Ceramic-Polymer Composite Membranes for Water and Wastewater Treatment: Bridging the Big Gap between Ceramics and Polymers
Abstract
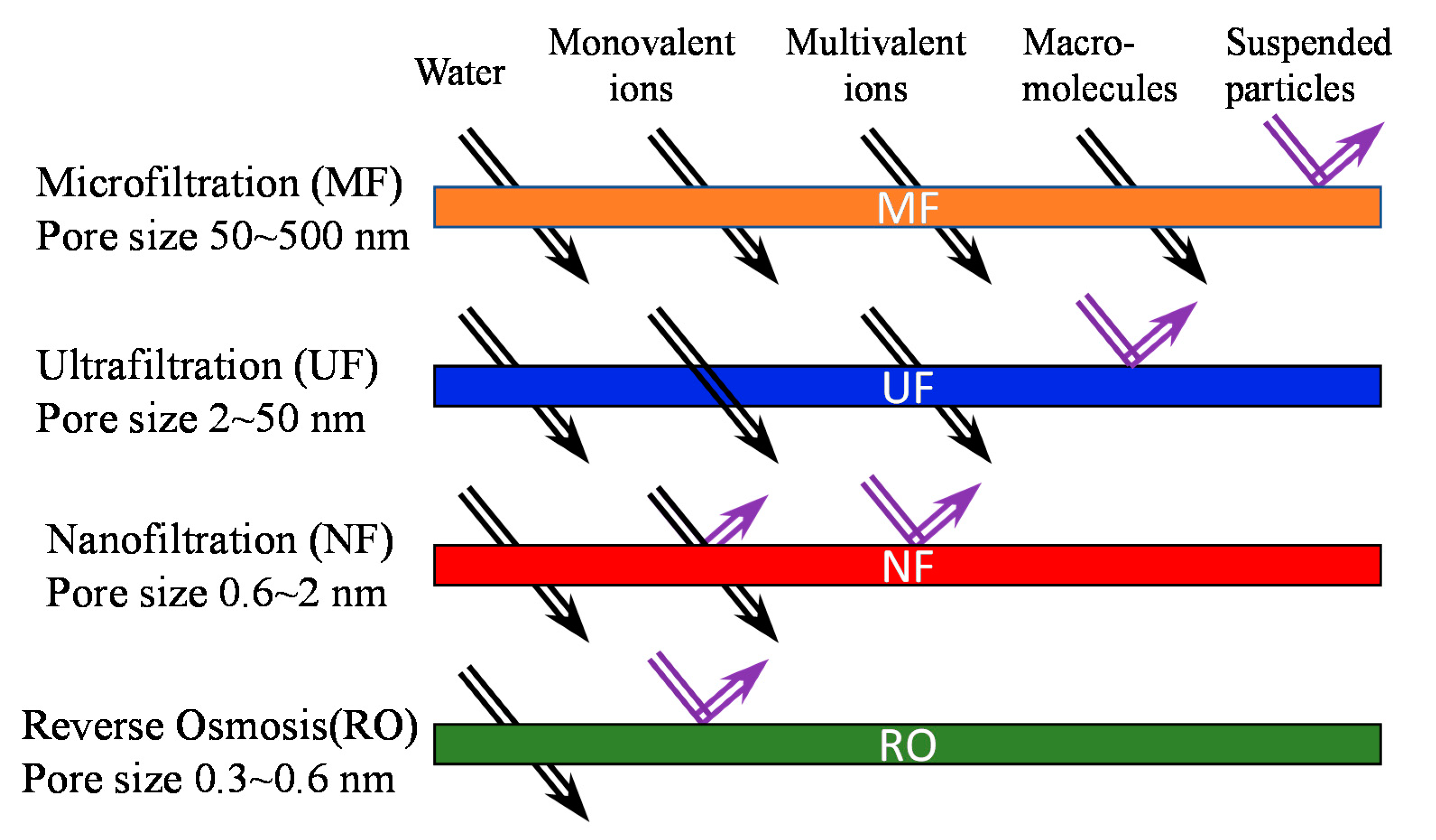
1. Introduction
2. Composite Membranes
2.1. Advantages of Inorganic-Polymer Composite Membranes
- Simultaneous enhancement of flux and rejection
- 2.
- Addition of antibacterial and photocatalytic properties
- 3.
- Modification of morphology of polymeric membrane
- 4.
- Improvement of mechanical properties and thermal stability
2.2. Disadvantages of Composite Membranes
- Compatibility between inorganic and organic components
- 2.
- Manufacturing cost
3. Ceramic-Polymer Composite Membrane
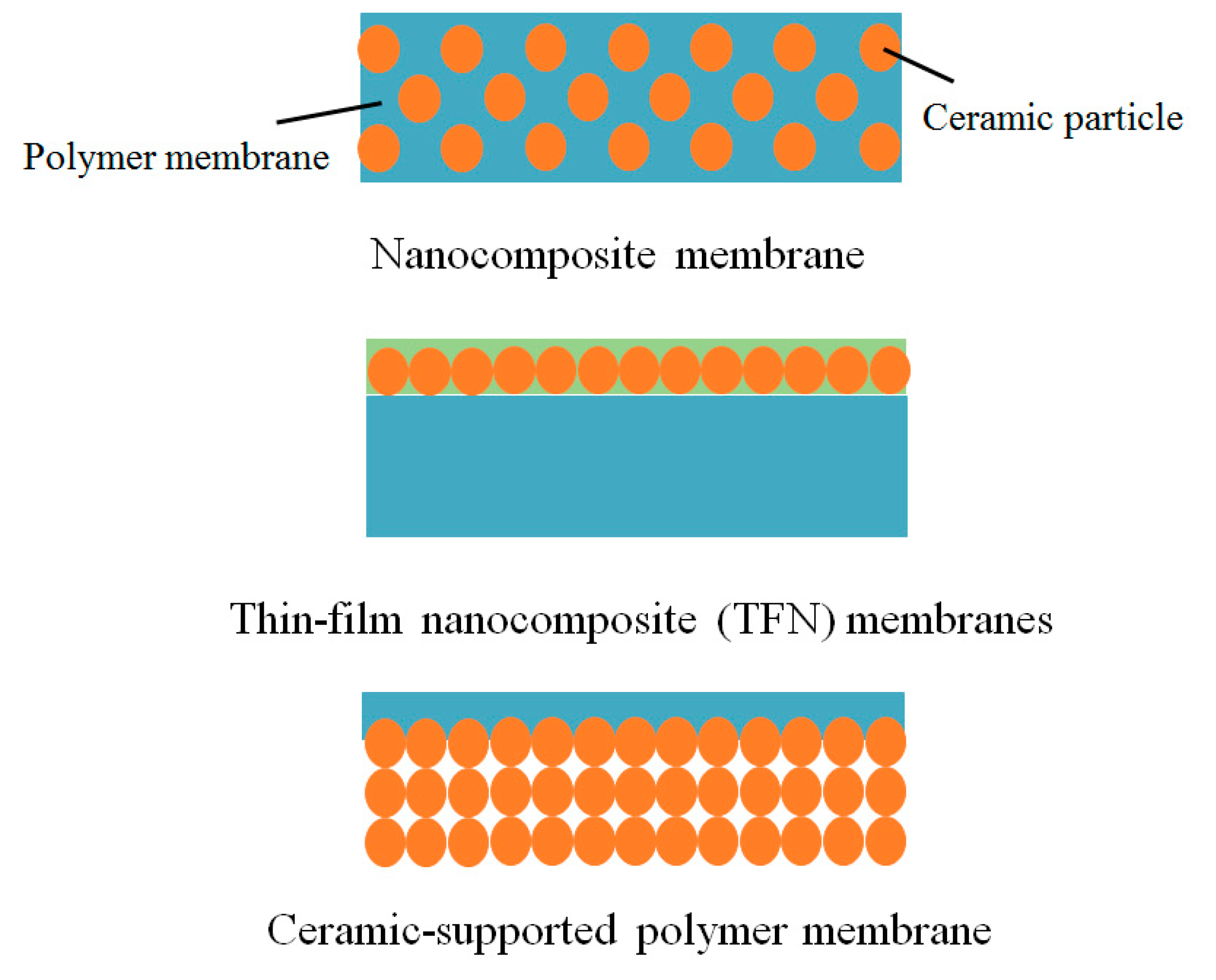
3.1. Ceramics in Polymer (Nanocomposite) Membranes
3.2. Thin Film Nanocomposite (TFN) Membranes
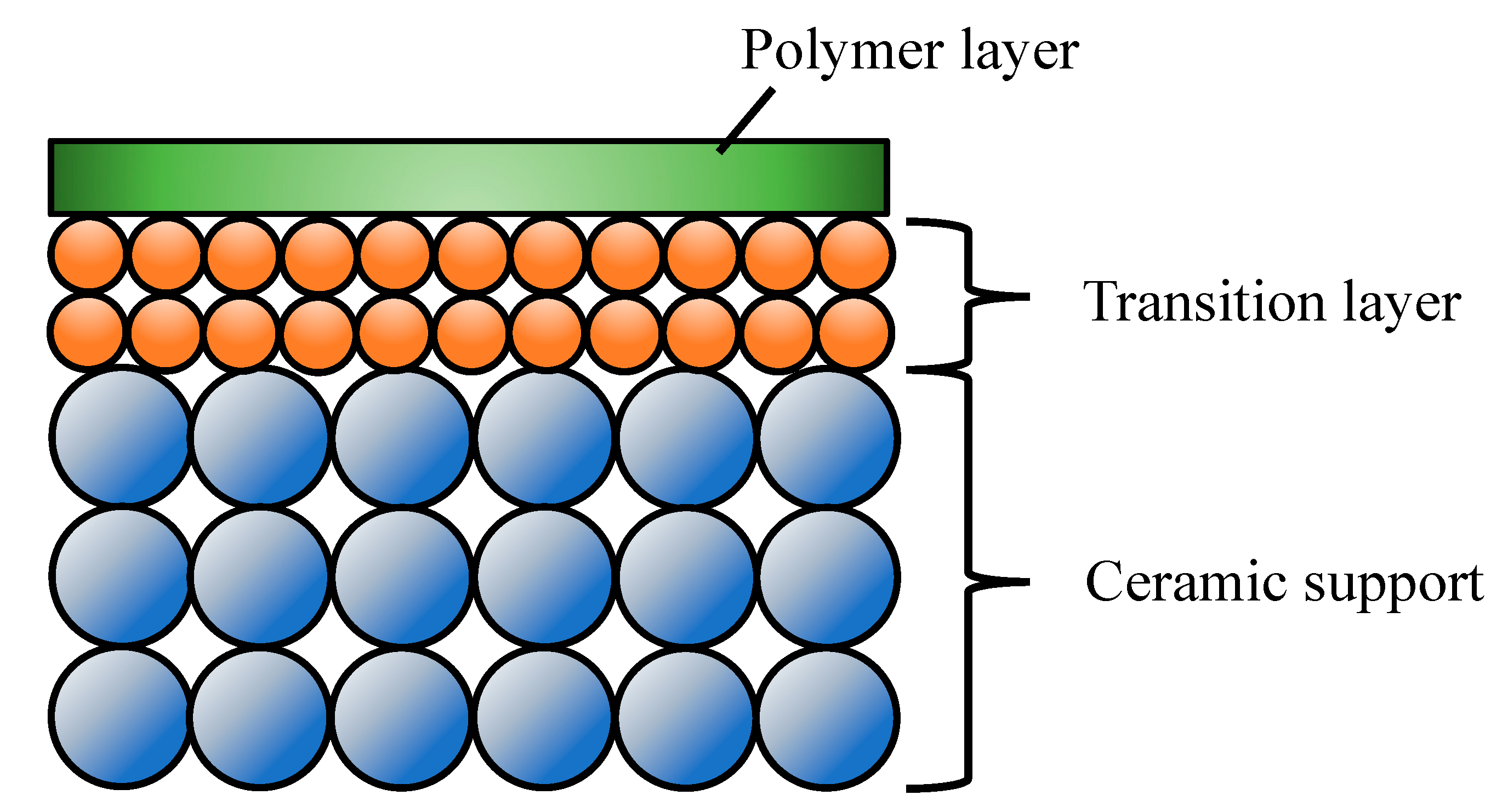
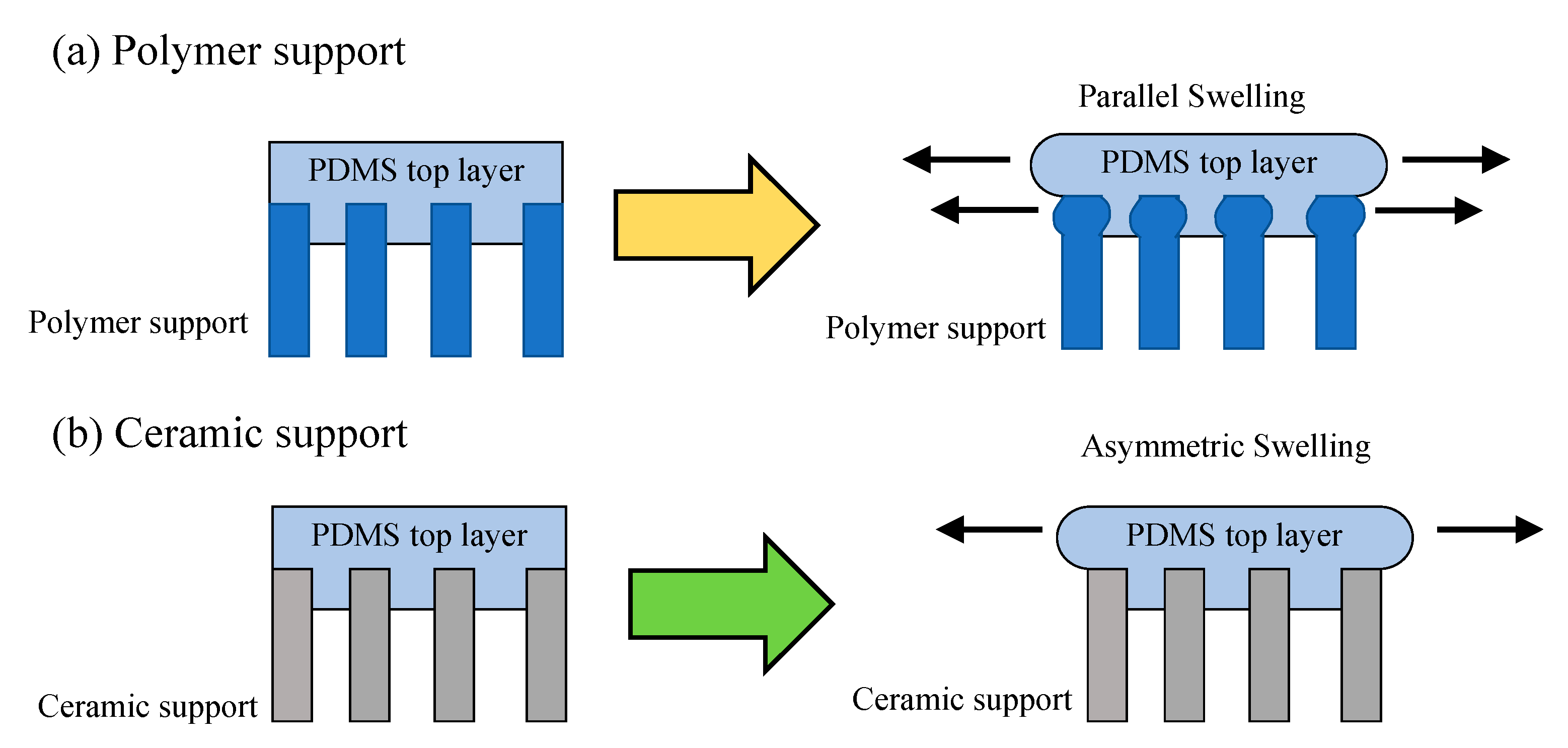
3.3. Ceramic-Supported Polymer Membranes
4. Strategies to Fabricate Ceramic-Polymer Composite Membranes
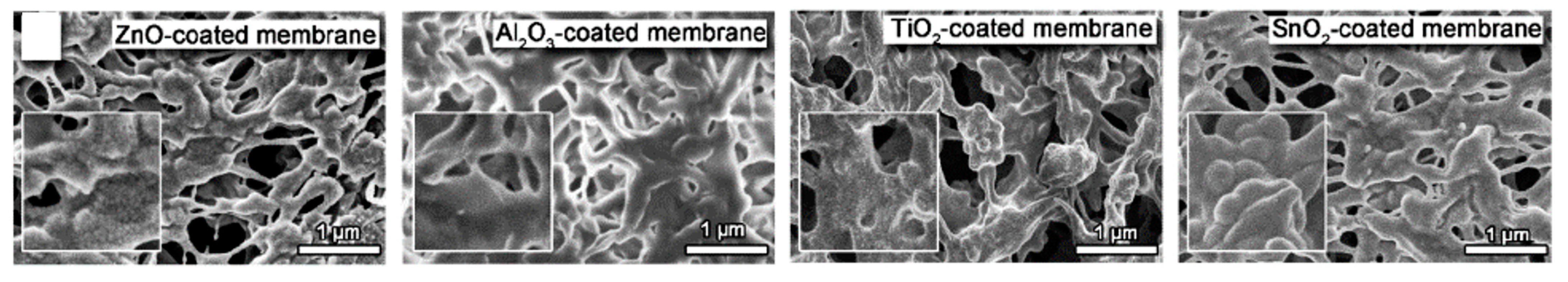
4.1. Modification of Ceramic Nanoparticles
4.1.1. Surface Modification
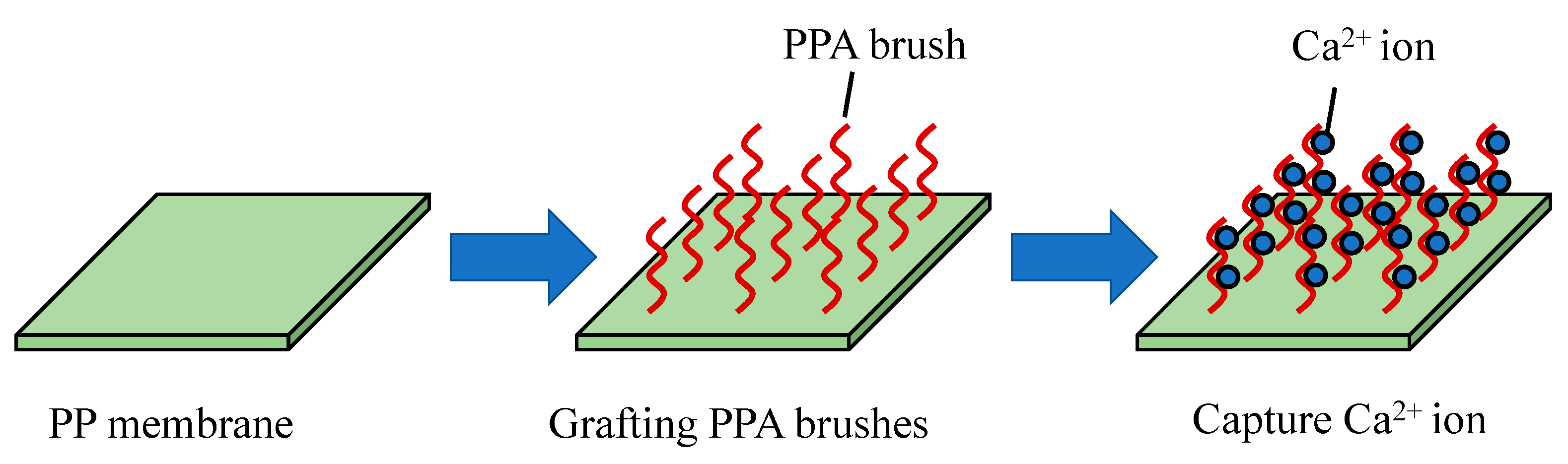
4.1.2. Surface Functionalization on Ceramic Nanoparticles
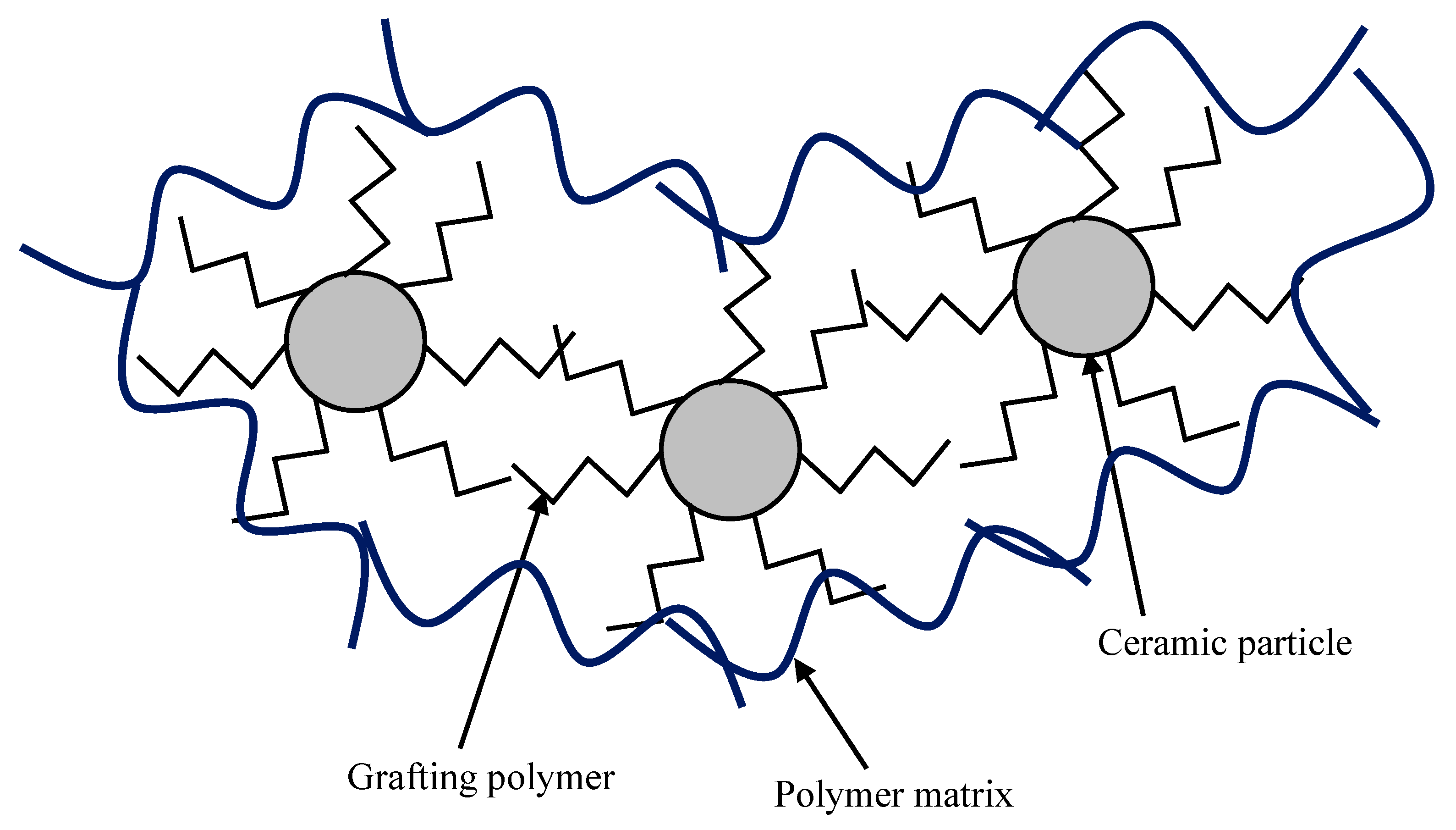
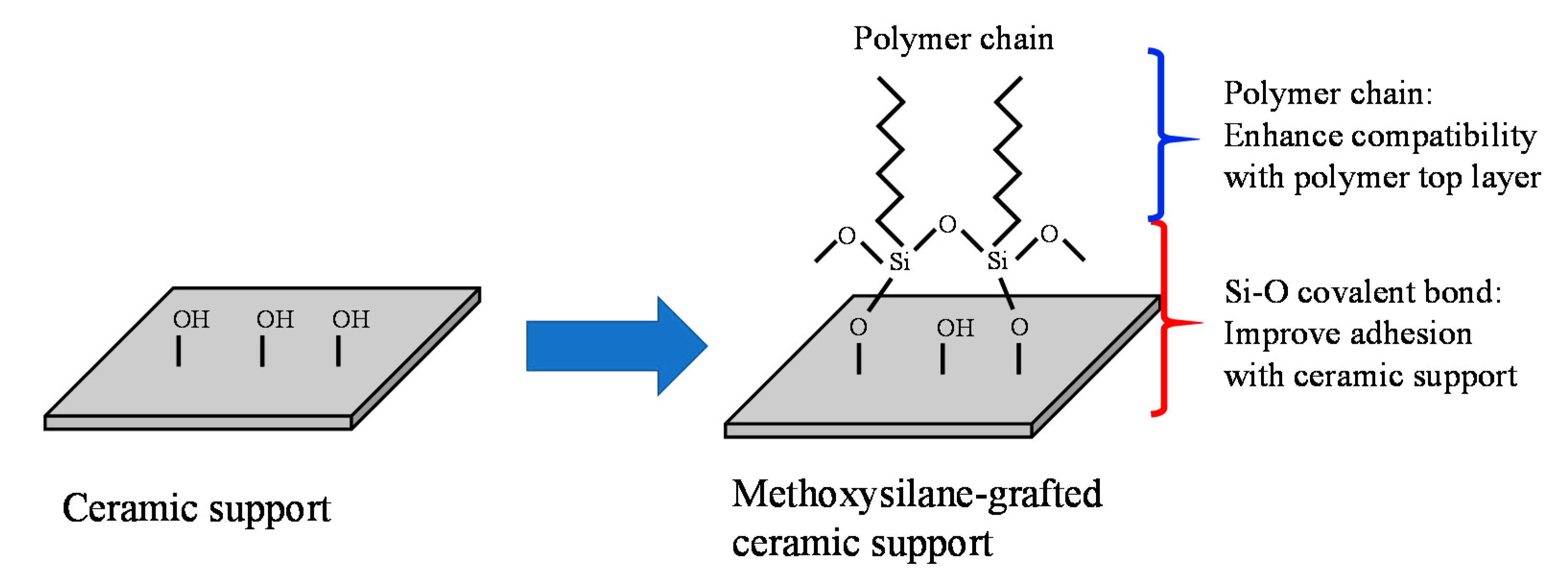
4.1.3. Organic Grafting
4.2. Modification of Polymers
4.2.1. Modification of Bulk Polymer Matrix
4.2.2. Surface Modification of Polymer Matrixes
4.3. Direct Deposition of Ceramic NPs in Polymer Matrixes
4.4. Ceramic-Supported Polymer Membranes
5. Perspective
- Fundamental study: Fundamental understandings for the influence of certain ceramic NPs on membrane structures and membrane performance is still unclear, in several cases. There have been several studies reporting influence of ceramic NPs on polymeric matrixes; however, the origin of the influence has not been researched in detail. As shown in Table 4, the influence of TiO2, Al2O3 and ZrO2 on the level of porosity and contact angle of polymeric matrix were different. However, it is still unclear how and what properties of the ceramic NPs influence the polymeric matrix. Detail studies on a relationship between surface properties of ceramic NPs, structures and performance of the membrane would be needed. This will help design new composite membranes.
- Structural study: Structural studies of the composite membranes have mostly been carried out by observation of morphology using SEM and sometimes by EDS, FT-IR and XPS. These rather simple structural analyses can lead to a wrong conclusion. For example, Fe-Boemite-PVB/PVDF membrane was prepared by (i) casting Boehmite-PVDF-PVB solution, (ii) immersing Boehmite-PVB-PVDF membrane into FeSO4 solution, and (iii) Reduction of Fe2+ on the Boehmite-PVB-PVDF membrane by KBH4 [234]. In the structural analysis of the membrane made by using SEM and FT-IR, the TFN-type membrane structure was envisioned. In the solution casting, the boemite particles could exist not only surface of the PVDF-PVB membrane, inside the PVDF-PVB membrane as well. Fe2+ ion would be captured by OH groups of boehmite inside the polymeric matrix. Thus, the membrane would be concluded as nanocomposite-type membrane, not TFN-type. The lack of structural analysis of membrane could cause misunderstanding of membrane structure, leading to the wrong conclusions.
- Long-term stability: there are several research works conducted in improving the compatibility between ceramic NPs and polymeric matrix, as described. However, knowledge of long-term compatibility is still not enough. In short term studies, there have been suggestions to suppress the aggregation of NPs in composite membranes and nanoparticle leakage. Studies on long-term compatibility between ceramic NPs and polymeric matrix, change of the membrane properties and environmental impact by the nanoparticle leakage would be required. Particularly, in addition to photocatalytic properties by TiO2, photocurrent would oxidize and damage/change the polymeric matrix. This should become more prominent after any long-term usage. However, studies on extended long-term stability of the photocatalytic membranes have not been performed. To understand the stability of the membranes and fate of nanoparticles, several characterizations, e.g., leaching tests, scanning electron microphotographs of the membrane surface and cross-section, roughness and FTIR with attenuated total reflectance (ATR) scans of used membranes must be carried out.
- Production cost: the expected application of the ceramic-polymer composite membranes for water treatment is still at a rather early stage. There are numerous laboratory-based works, but studies on large-scale production and industrial application have not been properly conducted [235]. More efforts have to be made to evaluate the long-term durability under the application conditions and cost-effectiveness including the supply of nanoparticles and methods for nanoparticle incorporation. Compared to current polymetric membranes, composite membranes require additional production processes, leading to higher production costs. More recently, novel fabrication techniques such as 3DP are emerging for membrane fabrication. Some of the new techniques have been applied for ceramic and polymeric membranes so far [236,237,238]; however, there were fewer for ceramic-polymer composite membranes. The 3DP would be able to reduce the production cost for the composite membranes, when properly developed. Give the Al2O3 membrane is several times more expensive than the PES polymeric membrane [164], development of cost-effective new ceramic NPs and the corresponding preparation process of composite membranes would be needed. Natural polymers, such as cellulose acetate and polysaccharide, normally possess polar groups in their structures, which can provide interacting sites for ceramic particles [239,240]. Additionally, the material cost of natural minerals such as kaolin, natural clay, etc. is much lower than Al2O3 [167]. Additionally, these natural minerals possess high hydrophilicity, which is expected to improve the hydrophilicity of composite membranes, if they are incorporated into the polymeric matrix. The usage of natural polymers and natural minerals would be a strategy to reduce the production cost of the composite membranes. Further study must be performed to reduce the production cost of the nanocomposite membranes.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef]
- Karakulski, K.; Gryta, M.; Morawski, A. Membrane processes used for potable water quality improvement. Desalination 2002, 145, 315–319. [Google Scholar] [CrossRef]
- Humes, H.D.; Fissell, W.H.; Tiranathanagul, K. The future of hemodialysis membranes. Kidney Int. 2006, 69, 1115–1119. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K. Recent developments on ion-exchange membranes and electro-membrane processes. Adv. Colloid Interface Sci. 2006, 119, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Darwano, H.; Vo Duy, S.; Sauve, S. A new protocol for the analysis of pharmaceuticals, pesticides, and hormones in sediments and suspended particulate matter from rivers and municipal wastewaters. Arch. Environ. Contam. Toxicol. 2014, 66, 582–593. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Tech. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Padaki, M.; Murali, R.S.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil-water separation: A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Geise, G.M.; Lee, H.-S.; Miller, D.J.; Freeman, B.D.; McGrath, J.E.; Paul, D.R. Water purification by membranes: The role of polymer science. J. Polym. Sci. B Polume Phys. 2010, 48, 1685–1718. [Google Scholar] [CrossRef]
- Gin, D.L.; Noble, R.D. Designing the next generation of chemical separation membranes. Science 2011, 332, 674–676. [Google Scholar] [CrossRef]
- Zhu, J.; Tian, M.; Hou, J.; Wang, J.; Lin, J.; Zhang, Y.; Liu, J.; Van der Bruggen, V. Surface zwitterionic functionalized graphene oxide for a novel loose nanofiltration membrane. J. Mater. Chem. A 2016, 4, 1980–1990. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Zoubi, H.; Darwish, N.A.; Mohamma, A.W.; Abu Arabi, M. A comprehensive review of nanofiltration membranes: Treatment, pretreatment, modelling, and atomic force microscopy. Desalination 2004, 170, 281–308. [Google Scholar] [CrossRef]
- Geise, G.M.; Paul, D.R.; Freeman, B.D. Fundamental water and salt transport properties of polymeric materials. Prog. Polym. Sci. 2014, 39, 1–42. [Google Scholar] [CrossRef]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination-Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Pant, D. The near-future integration of microbial desalination cells with reverse osmosis technology. Energy Environ. Sci. 2014, 7, 3921–3933. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids Surf. A 2019, 578, 123513. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Gitis, V.; Rothenberg, G. Ceramic Membranes: New Opportunities and Practical Applications; Willey-VCH Verlag GmbH&Co.: Weinheim, Germany, 2016. [Google Scholar]
- DeFriend, K.A.; Wiesner, M.R.; Barron, A.R. Alumina and aluminate ultrafiltration membranes derived from alumina nanoparticles. J. Membr. Sci. 2003, 224, 11–28. [Google Scholar] [CrossRef]
- Li, F.B.; Yang, Y.; Fan, Y.Q.; Xing, W.H.; Wang, Y. Modification of ceramic membranes for pore structure tailoring: The atomic layer deposition route. J. Membr. Sci. 2012, 397, 17–23. [Google Scholar] [CrossRef]
- Wen, Q.; Di, J.; Zhao, Y.; Wang, Y.; Jiang, L.; Yu, J. Flexible inorganic nanofibrous membranes with hierarchiral porosity for efficient water purification. Chem. Sci. 2013, 4, 4378–4382. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.Y.; Bai, H.W.; Sun, D.D. Concurrent filtration and solar photocatalytic disinfection/degradation using high performance Ag/TiO2 nanofiber membrane. Water Res. 2012, 46, 1101–1112. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Abdulkarim, A.A.; Ooi, B.S.; Ismail, S. Recent development in additives modifications of polyethersulfone membrane for flux enhancement. Chem. Eng. J. 2013, 223, 246–267. [Google Scholar] [CrossRef]
- Saljoughi, E.; Mousavi, S.M. Preparation and characterization of novel polysulfone nanofiltration membranes for removal of cadmium from contaminated water. Sep. Purif. Tech. 2012, 90, 22–30. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F. Effect of additives concentration on the surface properties and performance of PVDF ultrafiltration membranes for refinery produced wastewater treatment. Desalination 2011, 273, 226–234. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, X.; Li, F.; Zhao, X. Poly(vinyl pyrrolidone) modified poly(vinylidene fluoride) ultrafiltration membrane via a two-step surface grafting for radioactive wastewater treatment. Sep. Purif. Techol. 2018, 194, 404–409. [Google Scholar] [CrossRef]
- Lohokare, H.R.; Muthu, M.R.; Agarwal, G.P.; Kharul, U.K. Effective arsenic removal using polyacrylonitrile-based ultrafiltration (UF) membrane. J. Membr. Sci. 2008, 320, 159–166. [Google Scholar] [CrossRef]
- Kim, I.C.; Yun, H.G.; Lee, K.H. Preparation of asymmetric polyacrylonitrile membrane with small pore size by phase inversion and post-treatment process. J. Membr. Sci. 2002, 199, 75–84. [Google Scholar] [CrossRef]
- Unlu, D. Recovery of cutting oil from wastewater by pervaporation process using natural clay modified PVA membrane. Water Sci. Tech. 2019, 80, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Moradi, E.; Ebahimzdeh, H.; Mehrani, Z.; Asgharinezhad, A.A. The efficient removal of methylene blue from water samples using three-dimensional poly(vinyl alcohol)/starch nanofiber membrane as a green nanosorbent. Environ. Sci. Poll. Res. 2019, 26, 35071–35081. [Google Scholar] [CrossRef]
- Esmaeili, N.; Boyd, S.E.; Brown, C.L.; Gray, E.M.A.; Webb, C.J. Improving the gas-separation properties of PVAc-Zeolite 4A mixed-matrix membranes through nano-sizing silanation of the Zeolite. Chem. Phys. Chem. 2019, 20, 1590–1606. [Google Scholar]
- Goossens, I.; Van Haute, A. The influence of mineral fillers on the membrane properties of high flux asymmetric cellulose acetate reverse osmosis membranes. Desalination 1976, 18, 203–214. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed matrix membranes using carbon molecular sieves: I. Preparation and experimental results. J. Membr. Sci. 2003, 211, 311–334. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Matsuura, T.; Montazer-Rahmati, M.M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Tech. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Nambikkattu, J.; Kaleekkal, N.J.; Jacob, J.P. Metal ferrite incorporated polysulfone thin-film nanocomposite membranes for wastewater treatment. Environ. Sci. Poll. Res. 2021, 28, 11915–11927. [Google Scholar] [CrossRef]
- Kugarajah, V.; Dharmalingam, S. Effect of silver incorporated sulphonated poly ether ether ketone membranes on microbial fuel cell performance and microbial community analysis. Chem. Eng. J. 2021, 415, 128961. [Google Scholar] [CrossRef]
- Mehta, R.; Brahmbhatt, H.; Bhojani, G.; Mukherjee, M.; Bhattacharya, A. Poly(piperizinamide) with copper ion composite membranes: Application for mitigation of hexaconazole from water and combat microbial contamination. J. Hazard. Mater. 2019, 376, 102–111. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.; Li, J.; Cao, Y.; Feng, L. Hierarchical architectures of Ag cluster deposited biomimetic membrane: Synthesis, emulsion separation, catalytic and antibacterial performance. Sep. Purif. Tech. 2020, 241, 116733. [Google Scholar] [CrossRef]
- Liu, G.; Wei, W.; Jin, W.; Xu, N. Polymer/ceramic composite membranes and their application in pervaporation process. Chin. J. Chem. Eng. 2012, 20, 62–70. [Google Scholar] [CrossRef]
- Shen, L.; Huang, Z.; Liu, Y.; Li, R.; Xu, Y.; Jakaj, G.; Lin, H. Polymeric membranes incorporated with ZnO nanoparticles for membrane fouling mitigation: A brief review. Front. Chem. 2020, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Errahmani, K.B.; Benhabiles, O.; Bellebia, S.; Bengharez, Z.; Goosen, M.; Mahmoudi, H. Photocatalytic nanocomposite polymer-TiO2 membranes for pollutant removal from wastewater. Catalysts 2021, 11, 402. [Google Scholar] [CrossRef]
- Soria, R.B.; Zhu, J.; Gonza, I.; Bruggen, B.V.; Luis, P. Effect of (TiO2:ZnO) ratio on the anti-fouling properties of bio-inspired nanofiltration membranes. Sep. Purif. Tech. 2020, 251, 117280. [Google Scholar] [CrossRef]
- Darabi, R.R.; Jahanshahi, M.; Peyravi, M. A support assisted by photocatalytic Fe3O4/ZnO nanocomposite for thin-film forward osmosis membrane. Chem. Eng. Res. Des. 2018, 133, 11–25. [Google Scholar] [CrossRef]
- Ghanbari, M.; Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Davoody, M.; Ismail, A.F. Super hydrophilic TiO2/HNT nanocomposites as a new approach for fabrication of high performance thin film nanocomposite membranes for FO application. Desalination 2015, 371, 104–114. [Google Scholar] [CrossRef]
- Ma, N.; Wei, J.; Liao, R.; Tang, C.Y. Zeolite-polyamide thin film nanocomposite membranes: Towards enhanced performance for forward osmosis. J. Membr. Sci. 2012, 405–406, 149–157. [Google Scholar]
- Hoek, E.M.V.; Ghosh, A.K.; Huang, X.; Liong, M.; Zink, J.I. Physical-chemical properties, separation performance, and fouling resistance of mixed-matrix ultrafiltration membranes. Desalination 2011, 283, 89–99. [Google Scholar] [CrossRef]
- Samei, M.; Iravaninia, M.; Mohammadi, T.; Asadi, A.A. Solution diffusion modeling of a composite PVA/fumed silica ceramic supported membrane. Chem. Eng. Process. 2016, 109, 11–19. [Google Scholar] [CrossRef]
- Perera, M.G.N.; Galagedara, Y.R.; Ren, Y.; Jayaweera, M.; Zhao, Y.; Weerasooriya, R. Fabrication of fullerenol-incorporated thin-film nanocomposite forward osmosis membranes for improved desalination performances. J. Polym. Res. 2018, 25, 199. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, W.; Yu, Y.; Deng, B.; Li, J.; Jin, J. Sol-gel preparation of PAA-g-PVDF/TiO2 nanocomposite hollow fiber membranes with extremely high water flux and improved antifouling property. J. Membr. Sci. 2013, 432, 25–32. [Google Scholar] [CrossRef]
- Salazar, H.; Martins, P.M.; Santos, B.; Fernandes, M.M.; Reizabal, A.; Sebastian, V.; Batelho, G.; Tavares, C.J.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Photocatalytic and antimicrobial multifunctional nanocomposite membranes for emerging pollutants water treatment applications. Chemosphere 2020, 250, 126299. [Google Scholar] [CrossRef] [PubMed]
- Ahsani, M.; Hazrati, H.; Javadi, M.; Ulbricht, M.; Yegani, R. Preparation of antibiofouling nanocomposite PVDF/Ag-SiO2 membrane and long-term performance evaluation in the MBR system fed by real pharmaceutical wastewater. Sep. Purif. Tech. 2020, 249, 116938. [Google Scholar] [CrossRef]
- Liu, G.; Xiangli, F.; Wei, W.; Liu, S.; Jin, W. Improved performance of PDMS/ceramic composite pervaporation membranes by ZSM-5 homogeneously dispersed in PDMS via a surface graft/coating approach. Chem. Eng. J. 2011, 174, 495–503. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, Y.; Li, Y.; Zhang, P.; Cao, Z.; Yang, H.; Liu, C.; Tao, G.; Gong, F.; Matsuyama, H. Synthesis of hydrophilic carbon nanotubes by grafting poly(methyl methacrylate) via click reaction and its effect on poly(vinylidene fluoride)-carbon nanotube composite. Appl. Surf. Sci. 2018, 435, 79–90. [Google Scholar] [CrossRef]
- Chung, Y.T.; Mahmoudi, E.; Mohammad, A.W.; Benamor, A.; Johnson, D.; Hilal, N. Development of polysulfone-nanohybrid membranes using ZnO-GO composite for enhanced antifouling and antibacterial control. Desalination 2017, 402, 123–132. [Google Scholar] [CrossRef]
- Kim, H.J.; Pant, H.R.; Kin, J.H.; Choi, N.J.; Kim, C.S. Fabrication of multifunctional TiO2-fly ash/polyurethane nanocomposite membrane via electrospinning. Ceram. Int. 2014, 40, 3023–3029. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Zhang, X.; Li, J.; Wang, J.; Li, N. Polyvinylamine/amorphous metakaolin mixed-matrix composite membranes with facilitated transport carriers for highly efficient CO2/N2 separation. J. Membr. Sci. 2020, 599, 117828. [Google Scholar] [CrossRef]
- Daraei, P.; Ghaemi, N. Synergistic effect of Cloisite 15A and 30B nanofillers on the characteristics of nanocomposite polyethersulfone membrane. Appl. Clay Sci. 2019, 172, 96–105. [Google Scholar] [CrossRef]
- Shokri, E.; Shahed, E.; Hermani, M.; Etemadi, H. Towards enhanced fouling resistance of PVC ultrafiltration membrane using modified montmorillonite with folic acid. Appl. Clay Sci. 2021, 200, 105906. [Google Scholar] [CrossRef]
- Paz, Y. Application of TiO2 photocatalysis for air treatment: Patents’ overview. Appl. Catal. B 2010, 99, 448–460. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Taheri, A.H.; Mansourpanah, Y. Coupling TiO2 nanoparticles with UV irradiation for modification of polyethersulfone ultrafiltration membranes. J. Membr. Sci. 2008, 313, 158–169. [Google Scholar] [CrossRef]
- Damodar, R.A.; You, S.-J.; Chou, H.-H. Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes. J. Hazard. Mater. 2009, 172, 1321–1328. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Shen, H.-M.; Xu, Z.-L. PVDF-TiO2 composite hollow fiber ultrafiltration membranes prepared by TiO2 sol-gel method and blending method. J. Appl. Polym. Sci. 2009, 113, 1763–1772. [Google Scholar] [CrossRef]
- Zhou, A.; Jia, R.; Wang, Y.; Sun, S.; Xin, X.; Wang, M.; Zhao, Q.; Zhu, H. Abatement of sulfadiazine in water under a modified ultrafiltration membrane (PVDF-PVP-TiO2-dopamine) filtration-photocatalysis system. Sep. Purif. Tech. 2020, 234, 116099. [Google Scholar] [CrossRef]
- Razmjou, A.; Mansouri, J.; Chen, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Zinadini, S.; Vatanpour, V. A new approach to improve antifouling property of PVDF membrane using in situ polymerization of PAA functionalized TiO2 nanoparticles. J. Membr. Sci. 2011, 380, 155–162. [Google Scholar] [CrossRef]
- Sotto, A.; Boromand, A.; Balta, S.; Kim, J.; Van der Bruggen, B. Doping of polyethersulfone nanofiltration membranes: Antifouling effect observed at ultralow concentrations of TiO2 nanoparticles. J. Mater. Chem. 2011, 21, 10311–10320. [Google Scholar] [CrossRef]
- Hamid, N.A.A.; Ismail, A.F.; Matsuura, T.; Zularisam, A.W.; Lau, W.J.; Yuliwati, E.; Abdullah, M.S. Morphological and separation performance study of polysulfone/titanium dioxide (PSF/TiO2) ultrafiltration membranes for humic acid removal. Desalination 2011, 273, 85–92. [Google Scholar] [CrossRef]
- Abedini, R.; Mousavi, S.M.; Aminzadeh, R. A novel cellulose acetate (CA) membrane using TiO2 nanoparticles: Preparation, characterization and permeation study. Desalination 2011, 277, 40–45. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Rajaeian, B.; Rahimnejad, M. TiO2 entrapped nano-composite PVDF/SPES membranes: Preparation, characterization, antifouling and antibacterial properties. Desalination 2011, 278, 343–353. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, N.; Lee, Y.T. Preparation and characterization of PVDF/TiO2 organic-inorganic composite membranes for fouling resistance improvement. J. Membr. Sci. 2009, 345, 13–20. [Google Scholar] [CrossRef]
- Ngang, H.P.; Ooi, D.S.; Ahmad, A.L.; Lai, S.O. Preparation of PVDF-TiO2 mixed-matrix membrane and its evaluation on dye adsorption and UV-cleaning properties. Chem. Eng. J. 2012, 197, 359–367. [Google Scholar] [CrossRef]
- Sun, F.; Ren, H.-T.; Li, T.-T.; Huang, S.-Y.; Zhang, Y.; Lou, C.-W.; Lin, J.-H. Bioinspired design of underwater superoleophobic Poly(N-isopropylacrylamide)/polyeacrylonitrile/TiO2 nanofibrous membranes for highly efficient oil/water separation and photocatalysis. Environ. Res. 2020, 186, 109494. [Google Scholar] [CrossRef]
- Yaacob, N.; Goh, P.S.; Ismail, A.F.; Nazri, N.A.M.; Ng, B.C.; Abidin, M.N.Z.; Yogarathinam, L.T. ZrO2-TiO2 incorporated PVDF dual-layer hollow fiber membrane for oily wastewater treatment: Effect of air gap. Membrane 2020, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Gharebakhsh, H.; Panahi, H.A.; Toosi, M.R.; Hassani, A.H.; Moniri, E. Application of polyamide thin-film composite layered on polysulfone-GO/TiO2 mixed membranes for removal of nitrotoluene derivatives from petrochemical wastewaters. Environ. Sci. Poll. Res. 2020, 27, 42481–42494. [Google Scholar] [CrossRef]
- Sun, M.; Su, Y.; Mu, C.; Jiang, Z. Improved antifouling property of PES ultrafiltration membranes using additive of silica-PVP nanocomposite. Ind. Eng. Chem. Res. 2010, 49, 790–796. [Google Scholar] [CrossRef]
- Jomekian, A.; Mansoori, S.A.A.; Monirimanesh, N. Synthesis and characterization of novel PEO-MCM-41/PVDC nanocomposite membrane. Desalination 2011, 276, 239–245. [Google Scholar] [CrossRef]
- Shen, J.N.; Ruan, H.M.; Wu, L.G.; Gao, C.J. Preparation and characterization of PES-SiO2 organic-inorganic composite ultrafiltration membrane for raw water pretreatment. Chem. Eng. J. 2011, 168, 1272–1278. [Google Scholar] [CrossRef]
- Muriithi, B.; Loy, D.A. Processing, morphology, and water uptake of nafion/Ex-situ stöber silica nanocomposite membranes as a function of particle size. ACS Appl. Mater. Interfaces 2012, 4, 6766–6773. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, K.; Wang, K.; Xie, Z.; Ladewig, B.; Wang, H. Fabrication of polyethersulfone-mesoporous silica nanocomposite ultrafiltration membranes with antifouling properties. J. Membr. Sci. 2012, 423–424, 362–370. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Development of novel SiO2-GO nanohybrid/poly-sulfone membrane with enhanced performance. J. Membr. Sci. 2014, 451, 94–102. [Google Scholar] [CrossRef]
- Yan, L.; Hong, S.; Li, M.L.; Li, Y.S. Application of the Al2O3-PVDF nanocomposite tubular ultrafiltration (UF) membrane for oily wastewater treatment and its antifouling research. Sep. Purif. Tech. 2009, 66, 347–352. [Google Scholar] [CrossRef]
- Maximous, N.; Nakhla, G.; Wan, W.; Wong, K. Preparation, characterization and performance of Al2O3/PES membrane for wastewater filtration. J. Membr. Sci. 2009, 341, 67–75. [Google Scholar] [CrossRef]
- Maximous, N.; Nakhla, G.; Wong, K.; Wan, W. Optimization of Al2O3/PES membranes for wastewater filtration. Sep. Purif. Tech. 2010, 73, 294–301. [Google Scholar] [CrossRef]
- Maximous, N.; Nakhla, G.; Wan, W.; Wong, K. Effect of the metal oxide particle distributions on modified PES membranes characteristics and performance. J. Membr. Sci. 2010, 361, 213–222. [Google Scholar] [CrossRef]
- Csetneki, I.; Filipcsei, G.; Zrínyi, M. Smart nanocomposite polymermembranes with on/off switching control. Macromolecules 2006, 39, 1939–1942. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Salehi, E.; Khadivi, M.A.; Moradian, R.; Astinchap, B. Novel polyethersulfone nanocomposite membrane prepared by PANI/Fe3O4 nanoparticles with enhanced performance for Cu(II) removal from water. J. Membr. Sci. 2012, 415–416, 250–259. [Google Scholar] [CrossRef]
- Gholami, A.; Moghadassi, A.R.; Hosseini, S.M.; Shabani, S.; Gholami, F. Preparation and characterization of polyvinylchloride based nanocomposite nanofiltration-membrane modified by iron oxide nanoparticles for lead removal from water. J. Ind. Eng. Chem. 2014, 20, 1517–1522. [Google Scholar] [CrossRef]
- Alam, J.; Dass, L.A.; Ghasemi, M.; Alhoshan, M. Synthesis and optimization of PES-Fe3O4 mixed matrix nanocomposite membrane: Application studies in water purification. Polym. Compos. 2013, 34, 1870–1877. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Khadivi, M.A.; Astinchap, B.; Moradian, R. Fouling resistant mixed matrix polyethersulfone membranes blended with magnetic nanoparticles: Study of magnetic field induced casting. Sep. Purif. Tech. 2013, 109, 111–121. [Google Scholar] [CrossRef]
- María Arsuaga, J.; Sotto, A.; del Rosario, G.; Martínez, A.; Molina, S.; Teli, S.B.; de Abajo, J. Influence of the type, size, and distribution of metal oxide particles on the properties of nanocomposite ultrafiltration membranes. J. Membr. Sci. 2013, 428, 131–141. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, P.; Wang, C.; Sun, X.; Zhang, L. Thermostable PPESK/TiO2 nanocomposite ultrafiltration membrane for high temperature condensed water treatment. Desalination 2012, 299, 35–43. [Google Scholar] [CrossRef]
- Teli, S.B.; Molina, S.; Sotto, A.; Calvo, E.G.; De Abajo, J. Fouling resistant polysulfone-PANI/TiO2 ultrafiltration nanocomposite membranes. Ind. Eng. Chem. Res. 2013, 52, 9470–9479. [Google Scholar] [CrossRef]
- Abedini, R.; Mousavi, S.M.; Aminzadeh, R. Effect of sonochemical synthesized TiO2 nanoparticles and coagulation bath temperature on morphology, thermal stability and pure water flux of asymmetric cellulose acetate nanocomposite membranes prepared via phase inversion method. Chem. Ind. Chem. Eng. Q. 2012, 18, 385–398. [Google Scholar] [CrossRef]
- Rajaeian, B.; Rahimpour, A.; Tade, M.O.; Liu, S. Fabrication and characterization of polyamide thin film nanocomposite (TFN) nanofiltration membrane impregnated with TiO2 nanoparticles. Desalination 2013, 313, 176–188. [Google Scholar] [CrossRef]
- Ghanbari, M.; Emadzadeh, D.; Lau, W.J.; Lai, S.O.; Matsuura, T.; Ismail, A.F. Synthesis and characterization of novel thin film nanocomposite TFN) membranes embedded with halloysite nanotubes (HNTs) for water deslination. Desalination 2015, 358, 33–41. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Ghanbari, M.; Lau, W.J.; Rahbari-Sisakht, M.; Matsuura, T.; Ismail, A.F.; Kruczek, B. Solvothermal synthesis of nanoporous TiO2: The impact on thin-film composite membranes for engineered osmosis application. Nanotechnology 2016, 27, 345702. [Google Scholar] [CrossRef]
- Niksefat, N.; Jahanshahi, M.; Rahimpour, A. The effect of SiO2 nanoparticles on morphology and performance of thin film composite membranes for forward osmosis application. Desalination 2014, 343, 140–146. [Google Scholar] [CrossRef]
- Ding, W.; Li, Y.; Bao, M.; Zhang, J.; Zhang, C.; Lu, J. Highly permeable and stable forward osmosis (FO) membrane based on the incorporation of Al2O3 nanoparticles into both substrate and polyamide active layer. RSC Adv. 2017, 7, 40331. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Optimizing polyamide thin film composite membrane covalently bonded with modified mesoporous silica nanoparticles. J. Membr. Sci. 2013, 428, 341–348. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, W.; Cui, J.; Wu, S.; Han, J.; Zou, Y.; Huang, C. Hydrothermal synthesized UV-resistance and transparent coating composited superoleophilic electrospun membrane for high efficiency oily wastewater treatment. J. Hazard. Mater. 2020, 383, 121152. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Salehi, E.; Ghaemi, N.; Ghari, H.S.; Khadivi, M.A.; Rostami, E. Novel thin film composite membrane fabricated by mixed matrix nanoclay/chitosan on PVDF microfiltration support: Preparation, characterization and performance in dye removal. J. Membr. Sci. 2013, 436, 97–108. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, S.; Meng, L.; Chen, G.; Luo, X.; Huang, M. Comparison of novel functionalized nanofiber forward osmosis membranes for application in antibacterial activity and TRGs rejection. J. Hazard. Mater. 2020, 392, 12250. [Google Scholar] [CrossRef]
- Li, J.; Yuan, S.; Zhu, J.; Bruggen, B.V. High-flux antibacterial composite membranes via polydopamine-assisted PEI-TiO2/Ag modification for dye removal. Chem. Eng. J. 2019, 373, 275–284. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, Z.; Li, F.; Chen, Q.; Yin, D.; Min, X. A novel reduced graphene oxide-based composite membrane prepared via a facile deposition method for multifunctional applications: Oil/water separation and cationic dyes removal. Sep. Purif. Tech. 2018, 200, 130–140. [Google Scholar] [CrossRef]
- Yu, Z.; Min, X.; Li, F.; Yin, D.; Peng, Y.; Zeng, G. A mussel-inspired method to fabricate a novel reduced graphene oxide/Bi12O17Cl2 composites membrane for catalytic degradation and oil/water separation. Polym. Adv. Tech. 2019, 30, 101–109. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, J.-L.; Zeng, G.-M.; Song, B.; Cao, W.; Liu, H.-Y.; Huan, S.-Y.; Peng, P. Novel “loose” GO/MoS2 composites membranes with enhanced permeability for effective salts and dyes rejection at low pressure. J. Membr. Sci. 2019, 574, 112–123. [Google Scholar] [CrossRef]
- Zhao, G.; Zou, J.; Chen, X.; Zhang, T.; Yu, J.; Zhou, S.; Li, C.; Jiao, F. Integration of microfiltration and visible-light-driven photocatalysis on a ZnWO4 nanoparticle/nickel-aluminum-layered double hydroxide membrane for enhanced water purification. Ind. Eng. Chem. Res. 2020, 59, 6479–6487. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, H.; Ying, G.; Zhang, J.; Wu, Y.; Wu, S.; Yang, Y. Flexible, fouling-resistant and self-cleaning Ti3C2Tx-derivated hydrophilic nanofiltration membrane for highly efficient rejection of organic molecules from wastewater. J. Mater. Res. Tech. 2020, 9, 11675–11686. [Google Scholar] [CrossRef]
- Amoli-Diva, M.; Irani, E.; Pourghazi, K. Photocatalytic filtration reactors equipped with bi-plasmonic nanocomposite/poly acrylic acid-modified polyamide membranes for industrial wastewater treatment. Sep. Purif. Tech. 2020, 236, 116257. [Google Scholar] [CrossRef]
- Guan, J.; Fan, L.; Liu, Y.-N.; Shi, B.; Yuan, J.; Zhang, R.; You, X.; He, M.; Su, Y.; Jiang, Z. Incorporating arginine-FeIII complex into polyamide membranes for enhanced water performance and antifouling performance. J. Membr. Sci. 2020, 602, 117980. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Tang, C.Y.; Wang, Z.; Gao, C. Fabrication of carbon nanotubes incorporated double-skinned thin film nanocomposite membranes for enhanced separation performance and antifouling capability in forward osmosis process. Desalination 2015, 369, 1–9. [Google Scholar] [CrossRef]
- Amini, M.; Jahanshahi, M.; Rahimpour, A. Synthesis of novel thin film nanocomposite (TFN) forward osmosis membranes using functionalized multi-walled carbon nanotubes. J. Membr. Sci. 2013, 435, 233–241. [Google Scholar] [CrossRef]
- Zhao, H.; Qiu, S.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Improving the performance of polyamide reverse osmosis membrane by incorporation of modified multi-walled carbon nanotubes. J. Membr. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Shen, L.; Xiong, S.; Wang, Y. Graphene oxide incorporated thin-film composite membranes for forward osmosis applications. Chem. Eng. Sci. 2016, 143, 194–205. [Google Scholar] [CrossRef]
- Wu, X.; Field, R.W.; Wu, J.J.; Zhang, K. Polyvinylpyrrolidone modified graphene oxide as a modifier for thin film composite forward osmosis membranes. J. Membr. Sci. 2017, 540, 251–260. [Google Scholar] [CrossRef]
- Rastgar, M.; Shakeri, A.; Bozong, A.; Salehi, H.; Saadattalab, V. Highly-efficient forward osmosis membrane tailored by magnetically responsive graphene oxide/Fe3O4 nanohybrid. Appl. Surf. Sci. 2018, 441, 923–935. [Google Scholar] [CrossRef]
- Eslah, S.S.; Shokrollahzadeh, S.; Jazani, O.M.; Samimi, A. Fprward osmosis water desalination: Fabrication of graphene oxide-polyamide/polysulfone thin-film nanocomposite membrane with high water flux and low reverse salt diffusion. Sep. Sci. Tech. 2018, 53, 573–583. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, G.; Deng, B. Graphene oxide (GO) enhanced polyamide (PA) thin-film nanocomposite (TFN) membrane for water purification. Desalination 2016, 379, 93–101. [Google Scholar] [CrossRef]
- Majooni, Y.; Mortaheb, H.R.; Dizaji, A.K. Enhancement in pervaporative performance of PDMS membrane for separation of styrene from wastewater by hybridizing with reduced graphene oxide. J. Environ. Manag. 2020, 261, 110189. [Google Scholar] [CrossRef] [PubMed]
- Seyedpour, S.F.; Rahimpour, A.; Shamsabadi, A.A.; Soroush, M. Improved performance and antifouling properties of thin-film composite polyamide membranes modified with nano-sized bactericidal graphene quantum dots for forward osmosis. Chem. Eng. Res. Des. 2018, 139, 321–334. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Ng, B.C. Carbon nanotubes for desalination: Performance evaluation and current hurdles. Desalination 2013, 308, 2–14. [Google Scholar] [CrossRef]
- Goh, K.; Karahan, H.E.; Wei, L.; Bae, T.-H.; Fane, A.G.; Wang, R.; Chen, Y. Carbon nanomaterials for advancing separation membranes: A strategic perspective. Carbon 2016, 109, 694–710. [Google Scholar] [CrossRef]
- Mattia, D.; Lee, K.P.; Calabro, F. Water permeation in carbon nanotube membranes. Curr. Opin. Chem. Eng. 2014, 4, 32–37. [Google Scholar] [CrossRef]
- Das, R.; Hamid, S.B.A.; Ali, M.E.; Ismail, A.F.; Annuar, M.S.M.; Ramakrishna, S. Multifunctional carbon nanotubes in water treatment: The present, past and future. Desalination 2014, 354, 160–179. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Cony, B. Designing carbon nanotube membranes for efficient water desalination. J. Phys. Chem. B 2008, 112, 1427–1434. [Google Scholar]
- Chen, C.; Yang, Q.-H.; Yang, Y.; Lv, W.; Wen, Y.; Hou, P.-X.; Wang, M.; Cheng, H.-M. Self-assembled free-standing graphite oxide membrane. Adv. Mater. 2009, 21, 3007–3011. [Google Scholar] [CrossRef]
- Backes, C.; Hauke, F.; Hirsch, A. The potential of perylene bisimide derivatives for the solubilization of carbon nanotubes and graphene. Adv. Mater. 2011, 23, 2588–2601. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Bao, Y.; Chai, J.; Zhang, Q.; Han, D.; Niu, L. Synthesis and application of widely soluble graphene sheets. Langmuir 2010, 26, 12314–12320. [Google Scholar] [CrossRef]
- Hung, W.-S.; An, Q.-F.; De Guzman, M.; Lin, H.-Y.; Huang, S.-H.; Liu, W.-R.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Pressure-assisted self-assembly technique for fabricating composite membranes consisting of highly ordered selective laminate layers of amphiphilic graphene oxide. Carbon 2014, 68, 670–677. [Google Scholar] [CrossRef]
- Hegab, H.M.; Zou, L. Graphene oxide-assisted membranes: Fabrication and potential applications in desalination and water purification. J. Membr. Sci. 2015, 484, 95–106. [Google Scholar] [CrossRef]
- Faria, A.F.; Liu, C.; Xie, M.; Perreault, F.; Nghiem, L.D.; Ma, J.; Elimelech, M. Thin-film composite forward osmosis membranes functionalized with graphene oxide-silver nanocomposites for biofouling control. J. Membr. Sci. 2017, 525, 146–156. [Google Scholar] [CrossRef]
- Inurria, A.; Cay-Durgun, P.; Rice, D.; Zhang, H.; Seo, D.-K.; Ling, M.L.; Perreault, F. Polyimide thin-film nanocomposite membrane with graphene oxide nanosheets: Balancing membrane performance and fouling propensity. Desalination 2018, 451, 139–147. [Google Scholar] [CrossRef]
- Perreault, F.; Jaramillo, H.; Xie, M.; Ude, M.; Nghiem, L.D.; Elimelech, M. Biofouling mitigation in forward osmosis using graphene oxide functionalized thin-film composite membranes. Environ. Sci. Tech. 2016, 50, 5840–5848. [Google Scholar] [CrossRef]
- Marchetti, P.; Solomon, M.F.J.; Szekely, G.; Livingston, A.G. Molecular separation with organic solvent nanofiltration: A critical review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Sudoh, M.; Suzuki, Y. Preparation of composite UF membranes of sulfonated polysulfone coated on ceramics. J. Membr. Sci. 1999, 158, 55–62. [Google Scholar] [CrossRef]
- Peters, T.A.; Poeth, C.H.S.; Benes, N.E.; Bujis, H.C.W.M.; Vercauteren, F.F.; Keurentjes, J.T.F. Ceramic-supported thin PVA pervaporation membranes combining high flux and high selectivity; contradicting the flux-selectivity paradigm. J. Membr. Sci. 2006, 276, 42–50. [Google Scholar] [CrossRef]
- Yoshida, W.; Cohen, Y. Removal of methyl tert-butyl ether from water by pervaporation using ceramic-supported polymer membranes. J. Membr. Sci. 2004, 229, 27–32. [Google Scholar] [CrossRef]
- Xiangli, F.; Chen, Y.; Jin, W.; Xu, N. Polydimethylsiloxane (PDMS)/ceramic composite membrane with high flux for pervaporation of ethanol-water mixtures. Ind. Eng. Chem. Res. 2007, 46, 2224–2230. [Google Scholar] [CrossRef]
- Zhu, Y.; Xia, S.; Liu, G.; Jin, W. Preparation of ceramic-supported poly(vinyl alcohol)-chitosan composite membranes and their applications in pervaporation dehydration of organic/water mixtures. J. Membr. Sci. 2010, 349, 341–348. [Google Scholar] [CrossRef]
- Chen, Y.; Xiangli, F.; Jin, W.; Xu, N. Organic-inorganic composite pervaporation membranes prepared by self-assembly of polyelectrolyte multilayers on microporous ceramic supports. J. Membr. Sci. 2007, 302, 78–86. [Google Scholar] [CrossRef]
- Randon, J.; Paterson, R. Preliminary studies on the potential for gas separation by mesoporous ceramic oxide membranes surface modified by alkyl phosphonic acids. J. Membr. Sci. 1997, 134, 219–223. [Google Scholar] [CrossRef]
- Lu, M.; Hu, M.Z. Novel porous ceramic tube-supported polymer layer membranes for acetic acid/water separation by pervaporation dewatering. Sep. Purif. Tech. 2020, 236, 116312. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, H.; Hang, Y.; Liu, Q.; Liu, G.; Jin, W. Simultaneously enhancing interfacial adhesion and pervaporation separation performance of PDMS/ceramic composite membrane via a facile substrate surface grafting approach. AIChE J. 2019, 65, 16773. [Google Scholar] [CrossRef]
- Xia, L.; Ren, J.; Weyd, M.; McCutcheon, J.R. Ceramic-supported thin film composite membrane for organic solvent nanofiltration. J. Membr. Sci. 2018, 563, 857–863. [Google Scholar] [CrossRef]
- Chong, J.Y.; Wang, R. From micro to nano: Polyamide thin film on microfiltration ceramic tubular membranes for nanofiltration. J. Membr. Sci. 2019, 587, 117161. [Google Scholar] [CrossRef]
- Xu, R.; Liu, G.; Dong, X.; Jin, W. Pervaporation separation of n-octane/thiophene mixtures using polydimethylsiloxane/ceramic composite membranes. Desalination 2010, 258, 106–111. [Google Scholar] [CrossRef]
- Amirilargani, M.; Yokota, G.N.; Vermeji, G.H.; Merlet, R.B.; Delen, G.; Mandemaker, L.D.B.; Weckhuysen, B.M.; Winnubst, L.; Nijmeijer, A.; de Smet, L.C.P.M.; et al. Melamine-based microporous organic framework thin films on an alumina membrane for high-flux organic solvent nanofiltration. ChemSusChem 2020, 13, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Menne, D.; Uzum, C.; Koppelmann, A.; Wong, J.E.; van Foeken, C.; Borre, F.; Dahne, L.; Laakso, T.; Pihlajamaki, A.; Wessling, M. Regenerable polymer/ceramic hybrid nanofiltration membrane based on polyelectrolyte assembly by layer-by-layer technique. J. Membr. Sci. 2016, 520, 924–932. [Google Scholar] [CrossRef]
- Maaskant, E.; de Wit, P.; Benes, N.E. Direct interfacial polymerization onto thin ceramic hollow fibers. J. Membr. Sci. 2018, 550, 296–301. [Google Scholar] [CrossRef]
- Amirilargani, M.; Merlet, R.B.; Nijmeijer, A.; Winnubst, L.; de Smet, L.C.P.M.; Sudholter, E.J.R. Poly (maleic anhydride-alt-1-alkenes) directly grafted to γ-alumina for high-performance organic solvent nanofiltration membranes. J. Membr. Sci. 2018, 564, 259–266. [Google Scholar] [CrossRef]
- Wang, J.-W.; Li, X.-Z.; Fan, M.; Gu, J.-Q.; Hao, L.-Y.; Xu, X.; Chen, C.-S.; Wang, C.-M.; Hao, Y.-Z.; Agathopoulos, S. Porous β-sialon planar membrane with a robust polymer-derived hydrophobic ceramic surface. J. Membr. Sci. 2017, 535, 63–69. [Google Scholar] [CrossRef]
- Liu, G.; Wei, W.; Wu, H.; Dong, X.; Jiang, M.; Jin, W. Pervaporation performance of PDMS/ceramic composite membrane in acetone butanol ethanol (ABE) fermentation-PV coupled process. J. Membr. Sci. 2011, 373, 121–129. [Google Scholar] [CrossRef]
- Escorihuela, S.; Tena, A.; Shishatskiy, S.; Escolastico, S.; Brinkmann, T.; Serra, J.M.; Abetz, V. Gas separation properties of polyimide thin films on ceramic supports for high temperature application. Membranes 2018, 8, 16. [Google Scholar] [CrossRef]
- Faibish, R.S.; Cohen, Y. Fouling-resistant ceramic-supported polymer membranes for ultrafiltration of oil-in-water microemulsions. J. Membr. Sci. 2001, 185, 129–143. [Google Scholar] [CrossRef]
- Jana, S.; Saikia, A.; Purkait, M.K.; Mohanty, K. Chitosan based ceramic ultrafiltration membrane: Preparation, characterization and application to remove Hg(II) and As (III) using polymer enhanced ultrafiltration. Chem. Eng. J. 2011, 170, 209–219. [Google Scholar] [CrossRef]
- Wei, W.; Xia, S.; Liu, G.; Dong, X.; Jin, W.; Xu, N. Effects of polydimethylsiloxane (PDMS) molecular weight on performance of PDMS/ceramic composite membranes. J. Membr. Sci. 2011, 375, 334–344. [Google Scholar] [CrossRef]
- Guerra, K.; Pellegrino, J. Development of a Techno-Economic model to compare ceramic and polymeric membranes. Sep. Sci. Tech. 2013, 48, 51–65. [Google Scholar] [CrossRef]
- Arkell, A.; Olsson, J.; Wallberg, O. Process performance in lignin separation from softwood black liquor by membrane filtration. Chem. Eng. Res. Des. 2014, 92, 1792–1800. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-Y.; Liu, H.-L.; Ahmed, S.A.; Hanif, S.; Ren, S.-B.; Xu, J.-J.; Chen, H.-Y.; Xia, X.-H.; Wang, K. Insight into ion transfer through the sub-nanometer channels in zeolitic imidazolate frameworks. Angew. Chem. Int. Ed. 2017, 56, 4767–4771. [Google Scholar] [CrossRef]
- Benkhaya, S.; Achiou, B.; Ouammou, M.; Bennazha, J.; Younssi, S.A.; M’rabet, S.; Harfu, A.E. Preparation of low-cost composite membrane made of polysulfone/polyetherimide ultrafiltration layer and ceramic possolan support for dyes removal. Mater. Today Comm. 2019, 19, 212–219. [Google Scholar] [CrossRef]
- Lee, S.J.; Han, S.W.; Yoon, M.; Kim, K. Adsorption characteristics of 4-dimethylaminobenzoic acid on silver and titania: Diffuse reflectance infrared Fourier transform spectroscopy. Vib. Spectrosc. 2000, 24, 265–275. [Google Scholar] [CrossRef]
- Luo, M.-L.; Zhao, J.-Q.; Tang, W.; Pu, C.-S. Hydrophilic modification of poly(ether sulfone) ultrafiltration membrane surface by self-assembly of TiO2 nanoparticles. Appl. Surf. Sci. 2005, 249, 76–84. [Google Scholar] [CrossRef]
- Wang, X.-M.; Li, X.-Y.; Shih, K. In situ embedment and growth of anhydrous and hydrated aluminum oxide particles on polyvinylidene fluoride (PVDF) membranes. J. Membr. Sci. 2011, 368, 134–143. [Google Scholar] [CrossRef]
- Gohari, R.J.; Halakoo, E.; Nazri, N.A.M.; Lau, W.J.; Matsuura, T.; Ismail, A.F. Improving performance and antifouling capability of PES UF membranes via blending with highly hydrophilic hydrous manganese dioxide nanoparticles. Desalination 2014, 335, 87–95. [Google Scholar] [CrossRef]
- Yogarathinam, L.T.; Gangasalam, A.; Ismail, A.F.; Arumugam, S.; Narayanan, A. Concentration of whey protein from cheese whey effluent using ultrafiltration by combination of hydrophilic metal oxides and hydrophobic polymer. J. Chem. Tech. Biotech. 2018, 93, 2576–2591. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, B.; Zheng, X.; Wang, J.; Yuan, W.; Jiang, Z. Surface-modified Y zeolite-filled chitosan membrane for direct methanol fuel cell. J. Power Sources 2007, 173, 842. [Google Scholar] [CrossRef]
- Sun, H.; Lu, L.; Chen, X.; Jiang, Z. Surface-modified zeolite-filled chitosan membranes for pervaporation dehydration of ethanol. Appl. Surf. Sci. 2008, 254, 5367–5374. [Google Scholar] [CrossRef]
- Ayyaru, S.; Ahn, Y.-H. Fabrication and separation performance of polyethersulfone/sulfonated TiO2 (PES-STiO2) ultrafiltration membranes for fouling mitigation. J. Ind. Eng. Chem. 2018, 67, 199–209. [Google Scholar] [CrossRef]
- Bae, T.-H.; Liu, J.; Lee, J.S.; Koros, W.J.; Jones, C.W.; Nair, S. Facile high-yield solvothermal deposition of inorganic nanostructures on zeolite crystals for mixed matrix membrane fabrication. J. Am. Chem. Soc. 2009, 131, 14662–14663. [Google Scholar] [CrossRef]
- Zhang, L.-P.; Liu, Z.; Faraj, Y.; Zhao, Y.; Zhuang, R.; Xie, R.; Ju, X.-J.; Wang, W.; Chu, L.-Y. High-flux efficient catalytic membranes incorporated with iron-based Fenton-like catalysts for degradation of organic pollutants. J. Membr. Sci. 2019, 573, 493–503. [Google Scholar] [CrossRef]
- Zakeritabar, S.F.; Jahanshahi, M.; Peyravi, M. Photocatalytic behavior of induced membrane by ZrO2-SnO2 nanocomposite for pharmaceutical wastewater treatment. Catal. Lett. 2018, 148, 882–893. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Zhang, L.; Guo, J.; Wei, J. Polysulfone ultrafiltration membrane promoted by brownmillerite SrCuxCo1−xO3−λ-deposited MCM-41 for industrial wastewater decontamination: Catalytic oxidation and antifouling properties. Ind. Eng. Chem. Res. 2020, 59, 7805–7815. [Google Scholar] [CrossRef]
- Zeng, G.; He, Y.; Xu, Z.; Zhan, Y.; Ma, L.; Zhang, L. Preparation and characterization of a novel PVDF ultrafiltration membrane by blending with TiO2-HNTs nanocomposites. Appl. Surf. Sci. 2016, 271, 624–632. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, R.; Shen, G.; Li, Y.; Li, Y.; Gou, J.; Cheng, X. Construction of CuO@CuS/PVDF composite membrane and its superiority for degradation of antibiotics by activation of persulfate. Chem. Eng. J. 2021, 405, 126990. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, S.; Zhang, L.; Meng, Q.; Shen, C.; Zhang, J. Novel polysulfone hybrid ultrafiltration membrane prepared with TiO2-g-HEMA and its antifouling characteristics. J. Membr. Sci. 2013, 436, 163–173. [Google Scholar] [CrossRef]
- Zhi, S.-H.; Xu, J.; Deng, R.; Wang, L.-S.; Xu, Z.-K. Poly(vinylidene fluoride) ultrafiltration membranes containing hybrid silica nanoparticles: Preparation, characterization and performance. Polymer 2014, 55, 1333–1340. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, J.; Wang, Z.; Wen, G.; Jiang, J.; Shi, F.; Sheng, L. Hyperbranched-polymer functionalized multi-walled carbon nanotubes for poly(vinylidene fluoride) membranes: From dispersion to blended fouling-control membrane. Desalination 2012, 303, 29–38. [Google Scholar] [CrossRef]
- Ilyas, H.; Shawuti, S.; Siddiq, M.; Niazi, J.H.; Qureshi, A. PEG functionalized graphene oxide-silver nano-additive for enhanced hydrophilicity, permeability and fouling resistance properties of PVDF-co-HFP membranes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123646. [Google Scholar] [CrossRef]
- Etemadi, H.; Yegani, R.; Seyfollahi, M.; Rabiee, M. Synthesis, characterization, and antifouling properties of cecllulose acetate/polyethylene glycol-grafted nanodiamond nanocomposite membranes for humic acid removal from contaminated water. Iran. Polym. J. 2018, 27, 381–393. [Google Scholar] [CrossRef]
- Khodadousti, S.; Ashtiani, F.Z.; Karimi, M.; Fouladitajar, A. Preparation and characterization of novel PES-(SiO2-g-PMAA) membranes with antifouling and hydrophilic properties for separation of oil-in-water emulsions. Polym. Adv. Tech. 2019, 30, 2221–2232. [Google Scholar] [CrossRef]
- Wang, H.; Lu, X.; Lu, X.; Wang, Z.; Ma, J.; Wang, P. Improved surface hydrophilicity and antifouling property of polysulfone ultrafiltration membrane with poly(ethylene glycol) methyl ether methacrylate grafted graphene oxide nanofillers. Appl. Surf. Sci. 2017, 425, 603–613. [Google Scholar] [CrossRef]
- Liang, X.; Wang, P.; Wang, J.; Zhang, Y.; Wu, W.; Liu, J.; Bruggen, B. Zwitterionic functionalized MoS2 nanosheets for a novel composite membrane with effective salt/dye separation performance. J. Membr. Sci. 2019, 573, 270–279. [Google Scholar] [CrossRef]
- Su, Y.-H.; Liu, Y.-L.; Sun, Y.-M.; Lai, J.-Y.; Wang, D.-M.; Gao, Y.; Liu, B.; Guiver, M.D. Proton exchange membranes modified with sulfonated silica nanoparticles for direct methanol fuel cells. J. Membr. Sci. 2007, 296, 21–28. [Google Scholar] [CrossRef]
- Gosalawit, R.; Chirachanchai, S.; Shishatskiy, S.; Nunes, S.P. Sulfonated montmorillonite/sulfonated poly(ether ether ketone)(SMMT/SPEEK) nanocomposite membrane for direct methanol fuel cells (DMFCs). J. Membr. Sci. 2008, 323, 337–346. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Bruggen, B.; Sun, X.; Shen, J.; Han, W.; Wang, L. Fouling behavior of polyethersulfone ultrafiltration membranes functionalized with sol-gel formed ZnO nanoparticles. RSC Adv. 2015, 5, 50711–50719. [Google Scholar] [CrossRef]
- Chen, W.; Su, Y.; Zhang, L.; Shi, Q.; Peng, J.; Jiang, Z. In situ generated silica nanoparticles as pore-forming agent for enhanced permeability of cellulose acetate membranes. J. Membr. Sci. 2010, 348, 75–83. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, H.-C.; Liang, H.-Q.; Wan, L.-S.; Xu, Z.-K. Novel nanofiltration membrane with ultrathin zirconia film as selective layer. J. Membr. Sci. 2016, 500, 265–271. [Google Scholar] [CrossRef]
- Tang, L.; Livi, K.J.T.; Chen, K.L. Polysulfone membranes modified with bioinspired polydopamine and silver nanoparticles formed in situ to mitigate biofouling. Environ. Sci. Tech. Lett. 2015, 2, 59–65. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Wang, Z.; Wu, J.; Wang, J.; Wang, S. In situ immobilization of silver nanoparticles for improving permeability, antifouling and anti-bacterial properties of ultrafiltration membrane. J. Membr. Sci. 2016, 499, 269–281. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, B.; Wang, Z.; Li, B. Fabrication of omniphobic PVDF composite membrane with dual-scale hierarchical structure via chemical bonding for robust membrane distillation. J. Membr. Sci. 2021, 622, 119038. [Google Scholar] [CrossRef]
- Derami, H.G.; Gupta, P.; Gupta, R.; Rathi, P.; Morrissey, J.J.; Singamaneni, S. Palladium nanoparticle-decorated mesoporous polydopamine/bacterial nanocellulose as a catalytically active universal dye removal ultrafiltration membrane. ACS Appl. Nano Mater. 2020, 3, 5437–5448. [Google Scholar] [CrossRef]
- Soyekwo, F.; Liu, C.; Wen, H.; Hu, Y. Construction of an electroneutral zinc incorporated polymer network nanocomposite membrane with enhanced selectivity for salt/dye separation. Chem. Eng. J. 2020, 380, 122560. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, S.; Zhu, C.; Cao, B.; Kausar, F.; Xu, A.; Yuan, W.Z.; Zhang, Y. Towards high-performance hybrid hydrophilic membranes: Chemical anchoring of hydroxyl-rich nanoparticles on PVDF membranes via a silane coupling agent. J. Mater. Sci. 2017, 52, 11737–11748. [Google Scholar] [CrossRef]
- Boo, C.; Lee, J.; Elimelech, M. Omniphobic polyvinylidene fluoride (PVDF) membrane for desalination of shale gas produced water by membrane distillation. Environ. Sci. Technol. 2016, 50, 12275–12282. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Z.; Shi, H.; Chen, Q.; Zeng, G.; Di, H.; Ren, X.; He, Y. A novel antifouling and antibacterial surface-functionalized PVDF ultrafiltration membrane via binding Ag/SiO2 nanocomposites. J. Chem. Technol. Biotechnol. 2017, 92, 562–572. [Google Scholar] [CrossRef]
- Lu, T.; Xu, X.; Liu, X.; Sun, T. Super hydrophilic PVDF based composite membrane for efficient separation of tetracycline. Chem. Eng. J. 2017, 308, 151–159. [Google Scholar] [CrossRef]
- Jaleh, B.; Etivand, E.S.; Mohazzab, B.F.; Nasrollahzadeh, M.; Varma, R.S. Improving wettability: Deposition of TiO2 nanoparticles on the O2 plasma activated polypropylene membrane. Int. J. Mol. Sci. 2019, 20, 3309. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, G.; An, C.; Feng, R.; Yao, Y.; Zhao, S.; Huang, C.; Wu, Y. Plasma-induced poly(acrylic acid)-TiO2 coated polyvinylidene fluoride membrane for produced water treatment: Synchrotron X-ray, optimization, and insight studies. J. Clean. Prod. 2019, 227, 772–783. [Google Scholar] [CrossRef]
- Liang, S.; Kang, Y.; Tiraferri, A.; Giannelis, E.P.; Huang, X.; Elimelech, M. Highly hydrophilic polyvinylidene fluoride (PVDF) ultrafiltration membranes via postfabrication grafting of surface-tailored silica nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 6694–6703. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Xu, Z.-K. Mineral-coated polymer membranes with superhydrophilicity and underwater superoleophobic for effective oil/water separation. Sci. Rep. 2013, 3, 2776. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, H.C.; Wan, L.S.; Liang, H.Q.; Li, H.; Xu, Z.K. Polydopamine-coated porous substrates as a platform for mineralized β-FeOOH nanorods with photocatalysis under sunlight. ACS Appl. Mater. Interfaces 2015, 7, 11567–11574. [Google Scholar] [CrossRef]
- Zhi, S.-H.; Wan, L.-S.; Xu, Z.-K. Poly(vinylidene fluoride)/poly(acrylic acid)/calcium carbonate composite membranes via mineralization. J. Membr. Sci. 2014, 454, 144–154. [Google Scholar] [CrossRef]
- Chen, P.-C.; Wan, L.-S.; Xu, Z.-K. Bio-inspired CaCO3 coating for superhydrophilic hybrid mambranes with high water permeability. J. Mater. Chem. 2012, 22, 22727–22733. [Google Scholar] [CrossRef]
- Yang, H.C.; Chen, Y.F.; Ye, C.; Jin, Y.N.; Li, H.; Xu, Z.K. Polymer membrane with a mineral coating for enhanced curling resistance and surface wettability. Chem. Commun. 2015, 51, 12779–12782. [Google Scholar] [CrossRef]
- Yang, H.C.; Pi, J.K.; Liao, K.J.; Huang, H.; Wu, Q.Y.; Huang, H.J.; Xu, Z.K. Silica-decorated polypropylene microfiltration membranes with a mussel-inspired intermediate layer for oil-in-water emulsion separation. ACS Appl. Mater. Interfaces 2014, 6, 12566–12572. [Google Scholar] [CrossRef]
- Yang, H.-C.; Liao, K.-J.; Huang, H.; Wu, Q.-Y.; Wan, L.-S.; Xu, Z.K. Mussel-inspired modification of a polymer membrane for ultra-high water permeability and oil-in-water emulsion separation. J. Mater. Chem. A 2014, 2, 10225–10230. [Google Scholar] [CrossRef]
- Pi, J.K.; Wu, G.P.; Yang, H.C.; Arges, C.G.; Xu, Z.K. Separators with biomineralized zirconia coatings for enhanced thermo- and electro-performance of lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 21971–21978. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, Z.; Xie, A.; Wang, Q.; Liu, S.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Facile preparation of grass-like structured NiCo-LDH/PVDF composite membrane for efficient oil-water emulsion separation. J. Membr. Sci. 2019, 573, 226–233. [Google Scholar] [CrossRef]
- Ding, W.; Zhuo, H.; Bao, M.; Li, Y.; Lu, J. Fabrication of organic-inorganic nanofiltration membrane using ordered stacking SiO2 thin film as rejection layer assisted with layer-by-layer method. Chem. Eng. J. 2017, 330, 337–344. [Google Scholar] [CrossRef]
- Wongchitphimon, S.; Rongwong, W.; Chuah, C.Y.; Wang, R.; Bae, T.-H. Polymer-fluorinated silica composite hollow fiber membranes for the recovery of biogas dissolved in anaerobic effluent. J. Membr. Sci. 2017, 540, 146–154. [Google Scholar] [CrossRef]
- Lin, Y.; Loh, C.H.; Shi, L.; Fan, Y.; Wang, R. Preparation of high-performance Al2O3/PES composite hollow fiber UF membranes via facile in-situ vapor induced hydrolyzation. J. Membr. Sci. 2017, 539, 65–75. [Google Scholar] [CrossRef]
- Qin, A.; Li, X.; Zhao, X.; Liu, D.; He, C. Engineering a highly hydrophilic PVDF membrane via binding TiO2 nanoparticles and a PVA layer onto a membrane surface. ACS Appl. Mater. Interfaces 2015, 7, 8427–8436. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.H.; Wang, R. Insight into the role of amphiphilic pluronic block copolymer as pore-forming additive in PVDF membrane formation. J. Membr. Sci. 2013, 446, 492–503. [Google Scholar] [CrossRef]
- Yang, H.C.; Waldman, R.Z.; Chen, Z.; Darling, S.B. Atomic layer deposition for membrane interface engineering. Nanoscale 2018, 10, 20505–20513. [Google Scholar] [CrossRef]
- Leskela, M.; Ritala, M. Atomic layer deposition chemistry: Recent developments and future challenges. Angew. Chem. Int. Ed. 2003, 42, 5548–5554. [Google Scholar] [CrossRef] [PubMed]
- Steven, G.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 1, 111–131. [Google Scholar]
- Yang, H.C.; Xie, Y.; Chan, H.; Narayanan, B.; Chen, L.; Waldman, R.Z.; Sankaranarayanan, S.; Elam, J.W.; Darling, S.B. Crude-oil-repellent membranes by atomic layer deposition: Oxide interface engineering. ACS Nano 2018, 12, 8678–8685. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, Y.; Yang, J.; Wang, X.; Wang, Z.; Wang, Y. Plasma activation of porous polytetrafluoroethylene membranes for superior hydrophilicity and separation performances via atomic layer deposition of TiO2. J. Membr. Sci. 2013, 443, 62–68. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, J.; Dai, J.; Yang, Y.; Chen, X.; Wang, Y. Hydrophilization of porous polypropylene membranes by atomic layer deposition of TiO2 for simultaneously improved permeability and selectivity. J. Membr. Sci. 2013, 448, 215–222. [Google Scholar] [CrossRef]
- Chen, H.; Kong, L.; Wang, Y. Enhancing the hydrophilicity and water permeability of polypropylene membranes by nitric acid activation and metal oxide deposition. J. Membr. Sci. 2015, 487, 109–116. [Google Scholar] [CrossRef]
- Wei, W.; Xia, S.; Liu, G.; Gu, X.; Jin, W.; Xu, N. Interfacial adhesion between polymer separation layer and ceramic support for composite membrane. AIChE J. 2010, 56, 1584–1592. [Google Scholar] [CrossRef]
- Hang, Y.; Liu, G.; Huang, K.; Jin, W. Mechanical properties and interfacial adhesion of composite membranes probed by in-situ nano-indentation/scratch technique. J. Membr. Sci. 2015, 494, 205–215. [Google Scholar] [CrossRef]
- Hamm, J.B.S.; Ambrosi, A.; Pollo, L.D.; Marcillo, N.R.; Tessaro, I.G. Thin polymer layer-covered porous alumina tubular membranes prepared via a dip-coating/phase-inversion process. Mater. Chem. Phys. 2021, 265, 124511. [Google Scholar] [CrossRef]
- Shi, G.M.; Chung, T.-S. Thin film composite membranes on ceramic for pervaporation dehydration isopropanol. J. Membr. Sci. 2013, 448, 34–43. [Google Scholar] [CrossRef]
- Faibish, R.S.; Cohen, Y. Fouling and rejection behavior of ceramic and polymer-modified ceramic membranes for ultrafiltration of oil-in-water emulsions and microemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2001, 191, 27–40. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Ma, J.; Liu, H.; Ning, P. Synthesis and characterization of a new hydrophilic boehmite-PVB/PVDF blended membrane supported nano zero-valent iron for removal of Cr(IV). Sep. Purif. Tech. 2018, 205, 74–83. [Google Scholar] [CrossRef]
- Lakhotia, S.R.; Mukhopadhyay, M.; Kumari, P. Surface-Modified Nanocomposite Membranes. Sep. Purif. Rev. 2018, 47, 288–305. [Google Scholar] [CrossRef]
- Issac, M.N.; Kandasubramanian, B. Review of manufacturing three-dimensional-printed membranes for water treatment. Environ. Sci. Poll. Res. 2020, 27, 36091–36108. [Google Scholar] [CrossRef] [PubMed]
- Al-Shimmery, A.; Mazinanni, S.; Ji, J.; Chew, Y.M.J.; Mattia, D. 3D printed composite membranes with enhanced anti-fouling behavior. J. Membr. Sci. 2019, 574, 76–85. [Google Scholar] [CrossRef]
- Lyu, Z.; Ng, T.C.A.; Tran-Duc, T.; Lim, G.J.H.; Gu, Q.; Zhang, L.; Zhang, Z.; Ding, J.; Phan-Thien, N.; Wang, J.; et al. 3D-printed surface-patterned ceramic membrane with enhanced performance in crossflow filtration. J. Membr. Sci. 2020, 606, 118138. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, H.A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Mohammadi, T.; Saljoughi, E. Effect of production conditions on morphology and permeability of asymmetric cellulose acetate membranes. Desalination 2009, 243, 1–7. [Google Scholar] [CrossRef]

| Polymer | Abbreviation | Structure |
|---|---|---|
| poly-acrylonitrile | PAN | 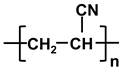 |
| poly-vinylpyrrolidone | PVP | 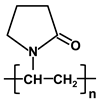 |
| poly-vinylidene fluoride | PVDF | 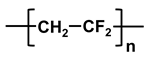 |
| poly-vinyl alcohol | PVA |  |
| poly-vinyl acetate | PVAc | 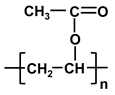 |
| Poly-ethersulfone | PES | 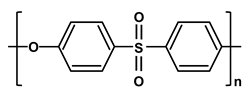 |
| Poly-sulfone | PSf |  |
| Ceramics | Polymer | Membrane | Improved Properties by Addition of Filler | Ref. |
|---|---|---|---|---|
| TiO2 (25 nm) | PVDF | Hollow fiber UF | Hydrophilicity | [68] |
| TiO2 | PVDF | Hollow fiber UF | Hydrophilicity | [28] |
| TiO2 (25 nm) + dopamine | PVDF + PVP | UF | Hydrophilicity, Photocatalycity | [69] |
| TiO2 (20 nm) | PES | UF | Hydrophilicity, antifouling performance | [70] |
| TiO2 (20 nm) | PVDF | MF | antifouling performance | [71] |
| TiO2 | PES | NF | Permeability, antifouling performance | [72] |
| TiO2 (21 nm) | PSf | Hollow fiber UF | antifouling performance | [73] |
| TiO2 (62 nm) | CA | - | Thermal stability, Water flux | [74] |
| TiO2 (20 nm) | PVDF-sulfonated-PES | Flat sheet UF | Hydrophilicity, Antifouling, photo-bactericidal effect | [75] |
| TiO2 (20 nm) | PVDF | UF | Hydrophilicity, antifouling performance | [76] |
| TiO2 (20 nm) | PVDF | UF | Hydrophilicity, Photocatalycity | [77] |
| TiO2 | PAN | - | Mechanical properties, Hydrophilicity, Photocatalycity | [78] |
| TiO2 | PVDF | - | Oil rejection, Water flux | [79] |
| TiO2 | PSf | - | Dye removal | [80] |
| Membrane | Porosity (%) | Pore Size (nm) | Water Flux (L m−2 h−1 at 200 kPa) |
|---|---|---|---|
| PES | 69.2 | 12.9 | 122 |
| 1wt% SiO2-PES | 74.8 | 13.7 | 145 |
| 2wt% SiO2-PES | 75.9 | 14.6 | 180 |
| 4wt% SiO2-PES | 74.9 | 12.8 | 137 |
| Membrane | Porosity (%) | Pore Size (nm) | Water Flux (L m−2 h−1 at 200 kPa) |
|---|---|---|---|
| PES | 17 | 89.3 | 5 |
| 1wt% Fe3O4-PES | 33 | 40.1 | 6 |
| 5wt% Fe3O4-PES | 44 | 3.9 | 12 |
| 10wt% Fe3O4-PES | 59 | 5.8 | 21 |
| Membrane | Contact Angle (°) | Porosity (%) | Water Flux (L m−2 h−1 at 300 kPa) |
|---|---|---|---|
| PES | 52.3 | 51.8 | 182 |
| TiO2-PES | 44.1 | 66.6 | 199 |
| Al2O3-PES | 37.8 | 62.1 | 209 |
| ZrO2-PES | 48.6 | 64.3 | 190 |
| Membrane | Contact Angle (°) | Porosity (%) | Ref. |
|---|---|---|---|
| PES | 52.3 | 51.8 | [96] |
| 0.4 wt.% TiO2-PES | 44.1 | 66.6 | [96] |
| PPESK | 50.7 | 80.5 | [97] |
| 1 wt.% TiO2-PPESK | 45.9 | 86.8 | [97] |
| PSf | 70.1 | 63.4 | [98] |
| 1 wt.% TiO2-PSf | 52.0 | 81.0 | [98] |
| CA | 69.3 | - | [99] |
| 5 wt.% TiO2-CA | 71.1 | - | [99] |
| PVDF | - | 72.2 | [54] |
| 1 wt.% TiO2-PVDF | - | 73.9 | [54] |
| Top Layer | Polymeric Support | Improved Properties | References |
|---|---|---|---|
| TiO2-PI | PES | Salt rejection, Water flux | [100] |
| Halloysite nanotube-PA | PSf | Antifouling performance, Water flux | [101] |
| TiO2-PA | PSf | Antifouling performance | [102] |
| Fe3O4/ZnO-PA | PSf | Hydrophilicity, Water flux | [48] |
| SiO2-PA | PSf | Hydrophilicity, Permeability, Salt rejection | [103] |
| Al2O3-PA | PSf | Antifouling performance, Water flux | [104] |
| TiO2/Halloysite nanotube-PA | PSf | Antifouling performance, Recovery | [49] |
| NaY-PA | PSf | Hydrophilicity, Permeability | [50] |
| Mesoporous-silica-PA | PSf | Antifouling performance, Water flux | [105] |
| ZnO-PDMS | PI | UV resistance, Superoleophilicity | [106] |
| Clay-Chitosan | PVDF | Dye adsorption | [107] |
| TiO2 | PS | Hydrophilicity, Water flix | [108] |
| TiO2 | PAN | Water flux, Dye rejection | [109] |
| SiO2 | PVDF | Dye rejection, Oil rejection | [110] |
| Bi12O17Cl2 | CA | Dye removal | [111] |
| MoS2 | PVDF | Salt rejection, Dye rejection | [112] |
| ZnWO4 | PVDF | Dye rejection | [113] |
| TiO2 | CA | Water flux, Dye rejection | [114] |
| Au-Ag-PAA | PA | Antifouling and antibiofouling performance | [115] |
| Arginine-Fe-PA | PES | Antifouling performance, Permeability | [116] |
| CNT-PA | PSf | Antifouling performance, Salt rejection | [117] |
| Amine-MWCNT-PA | PSf | Permeability, Salt rejection | [118] |
| MWCNTs-PA | PSf | Antifouling performance, Water flux | [119] |
| GO-PA | PAN | Antifouling performance, Hydrophilicity | [120] |
| PVP-GO-PA | PSf | Salt rejection, Water flux | [121] |
| GO/Fe3O4-PA | PES | Water flux, Antifouling performance | [122] |
| GO-PA | PSf | Water flux, Slat rejection | [123] |
| GO-PA | PSf | Water flux, Hydrophilicity | [124] |
| rGO-PDMS | PES | Thermal stability | [125] |
| Quantum dot graphene-PA | PES | Anti-bacterial property, long-term stability | [126] |
| Fullerenol (C60(OH)n)-PA | PSf | Hydrophilicity, antifouling property | [127] |
| Membrane | Pure Water Permeability (L m−2 h−1 bar−1) | NaCl Rejection (%) |
|---|---|---|
| PA/PSf | 2.94 | 72 |
| 0.01 wt.% SiO2-PA/PSf | 5.88 | 82 |
| 0.05 wt.% SiO2-PA/PSf | 9.52 | 89 |
| 0.1 wt.% SiO2-PA/PSf | 12.36 | 78 |
| Polymer Thin Film | Ceramic Support | Improved Properties | References |
|---|---|---|---|
| PDMS | Al2O3 hollow fiber | Butanol/water separation factor, long-term stability | [150] |
| PA | Al2O3 tubular UF membrane | Dye rejection, methanol permeability | [151] |
| PA | Al2O3 tubular | Salt rejection, water permeability | [152] |
| PDMS | ZrO2/Al2O3 | Sulfur removal efficiency | [153] |
| PVA | Fumed silica | Water selectivity, Pervaporation separation index | [52] |
| Melamine-terephthaldehyde | Al2O3 | n-heptane permeability, dye rejection | [154] |
| PDADMAC/poly(sodium 4-styrene sulfonate | Al2O3 monolith | Stability for backwashing, reusable ceramic support | [155] |
| PA | Al2O3 hollow fiber | Water flux | [156] |
| Poly (maleic anhydride-alt-1-alkenes) | γ-Al2O3 NF membrane | Dye rejection, permeability | [157] |
| Sulfonated polybenzimidazole | TiO2, TiO2/ZrO2 tubular | Mechanical ruggedness, flux | [148] |
| PDMS | β-sialon | Long-term stability, recovery ability | [158] |
| PDMS | ZrO2/Al2O3 tubular | High flux, recovery ability | [159] |
| PA | Al2O3 | High H2/CO2 selectivity | [160] |
| PVP | ZrO2 | Oil rejection, anti-fouling performance | [161] |
| Chitosan | Clay+Kaolin | Rejection of mercury and arsenic, cost of membrane | [162] |
| PDMS (polydimethylsiloxane) | ZrO2/Al2O3 tubular | High flux, Membrane stability | [163] |
| PVAc, PVP | Al2O3 tubular | Separation factor | [164] |
| PVAc | SiO2 tubular | Water flux | [165] |
| PDMS | γ-Al2O3 | IPA selectivity | [166] |
| PVA | ZrO2/Al2O3 tubular | Water permeability, Selectivity of water to ethyl acetate | [167] |
| PSf-PEI | Flat Pozzolan | Water permeability, Dye rejection | [168] |
| Sulfonated polybenzimidazole | TiO2/ZrO2 tubular | Flux, Pervaporation stability | [149] |
| Membrane | Porosity (%) | Contact Angle (°) | BSA Rejection (%) |
|---|---|---|---|
| PES | 68.4 | 75 | 88 |
| Non-sulfonated TiO2/PES | 77.3 | 60 | 92 |
| S-TiO2/PES | 87.6 | 49.2 | 99 |
| Grafted Ceramic NPs | Host Polymer | Reference |
|---|---|---|
| PHEMAb-PMMA@-SiO2 (1) | PVDF | [184] |
| silylated ZSM-5 | PDMS | [57] |
| Polyester-MWCNT | PVDF | [185] |
| PEG-GO | PVDF-co-HFP | [186] |
| PMMA-CNT | PVDF | [58] |
| PEG-nanodiamond | CA | [187] |
| PMMA-SiO2 | PES | [188] |
| P(PEGMA)-GO | PSf | [189] |
| PSBMA-MoS2 (2) | PES | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotobuki, M.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-Polymer Composite Membranes for Water and Wastewater Treatment: Bridging the Big Gap between Ceramics and Polymers. Molecules 2021, 26, 3331. https://doi.org/10.3390/molecules26113331
Kotobuki M, Gu Q, Zhang L, Wang J. Ceramic-Polymer Composite Membranes for Water and Wastewater Treatment: Bridging the Big Gap between Ceramics and Polymers. Molecules. 2021; 26(11):3331. https://doi.org/10.3390/molecules26113331
Chicago/Turabian StyleKotobuki, Masashi, Qilin Gu, Lei Zhang, and John Wang. 2021. "Ceramic-Polymer Composite Membranes for Water and Wastewater Treatment: Bridging the Big Gap between Ceramics and Polymers" Molecules 26, no. 11: 3331. https://doi.org/10.3390/molecules26113331
APA StyleKotobuki, M., Gu, Q., Zhang, L., & Wang, J. (2021). Ceramic-Polymer Composite Membranes for Water and Wastewater Treatment: Bridging the Big Gap between Ceramics and Polymers. Molecules, 26(11), 3331. https://doi.org/10.3390/molecules26113331








