao, S.; Zeng, K. Biocontrol ability and action mechanism of Metschnikowia citriensis against Geotrichum citri-aurantii causing sour rot of postharvest citrus fruit. Food Microbiol. 2020, 87, 103375. [Google Scholar] [CrossRef]
- Liu, X.; Fang, W.; Liu, L.; Yu, T.; Lou, B.; Zheng, X. Biological control of postharvest sour rot of citrus by two antagonistic yeasts. Lett. Appl. Microbiol. 2010, 51, 30–35. [Google Scholar] [CrossRef]
- Han, Q.; Wu, F.; Wang, X.; Qi, H.; Shi, L.; Ren, A.; Liu, Q.; Zhao, M.; Tang, C. The bacterial lipopeptide iturins induce Verticillium dahliae cell death by affecting fungal signalling pathways and mediate plant defence responses involved in pathogen-associated molecular pattern-triggered immunity. Environ. Microbiol. 2015, 17, 1166–1188. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Bie, X.; Lu, Z.; Lv, F.; Tao, Y.; Qu, X. Effects of fengycin from Bacillus subtilis fmbJ on apoptosis and necrosis in Rhizopus stolonifer. J. Microbiol. 2014, 52, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, J.; Sun, J.; Zhu, X.; Zhou, L.; Lu, Z.; Lu, Y. C16-Fengycin A affect the growth of Candida albicans by destroying its cell wall and accumulating reactive oxygen species. Appl. Microbiol. Biotechnol. 2019, 103, 8963–8975. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, L.; Bie, X.; Lu, Z.; Liu, H.; Zhang, C.; Lu, F.; Zhao, H. Identification of novel surfactin derivatives from NRPS modification of Bacillus subtilis and its antifungal activity against Fusarium moniliforme. BMC Microbiol. 2016, 16, 31. [Google Scholar] [CrossRef]
- Tao, Y.; Bie, X.M.; Lv, F.X.; Zhao, H.Z.; Lu, Z.X. Antifungal activity and mechanism of fengycin in the presence and absence of commercial surfactin against Rhizopus stolonifer. J. Microbiol. 2011, 49, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Ton, V.K.; Beaudry, V.; Rulli, S.; Cunningham, K.; Rao, R. Antifungal activity of amiodarone is mediated by disruption of calcium homeostasis. J. Biol. Chem. 2003, 278, 28831–28839. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Li, L.; Du, F.; Sun, L.; Shi, J.; Long, M.; Chen, Z. Activity and Mechanism of Action of Antifungal Peptides from Microorganisms: A Review. Molecules 2021, 26, 3438. https://doi.org/10.3390/molecules26113438
Li T, Li L, Du F, Sun L, Shi J, Long M, Chen Z. Activity and Mechanism of Action of Antifungal Peptides from Microorganisms: A Review. Molecules. 2021; 26(11):3438. https://doi.org/10.3390/molecules26113438
Chicago/Turabian StyleLi, Tianxi, Lulu Li, Fangyuan Du, Lei Sun, Jichao Shi, Miao Long, and Zeliang Chen. 2021. "Activity and Mechanism of Action of Antifungal Peptides from Microorganisms: A Review" Molecules 26, no. 11: 3438. https://doi.org/10.3390/molecules26113438
APA StyleLi, T., Li, L., Du, F., Sun, L., Shi, J., Long, M., & Chen, Z. (2021). Activity and Mechanism of Action of Antifungal Peptides from Microorganisms: A Review. Molecules, 26(11), 3438. https://doi.org/10.3390/molecules26113438






