Immobilization and Release Studies of Triazole Derivatives from Grafted Copolymer Based on Gellan-Carrying Betaine Units
Abstract
1. Introduction
- Synthesis of new bioactive compounds in which thiadiazole and triazole structures are grafted onto the aromatic heterocycle, 2-mercaptobenzoxazole, followed by the choice of the compound with the best biological properties in order to be immobilized on a polymeric support;
- Synthesis of new polymer-bioactive compound systems; and
- Detailed studies on the immobilization of the bioactive compound by adsorption on grafted copolymers and those containing the betaine structure.
2. Results and Discussion
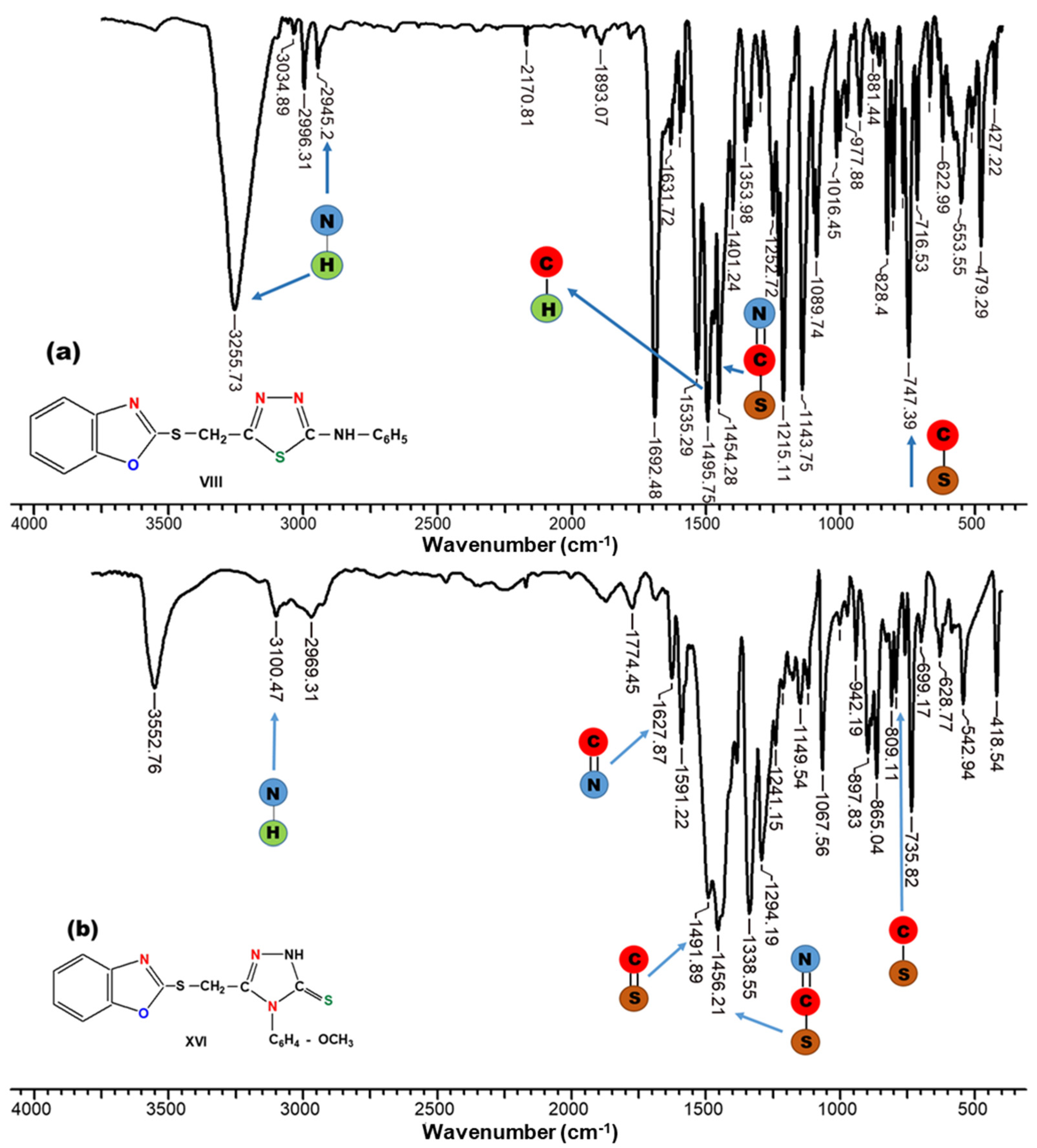
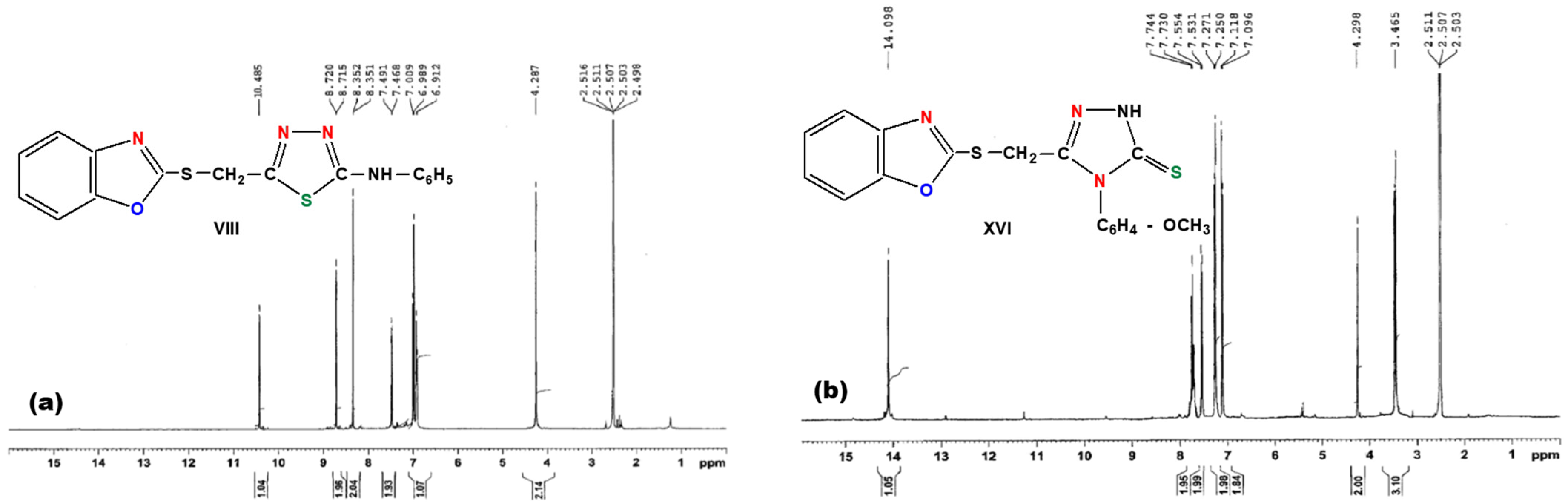
2.1. Synthesis of Thiadiazole and Triazole Derivatives
2.2. Antibacterial Activities of Bioactive Compounds
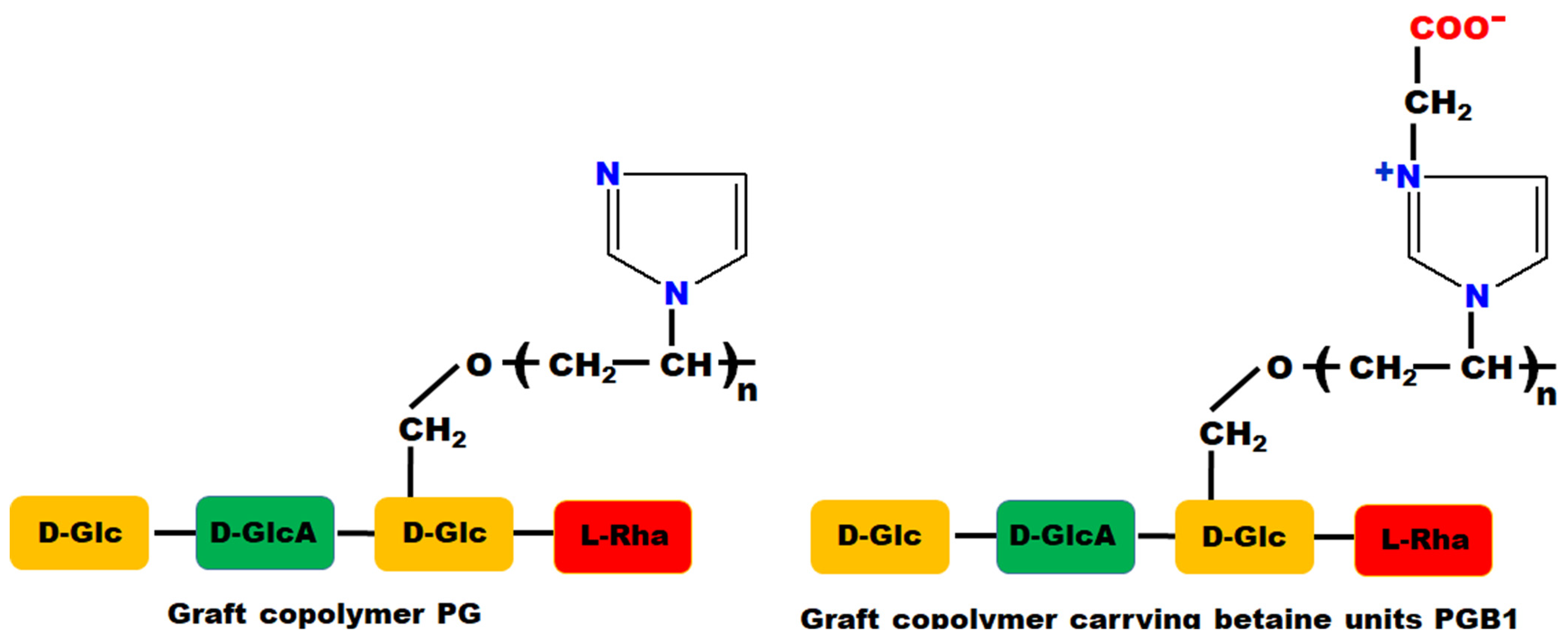
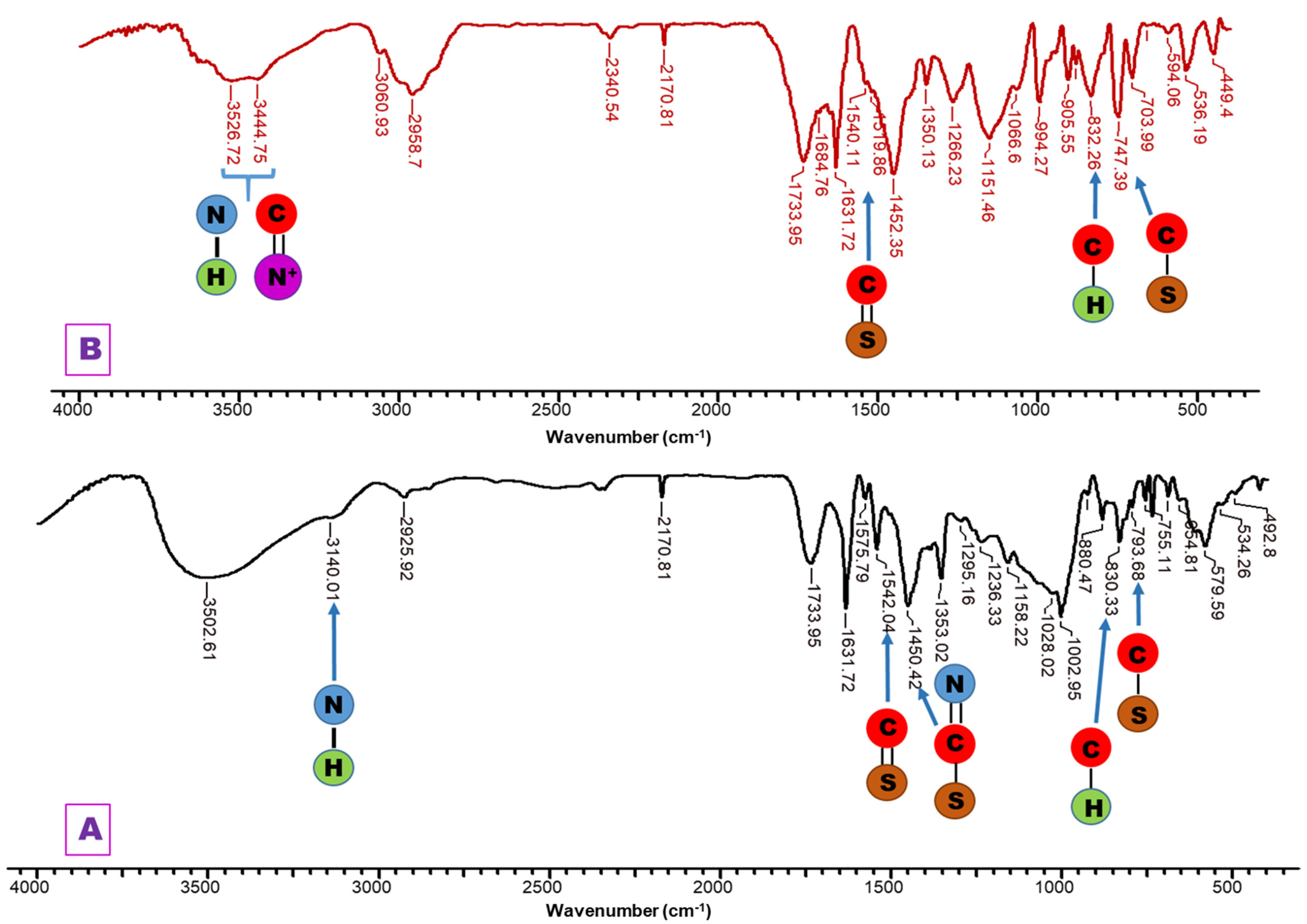
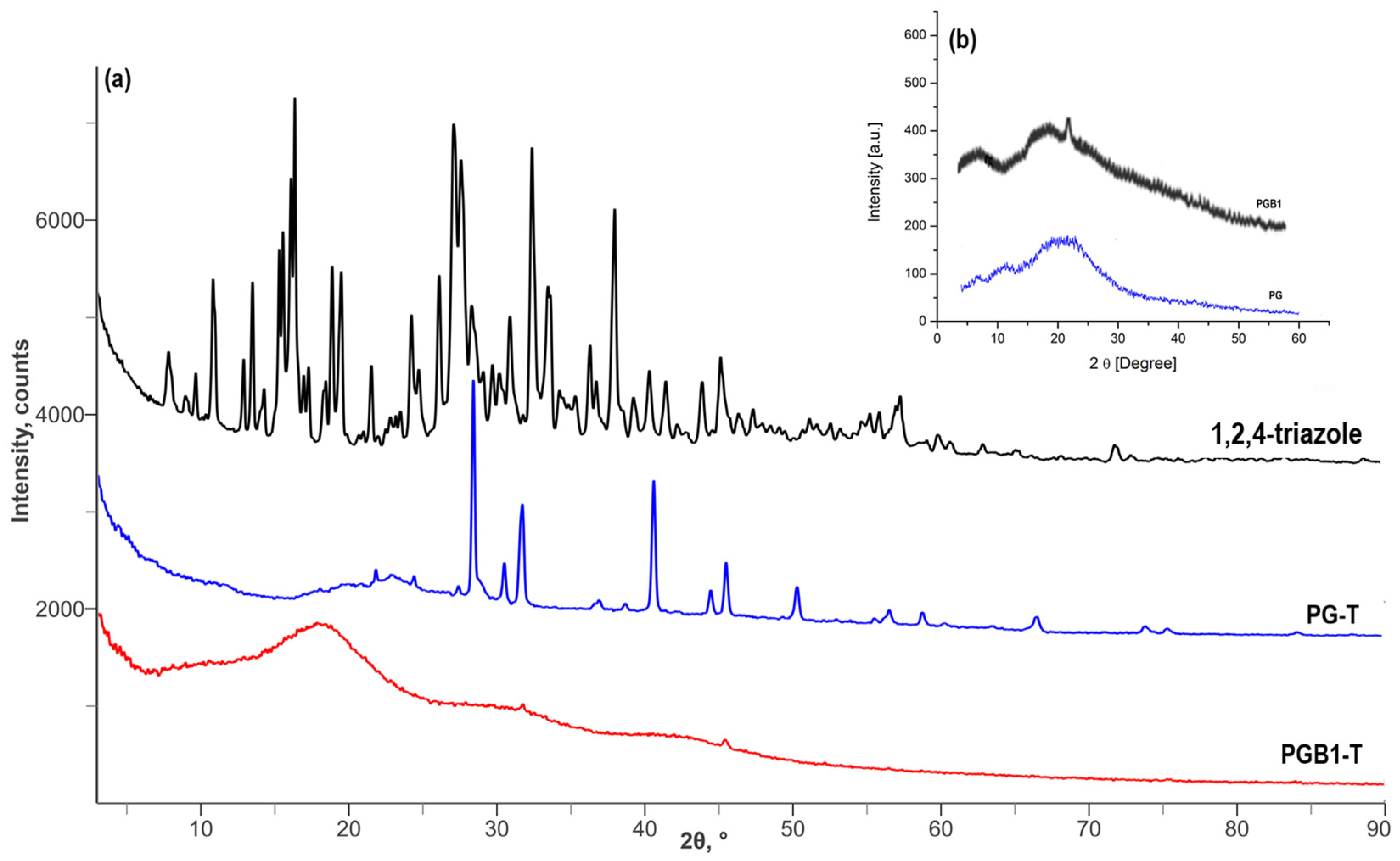
2.3. Immobilization Studies of Triazoles
- For PG—1,2,4-triazole systems (PG-T), it can be observed some new absorption bands as follows: the absorption band at 3140 cm−1 due to the stretching vibration of -NH group; 1542 cm−1 assigned to the stretching vibration of C=S group; 1450 cm−1 corresponding to the stretching vibration of -N=C-S group; and 793 cm−1 assigned to the stretching vibration of S-CH2 group.
- For PGB1—1,2,4-triazole systems (PGB1-T), the changes that occur in the FTIR spectrum are the following: the band vibration of 3439 cm−1 from PGB1 copolymer is shifted in PGB1-T spectra to 3445 cm−1 due to the overlap of the absorption band of -C=N+ group from PGB1 copolymer, with absorption band corresponding to the -NH group belonging to the 1,2,4-triazole; absorption band at 832 cm−1 is assigned to the stretching vibration of C-H out of plane bending.
2.3.1. Sorption Isotherms
- Langmuir isotherm
- Freundlich isotherm
- Dubinin–Radushkevich isotherm
- The theoretical values obtained for the maximum sorption capacity (qm) calculated on the basis of the Langmuir isotherm are close to the experimental values qc (457, 512 and 618 mg 1,2,4-triazole /g PG copolymer and 529, 601 and 672 mg of 1,2,4-triazole /g PGB1 copolymer);
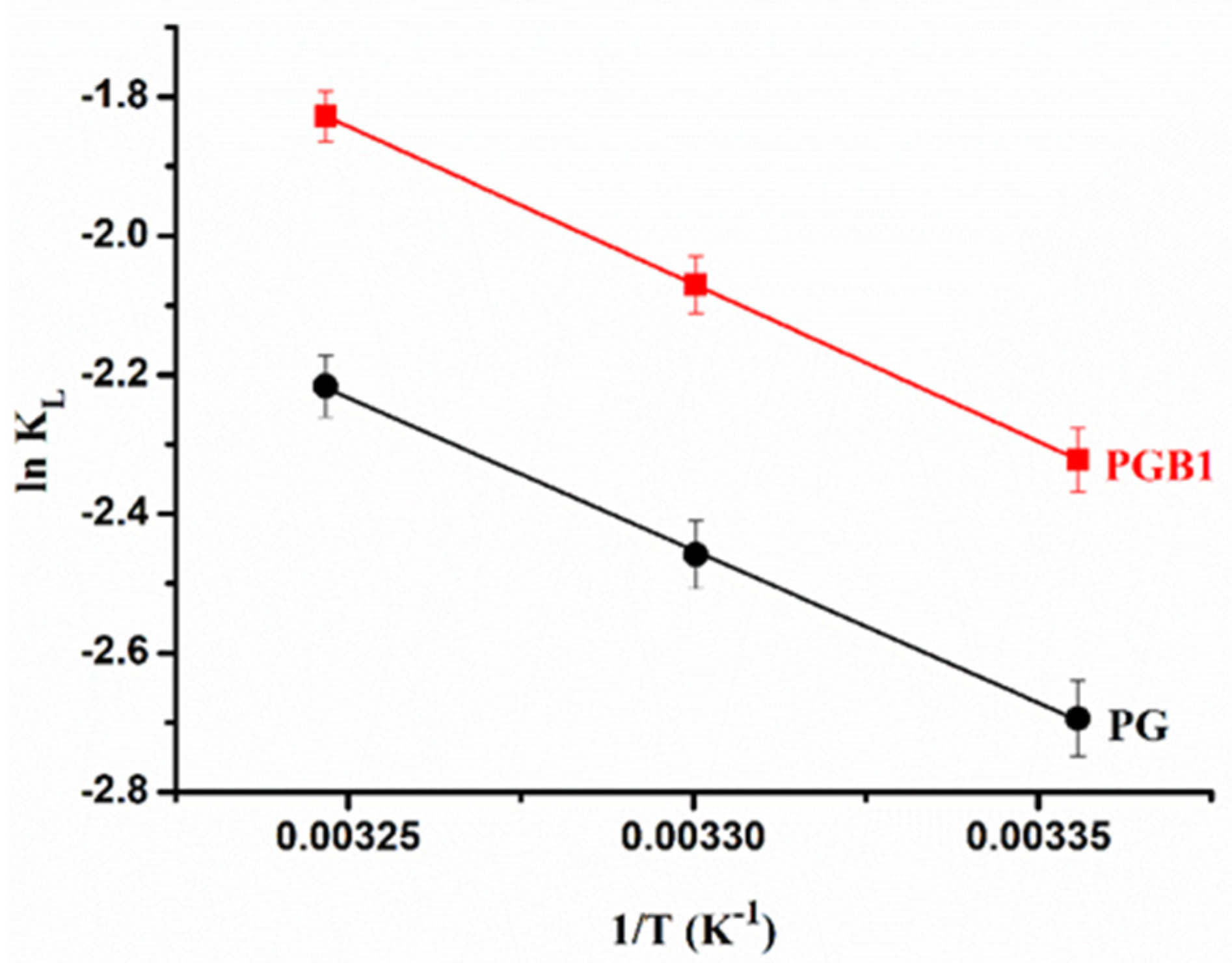
- The values of the equilibrium parameter, RL, were in the range between 0 and 1, thus confirming that the PG and PGB1 copolymers are favorable supports for the sorption of 1,2,4-triazoleat at the three temperatures studied. It is also observed that the KL values are higher in the case of the PGB1 copolymer than in the case of the PG copolymer, which indicates a higher affinity of the PGB1 copolymer for 1,2,4-triazole; this is in agreement with the highest sorption capacity obtained in the case of the PGB1 copolymer;
- The values for R2 and χ2 are in the range of 0992–0.997 and 2.010–3.429, respectively, indicating that the Langmuir isotherm describes well the experimental data;
- Although the values of 1/nf are in the range of 0–1, which indicates that the Freundlich isotherm is favorable in the case of 1,2,4-triazolesorption onto PG and PGB1 copolymers, the small values of R2 (0.911 to 0.921) associated with high values of χ2 (18.782–38.332) shows that the Freundlich isotherm does not describe well the experimental data;
- The analysis of the parameter values obtained by applying the Dubinin–Radushkevich isotherm shows that the qDR values are very close to the experimental values, which indicates that this isotherm describes well the experimental data. This is also supported by the fact that high values for R2 (0.997–0.999) and low values for χ2 (0.226–0.413) were obtained, confirming that the Dubinin–Radushkevich isotherm describes very well the sorption of 1,2,4-triazole onto PG and PGB1 copolymers;
- The calculated values of the average free energy of 1,2,4-triazole sorption onto PG and PGB1 copolymers are in the range of 1.099–3.714 kJ/mol, which indicates that the sorption process studied is physical in nature.
2.3.2. Thermodynamic Parameters
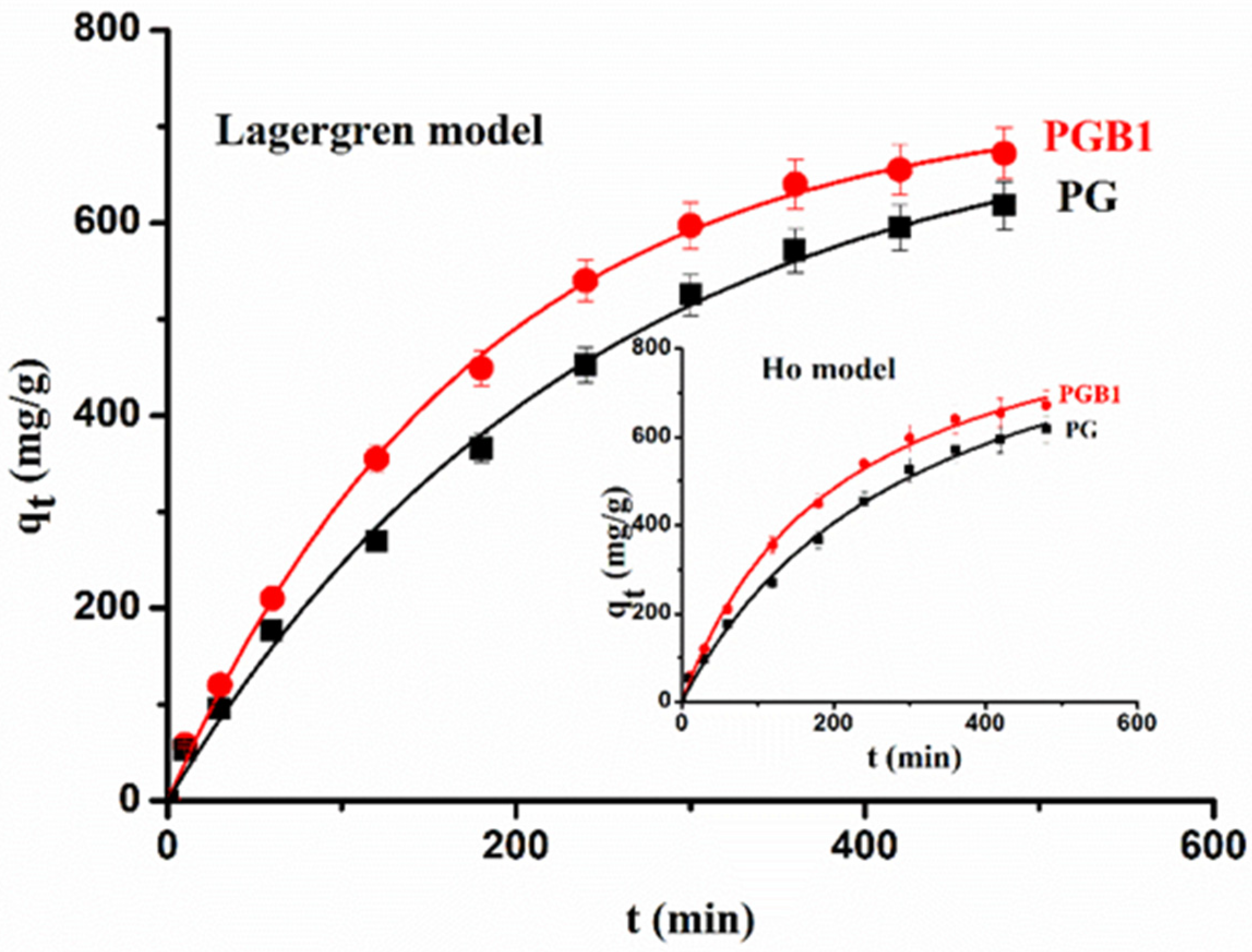
2.3.3. Sorption Kinetic Study
- Lagergren model [58]:
- Ho model [59]:
2.4. In Vitro Release Studies
- Higuchi model [60]:
- Korsmeyer–Peppas model [61]:
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Benzoxazole-2′-yl-mercapto-acetic Acid Hydrazide (Compound I)
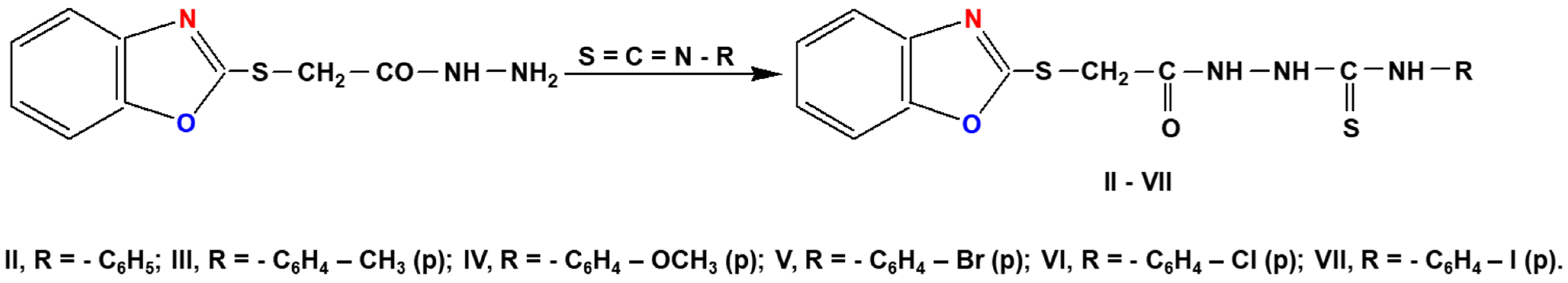
3.3. General Procedure for Synthesis of 1-(Benzoxazole-2′-yl-mercapto-acetyl)-4-aryl-thiosemicarbazide (Compounds II–VII)
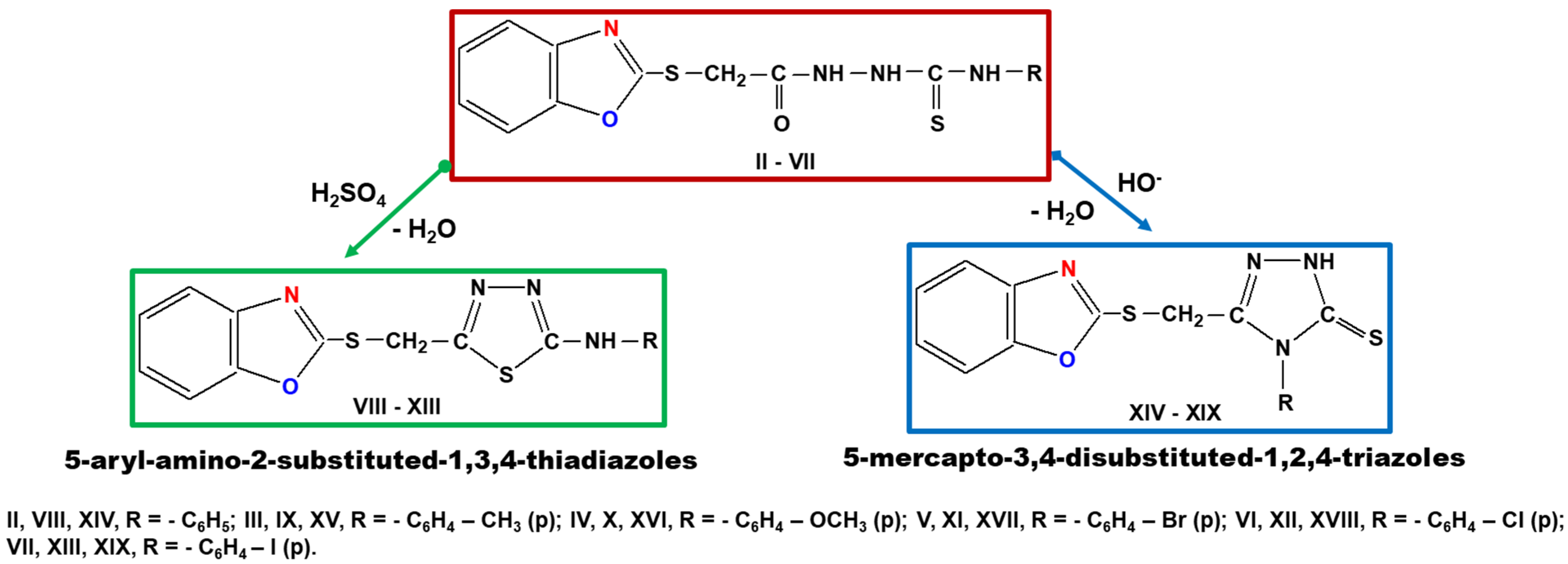
3.4. General Procedure for Synthesis of 1-(Benzoxazole-2′-yl-mercapto-methyl)-5-(aryl-amino)-1,3,4,thiadiazoles (Compounds VIII–XIII)
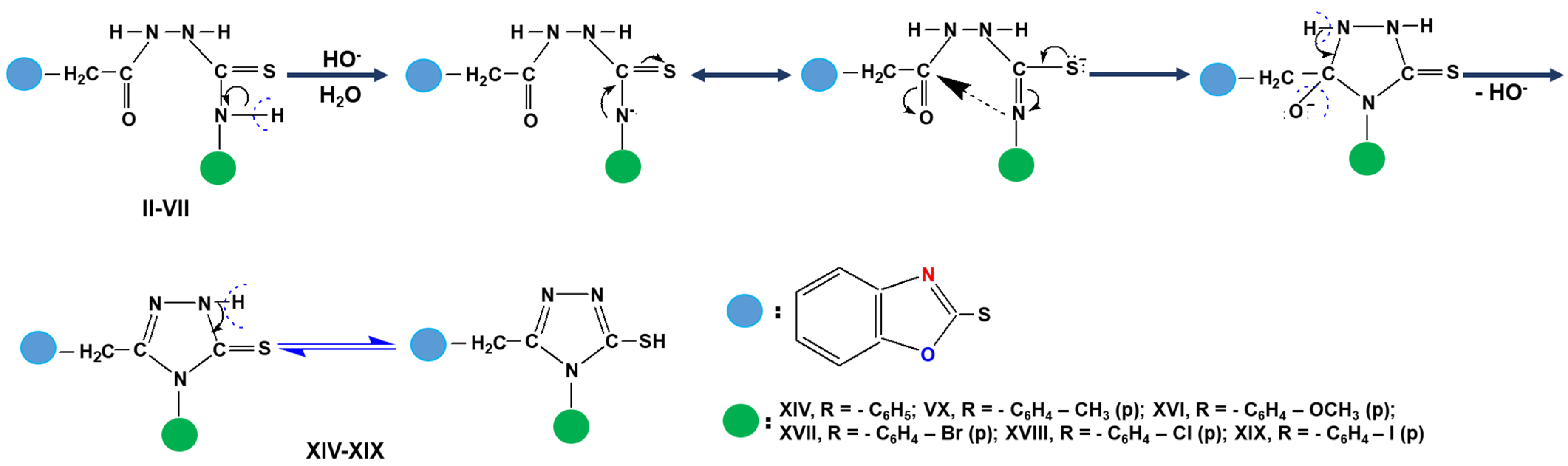
3.5. General Procedure for Synthesis of 3-(Benzoxazole-2′-yl-mercapto-methyl)-4-aryl-5mercapto-1,2,4-triazoles (Compounds XIV–XIX)
3.6. Antibacterial Activity
3.7. Immobilization of 1,2,4-Triazole
3.8. Release of 1,2,4-triazole
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Laraia, L.; Robke, L.; Waldmann, H. Bioactive compound collections: From design to target identification. Chem 2018, 4, 705–730. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Farshori, N.N.; Banday, M.R.; Ahmad, A.; Khan, A.U.; Rauf, A. Synthesis, characterization and in vitro antimicrobial activities of 5-alkenyl/hydroxyalkenyl-2-phenylamine-1,3,4-oxadiazoles and thiadiazoles. Bioorg. Med. Chem. Lett. 2010, 20, 1933–1938. [Google Scholar] [CrossRef] [PubMed]
- Pintilie, O.; Profire, L.; Sunel, V.; Popa, M.; Pui, A. Synthesis and antimicrobial activity of some new 1,3,4-thiadiazole and 1,2,4-triazole compounds having a D,L-methionine moiety. Molecules 2007, 12, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kadi, A.A.; El-Brollosy, N.R.; Al-Deeb, O.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial and antiinflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylomini)-5-substituted-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2007, 42, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.X.; Shi, Y.X.; Weng, J.Q.; Liu, X.H.; Zhao, W.G.; Li, B.J. Synthesis and antifungal activity of novel 1,2,4-triazole derivatives containing 1,2,3-thiadiazole moiety. J. Heterocycl. Chem. 2014, 51, 690–694. [Google Scholar] [CrossRef]
- Klip, N.T.; Capan, G.; Gursoy, A.; Uzun, M.; Satana, D. Synthesis, structure and antifungal evaluation of some novel 1,2,4-triazolylmercaptoacetyl thiosemicarbazide and 1,2,4-triazolylmercaptomethyl-1,3,4 thiadiazole analogs. J. Enzyme Inhib. Med. Chem. 2010, 25, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cao, Y.; Zhang, J.; Yu, S.; Zou, Y.; Chai, X.; Wu, Q.; Zhang, D.; Jiang, Y.; Sun, Q. Design, synthesis and antifugal activities of novel 1,2,4-triazole derivatives. Eur. J. Med. Chem. 2011, 46, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Jain, D.K. Synthesis, antifungal and antibacterial activity of novel 1,2,4-triazole derivatives. J. Adv. Pharm. Technol. Res. 2015, 6, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Solak, N.; Rollas, S. Synthesis and antituberculosis activity of 2-(aryl/alkylamino)-5-(4-aminophenyl)-1,3,4-thiadiazoles and their Schiff bases. Arkivoc 2006, 12, 173–181. [Google Scholar] [CrossRef]
- El-Reedy, A.A.M.; Soliman, N.K. Synthesis, biological activity and molecular modeling study of novel 1,2,4 triazolo[4,3-b] [1,2,4,5] tetrazines and 1,2,4-triazolo [4,3-b] [1,2,4] triazines. Sci. Rep. 2020, 10, 6137. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Kumar, H.; Javed, S.A. Condensed bridgehead nitrogen heterocyclic system: Synthesis and pharmacological activities of 1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazole derivatives of ibuprofen and biphenyl-4-yloxy acetic acid. Eur. J. Med. Chem. 2008, 43, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Javed, S.A.; Khan, S.A.; Amir, M. 1,3,4-Oxadiazole/thiadiazole and 1,2,4-triazole derivatives of bisphenyl-4-yloxy acetic acid: Synthesis and preliminary evaluation of biological properties. Eur. J. Med. Chem. 2008, 43, 2688–2698. [Google Scholar] [CrossRef] [PubMed]
- Salgin-Goksen, U.; Gokhan-Kelekci, N.; Goktas, O.; Koysa, Y.; Kilic, E.; Isik, S.; Aktay, G.; Ozalp, M. 1-Acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: Synthesis, analgesic-antiinflammatory and antimicrobial activities. Bioorg. Med. Chem. 2007, 15, 5738–5751. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Khan, M.S.Y.; Zaman, M.S. Synthesis, characterization and biological activities of substituted oxadiazole, triazole, thiadiazole and 4-thiazolidinone derivatives. Indian J. Chem. 2004, 43B, 2189–2194. [Google Scholar] [CrossRef]
- Clerici, F.; Pocar, D.; Guido, M.; Loche, A.; Perlini, V.; Brufani, M. Synthesis of 2-amino-5-sulfanyl-1,3,4-thiadiazole derivatives and evaluation of their antidepressant and anxiolytic activity. J. Med. Chem. 2001, 44, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Cressier, D.; Prouillac, C.; Hernandez, P.; Amourette, C.; Diserbo, M.; Lion, C.; Rima, G. Synthesis, antioxidant properties and radioprotective effects ofnew benzothiazoles and thiadiazoles. Bioorg. Med. Chem. 2009, 17, 5275–5284. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Bansal, K.K.; Sharma, A.; Sharma, D.; Deep, A. Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem. 2020, 188, 112016. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Abdelhamid, A.O.; Abdelrehem, N.A.; Kandeel, S.M. Efficient synthesis of new benzofuran-based thiazoles and investigation of their cytotoxic activity against human breast carcinoma cell lines. J. Heterocycl. Chem 2018, 55, 995–1001. [Google Scholar] [CrossRef]
- Padmavathi, V.; Reddy, G.S.; Padmaja, A.; Kondaiah, P.; Shazia, A. Synthesis, antimicrobial and cytotoxic activities of 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazoles. Eur. J. Med. Chem. 2009, 44, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Kheder, N.A.; Abdelaziz, M.R.; Mabkhot, Y.N.; Alhajoj, A.M. A facile synthesis and anticancer activity of some novel thiazoles carrying 1,3,4-thiadiazole moiety. Chem. Cent. J. 2017, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Choritos, G.; Trafalis, D.T.; Delezis, P.; Potamitis, C.; Sarli, V.; Zoumpoulakis, P.; Camoutsis, C. Synthesis and anticancer activity of novel 3,6-disubstituted 1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazole derivatives. Arabian J. Chem. 2019, 12, 4784–4794. [Google Scholar] [CrossRef]
- Jain, A.K.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R.K. 1,3,4-Thiadiazole and itas derivatives: A review on recent progress in biological activities. Chem. Biol. Drug Des. 2013, 81, 557–576. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, X.Y.; Chai, K.Y.; Lee, J.S.; Song, M.S.; Quan, Z.S. Synthesis and anticonvulsant evaluation of 4-(4-alkoxylphenyl)-3-ethyl-4H-1,2,4-triazoles as open-chain analogues of 7-alkoxyl-4,5-dihydro[1,2,4]triazolo[4,3-a]quinolines. Bioorg. Med. Chem. 2007, 15, 6775–6781. [Google Scholar] [CrossRef] [PubMed]
- Datar, P.A.; Deokule, T.A. Development of thiadiazole as an antidiabetic agent-A review. Mini Rev. Med. Chem. 2014, 14, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Van der Nieuwendijk, A.M.C.H.; Pietra, D.; Heitman, L.; Goblyos, A.; IJzerman, A.P. Synthesis and biological evaluation of 2,3,5 substituted [1,2,4] thiadiazoles as allosteric modulators of adenosine receptors. J. Med. Chem. 2004, 47, 663–672. [Google Scholar] [CrossRef]
- Leung-Toung, R.; Wodzinka, J.; Li, W.; Lowrie, J.; Kukreja, R.; Desilets, D.; Karimian, K.; Tam, T.F. 1,2,4 thiadiazole: A novel Cathepsin B inhibitor. Bioorg. Med. Chem. 2003, 11, 5529–5537. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, I.B.; Vishnuvardhan, M.V.P.S.; Nagarajan, A.; Kantevari, S.; Kamal, A. Imidazopyridine linked triazoles as tubulin inhibitors, effectively triggering apoptosis in lung cancer cell line. Bioorg. Chem. 2018, 80, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.; Desai, V.; Shingade, S. In-vitro Anti-cancer assay and apoptotic cell pathway of newly synthesized benzaxazole-N-heterocyclic hybrids as potent tyrosine kinase inhibitors. Bioorg. Chem. 2020, 94, 103382. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yang, Q.; Liu, T.; Li, K.; Liu, Y.; Zhu, C.; Zhang, Z.; Li, L.; Zhang, C.; Xie, M.; et al. Design, synthesis and in vitro evaluation of 6-amide-2-aryl benzoxazole/benzimidazole derivatives against tumor cells by inhibiting VEGFR-2 kinase. Eur. J. Med. Chem. 2019, 179, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Belal, A.; Abdelgawad, M.A. New benzothiazole/benzoxazole-pyrazole hybrids with potential as COX inhibitors: Design, synthesis and anticancer activity evaluation. Res. Chem. Intermed. 2017, 43, 3859–3872. [Google Scholar] [CrossRef]
- Padalkar, V.S.; Borse, B.N.; Gupta, V.D.; Phatangare, K.R.; Patil, V.S.; Umape, P.G.; Sekar, N. Synthesis and antimicrobial activity of novel 2-substituted benzimidazole, benzoxazole and benzothiazole derivatives. Arab. J. Chem. 2016, 9, S1125–S1130. [Google Scholar] [CrossRef]
- Seth, K.; Garg, S.K.; Kumar, R.; Purohit, P.; Meena, V.S.; Goyal, R.; Banerjee, U.C.; Chakraborti, A.K. 2-(2-Arylphenyl)benzoxazole as a novel anti-inflammatory scaffold: Synthesis and biological evaluation. ACS Med. Chem. Lett. 2014, 5, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Xu, H.; Wang, W.; Cao, Y.; Dong, G.; Wang, S.; Che, X.; Ji, H.; Miao, Z.; Yao, J.; et al. Design, synthesis and antifungal activity of isosteric analogues of benzoheterocyclic N-myristoyltransferase inhibitors. Eur. J. Med. Chem. 2010, 45, 3531–3540. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.K.; Lee, R.Y.; Kim, N.Y.; Kim, Y.H.; Song, A.L. Synthesis and antifungal activity of benzo[d]oxazole-4,7-diones. Bioorg. Med. Chem. Lett. 2009, 19, 5924–5926. [Google Scholar] [CrossRef]
- Jauhari, P.K.; Bhavani, A.; Varalwar, S.; Singhal, K.; Raj, P. Synthesis of some novel 2-substituted benzoxazoles as anticancer, antifugal and antimicrobial agents. Med. Chem. Res. 2008, 17, 412–424. [Google Scholar] [CrossRef]
- Haider, S.; Alam, M.S.; Hamid, H.; Shafi, S.; Nargotra, A.; Mahajan, P.; Nazreen, S.; Kalle, A.M.; Kharbanda, C.; Ali, Y.; et al. Synthesis of novel 1,2,3-triazole based benzoxazolinones: Their TNF-β based molecular docking with in-vivo anti-inflammatory, antinociceptive activities and ulcerogenic risk evaluation. Eur. J. Med. Chem. 2013, 70, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Kumar, S.; Narasimhan, B.; Lim, S.M.; Ramasamy, K.; Mani, V.; Shah, S.A.A. Design, synthesis and biological potential of heterocyclic benzoxazole scaffolds as promising antimicrobial and anticancer agents. Chem. Cent. J. 2018, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Duroux, R.; Renault, N.; Cuelho, J.E.; Agouridas, L.; Blum, D.; Lopes, L.V.; Melnyk, P.; Yous, S. Design, synthesis and evaluation of 2-aryl benzoxazoles as promising hit for the A2A receptor. J. Enzyme Inhib. Med. Chem. 2017, 32, 850–864. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.; Chavan, V. Exploration of the biological potential of benzoxazoles: An overview. Mini Rev. Org. Chem. 2019, 16, 111–126. [Google Scholar] [CrossRef]
- Padmavathi, V.; Kumara, C.P.; Venkatesh, B.C.; Padmaja, A. Synthesis and antimicrobial activity of amido linked pyrrolyl and pyrazolyl-oxazoles, thiazoles and imidazoles. Eur. J. Med. Chem. 2011, 46, 5317–5326. [Google Scholar] [CrossRef] [PubMed]
- Racovita, S.; Baranov, N.; Macsim, A.M.; Lionte, C.; Cheptea, C.; Sunel, V.; Popa, M.; Vasiliu, S.; Desbrieres, J. New grafted copolymers carrying betaine units based on gellan and N-vinylimidazole as precursors for design of drug delivery systems. Molecules 2020, 25, 5451. [Google Scholar] [CrossRef] [PubMed]
- Aparaschivei, R.; Holban, M.; Sunel, V.; Popa, M.; Desbrieres, J. Synthesis and characterization of new heterocyclic compounds with potential antituberculosis activity and their immobilization on polymer supports. Cellul. Chem. Technol. 2012, 46, 301–306. [Google Scholar]
- Cheptea, C.; Sunel, V.; Desbrieres, J.; Popa, M. Synthesis and antimicrobial activity of new derivatives of 1,3,4-thiadiazoles and 1,2,4-triazoles with 5-nitroindazole as support. J. Heterocycl. Chem. 2013, 50, 366–372. [Google Scholar] [CrossRef]
- Moise, M.; Sunel, V.; Profire, L.; Popa, M.; Desbrieres, J.; Peptu, C. 1,4-Disubstituted thiosemicarbazides with potential tuberculostatic action. Bull. Inst. Polit. Iasi. 2009, 55, 57–64. [Google Scholar]
- Moise, M.; Sunel, V.; Profire, L.; Popa, M.; Desbrieres, J.; Peptu, C. Synthesis and biological activity of some new 1,3,4-thiadiazole and 1,2,4-triazole compounds containing a phenylalanine moiety. Molecules 2009, 14, 2621–2631. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Sharma, J.; Amir, M. Synthesis and antimicrobial activities of 1,2,4-triazole and 1,3,4-thiadiazole derivatives of 5-amino-2-hydroxybenzoic acid. J. Chem. 2008, 5, 963–968. [Google Scholar] [CrossRef]
- Cheptea, C.; Sunel, V.; Morosanu, C.; Dorohoi, D.O. Derivatives of 1,2,4-triazole-3,4-disubstituted based on aminoaxids with potential biological activity. Rev. Chim. 2020, 71, 211–221. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1368. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravot, R.K. Pore and solid diffusion models for fixed bed adsorbents. AiChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Dubinin, M.M.; Zaverina, E.D.; Radushkevich, L.V. Sorption and structure of active carbons. I. Adsorption of organic vapors. Zhurnal Fizicheskoi Khimii. 1947, 21, 1351–1362. [Google Scholar]
- Chabani, M.; Amrane, A.; Bensmaili, A. Kinetic modelling of the adsorption of nitrates by ion exchange resin. Chem. Eng. J. 2006, 125, 111–117. [Google Scholar] [CrossRef]
- Yu, Y.; Zhuang, Y.Y.; Wang, Z.H.; Qiu, M.Q. Adsorption of water-soluble dyes onto modified resin. Chemosphere 2004, 54, 425–430. [Google Scholar] [CrossRef]
- Rodriguez, A.; Garcia, J.; Ovejero, G.; Mestanza, M. Adsorption of anionic and cationic dyes on activated carbon from aqueous solutions: Equilibrium and kinetics. J. Hazard. Mat. 2009, 172, 1311–1320. [Google Scholar] [CrossRef]
- Cigu, T.A.; Vasiliu, S.; Racovita, S.; Lionte, C.; Sunel, V.; Popa, M.; Cheptea, C. Adsorption and release studies of new cephalosporin from chitosan-g-poly(glycidyl methacrylate) microparticles. Eur. Polym. J. 2016, 82, 132–156. [Google Scholar] [CrossRef]
- Lagergren, S.; Svenska, B.K. Zur theorie der sogenannten adsorption geloester stoffe. Kungl. Sven. Vetenskapsakad. Handl. 1898, 24, 1–3. [Google Scholar]
- Ho, Y.S.; McKay, G. The kinetics of sorption of basic dyes from aqueous solutions by sphagnum moss peat. Can. J. Chem. Eng. 1998, 76, 822–827. [Google Scholar] [CrossRef]
- Higuchi, W.I. Diffusional models useful in biopharmaceutics. J. Pharm. Sci. 1967, 56, 315–324. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]




| Samples | Elemental Analysis, Calc (Found) (%) | Chemical Formula | Yield (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | Br | Cl | I | |||
| 1,4-disubstituted thiosemicarbazides (compounds II–VII) | ||||||||||
| II | 53.6 (53.9) | 3.9 (4.2) | 15.6 (16) | 17.9 (18.2) | 8.9 (7.7) | - | - | - | C16H14N4O2S2 | 65 |
| III | 54.8 (55.1) | 4.3 (4.5) | 15.1 (15.4) | 17.2 (17.6) | 8.6 (7.4) | - | - | - | C17H16N4O2S2 | 75 |
| IV | 52.6 (52.9) | 4.1 (4.3) | 14.4 (14.8) | 16.4 (16,9) | 12.5 (11.1) | - | - | - | C17H16N4O3S2 | 85 |
| V | 43.9 (44.1) | 2.9 (3.2) | 12.8 (12.9) | 14.6 (15) | 7.4 (6.3) | 18.3 (18.6) | - | - | C16H13N4O2S2Br | 80 |
| VI | 48.9 (49) | 3.3 (3.6) | 14.3 (14.6) | 16.3 (16.6) | 8.2 (6.8) | - | 9 (9.5) | - | C16H13N4O2S2Cl | 78 |
| VII | 39.7 (39.9) | 2.7 (2.9) | 11.6 (11.9) | 13.2 (13.6) | 6.6 (5.2) | - | - | 26.2 (26.6) | C16H13N4O2S2I | 77 |
| 1,3,4-thiadiazoles (compounds VIII–XIII) | ||||||||||
| VIII | 56.5 (56.6) | 3.5 (3.8) | 16.5 (16.8) | 18.8 (19.2) | 4.7 (3.6) | - | - | - | C16H12N4OS2 | 89 |
| IX | 57.6 (58) | 4 (4.1) | 15.8 (16.1) | 18.1 (18.5) | 4.5 (3.3) | - | - | - | C17H14N4OS2 | 83 |
| X | 52.1 (52.4) | 3.8 (4) | 15.1 (15.4) | 17.3 (17.6) | 11.7 (10.6) | - | - | - | C17H14N4O2S2 | 81 |
| XI | 45.8 (46.2) | 2.6 (2.8) | 13.4 (13.6) | 15.3 (15.7) | 22.9 (21.7) | 19 (19.4) | - | - | C16H11N4OS2Br | 79 |
| XII | 51.3 (51.4) | 2.9 (3.1) | 15 (15.3) | 17.1 (17.4) | 13.7 (12.8) | - | 9.5 (9.7) | - | C16H11N4OS2Cl | 74 |
| XIII | 41.2 (41.5) | 2.4 (2.6) | 12 (12.3) | 13.7 (14.1) | 30.7 (29.2) | - | - | 27.3 (27.5) | C16H11N4OS2I | 71 |
| 1,2,4-triazoles (compounds XIV–XIX) | ||||||||||
| XIV | 56.5 (56.7) | 3.5 (3.9) | 16.5 (16.9) | 18.8 (19.2) | 4.7 (3.3) | - | - | - | C16H12N4OS2 | 66 |
| XV | 57.6 (57.8) | 4 (4.3) | 15.8 (16.2) | 18.1 (18.3) | 4.5 (3.4) | - | - | - | C17H14N4OS2 | 74 |
| XVI | 55.1 (55.3) | 3.8 (4) | 15.1 (15.5) | 17.3 (17.7) | 8.7 (7.5) | - | - | - | C17H14N4O2S2 | 88 |
| XVII | 45.8 (46) | 2.6 (3) | 13.4 (13.7) | 15.3 (15.7) | 22.9 (21.6) | 19 (19.3) | - | - | C16H11N4OS2Br | 79 |
| XVIII | 51.3 (51.4) | 2.9 (3.3) | 15 (15.2) | 17.1 (17.4) | 13.7 (12.7) | - | 9.5 (9.9) | - | C16H11N4OS2Cl | 77 |
| XIX | 41.2 (41.4) | 2.4 (2.5) | 12 (12.4) | 13.7 (14) | 30.7 (29.7) | - | - | 27.3 (27.6) | C16H11N4OS2I | 74 |
| Sample Code | Time for Thermostating (h) | Staphylococcus aureus | Bacillus subtilis | Bacillus cereus | Salmonella enteritidis | Escherichia coli | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/mL | mg/mL | mg/mL | mg/mL | mg/mL | ||||||||||||
| 1 | 0.5 | 0.25 | 1 | 0.5 | 0.25 | 1 | 0.5 | 0.25 | 1 | 0.5 | 0.25 | 1 | 0.5 | 0.25 | ||
| VIII | 24 | - | + | • | - | - | • | + | + | + | + | + | + | - | - | • |
| 48 | - | - | - | - | - | • | - | + | + | + | + | + | - | • | • | |
| IX | 24 | • | • | - | • | • | + | + | + | + | + | + | + | - | • | • |
| 48 | • | • | - | • | • | + | + | + | + | + | + | + | • | • | • | |
| X | 24 | • | • | • | • | • | - | + | - | - | + | + | + | - | - | • |
| 48 | • | • | • | • | + | - | + | + | + | + | + | - | • | • | • | |
| XI | 24 | + | • | • | • | • | • | + | + | + | + | + | + | • | • | • |
| 48 | + | • | + | • | • | + | + | + | + | + | + | + | • | • | • | |
| XII | 24 | • | • | • | • | • | + | + | + | + | + | + | + | • | • | • |
| 48 | • | + | • | • | • | + | + | + | + | + | + | + | • | • | • | |
| XIII | 24 | • | + | • | • | • | + | + | + | + | + | + | + | • | • | • |
| 48 | • | + | • | • | • | + | + | + | + | + | + | + | • | • | • | |
| XIV | 24 | - | • | • | - | - | - | - | • | • | - | - | - | - | - | - |
| 48 | • | + | + | • | + | + | + | + | + | - | - | - | - | - | - | |
| XV | 24 | - | - | - | - | - | - | • | • | • | - | - | - | - | - | - |
| 48 | - | • | • | - | - | - | + | + | + | - | - | - | - | - | - | |
| XVI | 24 | - | - | - | - | - | - | • | • | • | - | - | - | - | - | - |
| 48 | - | - | • | - | - | - | + | + | + | - | - | - | - | - | - | |
| XVII | 24 | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| 48 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| XVIII | 24 | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| 48 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| XIX | 24 | • | • | • | • | • | • | • | • | • | • | • | • | - | • | • |
| 48 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Kanamycine | 24 | - | - | - | - | - | - | - | - | - | - | - | + | - | - | - |
| 48 | - | - | - | - | - | - | - | - | • | - | - | + | - | - | + | |
| PG | PGB1 | |||||
|---|---|---|---|---|---|---|
| 298 | 303 | 308 | 298 | 303 | 308 | |
| Langmuir model | ||||||
| qm (mg/g) | 478 | 533 | 636 | 537 | 616 | 685 |
| KL (L/mg) | 0.068 | 0.086 | 0.109 | 0.098 | 0.126 | 0.161 |
| RL | 0.01–0.29 | 0.01–0.25 | 0.10–0.20 | 0.01–0.22 | 0.01–0.18 | 0.004–0.15 |
| χ2 | 3.324 | 2.613 | 3.429 | 2.700 | 2.010 | 2.150 |
| R2 | 0.994 | 0.993 | 0.997 | 0.993 | 0.992 | 0.996 |
| Freundlich model | ||||||
| KF (L/g) | 0.457 | 0.512 | 0.617 | 0.528 | 0.602 | 0.673 |
| 1/nf | 0.939 | 0.738 | 0.729 | 0.686 | 0.540 | 0.383 |
| χ2 | 33.462 | 23.958 | 38.332 | 25.213 | 19.219 | 18.782 |
| R2 | 0.911 | 0.920 | 0.915 | 0.913 | 0.921 | 0.914 |
| Dubinin–Radushkevich model | ||||||
| qDR (mg/g) | 463 | 521 | 629 | 532 | 612 | 679 |
| E (kJ/mol) | 1.099 | 1.561 | 2.171 | 1.357 | 2.608 | 3.714 |
| χ2 | 0.402 | 0.359 | 0.413 | 0.371 | 0.226 | 0.277 |
| R2 | 0.998 | 0.997 | 0.998 | 0.998 | 0.999 | 0.998 |
| Sample Code | ΔH (kJ/mol) | ΔS (J/mol⋅K) | R2 | ΔG (kJ/mol) | ||
|---|---|---|---|---|---|---|
| 298 K | 303 K | 308 K | ||||
| PG | 36.49 | 100.05 | 0.995 | −29.78 | −30.278 | −30.78 |
| PGB1 | 37.67 | 107.14 | 0.998 | −31.89 | −32.425 | −32.96 |
| C1,2,4-triazole (g/mL) | PG | PGB1 | |||||
|---|---|---|---|---|---|---|---|
| 298 | 303 | 308 | 298 | 303 | 308 | ||
| qe,exp (mg/g) | 325 | 394 | 473 | 412 | 496 | 587 | |
| 3.6 × 10−4 | Lagergren model | ||||||
| qe,calc (mg/g) | 346.29 | 415.91 | 491.95 | 428.82 | 511.76 | 600.94 | |
| k1 (×103 min−1) | 2.19 | 3.01 | 3.31 | 3.83 | 3.92 | 4.14 | |
| χ2 | 1.738 | 2.263 | 1.119 | 1.119 | 1.682 | 1.103 | |
| R2 | 0.998 | 0.998 | 0.998 | 0.999 | 0.998 | 0.999 | |
| Ho model | |||||||
| qe,calc (mg/g) | 449.72 | 482.38 | 564.84 | 579.40 | 616.92 | 696.22 | |
| k2 [×106 (g/mg⋅ min)] | 1.26 | 1.62 | 2.43 | 2.89 | 3.51 | 3.92 | |
| χ2 | 4.014 | 4.686 | 5.976 | 2.411 | 5.499 | 5.040 | |
| R2 | 0.997 | 0.997 | 0.997 | 0.998 | 0.997 | 0.998 | |
| 3.6 × 10−3 | qe,exp (mg/g) | 432 | 491 | 593 | 507 | 583 | 659 |
| Lagergren model | |||||||
| qe,calc (mg/g) | 473.63 | 523.96 | 622.21 | 531.87 | 606.53 | 679.53 | |
| k1 (×103 min−1) | 3.10 | 3.52 | 4.01 | 4.13 | 4.27 | 4.87 | |
| χ2 | 1.456 | 1.446 | 1.569 | 1.312 | 1.852 | 1.786 | |
| R2 | 0.998 | 0.999 | 0.998 | 0.997 | 0.998 | 0.997 | |
| Ho model | |||||||
| qe,calc (mg/g) | 546.70 | 621.17 | 709.71 | 649.28 | 694.28 | 766.03 | |
| k2 [×106 (g/mg⋅ min)] | 2.19 | 2.46 | 3.11 | 3.07 | 3.72 | 4.01 | |
| χ2 | 2.071 | 2.539 | 2.390 | 2.772 | 2.083 | 2.310 | |
| R2 | 0.997 | 0.996 | 0.997 | 0.995 | 0.997 | 0.996 | |
| 7 × 10−3 | qe,exp (mg/g) | 449 | 504 | 606 | 519 | 594 | 667 |
| Lagergren model | |||||||
| qe,calc (mg/g) | 463.71 | 527.91 | 622.32 | 533.86 | 621.43 | 681.07 | |
| k1 (×103 min−1) | 3.42 | 3.96 | 4.43 | 4.60 | 4.90 | 5.10 | |
| χ2 | 1.722 | 1.949 | 1.654 | 1.034 | 1.326 | 1.764 | |
| R2 | 0.997 | 0.997 | 0.998 | 0.998 | 0.997 | 0.999 | |
| Ho model | |||||||
| qe,calc (mg/g) | 567.15 | 613.04 | 713.83 | 621.28 | 734.63 | 796.60 | |
| k2 [×106 (g/mg min)] | 3.09 | 3.87 | 4.11 | 3.35 | 4.19 | 5.17 | |
| χ2 | 2.609 | 2.913 | 1.895 | 2.776 | 2.864 | 3.775 | |
| R2 | 0.996 | 0.997 | 0.996 | 0.997 | 0.997 | 0.998 | |
| 15 × 10−3 | qe,exp (mg/g) | 457 | 512 | 618 | 529 | 601 | 672 |
| Lagergren model | |||||||
| qe,calc (mg/g) | 483.63 | 532.96 | 644.21 | 544.25 | 627.05 | 694.28 | |
| k1 (×103 min−1) | 3.67 | 4.67 | 4.81 | 4.92 | 5.14 | 5.48 | |
| χ2 | 1.466 | 1.343 | 1.691 | 1.144 | 1.671 | 1.024 | |
| R2 | 0.997 | 0.999 | 0.998 | 0.998 | 0.999 | 0.999 | |
| Ho model | |||||||
| qe,calc (mg/g) | 568.17 | 624.11 | 754.83 | 635.77 | 729.27 | 783.34 | |
| k2 [×106 (g/mg min)] | 3.85 | 4.38 | 5.83 | 4.23 | 4.97 | 5.73 | |
| χ2 | 3.050 | 3.208 | 3.903 | 3.887 | 4.321 | 3.678 | |
| R2 | 0.996 | 0.997 | 0.996 | 0.998 | 0.997 | 0.997 | |
| Sample Code | Higuchi Model | Korsmeyer–Peppas Model | |||
|---|---|---|---|---|---|
| kH (h−1/2) | R2 | kr (min−n) | n | R2 | |
| PG-T | 0.324 | 0.993 | 0.014 | 0.534 | 0.995 |
| PGB1-T | 0.294 | 0.994 | 0.017 | 0.634 | 0.996 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranov, N.; Racovita, S.; Vasiliu, S.; Macsim, A.M.; Lionte, C.; Sunel, V.; Popa, M.; Desbrieres, J.; Cheptea, C. Immobilization and Release Studies of Triazole Derivatives from Grafted Copolymer Based on Gellan-Carrying Betaine Units. Molecules 2021, 26, 3330. https://doi.org/10.3390/molecules26113330
Baranov N, Racovita S, Vasiliu S, Macsim AM, Lionte C, Sunel V, Popa M, Desbrieres J, Cheptea C. Immobilization and Release Studies of Triazole Derivatives from Grafted Copolymer Based on Gellan-Carrying Betaine Units. Molecules. 2021; 26(11):3330. https://doi.org/10.3390/molecules26113330
Chicago/Turabian StyleBaranov, Nicolae, Stefania Racovita, Silvia Vasiliu, Ana Maria Macsim, Catalina Lionte, Valeriu Sunel, Marcel Popa, Jacques Desbrieres, and Corina Cheptea. 2021. "Immobilization and Release Studies of Triazole Derivatives from Grafted Copolymer Based on Gellan-Carrying Betaine Units" Molecules 26, no. 11: 3330. https://doi.org/10.3390/molecules26113330
APA StyleBaranov, N., Racovita, S., Vasiliu, S., Macsim, A. M., Lionte, C., Sunel, V., Popa, M., Desbrieres, J., & Cheptea, C. (2021). Immobilization and Release Studies of Triazole Derivatives from Grafted Copolymer Based on Gellan-Carrying Betaine Units. Molecules, 26(11), 3330. https://doi.org/10.3390/molecules26113330









