Characterization of a Hg2+-Selective Fluorescent Probe Based on Rhodamine B and Its Imaging in Living Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. pH Effect on the Fluorescent Response of P and a P-Hg2+ System
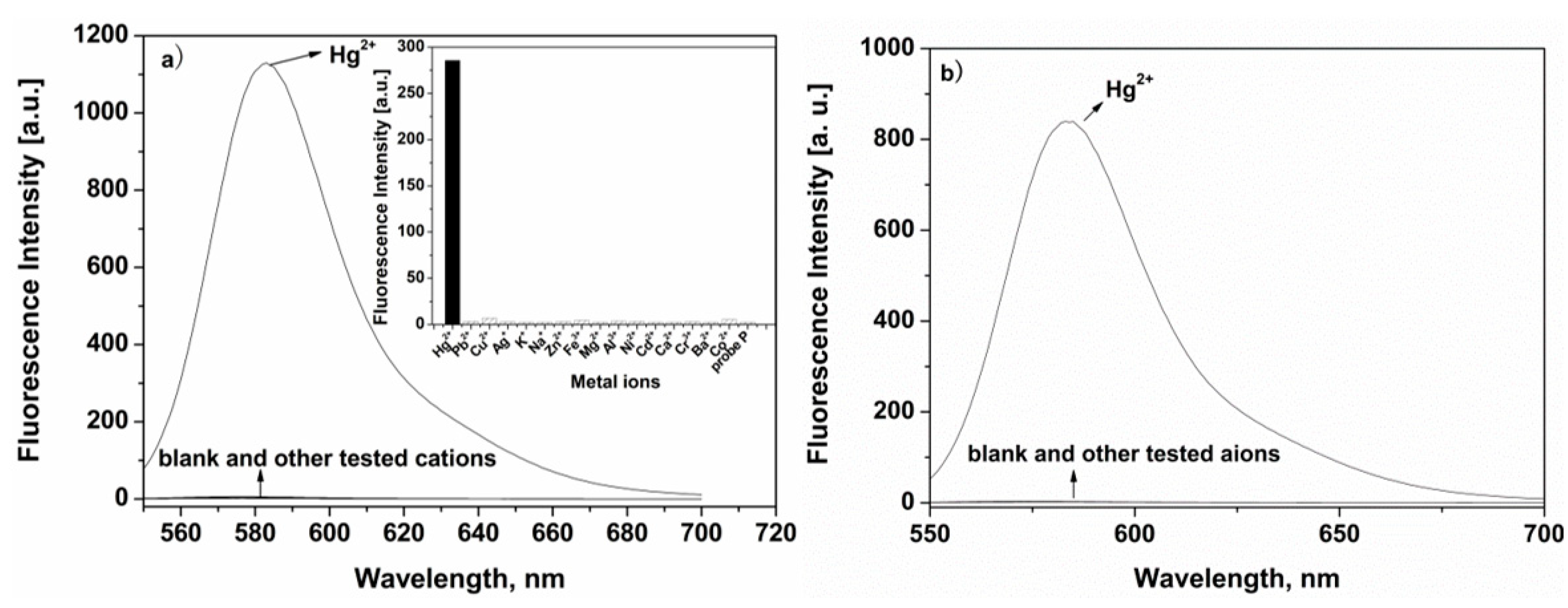
2.2. Selectivity and Sensitivity Measurement of P
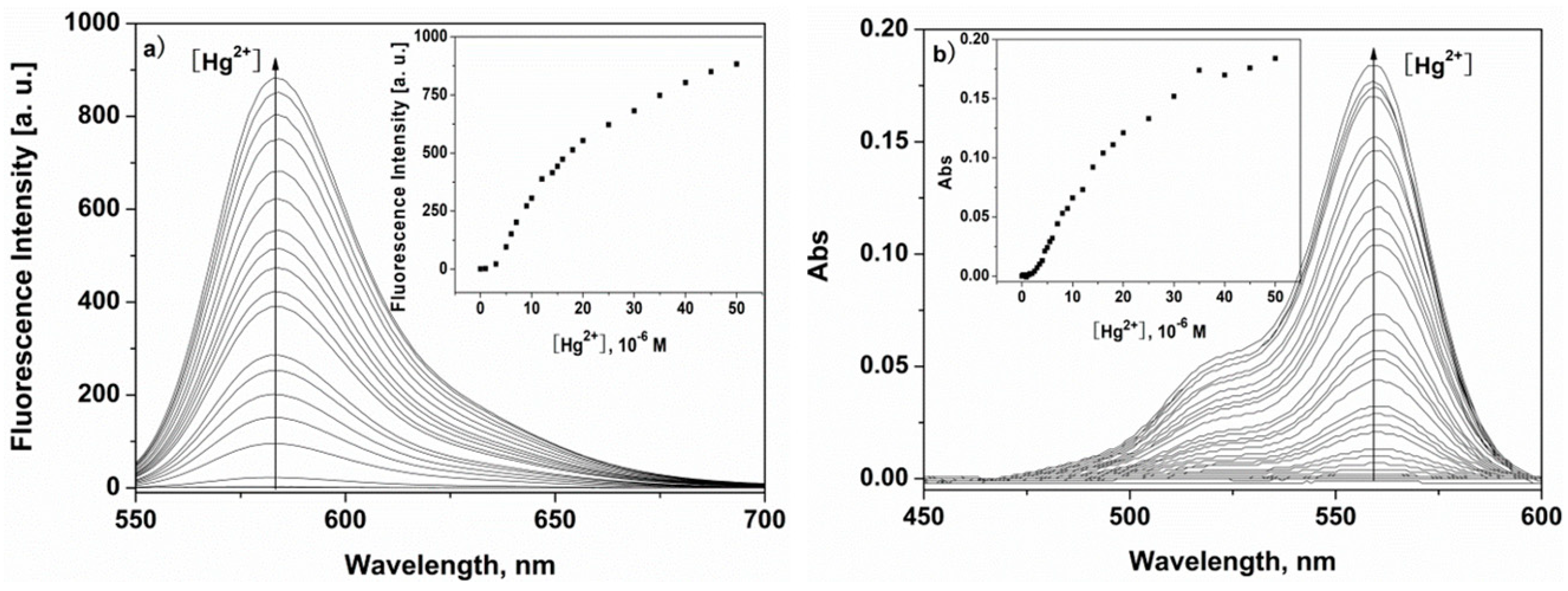
2.3. Fluorescent and UV-Vis Titration Experiments of P to Hg2+
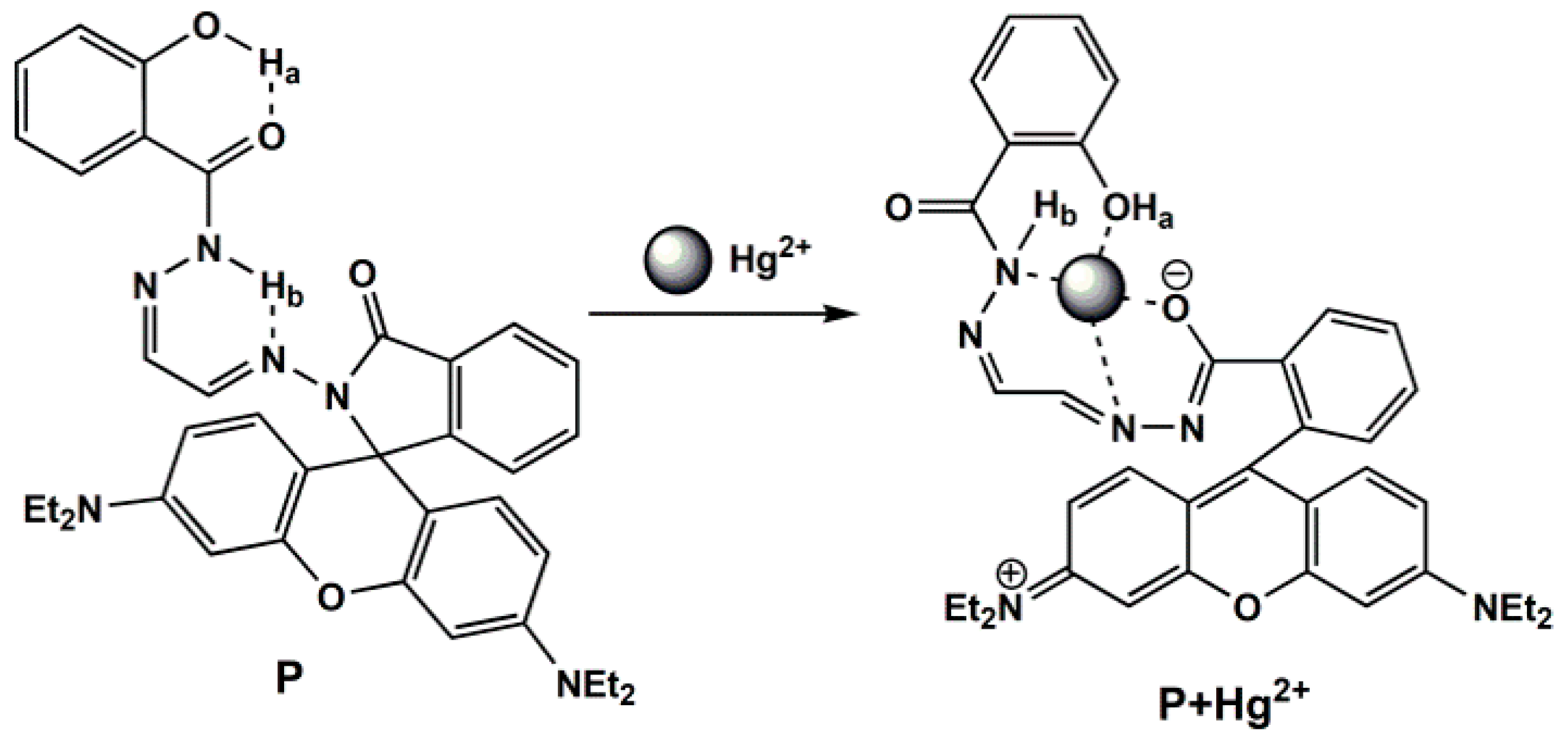
2.4. Coordination Mechanism of P with Hg2+
2.5. Preliminary Application of P in Cell Imaging
3. Materials and Methods
3.1. Main Reagents and Instruments
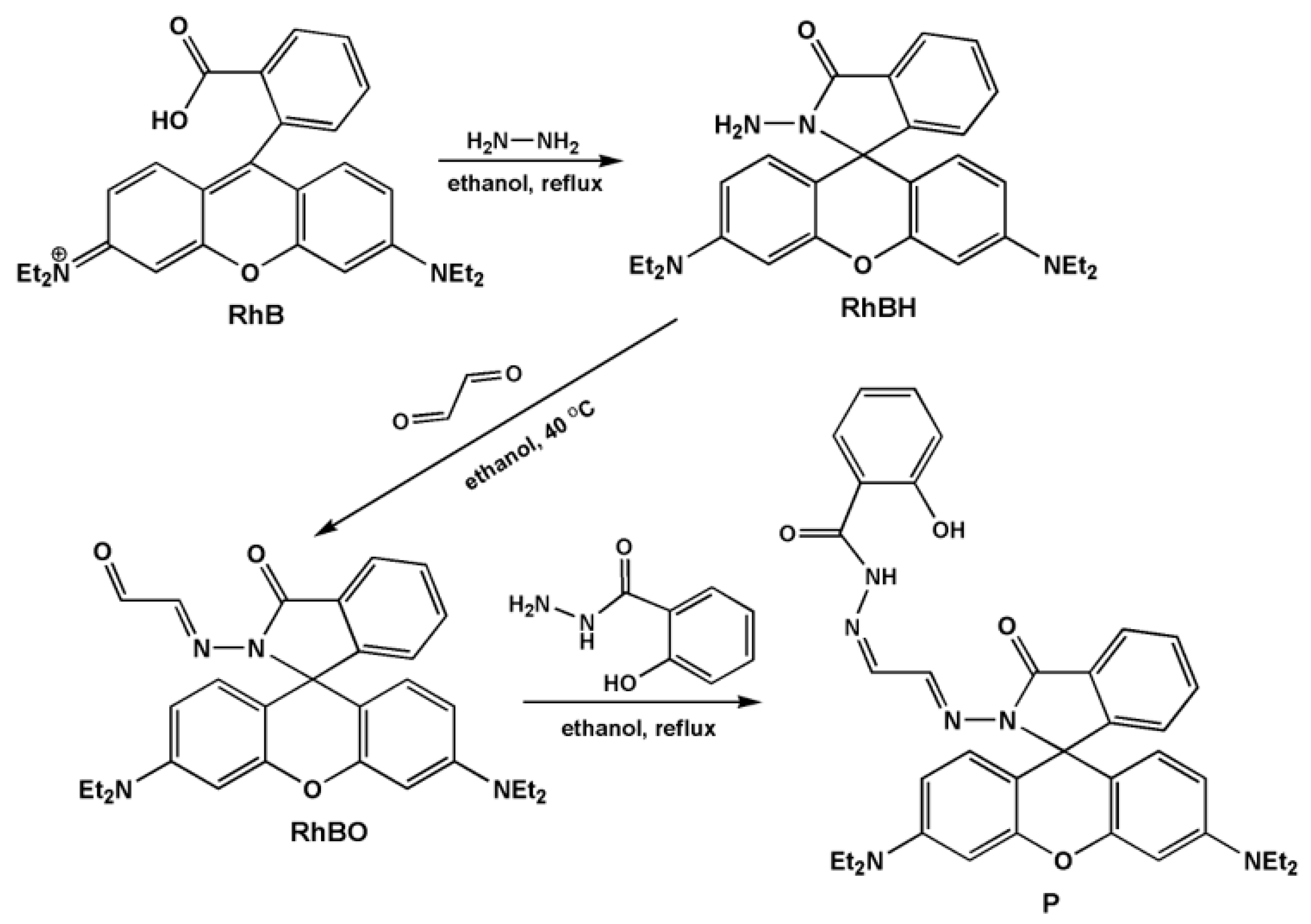
3.2. Synthesis of Probe P
3.3. General Spectroscopic Methods
3.4. Cell Incubation and Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Eisler, R. Health Risks of Gold Miners: A Synoptic Review. Environ. Geochem. Health 2003, 25, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Boening, D.W. Ecological effects, transport, and fate of mercury: A general review. Chemosphere 2000, 40, 1335–1351. [Google Scholar] [CrossRef]
- Ikenna, O.; Albert, R.N.; Erwin, B. Biomolecule—Mercury interactions: Modalities of DNA base—Mercury binding mecha-nisms. Remediation strategies. Chem. Rev. 2005, 104, 5911–5929. [Google Scholar]
- Antonio, C.; Vega, L.; David, C.; Alberto, T.; Arturo, E.; Rafaela, G.; José, V.G.; Concepció, R.; Klaus, W.; Pedro, M.; et al. Electroactive thiazole derivatives capped with ferrocenyl units showing charge-transfer transition and selective ion-sensing properties: A combined experimental and theoretical study. Inorg. Chem. 2007, 46, 825–838. [Google Scholar]
- Vallant, B.; Kadnar, R.; Goessler, W. Development of a new HPLC method for the determination of inorganic and methyl-mercury in biological samples with ICP-MS detection. J. Anal. At. Spectrom. 2007, 22, 322–325. [Google Scholar] [CrossRef]
- Tan, J.; Yan, X.-P. 2,1,3-Benzoxadiazole-based selective chromogenic chemosensor for rapid naked-eye detection of Hg2+ and Cu2+. Talanta 2008, 76, 9–14. [Google Scholar] [CrossRef]
- Ji, H.F.; Ma, F.L.; Dai, Y.P.; Zhao, X.X.; Xue, K.; Misal, S.; Zhang, P.; Qi, Z.J.; Zhu, H.Y. A near-infrared ratiometric fluores-cent probe for selectively tracking human cytochrome P450 3A5 in living cells and tumor-bearing mice. Sens. Actuators B Chem. 2021, 331, 129372. [Google Scholar] [CrossRef]
- Avirah, R.R.; Jyothish, K.; Ramaiah, D. Dual-Mode Semisquaraine-Based Sensor for Selective Detection of Hg2+ in a Micellar Medium. Org. Lett. 2007, 9, 121–124. [Google Scholar] [CrossRef]
- Guo, X.; Qian, X.; Jia, L. A Highly Selective and Sensitive Fluorescent Chemosensor for Hg2+ in Neutral Buffer Aqueous Solution. J. Am. Chem. Soc. 2004, 126, 2272–2273. [Google Scholar] [CrossRef]
- Ma, X.; Wang, J.; Shan, Q.L.; Tan, Z.W.; Wei, G.H.; Wei, D.B.; Du, Y.G. A “turn-on” fluorescent Hg2+ chemosensor based on ferrier carbocyclization. Org. Lett. 2012, 14, 820–823. [Google Scholar] [CrossRef]
- Li, A.-L.; Wang, Z.-L.; Wang, W.-Y.; Liu, Q.-S.; Sun, Y.; Wang, S.-F.; Gu, W. A novel dehydroabietic acid-based fluorescent probe for detection of Fe3+ and Hg2+ ions and its application in live-cell imaging. Microchem. J. 2021, 160, 105682. [Google Scholar] [CrossRef]
- Pandey, S.; Azam, A.; Pandey, S.; Chawla, H.M. Novel dansyl-appended calix[4]arene frameworks: Fluorescence properties and mercury sensing. Org. Biomol. Chem. 2009, 7, 269–279. [Google Scholar] [CrossRef]
- Bhatt, S.; Vyas, G.; Paul, P. A New Molecular Probe for Colorimetric and Fluorometric Detection and Removal of Hg2+ and its Application as Agarose Film-Based Sensor for On-Site Monitoring. J. Fluoresc. 2020, 30, 1531–1542. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, M.H.; Kim, H.J.; Kim, J.S.; Yoon, J. A new trend in rhodamine-based chemosensors: Application of spirolac-tam ring-opening to sensing ions. Chem. Soc. Rev. 2008, 37, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Ghezelsefloo, S.; Rad, J.K.; Hajiali, M.; Mahdavian, A.R. Rhodamine-based fluorescent polyacrylic nanoparticles: A highly selective and sensitive chemosensor for Fe (II) and Fe (III) cations in water. J. Environ. Chem. Eng. 2021, 9, 105082. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, H.; Kang, H.; Fan, C.; Liu, G.; Pu, S. A solvent-dependent chemosensor for fluorimetric detection of Hg2+ and colorimetric detection of Cu2+ based on a new diarylethene with a rhodamine B unit. RSC Adv. 2019, 9, 42155–42162. [Google Scholar] [CrossRef]
- Chen, X.; Jia, J.; Ma, H.; Wang, S.; Wang, X. Characterization of rhodamine B hydroxylamide as a highly selective and sensitive fluorescence probe for copper(II). Anal. Chim. Acta 2009, 632, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Zhang, X.; Ren, T.; Yuan, L. Molecular engineering of ultra-sensitive fluorescent probe with large Stokes shift for imaging of basal HOCl in tumor cells and tissues. Chin. Chem. Lett. 2020, 31, 2980–2984. [Google Scholar] [CrossRef]
- Wu, J.S.; Hwang, I.-C.; Kim, K.S.; Kim, J.S. Rhodamine-based Hg2+-selective chemodosimeter in aqueous solution: Fluo-rescent off-on. Org. Lett. 2007, 9, 907–910. [Google Scholar] [CrossRef]
- Du, J.; Fan, J.; Peng, X.; Sun, P.; Wang, J.; Li, H.; Sun, S. A New Fluorescent Chemodosimeter for Hg2+: Selectivity, Sensitivity, and Resistance to Cys and GSH. Org. Lett. 2010, 12, 476–479. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, P.; Yan, W.B.; He, C.; Xiong, L.Q.; Li, F.Y.; Duan, C.Y. A bright water-compatible sugar-rhodamine fluo-rescence sensor for selective detection of Hg2+ in natural water and living cells. J. Environ. Monit. 2009, 11, 330–335. [Google Scholar] [CrossRef]
- Ma, Q.-J.; Zhang, X.-B.; Zhao, X.-H.; Jin, Z.; Mao, G.-J.; Shen, G.-L.; Yu, R.-Q. A highly selective fluorescent probe for Hg2+ based on a rhodamine–coumarin conjugate. Anal. Chim. Acta 2010, 663, 85–90. [Google Scholar] [CrossRef]
- Singh, S.; Coulomb, B.; Boudenne, J.-L.; Bonne, D.; Dumur, F.; Simon, B.; Robert-Peillard, F. Sub-ppb mercury detection in real environmental samples with an improved rhodamine-based detection system. Talanta 2021, 224, 121909. [Google Scholar] [CrossRef]
- Wen, D.; Yu, Y.H. A novel turn-on fluorescent probe for Hg2+ detection based on rhodamine B spirolactam derivative. Int. J. Environ. Anal. Chem. 2019, 99, 1515–1527. [Google Scholar]
- Petdum, A.; Panchan, W.; Sirirak, J.; Promarak, V.; Sooksimuang, T.; Wanichacheva, N. Colorimetric and fluorescent sensing of a new FRET system via [5]helicene and rhodamine 6G for Hg2+ detection. New J. Chem. 2018, 42, 1396–1402. [Google Scholar] [CrossRef]
- Vanjare, B.D.; Mahajan, P.G.; Ryoo, H.-I.; Dige, N.C.; Choi, N.G.; Han, Y.; Kim, S.J.; Kim, C.-H.; Lee, K.H. Novel rhodamine based chemosensor for detection of Hg2+: Nanomolar detection, real water sample analysis, and intracellular cell imaging. Sens. Actuators B Chem. 2021, 330, 129308. [Google Scholar] [CrossRef]
- Shi, W.; Ma, H. Rhodamine B thiolactone: A simple chemosensor for Hg2+ in aqueous media. Chem. Commun. 2008, 16, 1856–1858. [Google Scholar] [CrossRef]
- Zheng, H.; Qian, Z.; Xu, L.; Yuan, F.; Lan, L.; Xu, J. Switching the recognition preference of rhodamine B spirolactam by re-placing one atom: Design of rhodamine B thiohydrazide for recognition of Hg(II) in aqueous solution. Org. Lett. 2006, 8, 859–861. [Google Scholar] [CrossRef]
- Zhou, Z.L.; Tang, H.H.; Chen, S.Y.; Huang, Y.H.; Zhu, X.H.; Li, H.T.; Zhang, Y.Y.; Yao, S.Z. A turn-on red-emitting fluores-cent probe for determination of copper(II) ions in food samples and living zebrafish. Food Chem. 2021, 343, 128513. [Google Scholar] [CrossRef]
- Yu, C.W.; Jian, L.; Ji, Y.X.; Zhang, J. Al(III)-Responsive “off-on” chemosensor based on rhodamine derivative and its application in cell imaging. RSC Adv. 2018, 8, 31106–31112. [Google Scholar] [CrossRef]
- Yu, C.; Cui, S.; Ji, Y.; Wen, S.; Jian, L.; Zhang, J. A pH tuning single fluorescent probe based on naphthalene for dual-analytes (Mg2+ and Al3+) and its application in cell imaging. RSC Adv. 2020, 10, 21399–21405. [Google Scholar] [CrossRef]
- Ji, Y.; Yu, C.; Wen, S.; Zhang, J. Characterization of an Al3+-selective fluorescent probe based on a benzoyl hydrazine derivative and its application in cell imaging. Turk. J. Chem. 2016, 40, 625–630. [Google Scholar] [CrossRef]
- He, H.; Cheng, Z.; Zheng, L. Aqueous Zn2+ analysis: Specific recognition and instant imaging by Schiff base fluorescent probes. J. Mol. Struct. 2021, 1227, 129522. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, J.; Chen, C.; Wei, Z.; Tian, L. Synthesis, Characterization and Antibacterial Activities of Manganese(II), Nickel(II), Copper(II) and Zinc(II) Complexes of the Hydrazone Compounds Derived from 1-Phenyl-3-methyl-4-acyl-pyrazole and Benzoyl Hydrazine. Asian J. Chem. 2013, 25, 8444–8446. [Google Scholar] [CrossRef]
- Rodríguez-Cáceres, M.I.; Agbaria, R.A.; Warner, I.M. Fluorescence of Metal–Ligand Complexes of Mono- and Di-Substituted Naphthalene Derivatives. J. Fluoresc. 2005, 15, 185–190. [Google Scholar] [CrossRef]
- Li, N.; Yu, C.; Ji, Y.; Zhang, J. Characterization of a Cu2+-selective fluorescent probe derived from rhodamine B with 1,2,4-triazole as subunit and its application in cell imaging. Turk. J. Chem. 2015, 39, 660–666. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Yu, C.; Yang, M.; Wen, S.; Zhang, J. Characterization of a Hg2+-Selective Fluorescent Probe Based on Rhodamine B and Its Imaging in Living Cells. Molecules 2021, 26, 3385. https://doi.org/10.3390/molecules26113385
Zhang W, Yu C, Yang M, Wen S, Zhang J. Characterization of a Hg2+-Selective Fluorescent Probe Based on Rhodamine B and Its Imaging in Living Cells. Molecules. 2021; 26(11):3385. https://doi.org/10.3390/molecules26113385
Chicago/Turabian StyleZhang, Wenting, Chunwei Yu, Mei Yang, Shaobai Wen, and Jun Zhang. 2021. "Characterization of a Hg2+-Selective Fluorescent Probe Based on Rhodamine B and Its Imaging in Living Cells" Molecules 26, no. 11: 3385. https://doi.org/10.3390/molecules26113385
APA StyleZhang, W., Yu, C., Yang, M., Wen, S., & Zhang, J. (2021). Characterization of a Hg2+-Selective Fluorescent Probe Based on Rhodamine B and Its Imaging in Living Cells. Molecules, 26(11), 3385. https://doi.org/10.3390/molecules26113385




