Strategies for the Removal of Polysaccharides from Biorefinery Lignins: Process Optimization and Techno Economic Evaluation
Abstract
1. Introduction
2. Results and Discussion
2.1. Alkaline Hydrolysis-Acid Precipitation Treatment
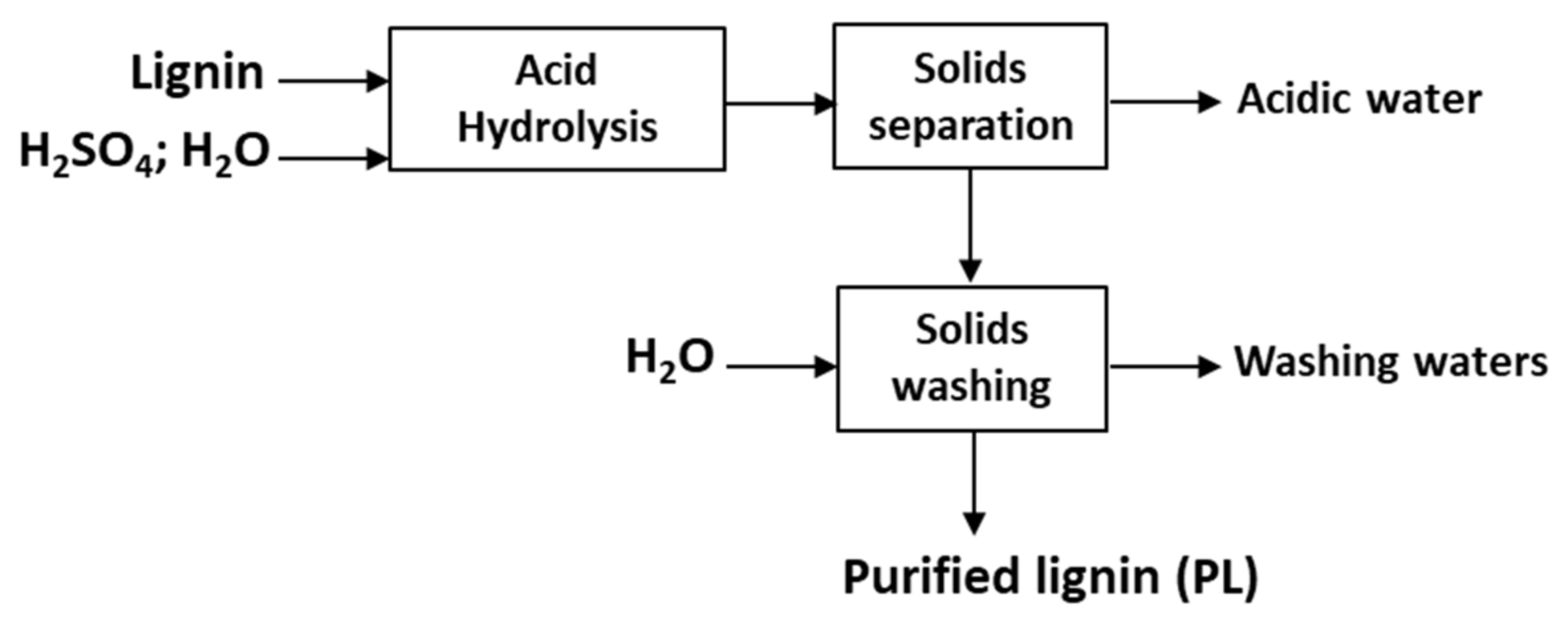
2.2. Acid Hydrolysis Treatment
2.3. Assessment of Industrial Viability
2.3.1. Alkaline Hydrolysis-Acid Precipitation
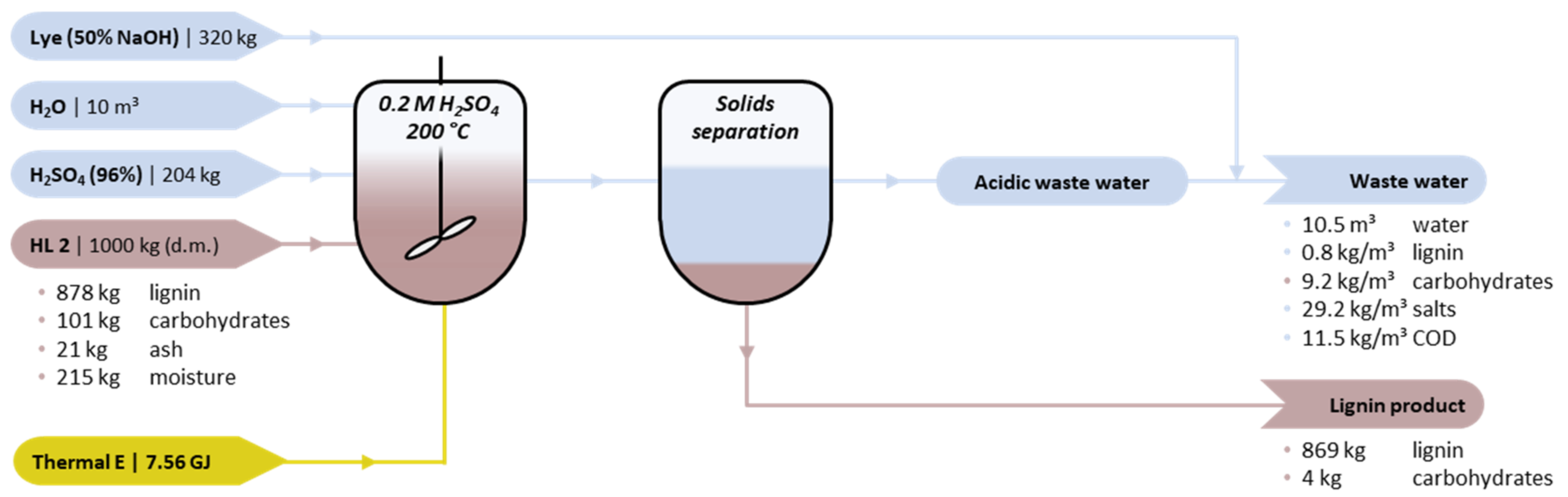
2.3.2. Acid Hydrolysis
3. Materials and Methods
3.1. Chemicals
3.2. Alkaline Hydrolysis-Acid Precipitation
3.3. Acid Hydrolysis
3.4. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mussatto, S.I. Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier Science: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Ek, M.; Gellerstedt, G.; Henriksson, G. Wood Chemistry and Wood Biotechnology; Walter de Gruyter GmbH: Berlin, Germany, 2016. [Google Scholar]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Pulkki, R.; Xu, C. Global lignin supply overview and kraft lignin potential as an alternative for petroleum-based polymers. Renew. Sust. Energ. Rev. 2020, 123, 109768. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. Chemsuschem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- Tribot, A.; Amer, G.; Alio, M.A.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.D.; Callois, J.M.; Vial, C.; Michaud, P.; et al. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Obydenkova, S.V.; Kouris, P.D.; Hensen, E.J.M.; Smeulders, D.M.J.; van der Meer, Y.; Boot, M.D. Industrial lignin from 2G biorefineries—Assessment of availability and pricing strategies. Bioresour. Technol. 2019, 291. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Takkellapati, S. The current and emerging sources of technical lignins and their applications. Biofuel Bioprod. Biorefin. 2018, 12, 756–787. [Google Scholar] [CrossRef]
- Miettinen, M. Method for Making a Lignin Component, a Lignin Component and Its Use and a Product. U.S. Patent Application No. 14/363976, 20 November 2014. [Google Scholar]
- Arbiom Inc. Method for Fractionating a Lignocellulosic Biomass. U.S. Patent Application No. 15/368,648, 8 June 2017. [Google Scholar]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. Bioresources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Yin, E.Q.; Zhou, Z.; Wang, S.C.; Zhu, M.F. Effects of Purification on the Structure and Properties of Hardwood Kraft Lignin; Springer: Singapore, 2018; pp. 741–752. [Google Scholar]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crops Prod. 2019, 139. [Google Scholar] [CrossRef]
- Nirmale, T.C.; Kale, B.B.; Varma, A.J. A review on cellulose and lignin based binders and electrodes: Small steps towards a sustainable lithium ion battery. Int. J. Biol. Macromol. 2017, 103, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Delgado, N.; Ysambertt, F.; Chavez, G.; Bravo, B.; Garcia, D.E.; Santos, J. Valorization of Kraft Lignin of Different Molecular Weights as Surfactant Agent for the Oil Industry. Waste Biomass Valori. 2019, 10, 3383–3395. [Google Scholar] [CrossRef]
- Ouyang, X.P.; Ke, L.X.; Qiu, X.Q.; Guo, Y.X.; Pang, Y.X. Sulfonation of Alkali Lignin and Its Potential Use in Dispersant for Cement. J. Dispers. Sci. Technol. 2009, 30, 1–6. [Google Scholar] [CrossRef]
- Souto, F.; Calado, V.; Pereira, N. Lignin-based carbon fiber: A current overview. Mater. Res. Express 2018, 5. [Google Scholar] [CrossRef]
- Nunes, K.S.D.; Pardini, L.C. Purification and characterization methods for lignin biomass as a potential precursor for carbon materials. Cellul. Chem. Technol. 2019, 53, 227–242. [Google Scholar] [CrossRef]
- Solt, P.; Rossiger, B.; Konnerth, J.; van Herwijnen, H.W.G. Lignin Phenol Formaldehyde Resoles Using Base-Catalysed Depolymerized Kraft Lignin. Polymers 2018, 10, 1162. [Google Scholar] [CrossRef]
- Ortiz, P.; Vendamme, R.; Eevers, W. Fully Biobased Epoxy Resins from Fatty Acids and Lignin. Molecules 2020, 25, 1158. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwenhove, I.; Renders, T.; Lauwaert, J.; De Roo, T.; De Clercq, J.; Verberckmoes, A. Biobased Resins Using Lignin and Glyoxal. ACS Sustain. Chem. Eng. 2020, 8, 18789–18809. [Google Scholar] [CrossRef]
- Vendamme, R.; de Bueren, J.B.; Gracia-Vitoria, J.; Isnard, F.; Mulunda, M.M.; Ortiz, P.; Wadekar, M.; Vanbroekhoven, K.; Wegmann, C.; Buser, R.; et al. Aldehyde-Assisted Lignocellulose Fractionation Provides Unique Lignin Oligomers for the Design of Tunable Polyurethane Bioresins. Biomacromolecules 2020, 21, 4135–4148. [Google Scholar] [CrossRef]
- Wang, C.; Kelley, S.S.; Venditti, R.A. Lignin-Based Thermoplastic Materials. Chemsuschem 2016, 9, 770–783. [Google Scholar] [CrossRef]
- European Commission. The European Green Deal; Brussels, 11.12.2019, COM (2019) 640 Final; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Sazanov, Y.N.; Popova, E.N.; Sumerskii, I.V.; Mokeev, M.V.; Kulikova, E.M.; Litvinova, L.S.; Kever, E.E.; Krutov, S.M.; Gribanov, A.V. Thermochemical Transformations of Hydrolysis Lignin. Russ. J. Appl. Chem. 2010, 83, 1607–1614. [Google Scholar] [CrossRef]
- Fang, W.W.; Alekhina, M.; Ershova, O.; Heikkinen, S.; Sixta, H. Purification and characterization of kraft lignin. Holzforschung 2015, 69, 943–950. [Google Scholar] [CrossRef]
- Prado, R.; Erdocia, X.; Serrano, L.; Labidi, J. Lignin purification with green solvents. Cellul. Chem. Technol. 2012, 46, 221–225. [Google Scholar]
- Servaes, K.; Varhimo, A.; Dubreuil, M.; Bulut, M.; Vandezande, P.; Siika-aho, M.; Sirvio, J.; Kruus, K.; Porto-Carrero, W.; Bongers, B. Purification and concentration of lignin from the spent liquor of the alkaline oxidation of woody biomass through membrane separation technology. Ind. Crops Prod. 2017, 106, 86–96. [Google Scholar] [CrossRef]
- Mbotchak, L.; Le Morvan, C.; Duong, K.L.; Rousseau, B.; Tessier, M.; Fradet, A. Purification, Structural Characterization, and Modification of Organosolv Wheat Straw Lignin. J. Agric. Food Chem. 2015, 63, 5178–5188. [Google Scholar] [CrossRef] [PubMed]
- Green, D.W.; Southard, M.Z. Perry’s Chemical Engineers’ Handbook, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2019; Chapter 9. [Google Scholar]
- Pintaric, Z.N.; Kravanja, Z. The Importance of using Discounted Cash Flow Methodology in Techno-economic Analyses of Energy and Chemical Production Plants. J. Sustain. Dev. Energy Water Environ. Syst. 2017, 5, 163–176. [Google Scholar] [CrossRef][Green Version]
- Zoia, L.; Salanti, A.; Tolppa, E.L.; Ballabio, D.; Orlandi, M. Valorization of Side-Streams from a SSF Biorefinery Plant: Wheat Straw Lignin Purification Study. Bioresources 2017, 12, 1680–1696. [Google Scholar] [CrossRef]
- Klamrassamee, T.; Tana, T.; Laosiripojana, N.; Moghaddam, L.; Zhang, Z.Y.; Rencoret, J.; Gutierrez, A.; del Rio, J.C.; Doherty, W.O.S. Effects of an alkali-acid purification process on the characteristics of eucalyptus lignin fractionated from a MIBK-based organosolv process. RSC Adv. 2016, 6, 92638–92647. [Google Scholar] [CrossRef]
- Evstigneev, E.I. Factors affecting lignin solubility. Russ. J. Appl. Chem. 2011, 84, 1040–1045. [Google Scholar] [CrossRef]
- Evstigneyev, E.I.; Shevchenko, S.M. Structure, chemical reactivity and solubility of lignin: A fresh look. Wood Sci. Technol. 2019, 53, 7–47. [Google Scholar] [CrossRef]
- Dai, L.X.; Lu, H.M.; Zhang, L.P. The Purification of Industrial Alkali Lignin with Xylanase. In Biotechnology, Chemical and Materials Engineering III, Pts 1 and 2; Sung, W.P., Chen, R., Eds.; Advanced Materials Research: Stafa-Zurich, Switzerland, 2014; Volume 884–885, pp. 598–602. [Google Scholar]
- Singh, R.; Singh, S.; Trimukhe, K.D.; Pandare, K.V.; Bastawade, K.B.; Gokhale, D.V.; Varma, A.J. Lignin-carbohydrate complexes from sugarcane bagasse: Preparation, purification, and characterization. Carbohydr. Polym. 2005, 62, 57–66. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Chen, W.S.; Xia, Q.Q.; Guo, B.T.; Wang, Q.W.; Liu, S.X.; Liu, Y.X.; Li, J.; Yu, H.P. Efficient Cleavage of Lignin-Carbohydrate Complexes and Ultrafast Extraction of Lignin Oligomers from Wood Biomass by Microwave-Assisted Treatment with Deep Eutectic Solvent. Chemsuschem 2017, 10, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Chen, H.Z. Carbohydrate elimination of alkaline-extracted lignin liquor by steam explosion and its methylolation for substitution of phenolic adhesive. Ind. Crops Prod. 2014, 53, 93–101. [Google Scholar] [CrossRef]
- De Carvalho, D.M.; Colodette, J.L. Comparative Study of Acid Hydrolysis of Lignin and Polysaccharides in Biomasses. Bioresources 2017, 12, 6907–6923. [Google Scholar] [CrossRef]
- Wei, Z.M.; Li, Y.M.; Cai, F.J.; Hou, Y. Contribution of Lignin from Different Bioresources to the Pollution Load. Bioresources 2018, 13, 9053–9065. [Google Scholar] [CrossRef]
- Hodasova, L.; Jablonsky, M.; Skulcova, A.; Haz, A. Lignin, potential products and their market value. Wood Res. 2015, 60, 973–986. [Google Scholar]
- Barker, C. Europe Caustic Soda Q1 Price Falls 10% as Market Readjusts. ICIS. Available online: https://www.icis.com/explore/resources/news/2020/01/28/10461663/europe-caustic-soda-q1-price-falls-10-as-market-readjusts (accessed on 28 January 2020).
- Little, A. Price and Market Trends: US Sulphuric Acid Prices Hit 6-Year Highs. ICIS. Available online: https://www.icis.com/explore/resources/news/2018/04/06/10209494/price-and-market-trends-us-sulphuric-acid-prices-hit-6-year-highs/ (accessed on 6 April 2018).
- Baghel, S.; Sahariah, B.P.; Anandkumar, J. Bioremediation of Lignin-Rich Pulp and Paper Industry Effluent; Springer: Singapore, 2020. [Google Scholar]
- European Comission. Quarterly report on European Gas Markets. Mark. Obs. Energy Eur Comm. 2019, 12, 1–43. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Laboratory Analytical Procedure. NREL 2008, 1617, 1–6. [Google Scholar]
- Meng, X.; Crestini, C.; Ben, H.; Hao, N.; Pu, Y.; Ragauskas, A.J.; Argyropoulos, D.S. Determination of hydroxyl groups in biorefinery resources via quantitative 31P NMR spectroscopy. Nat. Protoc. 2019, 14, 2627–2647. [Google Scholar] [CrossRef] [PubMed]
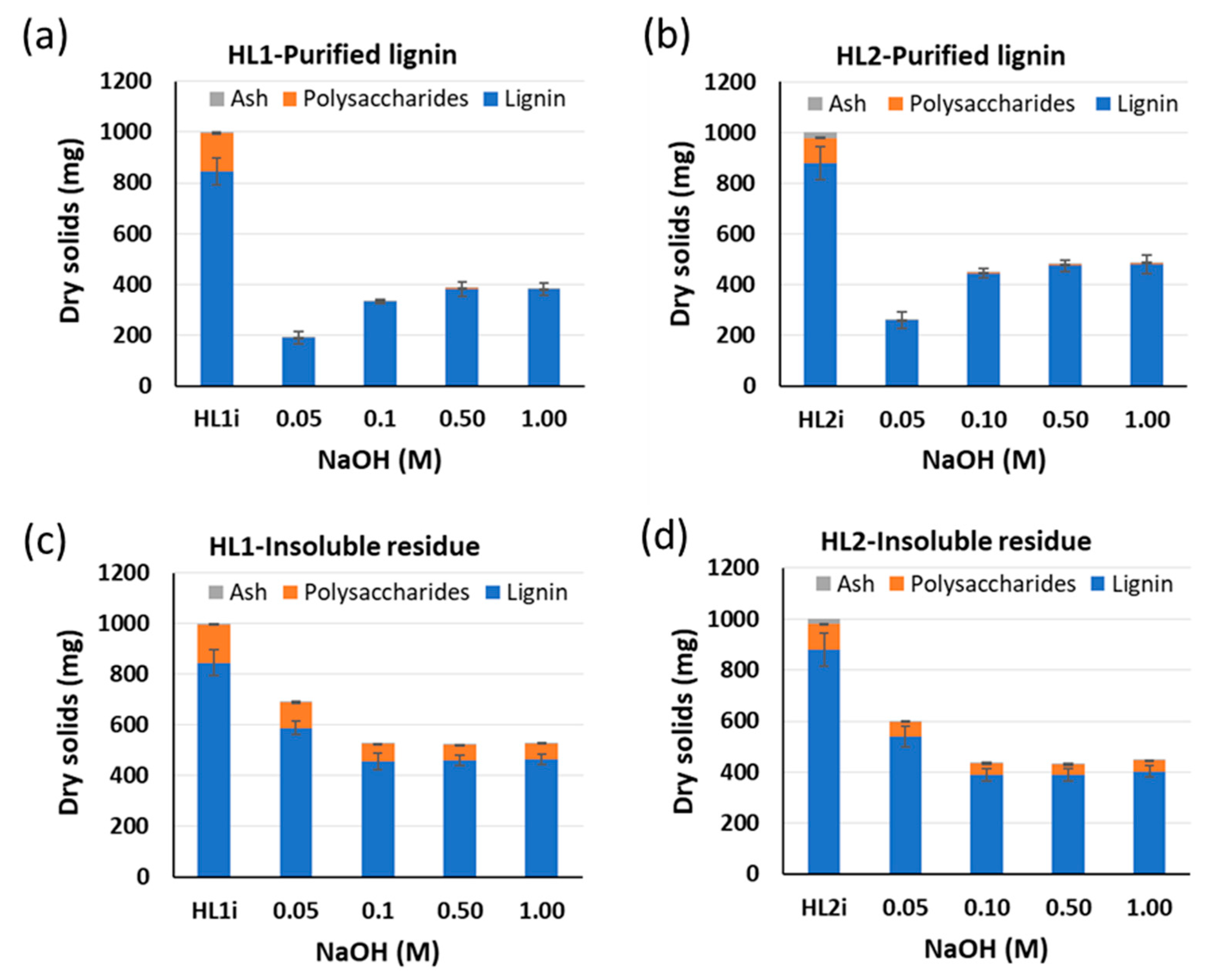
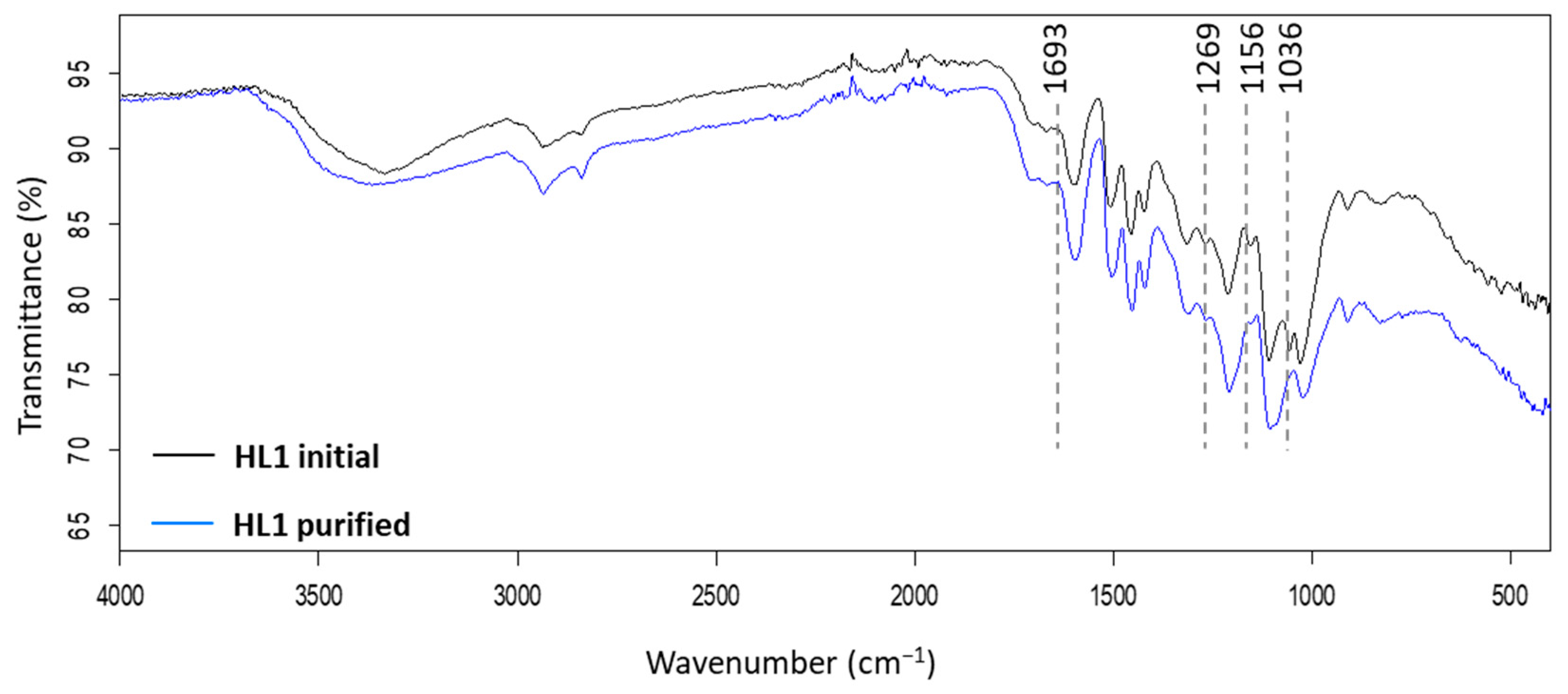
 120 °C). Yields of purified lignins ≥ 95%. Standard deviation error bars of duplicate experiments.
120 °C). Yields of purified lignins ≥ 95%. Standard deviation error bars of duplicate experiments.
 120 °C). Yields of purified lignins ≥ 95%. Standard deviation error bars of duplicate experiments.
120 °C). Yields of purified lignins ≥ 95%. Standard deviation error bars of duplicate experiments.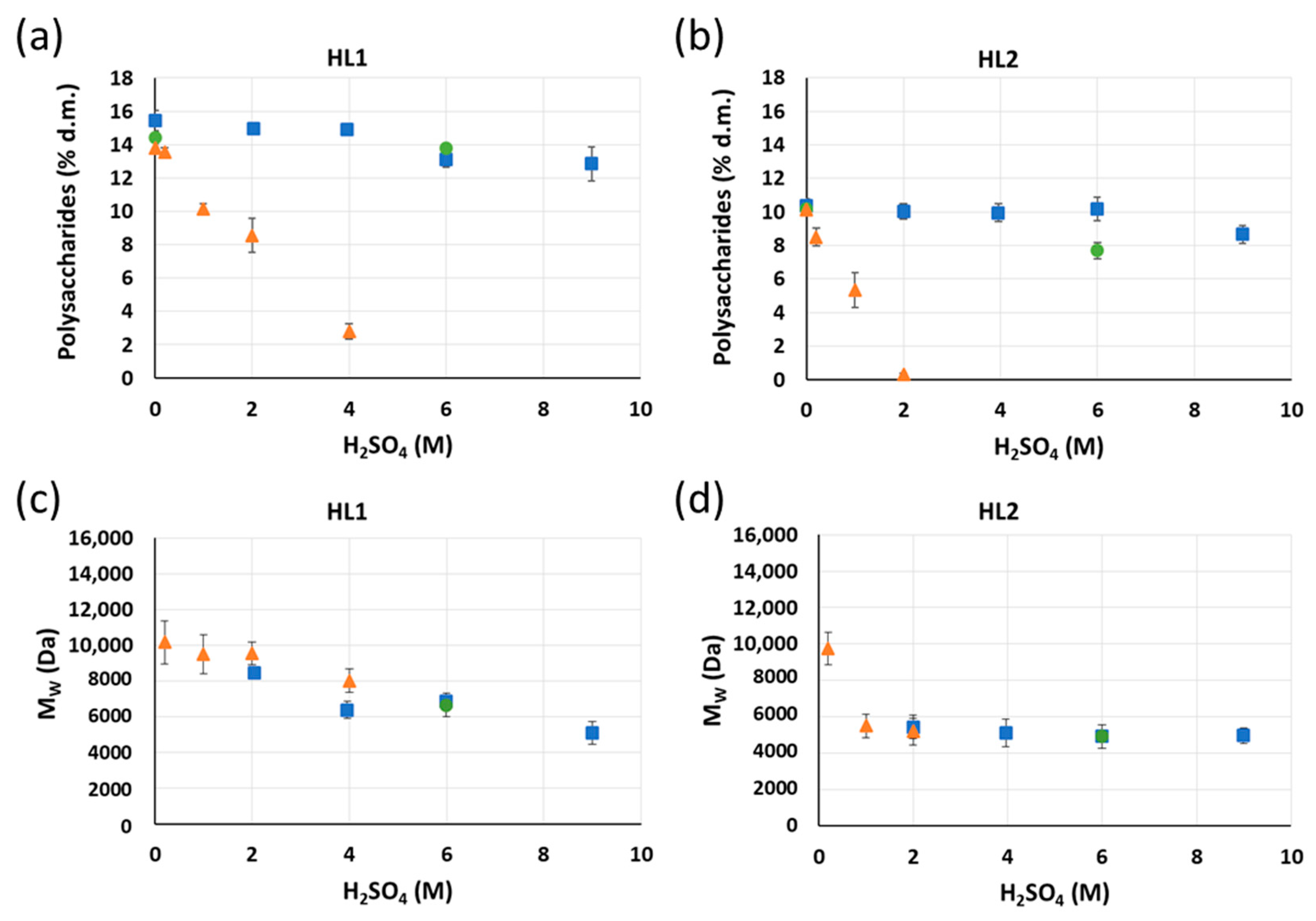
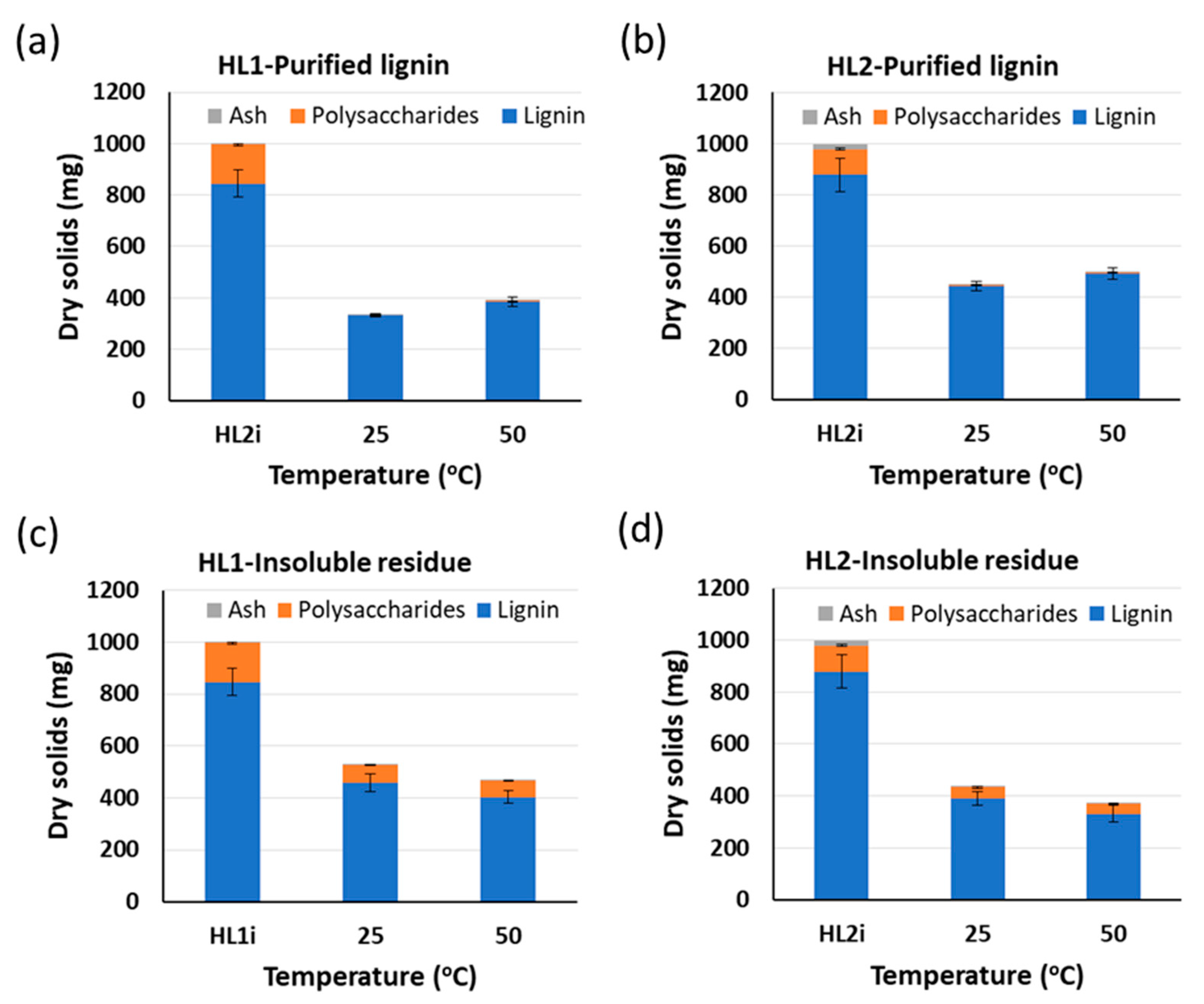
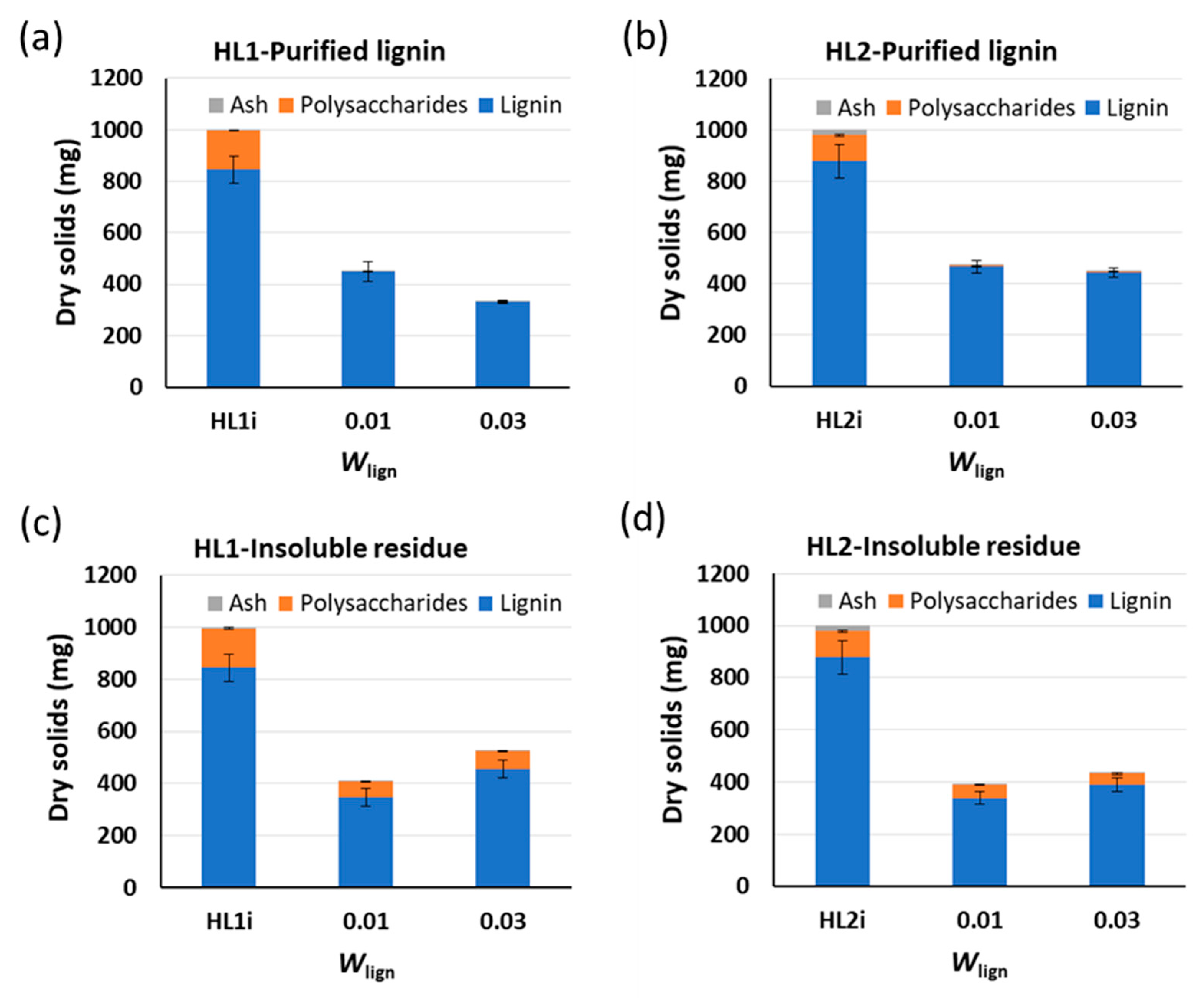
 6 h;
6 h;  22 h). Yields of purified lignins ≥ 95%. Standard deviation error bars of duplicate experiments.
22 h). Yields of purified lignins ≥ 95%. Standard deviation error bars of duplicate experiments.
 6 h;
6 h;  22 h). Yields of purified lignins ≥ 95%. Standard deviation error bars of duplicate experiments.
22 h). Yields of purified lignins ≥ 95%. Standard deviation error bars of duplicate experiments.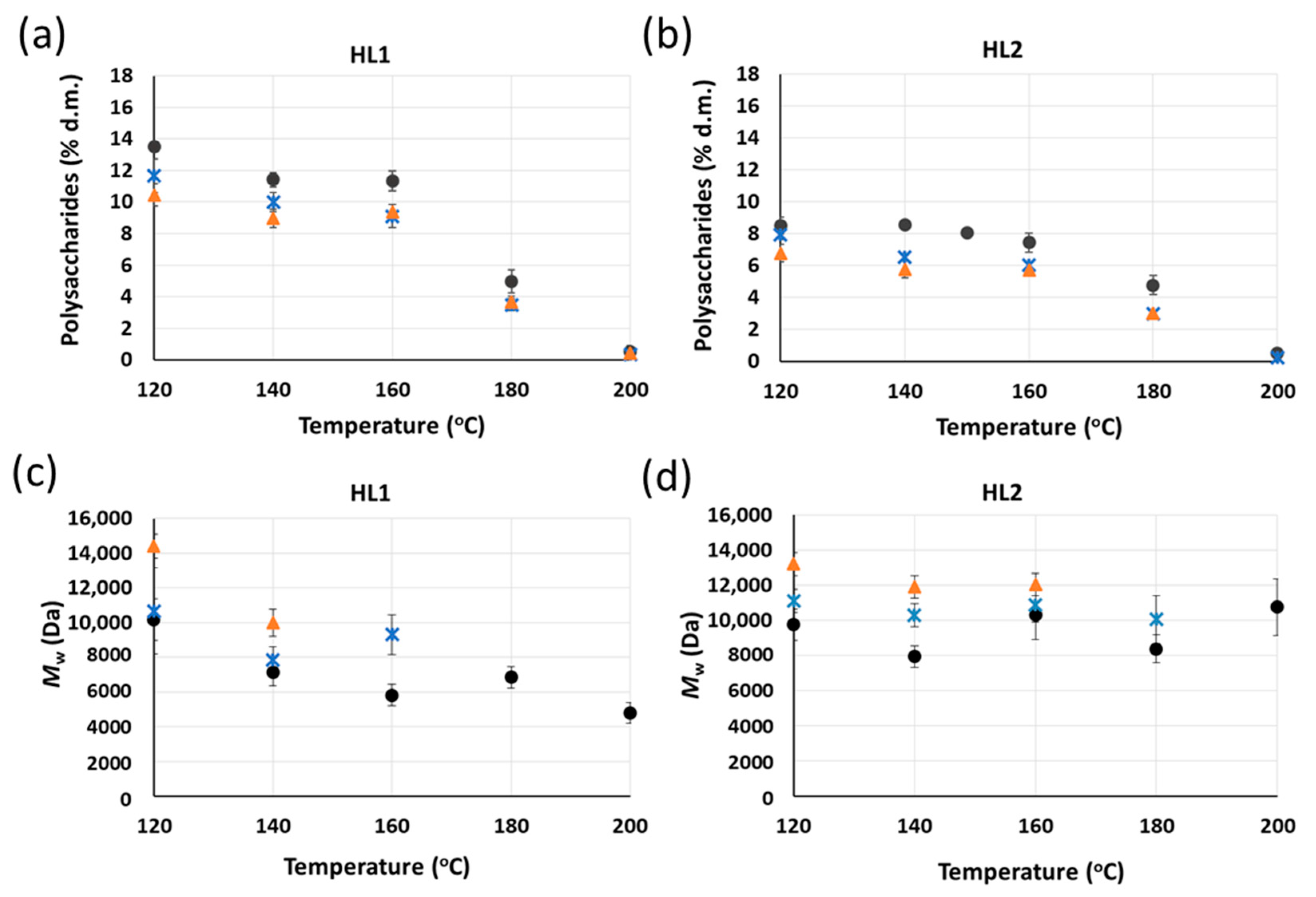

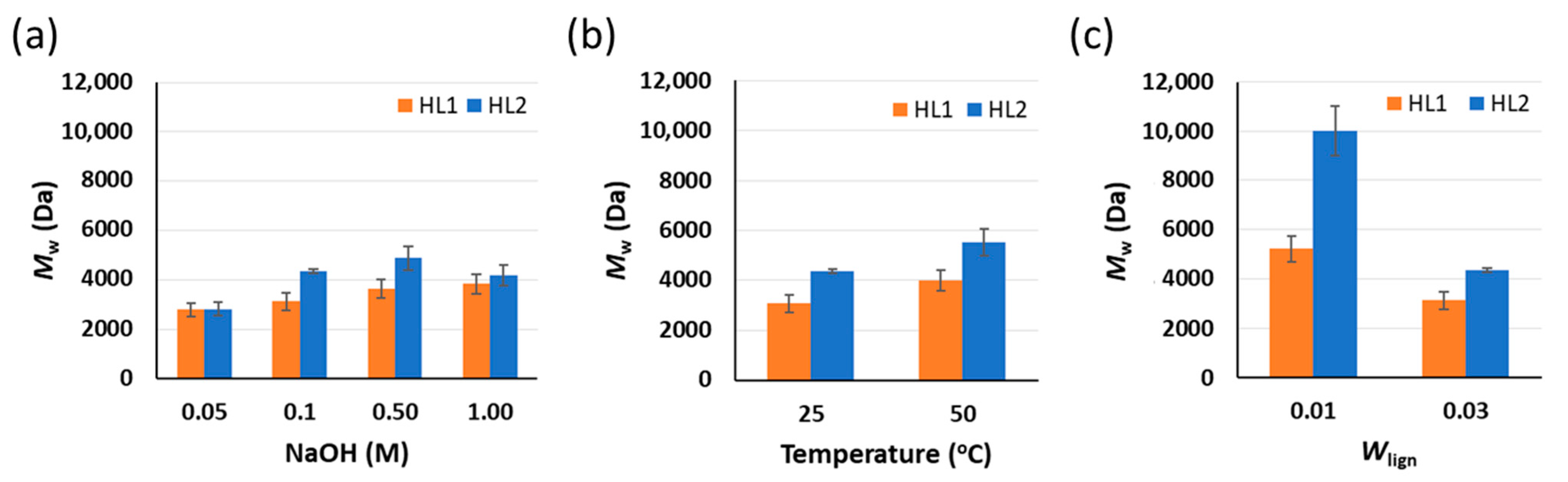
| Lignin | Wlig | T (°C) | NaOH (M) | Aliphatic OH | Aromatic OH | Carboxylic Acid | Total OH |
|---|---|---|---|---|---|---|---|
| (mmol/g) | (mmol/g) | (mmol/g) | (mmol/g) | ||||
| HL1 | 0.03 | 25 | 0.1 | 0.9 | 3.2 | 0.3 | 4.4 |
| 0.03 | 25 | 0.5 | 1.3 | 3.5 | 0.3 | 5.0 | |
| 0.03 | 25 | 1.0 | 1.1 | 3.8 | 0.3 | 5.3 | |
| 0.03 | 50 | 0.1 | 0.9 | 3.0 | 0.3 | 4.2 | |
| 0.01 | 25 | 0.1 | 1.1 | 3.2 | 0.4 | 4.7 | |
| HL2 | 0.03 | 25 | 0.1 | 2.0 | 2.0 | 0.2 | 4.2 |
| 0.03 | 25 | 0.5 | 2.1 | 2.4 | 0.3 | 4.8 | |
| 0.03 | 25 | 1.0 | 2.0 | 2.6 | 0.3 | 4.9 | |
| 0.03 | 50 | 0.1 | 2.1 | 2.5 | 0.3 | 4.9 | |
| 0.01 | 25 | 0.1 | na | na | na | na |
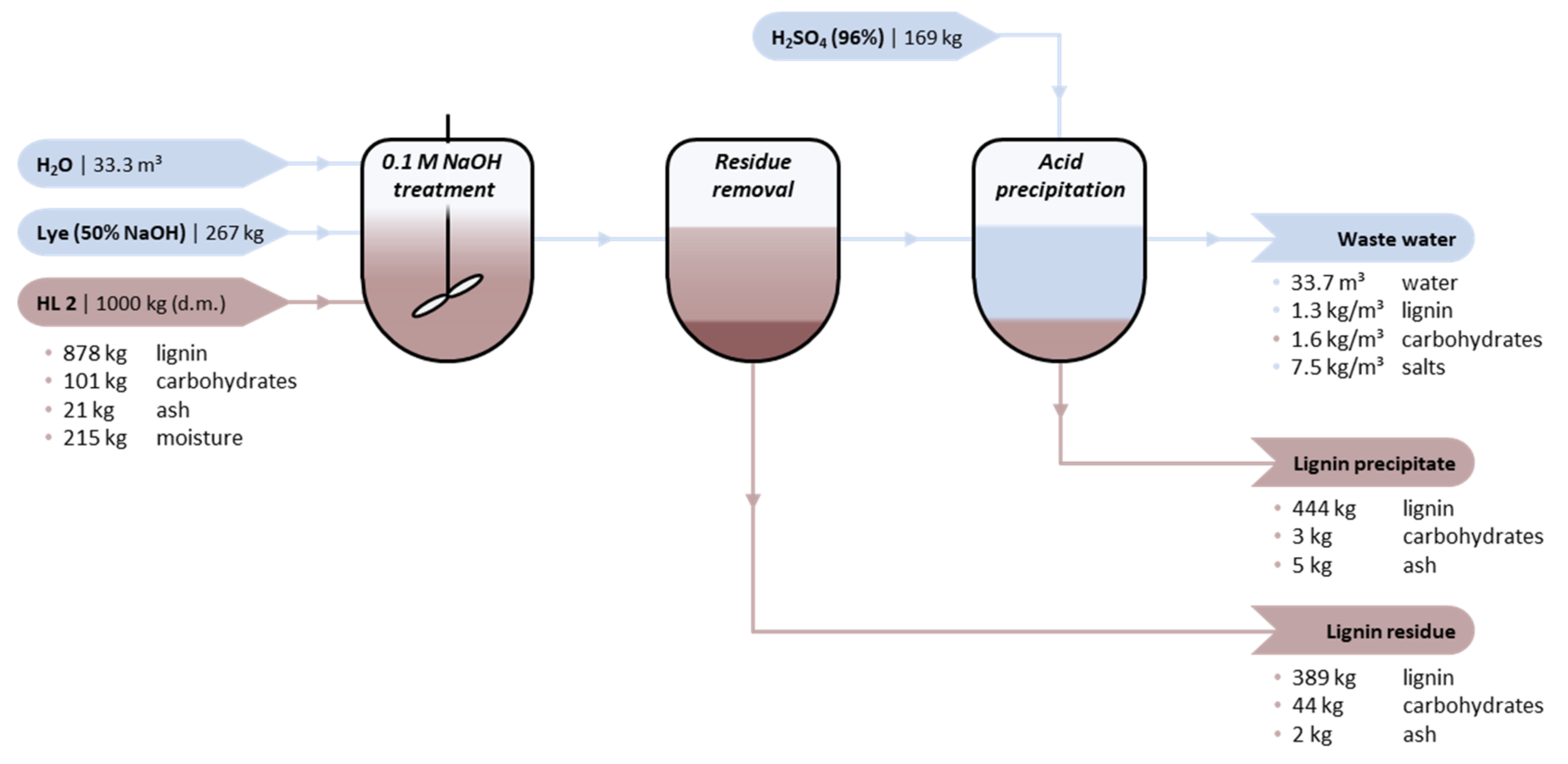
| Variable Costs | HL1 | Cost | HL2 | Cost | Indicative Unit Cost; Info |
|---|---|---|---|---|---|
| Lignin feedstock | 1000 kg HL1 845 kg lignin | €254 | 1000 kg HL2 878 kg lignin | €263 | €300/t lignin [42] |
| Fresh water | 33 m3 | €33 | 33 m3 | €33 | €1/m3; WLign = 0.03 |
| Lye (50% NaOH) | 264 kg | €79 | 264 kg | €79 | €300/t [43]; [NaOH] = 0.1 M |
| H2SO4 (96%) | 169 kg | €13 | 169 kg | €13 | €75/t [44]; stoichiometric |
| Waste water treatment | 33.4 m3 | min. €50 | 33.2 m3 | min. €50 | €1.5/m3 (excluding primary treatment) a |
| Total | €428 | €438 | |||
| Revenues | HL1 | Revenue | HL2 | Revenue | Indicative unit cost; Info |
| Lignin precipitate (LP) | 337 kg LP 331 kg lignin | €331 | 452 kg LP 444 kg lignin | €444 | €1000/t lignin content [42] |
| Lignin residue (LR) | 530 kg LR 457 kg lignin | €114 | 435 kg LR389 kg lignin | €97 | €250/t lignin (expected to be of lower value than the respective feedstock) |
| Total | €446 | €541 | |||
| Balance | €17 | €103 |
| Variable Costs | HL 1 | Cost | HL 2 | Cost | Indicative Unit Cost; Info |
|---|---|---|---|---|---|
| Lignin feedstock | 1000 kg HL1 845 kg lignin | €254 | 1000 kg HL2 878 kg lignin | €263 | €300/t lignin [42] |
| Fresh water | 10 m3 | €10 | 10 m3 | €10 | €1/m3; WLign = 0.10 |
| Lye (50% NaOH) | 320 kg | €96 | 320 kg | €96 | €300/t [43] (stoichiometric) |
| H2SO4 (96%) | 204 kg | €15 | 204 kg | €15 | €75/t [44]; [H2SO4] = 0.2 M |
| Natural gas | 2.58 MWh a | €39 | 2.58 MWh a | €39 | €15/MWh a nat. gas [46] (90% boiler efficiency) |
| Waste water treatment | 10.3 m3 | min. €15 | 10.5 | min. €16 | €1.5 EUR/m3 (excluding primary treatment) b |
| Total | €439 | €449 | |||
| Revenues | HL 1 | Revenue | HL 2 | Revenue | Indicative Unit Cost; Info |
| Lignin product | 841 kg product 837 kg lignin | €837 | 873 kg product 869 kg lignin | €869 | €1000/t lignin [42] |
| Total | €837 | €869 | |||
| Balance | €397 | €420 |
| Parameter | HL1 | HL2 |
|---|---|---|
| Dry matter content (d.m.; wt%) | 97.0 | 82.3 |
| Ash (wt% of d.m.) | 0.4 | 2.1 |
| Physical state | Powder | Powder |
| Sodium, Na (wt% of d.m.) | 0.01 | 0.59 |
| Sulfur, S (wt% of d.m.) | 0.03 | 0.29 |
| Carbohydrates (wt% of d.m.) | 15.1 ± 0.5 | 10.1 ± 0.4 |
| Feedstock | Hardwood (mix) | Hardwood (birch) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corderi, S.; Renders, T.; Servaes, K.; Vanbroekhoven, K.; De Roo, T.; Elst, K. Strategies for the Removal of Polysaccharides from Biorefinery Lignins: Process Optimization and Techno Economic Evaluation. Molecules 2021, 26, 3324. https://doi.org/10.3390/molecules26113324
Corderi S, Renders T, Servaes K, Vanbroekhoven K, De Roo T, Elst K. Strategies for the Removal of Polysaccharides from Biorefinery Lignins: Process Optimization and Techno Economic Evaluation. Molecules. 2021; 26(11):3324. https://doi.org/10.3390/molecules26113324
Chicago/Turabian StyleCorderi, Sandra, Tom Renders, Kelly Servaes, Karolien Vanbroekhoven, Tony De Roo, and Kathy Elst. 2021. "Strategies for the Removal of Polysaccharides from Biorefinery Lignins: Process Optimization and Techno Economic Evaluation" Molecules 26, no. 11: 3324. https://doi.org/10.3390/molecules26113324
APA StyleCorderi, S., Renders, T., Servaes, K., Vanbroekhoven, K., De Roo, T., & Elst, K. (2021). Strategies for the Removal of Polysaccharides from Biorefinery Lignins: Process Optimization and Techno Economic Evaluation. Molecules, 26(11), 3324. https://doi.org/10.3390/molecules26113324







