Solar Water Disinfection to Produce Safe Drinking Water: A Review of Parameters, Enhancements, and Modelling Approaches to Make SODIS Faster and Safer
Abstract
1. Introduction
- Low-cost: The most impoverished communities are the most affected.
- User-friendly: Everybody should easily produce safe drinking water.
- Sustainable: To avoid the need for consumables that are expensive or hard to obtain.
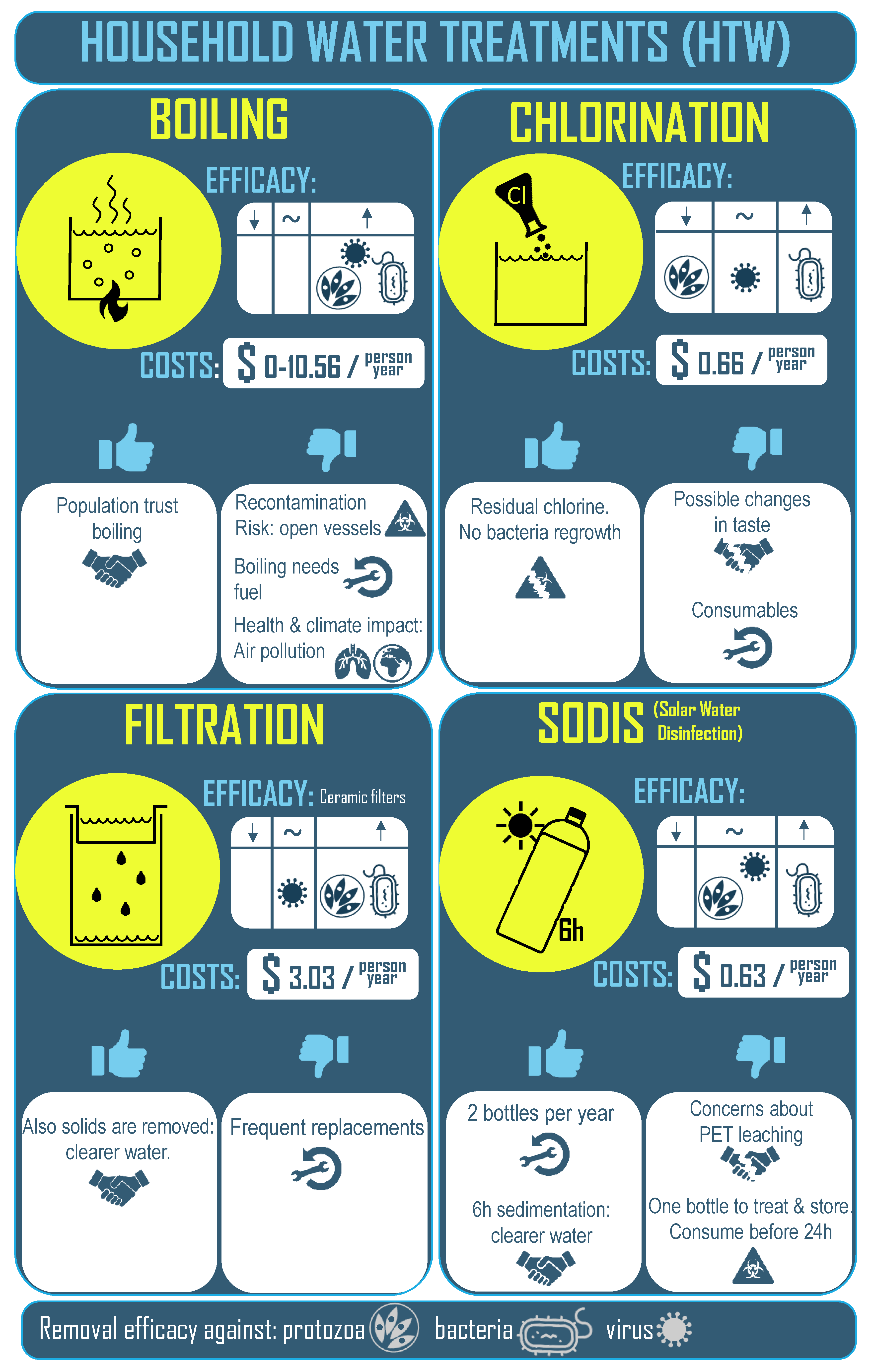
- Boiling: This process is highly effective against all classes of microbial pathogens [13]. People universally accept that boiling makes water safer to drink, so they trust the treatment and adopt it readily. Boiling requires large amounts of fuel with estimated costs of up to $10.56 per person per year [14], unless it is freely collected (in the form of firewood. However, boiling causes health risks due to indoor air pollution and boiled water is very vulnerable to recontamination since it usually is cooled in open containers [11,15].
- Chlorination: Chlorine can be applied in liquid or tablet form, is also easy to apply at household level, and is very inexpensive (estimated costs of $0.66 per person per year) [16]. A particular strength of chlorine is that residual chlorine in the water can protect against bacterial regrowth. However, chlorine is less effective against some viruses and ineffective against common protozoa. Disinfection by-products can be formed due to reactions with naturally occurring substances. These by-products can change the smell and taste of the treated water. Chlorination is sometimes rejected on these grounds. Furthermore, heavy use requires consumables that must be replaced periodically, and, in general, the population that demands HWT is in difficult to access, remote areas [12,17].
- Filtration: Generally, filters do not remove all pathogens since their filter pores are larger than the microorganisms. However, ceramic filters do retain protozoa, work well against bacteria, and some of them have efficacy against viruses (the smallest pathogen). Users have confidence in filters since they tend to clarify the water, and ceramic filters can also evaporatively cool the water [12,18]. Nevertheless, ceramic filters are fragile and maintenance can be expensive (estimated costs of $3.03 per person per year) [16].
- SODIS: Solar water disinfection, or SODIS, is based on the germicidal effect of UV light and its synergistic effect with rise in water temperature. The procedure is very user-friendly since it involves just filling a transparent container with water and placing it in direct sunlight for several hours. The treatment is cheap because only a transparent container such as a glass or plastic container is required (estimated costs of $0.63 per person per year) [16]. Generally, UVA radiation from the sun is lethal against bacteria, as is UVB radiation against bacteria, viruses, and protozoa [19,20,21]. No adverse effect on the water’s taste has been observed. However, it is recommended that the treated water is consumed within the 24 h following exposure since bacteria can regrow in the dark while the water is stored and cooling. Water turbidity decreases the solar disinfection efficiency and prolongs treatment time. An additional benefit is that since the water is generally treated and then stored in the same container, there is a decreased risk of recontamination [22].
2. SODIS: Variables
2.1. Radiation
2.1.1. Photoinactivation Mechanisms
2.1.2. Solar Spectrum
2.2. Container Material
2.2.1. Optical Properties
2.2.2. Mechanical Properties
2.2.3. Ageing of the Material
2.2.4. Accessibility
Affordability
Availability
Adoption
2.3. Water Quality
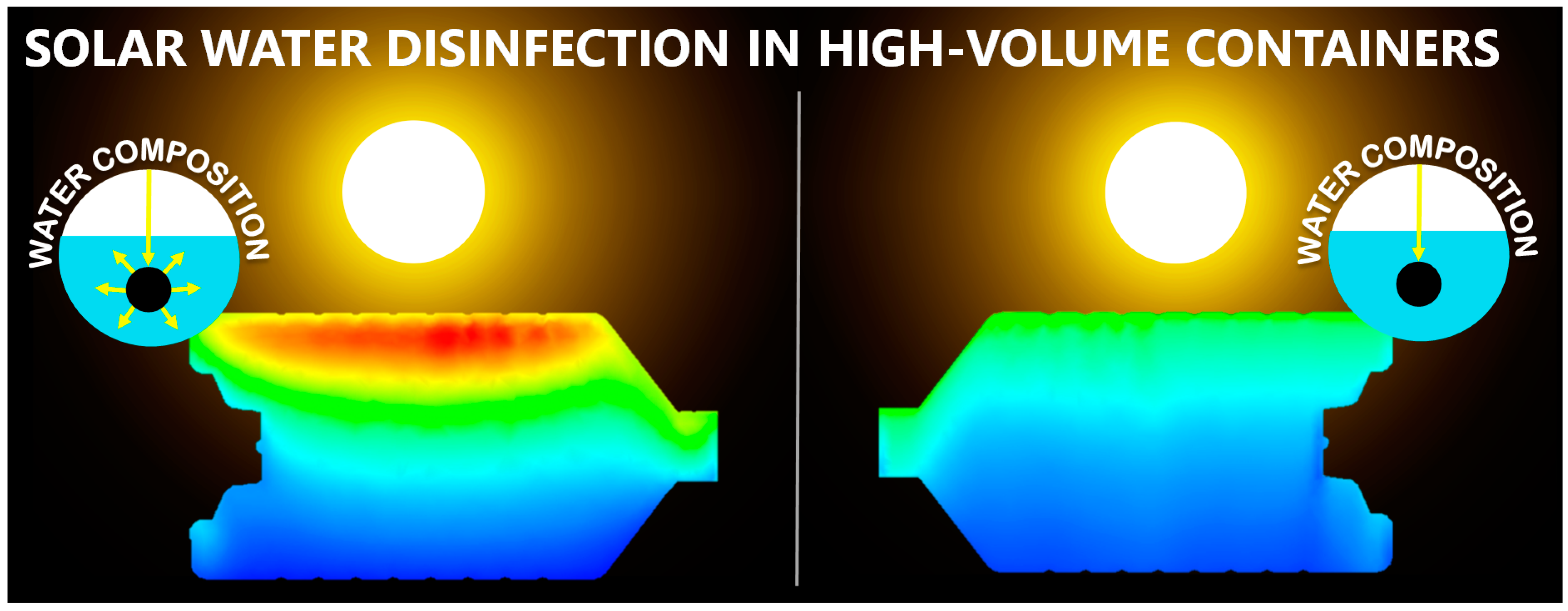
2.3.1. Chemical Composition
Radiation Attenuators
Sensitisers
2.3.2. Pathogens
Viruses
Bacteria
Protozoa
2.4. Temperature
2.4.1. Inactivation Effects
2.4.2. Enhancements
3. SODIS: Kinetic Modelling
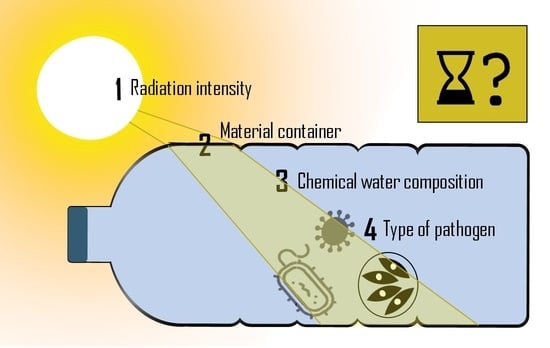
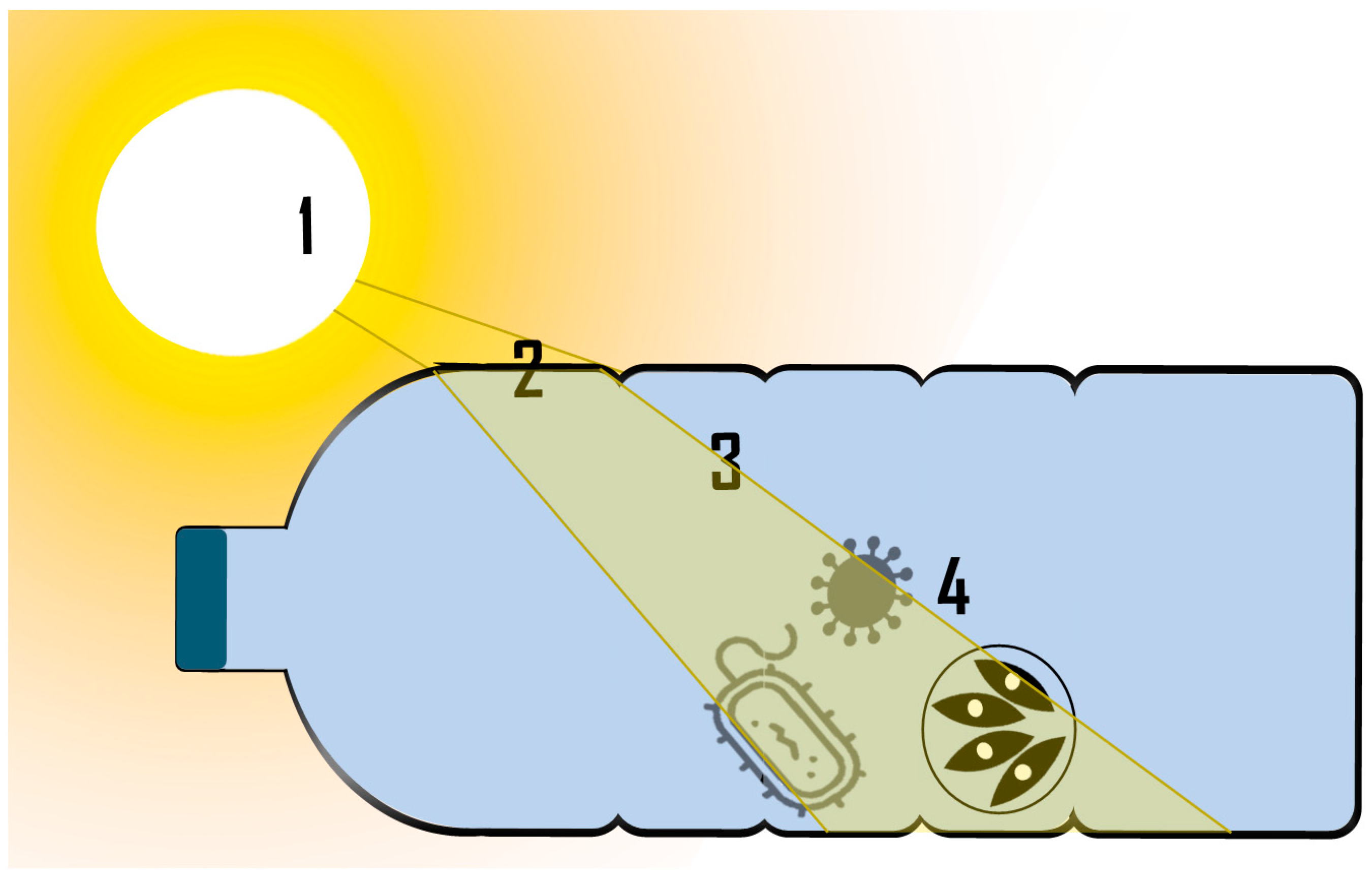
3.1. Spectral Irradiance Values
3.1.1. Radiation Source
3.1.2. Material Container
3.1.3. Water Composition
3.2. Temperature
3.3. Light Modelling
3.3.1. Endogenous Damage
3.3.2. Exogenous Damage
3.4. Temperature Modelling
3.5. Comprehensive Kinetic Models
3.5.1. Computational Fluid Dynamics (CFD)
4. Conclusions
- (1)
- Low batch volume of bottles.
- (2)
- Limited effectiveness of PET bottles against viruses and protozoa.
- (3)
- Overestimation of the recommended exposure time.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations (UN). A/RES/64/292; United Nations (UN): New York, NY, USA, 2010. [Google Scholar]
- United Nations (UN). The 2030 AGENDA for Sustainable Development; United Nations (UN): New York, NY, USA, 2015. [Google Scholar]
- United Nations (UN). Sustainable Development Goal 6. Synthesis Report on Water and Sanitation 2018; United Nations (UN): New York, NY, USA, 2018; ISBN 978-92-1-101370-2. [Google Scholar]
- UNU-INWEH/UNESCAP. Water Security & the Global Water Agenda: A UN-Water Analytical Brief; United Nations University (UNU): Hamilton, ON, Canada, 2013; ISBN 9789280860382. [Google Scholar]
- FAO. The Future of Food and Agriculture: Trends and Challenges; FAO: Rome, Italy, 2017; ISBN 9789251095515. [Google Scholar]
- IPCC. Summary for Policymakers. Global Warming of 1.5 °C; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Wada, Y.; van Beek, L.P.H.; Wanders, N. Sustainability of global water use: Past reconstruction and future projections Related content Human water consumption intensifies hydrological drought worldwide. Environ. Res. Lett. 2014, 9, 104003. [Google Scholar] [CrossRef]
- UNESCO/UN-Water. The United Nations World Water Development Report 2020: Water and Climate Change; UNESCO: Paris, France, 2020. [Google Scholar]
- Institute for Health Metrics and Evaluation. Global Burden of Disease (GBD) Compare. Available online: https://www.thelancet.com/gbd/gbd-compare-visualisation (accessed on 4 June 2020).
- McGuigan, K.G.; Conroy, R.M.; Mosler, H.-J.; du Preez, M.; Ubomba-Jaswa, E.; Fernandez-Ibañez, P. Solar water disinfection (SODIS): A review from bench-top to roof-top. J. Hazard. Mater. 2012, 235, 29–46. [Google Scholar] [CrossRef]
- Gadgil, A. Drinking water in developing countries. Annu. Rev. Energy Environ. 1998, 23, 253–286. [Google Scholar] [CrossRef]
- Pichel, N.; Vivar, M.; Fuentes, M. The problem of drinking water access: A review of disinfection technologies with an emphasis on solar treatment methods. Chemosphere 2019, 218, 1014–1030. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Boil Water. Available online: https://www.who.int/water_sanitation_health/dwq/Boiling_water_01_15.pdf (accessed on 13 April 2021).
- Clasen, T.; McLaughlin, C.; Nayaar, N.; Boisson, S.; Gupta, R.; Desai, D.; Shah, N. Microbiological effectiveness and cost of disinfecting water by boiling in semi-urban India. Am. J. Trop. Med. Hyg. 2008, 79, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Gilman, R.H.; Skillicorn, P. Boiling of drinking-water: Can a fuel-scarce community afford it? Bull. World Health Organ. 1985, 63, 157–163. [Google Scholar]
- Clasen, T.; Haller, L.; Walker, D.; Bartram, J.; Cairncross, S. Cost-effectiveness of water quality interventions for preventing diarrhoeal disease in developing countries. J. Water Health 2007, 5, 599–608. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Regional Office for South.-East. Asia Principles and Practices of Drinking-Water Chlorination: A Guide to Strengthening Chlorination Practices in Small-to Medium Sized Water Supplies; World Health Organization, Regional Office for South-East Asia: New Delhi, India, 2017; ISBN 978-92-9022-536-2. [Google Scholar]
- World Health Organization (WHO). Managing Water in the Home: Accelerated Health Gains from Improved Water Supply; World Health Organization (WHO): Geneva, Switzerland, 2002. [Google Scholar]
- García-Gil, Á.; Martinez, A.; Polo-López, M.I.; Marugán, J. Kinetic modeling of the synergistic thermal and spectral actions on the inactivation of viruses in water by sunlight. Water Res. 2020, 183, 116074. [Google Scholar] [CrossRef] [PubMed]
- García-Gil, Á.; Abeledo-Lameiro, M.J.; Gómez-Couso, H.; Marugán, J. Kinetic modeling of the synergistic thermal and spectral actions on the inactivation of Cryptosporidium parvum in water by sunlight. Water Res. 2020, 185, 116226. [Google Scholar] [CrossRef]
- Nelson, K.L.; Boehm, A.B.; Davies-Colley, R.J.; Dodd, M.C.; Kohn, T.; Linden, K.G.; Liu, Y.; Maraccini, P.A.; McNeill, K.; Mitch, W.A.; et al. Sunlight-mediated inactivation of health-relevant microorganisms in water: A review of mechanisms and modeling approaches. Environ. Sci. Process. Impacts 2018, 20, 1089–1122. [Google Scholar] [CrossRef] [PubMed]
- Rufener, S.; Mäusezahl, D.; Mosler, H.-J.; Weingartner, R. Quality of drinking-water at source and point-of-consumption-drinking cup as a high potential recontamination risk: A field study in Bolivia. J. Health Popul. Nutr. 2010, 28, 34–41. [Google Scholar] [CrossRef]
- Luzi, S.; Tobler, M.; Suter, F.; Meierhofer, R. SODIS Manual: Guidance on Solar Water Disinfection; EAWAG: Dibendorf, Switzerland, 2016; ISBN 9783906484594. [Google Scholar]
- Wegelin, M.; Canonica, S.; Alder, A.C.; Marazuela, D.; Suter, M.J.-F.; Bucheli, T.D.; Haefliger, O.P.; Zenobi, R.; McGuigan, K.G.; Kelly, M.T.; et al. Does sunlight change the material and content of polyethylene terephthalate (PET) bottles? Res. Technol. 2001, 50, 125–135. [Google Scholar] [CrossRef]
- Ubomba-Jaswa, E.; Fernández-Ibáñez, P.; McGuigan, K.G. A preliminary Ames fluctuation assay assessment of the genotoxicity of drinking water that has been solar disinfected in polyethylene terephthalate (PET) bottles. J. Water Health 2010, 8, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Ozores Diez, P.; Giannakis, S.; Rodríguez-Chueca, J.; Wang, D.; Quilty, B.; Devery, R.; McGuigan, K.; Pulgarin, C. Enhancing solar disinfection (SODIS) with the photo-Fenton or the Fe2+/peroxymonosulfate-activation process in large-scale plastic bottles leads to toxicologically safe drinking water. Water Res. 2020, 186, 116387. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Emergency Treatment of Drinking Water at Point-of-Use. WHO-Technical Notes for Emergencies No. 5; World Health Organization (WHO): Geneva, Switzerland, 2005. [Google Scholar]
- World Health Organization (WHO). Evaluating Household Water Treatment Options: Health-Based Targets and Microbiological Performance Specifications; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Center for Desease Control and Prevention (CDC). Household Water Treatment. Solar Disinfection. Available online: https://www.cdc.gov/safewater/pdf/solar2011final.pdf (accessed on 13 April 2021).
- Conroy, R.M.; Meegan, M.E.; Joyce, T.; McGuigan, K.; Barnes, J. Solar disinfection of drinking water protects against cholera in children under 6 years of age. Arch. Dis. Child. 2001, 85, 293–295. [Google Scholar] [CrossRef]
- Graf, J.; Togouet, S.Z.; Kemka, N.; Niyitegeka, D.; Meierhofer, R.; Pieboji, J.G. Health gains from solar water disinfection (SODIS): Evaluation of a water quality intervention in Yaoundé, Cameroon. J. Water Health 2010, 8, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.; Roy, S.; Abraham, V.; Holmgren, G.; George, K.; Balraj, V.; Abraham, S.; Muliyil, J.; Joseph, A.; Kang, G. Solar disinfection of water for diarrhoeal prevention in southern India. Arch. Dis. Child. 2006, 91, 139–141. [Google Scholar] [CrossRef]
- Kohn, T.; Mattle, M.J.; Minella, M.; Vione, D. A modeling approach to estimate the solar disinfection of viral indicator organisms in waste stabilization ponds and surface waters. Water Res. 2016, 88, 912–922. [Google Scholar] [CrossRef]
- Parker, K.M.; Mitch, W.A. Halogen radicals contribute to photooxidation in coastal and estuarine waters. Proc. Natl. Acad. Sci. USA 2016, 113, 5868–5873. [Google Scholar] [CrossRef] [PubMed]
- Silverman, A.I.; Tay, N.; Machairas, N. Comparison of biological weighting functions used to model endogenous sunlight inactivation rates of MS2 coliphage. Water Res. 2019, 151, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Busse, M.M.; Becker, M.; Applegate, B.M.; Camp, J.W.; Blatchley, E.R. Responses of Salmonella typhimurium LT2, Vibrio harveyi, and Cryptosporidium parvum to UVB and UVA radiation. Chem. Eng. J. 2019, 371, 647–656. [Google Scholar] [CrossRef]
- Mattle, M.J.; Vione, D.; Kohn, T. Conceptual model and experimental framework to determine the contributions of direct and indirect photoreactions to the solar disinfection of MS2, phiX174, and adenovirus. Environ. Sci. Technol. 2015, 49, 334–342. [Google Scholar] [CrossRef] [PubMed]
- García-Gil, Á.; Pablos, C.; García-Muñoz, R.A.; McGuigan, K.G.; Marugán, J. Material selection and prediction of solar irradiance in plastic devices for application of solar water disinfection (SODIS) to inactivate viruses, bacteria and protozoa. Sci. Total Environ. 2020, 730, 139126. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.B.; Iriarte, M.; Nelson, K.L. Solar water disinfection (SODIS) of Escherichia coli, Enterococcus spp., and MS2 coliphage: Effects of additives and alternative container materials. Water Res. 2012, 46, 1745–1754. [Google Scholar] [CrossRef]
- Lawrie, K.; Mills, A.; Figueredo-Fernández, M.; Gutiérrez-Alfaro, S.; Manzano, M.; Saladin, M. UV dosimetry for solar water disinfection (SODIS) carried out in different plastic bottles and bags. Sens. Actuators B Chem. 2015, 208, 608–615. [Google Scholar] [CrossRef]
- Ubomba-Jaswa, E.; Fernández-Ibáñez, P.; Navntoft, C.; Polo-López, M.I.; McGuigan, K.G. Investigating the microbial inactivation efficiency of a 25 L batch solar disinfection (SODIS) reactor enhanced with a compound parabolic collector (CPC) for household use. J. Chem. Technol. Biotechnol. 2010, 85, 1028–1037. [Google Scholar] [CrossRef]
- Reyneke, B.; Ndlovu, T.; Vincent, M.B.; Martínez-García, A.; Polo-López, M.I.; Fernández-Ibáñez, P.; Ferrero, G.; Khan, S.; McGuigan, K.G.; Khan, W. Validation of large-volume batch solar reactors for the treatment of rainwater in field trials in sub-Saharan Africa. Sci. Total Environ. 2020, 717, 137223. [Google Scholar] [CrossRef]
- García-Gil, Á.; Casado, C.; Pablos, C.; Marugán, J. Novel procedure for the numerical simulation of solar water disinfection processes in flow reactors. Chem. Eng. J. 2019, 376, 120194. [Google Scholar] [CrossRef]
- Mac Mahon, J.; Gill, L.W. Sustainability of novel water treatment technologies in developing countries: Lessons learned from research trials on a pilot continuous flow solar water disinfection system in rural Kenya. Dev. Eng. 2018, 3, 47–59. [Google Scholar] [CrossRef]
- Kalt, P.; Birzer, C.; Evans, H.; Liew, A.; Padovan, M.; Watchman, M. A solar disinfection water treatment system for remote communities. Procedia Eng. 2014, 78, 250–258. [Google Scholar] [CrossRef]
- Wegelin, M.; Sommer, B. Solar water disinfection (SODIS)-destined for worldwide use? Waterlines 1998, 16, 30–32. [Google Scholar] [CrossRef]
- Fagan, H.G.; Linnane, K.S.; McGuigan, K.G.; Rugamayo, A. Water is Life—Progress to Secure Water Provision in Rural Uganda; Practical Action Publishing: Rugby, UK, 2015; ISBN 185339890X. [Google Scholar]
- Martínez-García, A.; Vincent, M.; Rubiolo, V.; Domingos, M.; Canela, M.C.; Oller, I.; Fernández-Ibáñez, P.; Polo-López, M.I. Assessment of a pilot solar V-trough reactor for solar water disinfection. Chem. Eng. J. 2020, 399, 125719. [Google Scholar] [CrossRef]
- Gillen, K.T.; Celina, M. Predicting polymer degradation and mechanical property changes for bombined radiation-thermal aging environments. Rubber Chem. Technol. 2017, 91, 27–63. [Google Scholar] [CrossRef]
- Martin, J.R.; Gardner, R.J. Effect of long term humid aging on plastics. Polym. Eng. Sci. 1981, 21, 557–565. [Google Scholar] [CrossRef]
- White, J.R. Polymer ageing: Physics, chemistry or engineering? Time to reflect. Comptes Rendus Chim. 2006, 9, 1396–1408. [Google Scholar] [CrossRef]
- Rånby, B. Basic reactions in the photodegradation of some important polymers. J. Macromol. Sci. Part A Pure Appl. Chem. 1993, 30, 583–594. [Google Scholar] [CrossRef]
- Kircher, K. Chemical Reactions in Plastics Processing; C. Hanser: Munich, Germany, 1987. [Google Scholar]
- Kuvshinnikova, O.; Boven, G.; Pickett, J.E. Weathering of aromatic engineering thermoplastics: Comparison of outdoor and xenon arc exposures. Polym. Degrad. Stab. 2019, 160, 177–194. [Google Scholar] [CrossRef]
- Frischknecht, R.; Jungbluth, N.; Althaus, H.-J.; Doka, G.; Dones, R.; Heck, T.; Hellweg, S.; Hischier, R.; Nemecek, T.; Rebitzer, G.; et al. The ecoinvent database: Overview and methodological framework. Int. J. Life Cycle Assess. 2005, 10, 3–9. [Google Scholar] [CrossRef]
- WaterSPOUTT Project. Available online: www.waterspoutt.eu (accessed on 25 March 2021).
- García-Gil, Á.; Valverde, R.; García-Muñoz, R.A.; McGuigan, K.G.; Marugán, J. Solar Water Disinfection in high-volume containers: Are naturally occurring substances attenuating factors of radiation? Chem. Eng. J. 2020, 339, 125852. [Google Scholar] [CrossRef]
- Gall, M.P.; Davies-Colley, R.J.; Merrilees, R.A. Exceptional visual clarity and optical purity in a sub-alpine lake. Limnol. Oceanogr. 2013, 58, 443–451. [Google Scholar] [CrossRef]
- Vione, D.; Minella, M.; Maurino, V.; Minero, C. Indirect photochemistry in sunlit surface waters: Photoinduced production of reactive transient species. Chemistry 2014, 20, 10590–10606. [Google Scholar] [CrossRef]
- McNeill, K.; Canonica, S. Triplet state dissolved organic matter in aquatic photochemistry: Reaction mechanisms, substrate scope, and photophysical properties. Environ. Sci. Process. Impacts 2016, 18, 1381–1399. [Google Scholar] [CrossRef]
- Zafiriou, O.C. Sources and reactions of OH and daughter radicals in seawater. J. Geophys. Res. 1974, 79, 4491–4497. [Google Scholar] [CrossRef]
- Hoigné, J.; Faust, B.C.; Haag, W.R.; Scully, F.E.; Zepp, R.G. Aquatic humic substances as sources and sinks of photochemically produced transient reactants. In Aquatic Humic Substances; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1988; Volume 219, pp. 23–363. ISBN 9780841214286. [Google Scholar]
- Foote, C.S.; Selverstone Valentine, J.; Arthur, G. Active Oxygen in Chemistry; Springer: New York, NY, USA, 1995; Volume 2. [Google Scholar]
- Bodrato, M.; Vione, D. APEX (Aqueous Photochemistry of Environmentally occurring Xenobiotics): A free software tool to predict the kinetics of photochemical processes in surface waters. Environ. Sci. Process. Impacts 2014, 16, 732–740. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Gschwend, P.M.; Imboden, D.M. Environmental Organic Chemistry, 3rd ed.; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Vione, D. A critical view of the application of the APEX software (Aqueous Photochemistry of Environmentally-Occurring Xenobiotics) topredict photoreaction kinetics in surface freshwaters. Molecules 2020, 25, 9. [Google Scholar] [CrossRef]
- Smit, K.C. The Science of Photobiology, 2nd ed.; Smit, K.C., Ed.; Springer: New York, NY, USA, 1989. [Google Scholar]
- Davies, M.J. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003, 305, 761–770. [Google Scholar] [CrossRef]
- Boreen, A.L.; Edhlund, B.L.; Cotner, J.B.; McNeill, K. Indirect photodegradation of dissolved free amino acids: The contribution of singlet oxygen and the differential reactivity of DOM from various sources. Environ. Sci. Technol. 2008, 42, 5492–5498. [Google Scholar] [CrossRef]
- Lundeen, R.A.; Janssen, E.M.-L.; Chu, C.; Mcneill, K. Environmental Photochemistry of Amino Acids, Peptides and Proteins. Chimia 2014, 68, 812–817. [Google Scholar] [CrossRef]
- Michaeli, A.; Feitelson, J. Reactivity of singlet oxygen toward amino acids and peptides. Photochem. Photobiol. 1994, 59, 284–289. [Google Scholar] [CrossRef]
- Parashar, U.D.; Burton, A.; Lanata, C.; Boschi-Pinto, C.; Shibuya, K.; Steele, D.; Birmingham, M.; Glass, R.I. Global mortality associated with rotavirus disease among children in 2004. J. Infect. Dis. 2009, 200 (Suppl. 1), S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Love, D.C.; Silverman, A.; Nelson, K.L. Human virus and bacteriophage inactivation in clear water by simulated sunlight compared to bacteriophage inactivation at a Southern California beach. Environ. Sci. Technol. 2010, 44, 6965–6970. [Google Scholar] [CrossRef]
- Theitler, D.J.; Nasser, A.; Gerchman, Y.; Kribus, A.; Mamane, H. Synergistic effect of heat and solar UV on DNA damage and water disinfection of E. Coli and bacteriophage MS2. J. Water Health 2012, 10, 605–618. [Google Scholar] [CrossRef]
- Lytle, C.D.; Sagripanti, J.-L. Predicted inactivation of viruses of relevance to biodefense by solar radiation. J. Virol. 2005, 79, 14244–14252. [Google Scholar] [CrossRef]
- Kohn, T.; Nelson, K.L. Sunlight-mediated inactivation of MS2 coliphage via exogenous singlet oxygen produced by sensitizers in natural waters. Environ. Sci. Technol. 2007, 41, 192–197. [Google Scholar] [CrossRef]
- Kohn, T.; Grandbois, M.; Mcneill, K.; Nelson, K.L. Association with natural organic matter enhances the sunlight-mediated inactivation of MS2 coliphage by singlet oxygen. Environ. Sci. Technol. 2007, 41, 4626–4632. [Google Scholar] [CrossRef]
- Romero-Maraccini, O.C.; Sadik, N.J.; Rosado-Lausell, S.L.; Pugh, C.R.; Niu, X.-Z.; Croué, J.-P.; Nguyen, T.H. Sunlight-induced inactivation of human Wa and porcine OSU rotaviruses in the presence of exogenous photosensitizers. Environ. Sci. Technol. 2013, 47, 11004–11012. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Lausell, S.L.; Wang, H.; Gutiérrez, L.; Romero-Maraccini, O.C.; Niu, X.Z.; Gin, K.Y.H.; Croué, J.P.; Nguyen, T.H. Roles of singlet oxygen and triplet excited state of dissolved organic matter formed by different organic matters in bacteriophage MS2 inactivation. Water Res. 2013, 47, 4869–4879. [Google Scholar] [CrossRef] [PubMed]
- Jagger, J. Solar-UV Actions on Living Cells; Praeger: New York, NY, USA, 1985; ISBN 0030008484. [Google Scholar]
- Chen, S.; Schopfer, P. Hydroxyl-radical production in physiological reactions. Eur. J. Biochem. 1999, 260, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Seaver, L.C.; Imlay, J.A. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 2001, 183, 7173–7181. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Darakas, E.; Escalas-Cañellas, A.; Pulgarin, C. Solar disinfection modeling and post-irradiation response of Escherichia coli in wastewater. Chem. Eng. J. 2015, 281, 588–598. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.-P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef]
- Maraccini, P.A.; Wenk, J.; Boehm, A.B. Exogenous indirect photoinactivation of bacterial pathogens and indicators in water with natural and synthetic photosensitizers in simulated sunlight with reduced UVB. J. Appl. Microbiol. 2016, 121, 587–597. [Google Scholar] [CrossRef]
- Maraccini, P.A.; Wenk, J.; Boehm, A.B. Photoinactivation of eight health-relevant bacterial species: Determining the importance of the exogenous indirect mechanism. Environ. Sci. Technol. 2016, 50, 5050–5059. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Sow, S.O.; Muhsen, K.; Nasrin, D.; Blackwelder, W.C.; Wu, Y.; Farag, T.H.; Panchalingam, S.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; et al. The burden of Cryptosporidium diarrheal disease among children <24 months of age in moderate/high mortality regions of Sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS Negl. Trop. Dis. 2016, 10, e0004729. [Google Scholar] [CrossRef]
- Gómez-Couso, H.; Fontán-Sainz, M.; Navntoft, C.; Fernández-Ibáñez, P.; Ares-Mazás, E. Comparison of different solar reactors for household disinfection of drinking water in developing countries: Evaluation of their efficacy in relation to the waterborne enteropathogen Cryptosporidium parvum. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 645–652. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, S.; Kuhlenschmidt, M.S.; Kuhlenschmidt, T.B.; Drnevich, J.; Nguyen, T.H. Inactivation mechanisms of Cryptosporidium parvum oocysts by solar ultraviolet irradiation. Environ. Sci. Water Res. Technol. 2015, 1, 188–198. [Google Scholar] [CrossRef]
- Linden, K.G.; Shin, G.; Sobsey, M.D. Comparative effectiveness of UV wavelengths for the inactivation of Cryptosporidium parvum oocysts in water. Water Sci. Technol. 2001, 43, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.E.; Wright, H.B.; Hargy, T.M.; Larason, T.C.; Linden, K.G. Action spectra for validation of pathogen disinfection in medium-pressure ultraviolet (UV) systems. Water Res. 2015, 70, 27–37. [Google Scholar] [CrossRef]
- Leuenberger, P.; Ganscha, S.; Kahraman, A.; Cappelletti, V.; Boersema, P.J.; von Mering, C.; Claassen, M.; Picotti, P. Cell-wide analysis of protein thermal unfolding reveals determinants of thermostability. Science 2017, 355, eaai7825. [Google Scholar] [CrossRef]
- Mackey, B.M.; Miles, C.A.; Parsons, S.E.; Seymour, D.A. Thermal denaturation of whole cells and cell components of Escherichia coli examined by differential scanning calorimetry. J. Gen. Microbiol. 1991, 137, 2361–2374. [Google Scholar] [CrossRef] [PubMed]
- Fayer, R.; Nerad, T. Effects of low temperatures on viability of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 1996, 62, 1431. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Murphy, T.; Holden, N.M. Evaluation of the effect of temperature on the die-off rate for Cryptosporidium parvum oocysts in water, soils, and feces. Appl. Environ. Microbiol. 2008, 74, 7101–7107. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.B.; Eaglesham, B.S.; Anthony, L.C.; Kachlany, S.C.; Bowman, D.D.; Ghiorse, W.C. Significance of wall structure, macromolecular composition, and surface polymers to the survival and transport of Cryptosporidium parvum Oocysts. Appl. Environ. Microbiol. 2010, 76, 1926–1934. [Google Scholar] [CrossRef]
- King, B.J.; Keegan, A.R.; Monis, P.T.; Saint, C.P. Environmental temperature controls Cryptosporidium oocyst metabolic rate and associated retention of infectivity. Appl. Environ. Microbiol. 2005, 71, 3848–3857. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Couso, H.; Fontán-Sainz, M.; Fernández-Alonso, J.; Ares-Mazás, E. Excystation of Cryptosporidium parvum at temperatures that are reached during solar water disinfection. Parasitology 2009, 136, 393–399. [Google Scholar] [CrossRef]
- Smith, H.V.; Nichols, R.A.B.; Grimason, A.M. Cryptosporidium excystation and invasion: Getting to the guts of the matter. Trends Parasitol. 2005, 21, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Lee, J.E.U.N.; Lim, M.I.Y.; Ko, G. Effect of temperature, pH, and NaCl on the inactivation kinetics of murine norovirus. J. Food Prot. 2012, 75, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Šolić, M.; Krstulović, N. Separate and combined effects of solar radiation, temperature, salinity, and pH on the survival of faecal coliforms in seawater. Mar. Pollut. Bull. 1992, 24, 411–416. [Google Scholar] [CrossRef]
- McGuigan, K.G.; Joyce, T.M.; Conroy, R.M.; Gillespie, J.B.; Elmore-Meegan, M. Solar disinfection of drinking water contained in transparent plastic bottles: Characterizing the bacterial inactivation process. J. Appl. Microbiol. 1998, 84, 1138–1148. [Google Scholar] [CrossRef]
- Romero, O.C.; Straub, A.P.; Kohn, T.; Nguyen, T.H. Role of temperature and Suwannee River Natural Organic Matter on inactivation kinetics of rotavirus and bacteriophage MS2 by solar irradiation. Environ. Sci. Technol. 2011, 45, 10385–10393. [Google Scholar] [CrossRef] [PubMed]
- Castro-Alférez, M.; Polo-López, M.I.; Marugán, J.; Fernández-Ibáñez, P. Mechanistic modeling of UV and mild-heat synergistic effect on solar water disinfection. Chem. Eng. J. 2017, 316, 111–120. [Google Scholar] [CrossRef]
- Mani, S.K.; Kanjur, R.; Bright Singh, I.S.; Reed, R.H. Comparative effectiveness of solar disinfection using small-scale batch reactors with reflective, absorptive and transmissive rear surfaces. Water Res. 2006, 40, 721–727. [Google Scholar] [CrossRef]
- Casado, C.; García-Gil, Á.; van Grieken, R.; Marugán, J. Critical role of the light spectrum on the simulation of solar photocatalytic reactors. Appl. Catal. B Environ. 2019, 252, 1–9. [Google Scholar] [CrossRef]
- Kehoe, S.C.; Joyce, T.M.; Ibrahim, P.; Gillespie, J.B.; Shahar, R.A.; McGuigan, K.G. Effect of agitation, turbidity, aluminium foil reflectors and container volume on the inactivation efficiency of batch-process solar disinfectors. Water Res. 2001, 35, 1061–1065. [Google Scholar] [CrossRef]
- Rijal, G.K.; Fujioka, R.S. Use of reflectors to enhance the synergistic effects of solar heating and solar wavelengths to disinfect drinking water sources. Water Sci. Technol. 2004, 48, 481–488. [Google Scholar] [CrossRef]
- Martín-Sómer, M.; Moreno-SanSegundo, J.; Álvarez-Fernández, C.; van Grieken, R.; Marugán, J. High-performance low-cost solar collectors for water treatment fabricated with recycled materials, open-source hardware and 3d-printing technologies. Sci. Total Environ. 2021, 784, 147119. [Google Scholar] [CrossRef]
- Gueymard, C.A. Interdisciplinary applications of a versatile spectral solar irradiance model: A review. Energy 2005, 30, 1551–1576. [Google Scholar] [CrossRef]
- National Center for Atmospheric Research. Tropospheric Ultraviolet and Visible (TUV) Radiation Model. Available online: https://www2.acom.ucar.edu/modeling/tropospheric-ultraviolet-and-visible-tuv-radiation-model (accessed on 4 June 2021).
- Moreno-SanSegundo, J.; Giannakis, S.; Samoili, S.; Farinelli, G.; McGuigan, K.G.; Marugán, J. SODIS potential: A novel parameter to assess the suitability of solar water disinfection worldwide. Chem. Eng. J. 2021, 419, 129889. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory, Solar Position Algorithm|NREL. Available online: http://www.nrel.gov/mide/solpos.spa.html (accessed on 4 June 2021).
- Lambert, J.H. Photometria Sive de Mensura et Gradibus Luminis, Colorum et Umbrae; Eberhardt Klett: Augsburg, Germany, 1760. [Google Scholar]
- Beer, A. Bestimmung der Absorption des rothen Lichts in farbigen Flüssigkeiten. Ann. Phys. 1852, 162, 78–88. [Google Scholar] [CrossRef]
- Kirk, J. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambridge, UK, 1994; ISBN 9780511623370. [Google Scholar]
- Boyd, C.E. Solar radiation and water temperature. In Water Quality; Springer: Cham, Switzerland, 2020; pp. 21–39. [Google Scholar]
- Brutsaert, W. Heat and mass transfer to and from surfaces with dense vegetation or similar permeable roughness. Bound.-Layer Meteorol. 1979, 16, 365–388. [Google Scholar] [CrossRef]
- Rutherford, J.C.; Blackett, S.; Blackett, C.; Saito, L.; Davies-Colley, R.J. Predicting the effects of shade on water temperature in small streams. N. Z. J. Mar. Freshw. Res. 1997, 31, 707–721. [Google Scholar] [CrossRef]
- Chick, H. An investigation of the laws of disinfection. J. Hyg. 1908, 8, 92–158. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.E. A note on the variation of the rate of disinfection with change in the concentration of the disinfectant. J. Hyg. 1908, 8, 536–542. [Google Scholar] [CrossRef]
- Hom, L.W. Kinetics of chlorine disinfection in an ecosystem. J. Sanit. Eng. Div. 1972, 98, 183–194. [Google Scholar] [CrossRef]
- Chamberlin, C.E.; Mitchell, R. A decay model for enteric bacteria in natural waters. In Water Polution Microbiology; Mitchell, R., Ed.; Willey-Intercience Publication: Hoboken, NJ, USA, 1978; pp. 325–348. [Google Scholar]
- Severin, B.F.; Suidan, M.T.; Engelbrecht, R.S. Kinetic modeling of U.V. disinfection of water. Water Res. 1982, 17, 1669–1678. [Google Scholar] [CrossRef]
- Castro-Alférez, M.; Polo-López, M.I.; Marugán, J.; Fernández-Ibáñez, P. Mechanistic model of the Escherichia coli inactivation by solar disinfection based on the photo-generation of internal ROS and the photo-inactivation of enzymes: CAT and SOD. Chem. Eng. J. 2017, 318, 214–223. [Google Scholar] [CrossRef]
- Silverman, A.I.; Nguyen, M.T.; Schilling, I.E.; Wenk, J.; Nelson, K.L. Sunlight inactivation of viruses in open-water unit process treatment wetlands: Modeling endogenous and exogenous inactivation rates. Environ. Sci. Technol. 2015, 49, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.B.; Love, D.C.; Schuech, R.; Nelson, K.L. Simulated sunlight action spectra for inactivation of MS2 and PRD1 bacteriophages in clear water. Environ. Sci. Technol. 2011, 45, 9249–9255. [Google Scholar] [CrossRef]
- Silverman, A.I.; Nelson, K.L. Modeling the endogenous sunlight inactivation rates of laboratory strain and Wastewater E. coli and enterococci using biological weighting functions. Environ. Sci. Technol. 2016, 50, 12292–12301. [Google Scholar] [CrossRef]
- Lui, G.Y.; Roser, D.; Corkish, R.; Ashbolt, N.J.; Stuetz, R. Point-of-use water disinfection using ultraviolet and visible light-emitting diodes. Sci. Total Environ. 2016, 553, 626–635. [Google Scholar] [CrossRef]
- Vione, D. The modelling of Surface-Water photoreactions made easier: Introducing the concept of ‘equivalent monochromatic wavelengths’. Water Res. 2021, 190, 116675. [Google Scholar] [CrossRef] [PubMed]
- Mancini, J.L. Numerical estimates of coliform mortality rates under various conditions. Water Pollut. Control. Fed. 1978, 50, 2477–2484. [Google Scholar]
- Peleg, M.; Normand, M.D.; Corradini, M.G. The Arrhenius equation revisited. Crit. Rev. Food Sci. Nutr. 2012, 52, 830–851. [Google Scholar] [CrossRef]
- Ansys, I. ANSYS FLUENT Theory Guide; Ansys, Inc.: Canonsburg, PA, USA, 2012. [Google Scholar]
- Cassano, A.E.; Alfano, O.M. Reaction engineering of suspended solid heterogeneous photocatalytic reactors. Catal. Today 2000, 58, 167–197. [Google Scholar] [CrossRef]
- Moreno, J.; Casado, C.; Marugán, J. Improved discrete ordinate method for accurate simulation radiation transport using solar and LED light sources. Chem. Eng. Sci. 2019, 205, 151–164. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Gil, Á.; García-Muñoz, R.A.; McGuigan, K.G.; Marugán, J. Solar Water Disinfection to Produce Safe Drinking Water: A Review of Parameters, Enhancements, and Modelling Approaches to Make SODIS Faster and Safer. Molecules 2021, 26, 3431. https://doi.org/10.3390/molecules26113431
García-Gil Á, García-Muñoz RA, McGuigan KG, Marugán J. Solar Water Disinfection to Produce Safe Drinking Water: A Review of Parameters, Enhancements, and Modelling Approaches to Make SODIS Faster and Safer. Molecules. 2021; 26(11):3431. https://doi.org/10.3390/molecules26113431
Chicago/Turabian StyleGarcía-Gil, Ángela, Rafael A. García-Muñoz, Kevin G. McGuigan, and Javier Marugán. 2021. "Solar Water Disinfection to Produce Safe Drinking Water: A Review of Parameters, Enhancements, and Modelling Approaches to Make SODIS Faster and Safer" Molecules 26, no. 11: 3431. https://doi.org/10.3390/molecules26113431
APA StyleGarcía-Gil, Á., García-Muñoz, R. A., McGuigan, K. G., & Marugán, J. (2021). Solar Water Disinfection to Produce Safe Drinking Water: A Review of Parameters, Enhancements, and Modelling Approaches to Make SODIS Faster and Safer. Molecules, 26(11), 3431. https://doi.org/10.3390/molecules26113431