Oriental Hornet (Vespa orientalis) Larval Extracts Induce Antiproliferative, Antioxidant, Anti-Inflammatory, and Anti-Migratory Effects on MCF7 Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of V. orientalis Larval Extracts
2.2. HPLC Analysis of Flavonoids and Phenolic Compounds
2.3. DPPH Radical Scavenging Assay
2.4. MTT Cytotoxicity Assay
2.5. Experimental Design
2.6. Estimation of Intracellular Reactive Oxygen Species
2.7. Detection of Antioxidant Enzymes Activities
2.8. In Vitro Scratch (Wound Healing) Assay
2.9. Gene Expression Analysis by qPCR
2.10. Statistical Analysis
3. Results
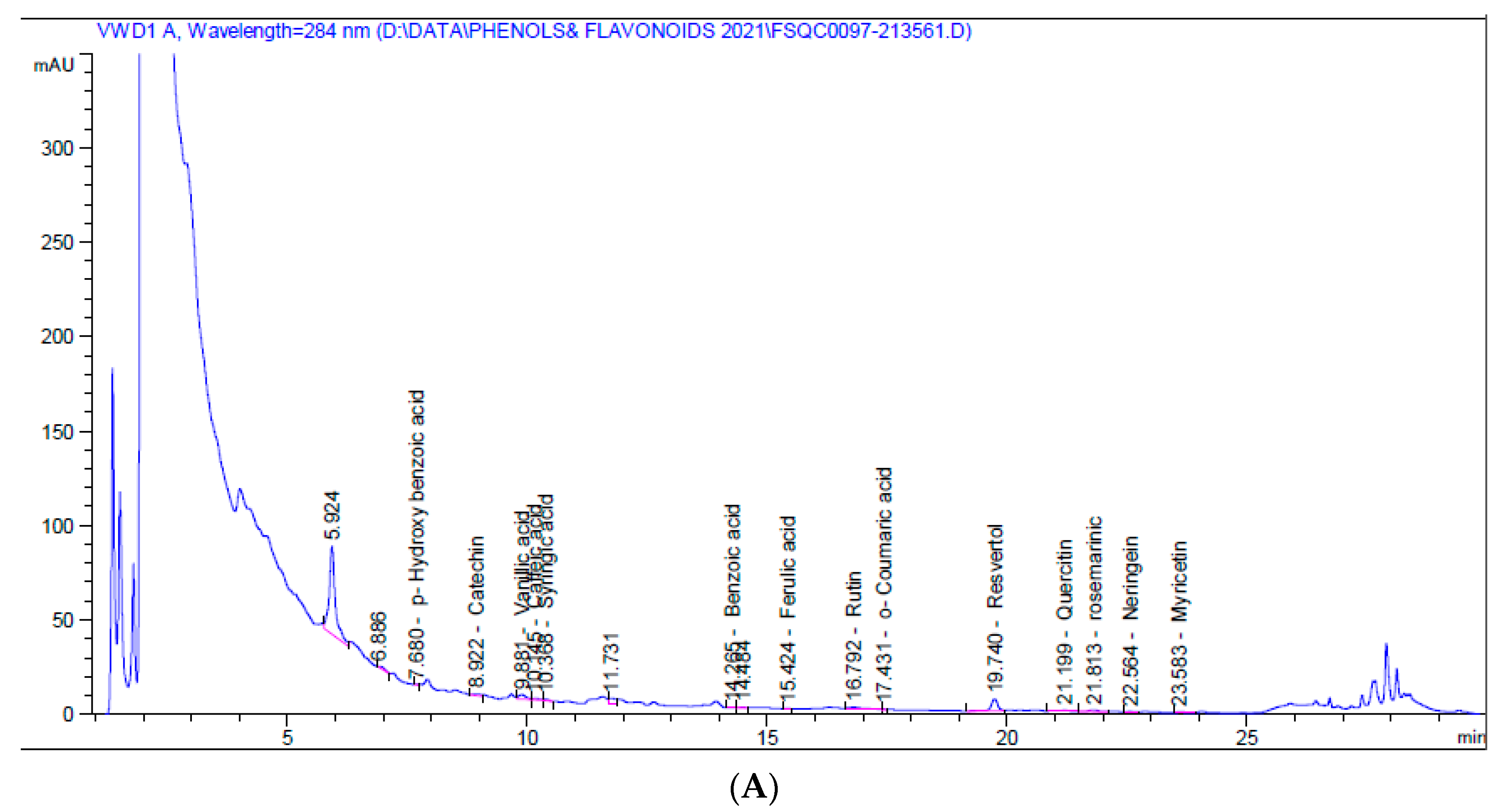
3.1. Larval Extract Had Numerous Flavonoids and Phenolic Compounds
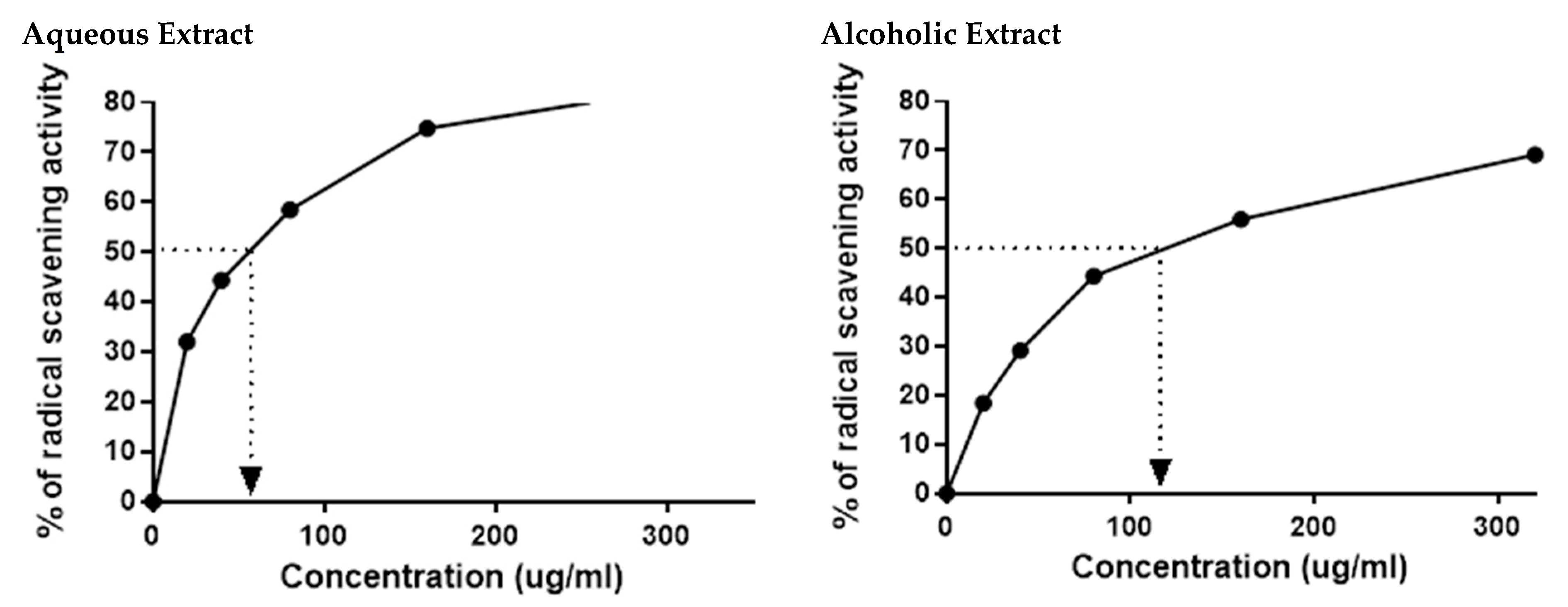
3.2. Larval Extract Had Potent Antioxidant Activities
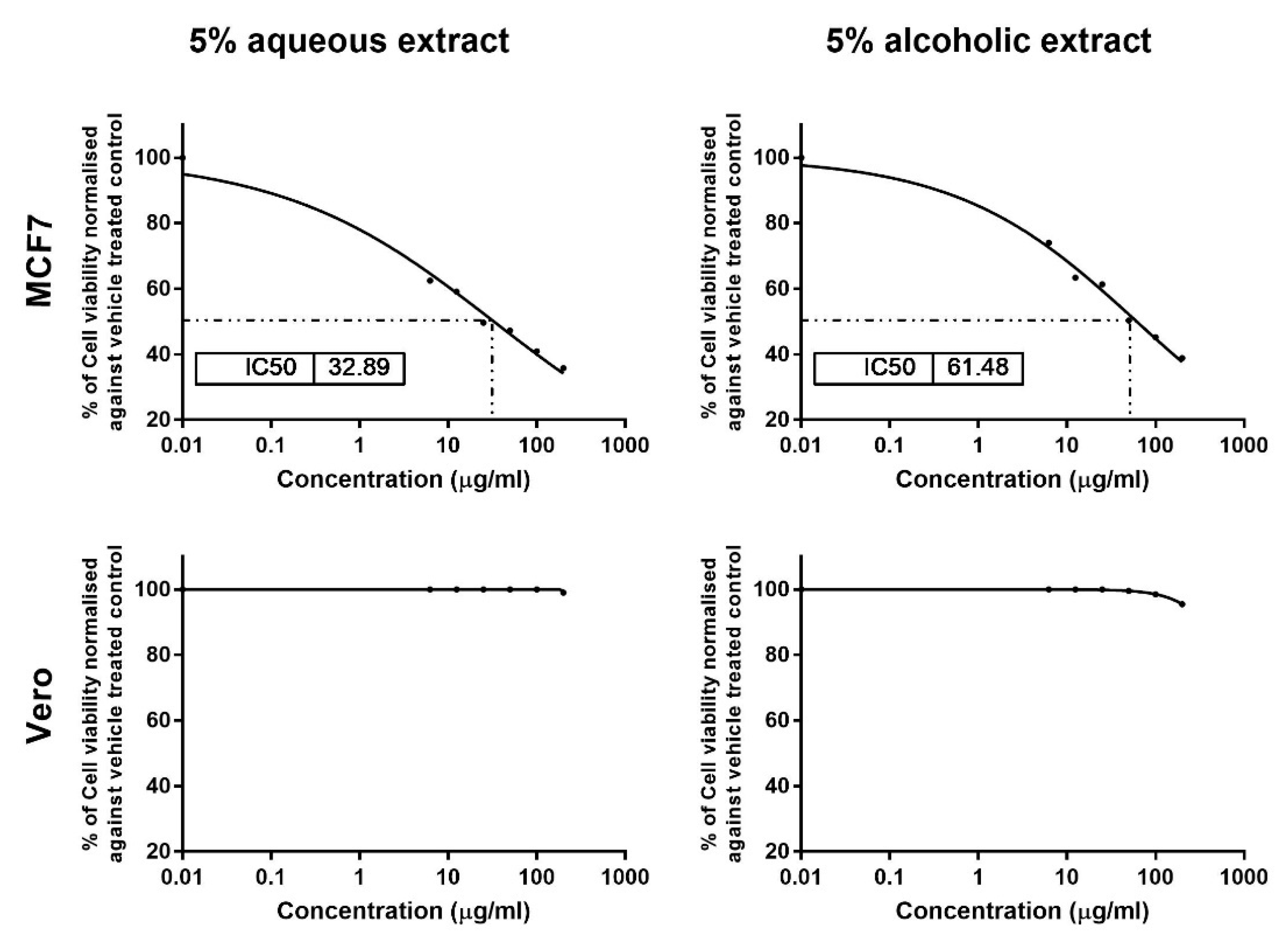
3.3. Larval Extracts Inhibited MCF7 Viability
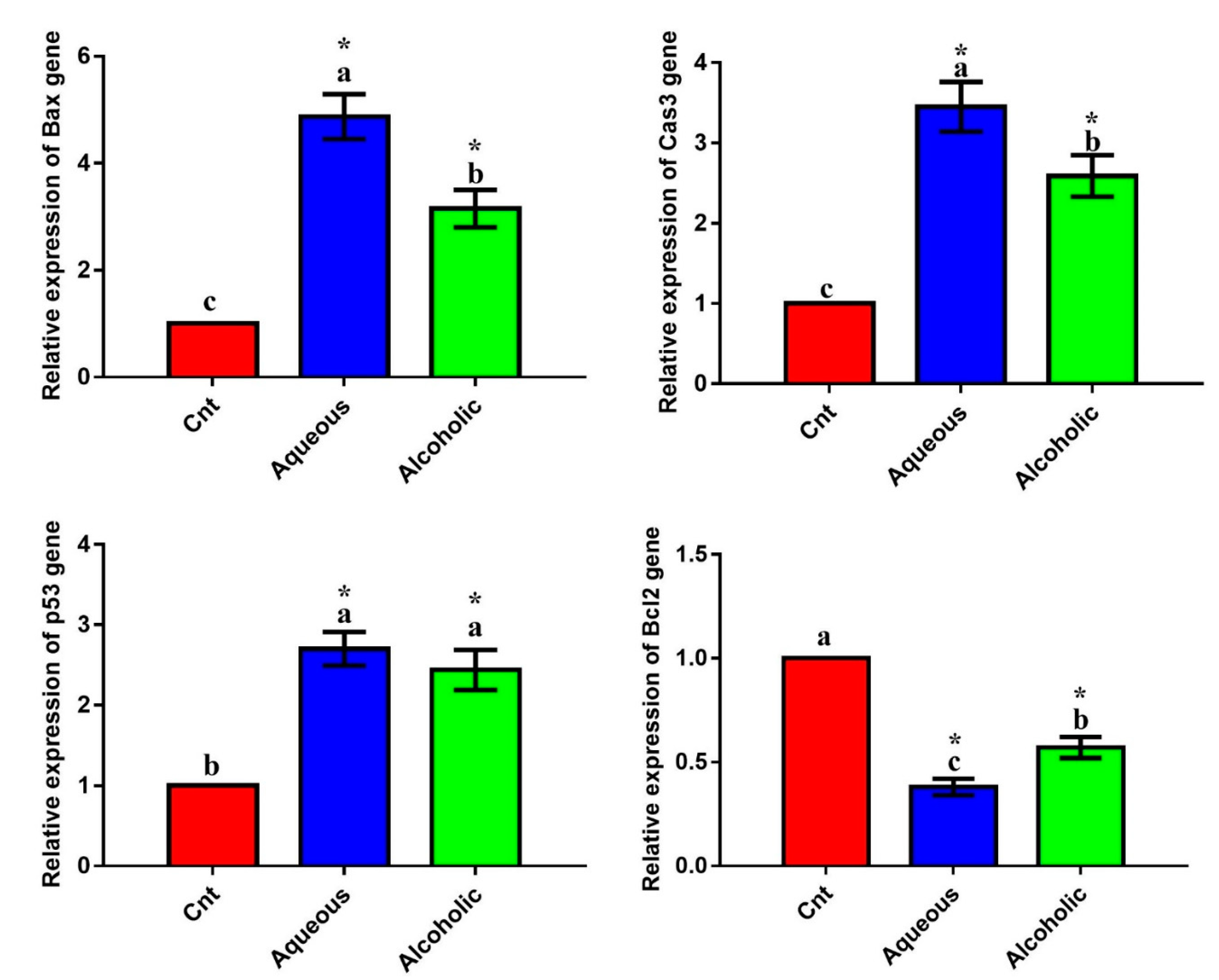
3.4. Larval Extracts Modulated the Expression of Apoptosis-Related Genes
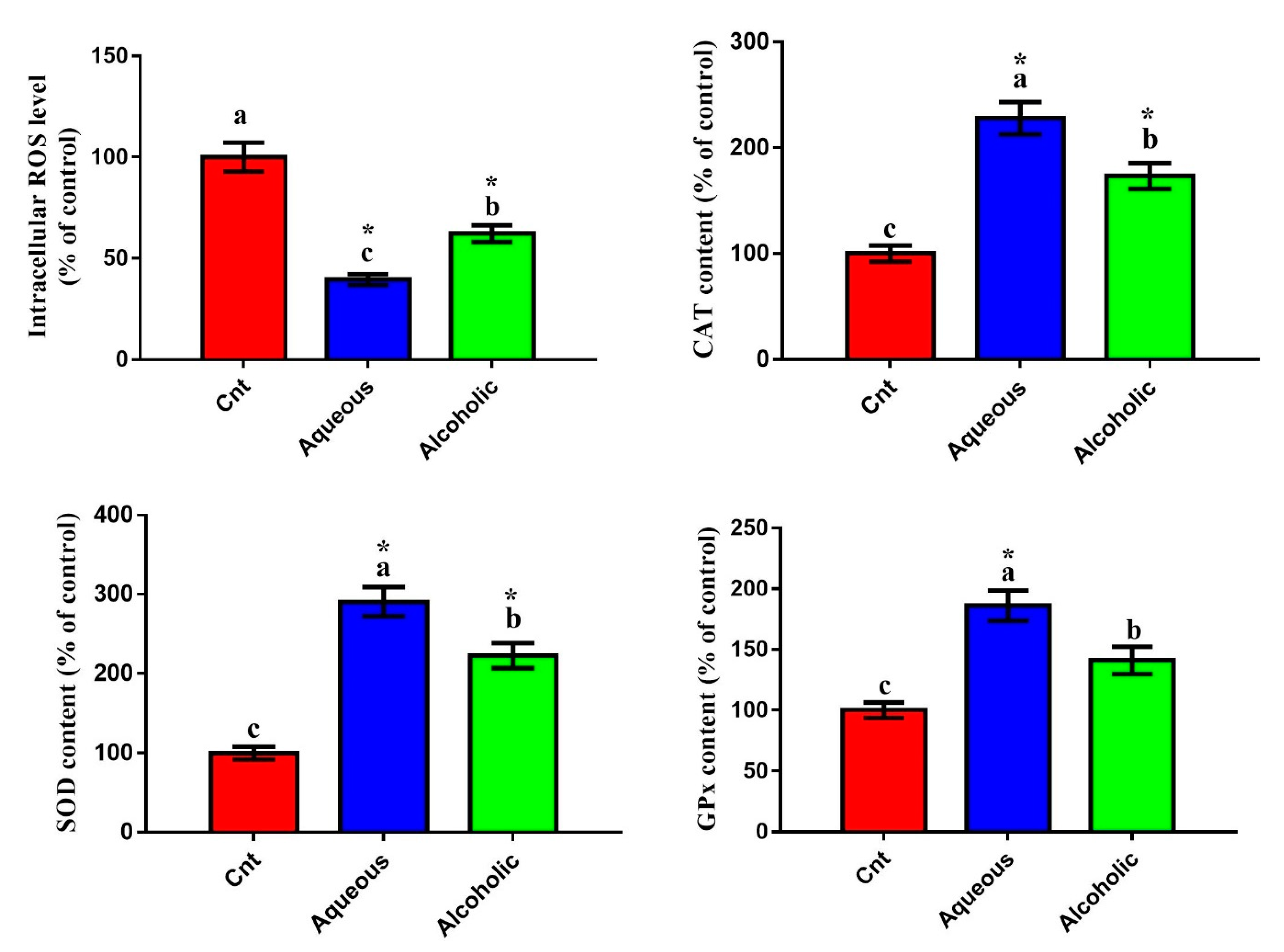
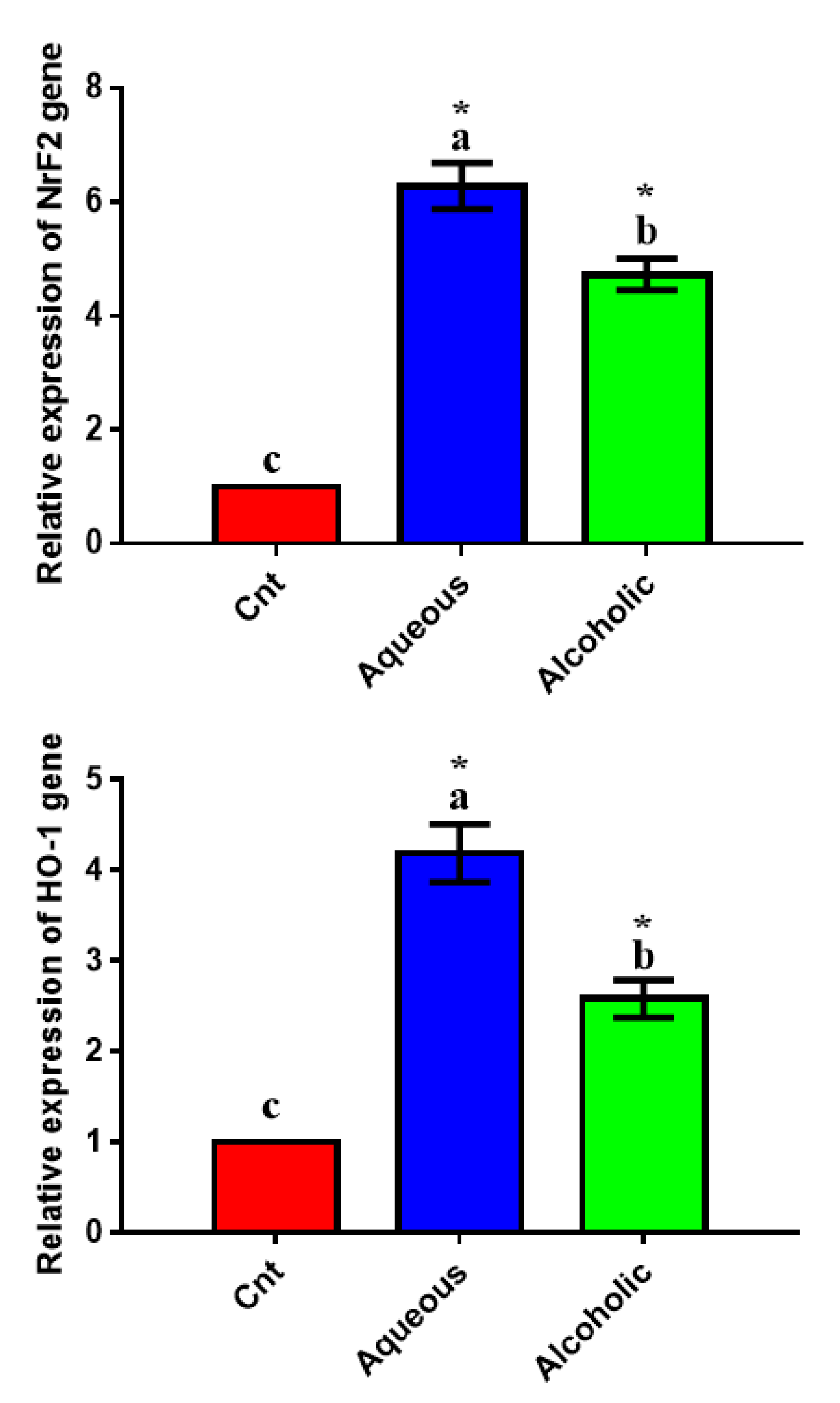
3.5. Larval Extracts Inhibited Intracellular ROS and Induced Antioxidant Status
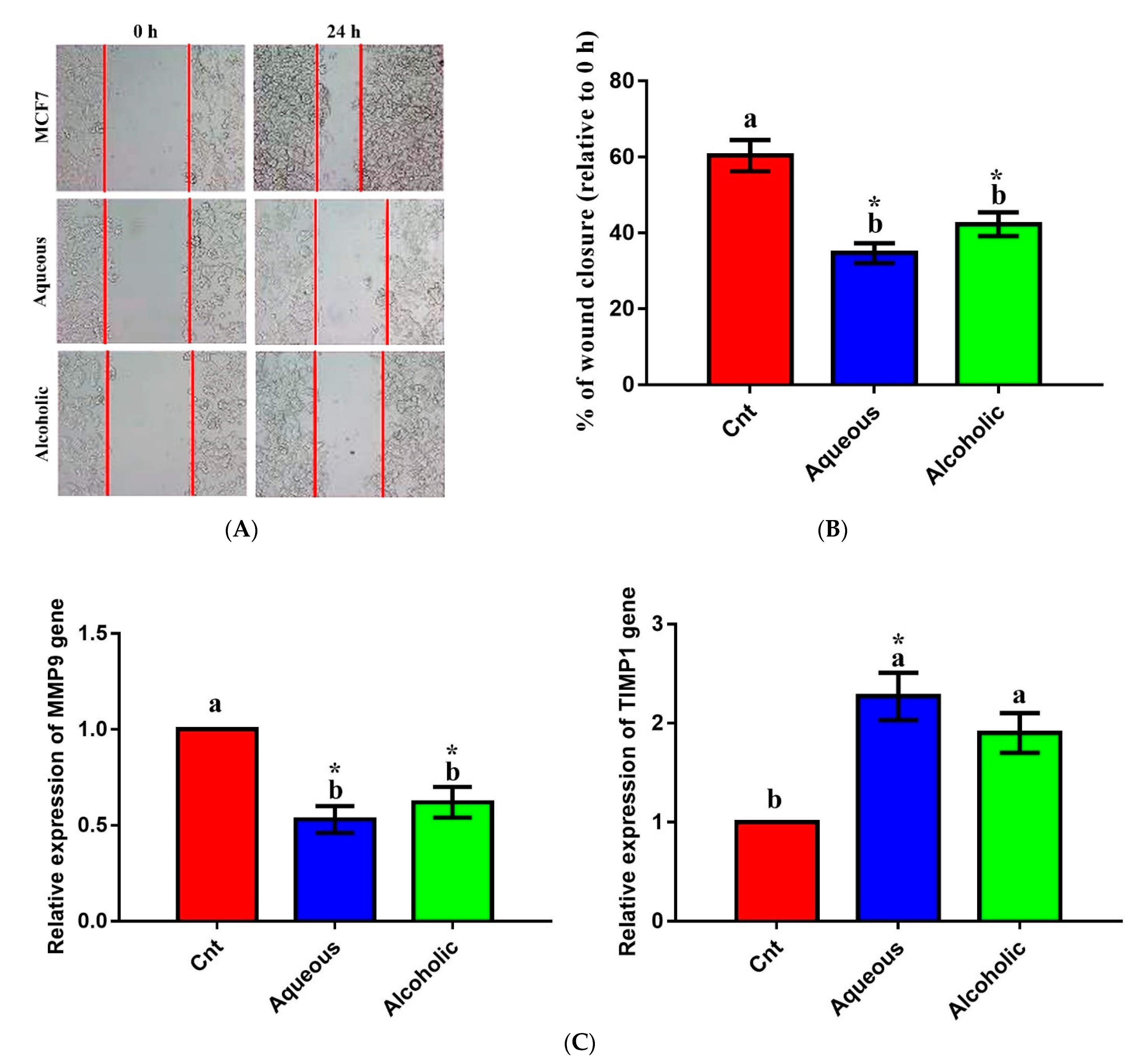
3.6. Larval Extracts Inhibited MCF7 Migration
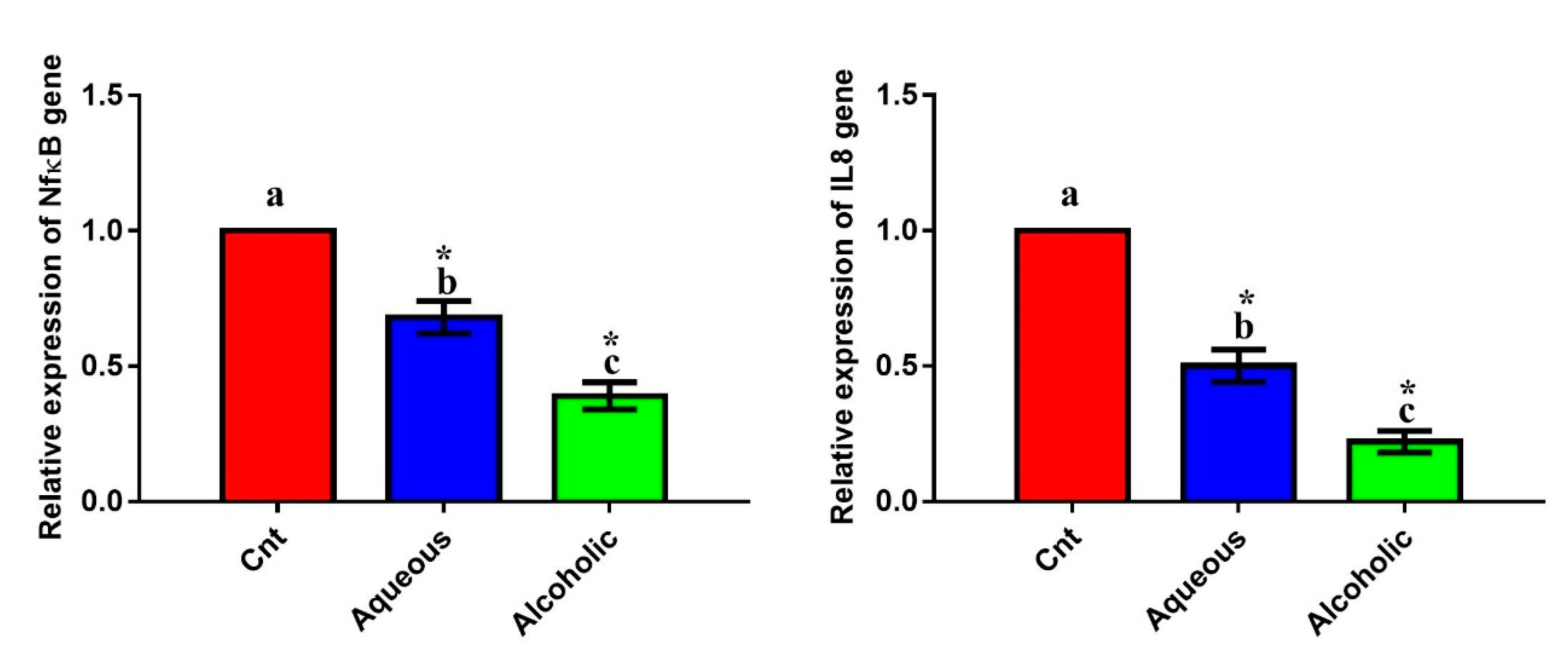
3.7. Larval Extracts Reduced the Expression of Inflammation-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Deng, Y.; Sriwiriyajan, S.; Tedasen, A.; Hiransai, P.; Graidist, P. Anti-cancer effects of piper nigrum via inducing multiple molecular signaling in vivo and in vitro. J. Ethnopharmacol. 2016, 188, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Abu Gazia, M.; El-Magd, M.A. Ameliorative effect of cardamom aqueous extract on doxorubicin-induced cardiotoxicity in rats. Cellstissuesorgans 2018, 206, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, J.; Kozłowska, A.; Kocki, J. Breast cancer metastasis—Insight into selected molecular mechanisms of the phenomenon. Postepy Hig. I Med. Dosw. 2015, 69, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Magdy, A.; Sadaka, E.; Hanafy, N.; El-Magd, M.A.; Allahloubi, N.; El Kemary, M. Green tea ameliorates the side effects of the silver nanoparticles treatment of ehrlich ascites tumor in mice. Mol. Cell. Toxicol. 2020, 16, 271–282. [Google Scholar] [CrossRef]
- El-Magd, M.A.; Mohamed, Y.; El-Shetry, E.S.; Elsayed, S.A.; Abo Gazia, M.; Abdel-Aleem, G.A.; Shafik, N.M.; Abdo, W.S.; El-Desouki, N.I.; Basyony, M.A. Melatonin maximizes the therapeutic potential of non-preconditioned mscs in a den-induced rat model of hcc. Biomed. Pharmacother. 2019, 114, 108732. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.G.; Ali, R.A.; Abd El-Monem, D.D.; El-Magd, M.A. Graviola leaves extract enhances the anticancer effect of cisplatin on various cancer cell lines. Mol. Cell. Toxicol. 2020, 16, 385–399. [Google Scholar] [CrossRef]
- Attia, A.M.; Khodair, A.I.; Gendy, E.A.; El-Magd, M.A.; Elshaier, Y.A. New 2-oxopyridine/2-thiopyridine derivatives tethered to a benzotriazole with cytotoxicity on mcf7 cell lines and with antiviral activities. Lett. Drug Des. Discov. 2020, 17, 124–137. [Google Scholar] [CrossRef]
- Pettit, G.R.; Meng, Y.; Herald, D.L.; Knight, J.C.; Day, J.F. Antineoplastic agents. 553. The texas grasshopper Brachystola magna. J. Nat. Prod. 2005, 68, 1256–1258. [Google Scholar] [CrossRef][Green Version]
- Lee, J.-E.; Jo, D.-E.; Lee, A.-J.; Park, H.-K.; Youn, K.; Yun, E.-Y.; Hwang, J.-S.; Jun, M.; Kang, B.H. Hepatoprotective and anticancer activities of allomyrina dichotoma larvae. J. Life Sci. 2015, 25, 307–316. [Google Scholar] [CrossRef]
- Kim, Y.M.; Ku, M.J.; Son, Y.-J.; Yun, J.-M.; Kim, S.H.; Lee, S.Y. Anti-metastatic effect of cantharidin in a549 human lung cancer cells. Arch. Pharm. Res. 2013, 36, 479–484. [Google Scholar] [CrossRef]
- El-Garawani, I.; El-Seedi, H.; Khalifa, S.; El Azab, I.H.; Abouhendia, M.; Mahmoud, S. Enhanced antioxidant and cytotoxic potentials of lipopolysaccharides-injected musca domestica larvae. Pharmaceutics 2020, 12, 1111. [Google Scholar] [CrossRef]
- Yan, Y.-M.; Li, L.-J.; Qin, X.-C.; Lu, Q.; Tu, Z.-C.; Cheng, Y.-X. Compounds from the insect blaps japanensis with cox-1 and cox-2 inhibitory activities. Bioorg. Med. Chem. Lett. 2015, 25, 2469–2472. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Li, D.; Du, R. Analysis of antibacterial-relative proteins and peptides in housefly larvae. Wei Sheng Yan Jiu J. Hyg. Res. 2004, 33, 86–88. [Google Scholar]
- Sun, H.-X.; Chen, L.-Q.; Zhang, J.; Chen, F.-Y. Anti-tumor and immunomodulatory activity of peptide fraction from the larvae of musca domestica. J. Ethnopharmacol. 2014, 153, 831–839. [Google Scholar] [CrossRef]
- Hou, L.; Shi, Y.; Zhai, P.; Le, G. Antibacterial activity and in vitro anti-tumor activity of the extract of the larvae of the housefly (Musca domestica). J Ethnopharmacol. 2007, 111, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Tao, R.; Li, Y.; Ma, H.; Xiu, J.; Fu, P.; Wu, J. Identification and characterization of a novel antimicrobial protein from the housefly Musca domestica. Biochem. Biophys. Res. Commun. 2017, 490, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Di Mattia, C.; Battista, N.; Sacchetti, G.; Serafini, M. Antioxidant activities in vitro of water and liposoluble extracts obtained by different species of edible insects and invertebrates. Front. Nutr. 2019, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Karaś, M.; Jakubczyk, A. Antioxidant activity of predigested protein obtained from a range of farmed edible insects. Int. J. Food Sci. Technol. 2017, 52, 306–312. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Abu Khudir, R.; El-Magd, M.A.; Salama, A.F.; Tousson, E.M.; El-Dsoki, S.M. Curcumin attenuated oxidative stress and inflammation on hepatitis induced by fluvastatin in female albino rats. Alex. J. Vet. Sci. 2019, 62, 102–115. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; El-Magd, M.A.; El-Sayed, R.A. Panax ginseng modulates oxidative stress, DNA damage, apoptosis, and inflammations induced by silicon dioxide nanoparticles in rats. Environ. Toxicol. 2021. [Google Scholar] [CrossRef]
- Khamis, A.A.A.; Ali, E.M.M.; El-Moneim, M.A.A.; Abd-Alhaseeb, M.M.; El-Magd, M.A.; Salim, E.I. Hesperidin, piperine and bee venom synergistically potentiate the anticancer effect of tamoxifen against breast cancer cells. Biomed. Pharmacother. 2018, 105, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J. Antioxidants and cancer: Molecular mechanisms. Free Radic. Diagn. Med. 1994, 366, 215–239. [Google Scholar]
- Haddad, N.J.; Fuchs, S.; Haddaden, J.; Kopelke, J.-P. Record of Sphecophaga vesparum Curtis, a natural enemy of Vespa orientalis in northern Jordan. Zool. Middle East 2005, 35, 114–116. [Google Scholar] [CrossRef]
- Bagriacik, N. Determination of some structural features of the nest paper of Vespa orientalis linneaus, 1771 and Vespa crabro linneaus, 1758 (hymenoptera: Vespinae) in Turkey. Arch. Biol. Sci. 2011, 63, 449–455. [Google Scholar] [CrossRef]
- Dehghani, R.; Kassiri, H.; Mazaheri-Tehrani, A.; Hesam, M.; Valazadi, N.; Mohammadzadeh, M. A study on habitats and behavioral characteristics of hornet wasp (hymenoptera: Vespidae: Vespa orientalis), an important medical-health pest. Biomed. Res. 2019, 30. [Google Scholar] [CrossRef]
- Ebrahimi, E.; Carpenter, J.M. Distribution pattern of the hornets vespa orientalis and v. Crabro in iran: (hymenoptera: Vespidae). Zool. Middle East 2012, 56, 63–66. [Google Scholar] [CrossRef]
- Ishak, H.D.; Miller, J.L.; Sen, R.; Dowd, S.E.; Meyer, E.; Mueller, U.G.J.S.R. Microbiomes of ant castes implicate new microbial roles in the fungus-growing ant trachymyrmex septentrionalis. Sci. Rep. 2011, 1, 204. [Google Scholar] [CrossRef]
- Papachristoforou, A.; Sueur, J.; Rortais, A.; Angelopoulos, S.; Thrasyvoulou, A.; Arnold, G.J.A. High frequency sounds produced by cyprian honeybees apis mellifera cypria when confronting their predator, the oriental hornet vespa orientalis. Apidologie 2008, 39, 468–474. [Google Scholar] [CrossRef]
- Ken, T.; Hepburn, H.R.; Radloff, S.E.; Yusheng, Y.; Yiqiu, L.; Danyin, Z.; Neumann, P. Heat-balling wasps by honeybees. Naturwissenschaften 2005, 92, 492–495. [Google Scholar] [CrossRef]
- Narzari, S.; Sarmah, J. Proximate composition of wild edible insects consumed by the bodo tribe of assam, india. Int. J. Bioassays 2015, 4, 4050–4054. [Google Scholar]
- Dutta, P.; Dey, T.; Manna, P.; Kalita, J. Antioxidant potential of Vespa affinis L., a traditional edible insect species of north east India. PLoS ONE 2016, 11, e0156107. [Google Scholar] [CrossRef]
- Jalaei, J.; Fazeli, M.; Rajaian, H.; Shekarforoush, S.S. In vitro antibacterial effect of wasp (Vespa orientalis) venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 22. [Google Scholar] [CrossRef]
- Jalaei, J.; Layeghi-Ghalehsoukhteh, S.; Hosseini, A.; Fazeli, M. Antibacterial effects of gold nanoparticles functionalized with the extracted peptide from vespa orientalis wasp venom. J. Pept. Sci. 2018, 24, e3124. [Google Scholar] [CrossRef]
- Lee, S.H.; Baek, J.H.; Yoon, K.A. Differential properties of venom peptides and proteins in solitary vs. Social hunting wasps. Toxins 2016, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Mukund, H.; Manjunath, P.M. Comparative enzyme activity of Vespa orientalis venom and its photooxidized venom products. J. Biochem. Biophys. 2017, 1, 104. [Google Scholar]
- Leite, N.B.; Aufderhorst-Roberts, A.; Palma, M.S.; Connell, S.D.; Neto, J.R.; Beales, P.A. Pe and ps lipids synergistically enhance membrane poration by a peptide with anticancer properties. Biophys. J. 2015, 109, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Zurita, E.; Giralt, E. Delivering wasp venom for cancer therapy. J. Control. Release 2014, 182, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Saidemberg, D.M.; da Silva-Filho, L.C.; Tognoli, L.M.C.; Tormena, C.F.; Palma, M.S. Polybioside, a neuroactive compound from the venom of the social wasp Polybia paulista. J. Nat. Prod. 2010, 73, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Barenholz-Paniry, V.; Ishay, J.S.; Pick, I.A.; Hammel, I. Mast cell activation by hornet (Vespa orientalis) venom. Int. Arch. Allergy Appl. Immunol. 1990, 93, 178–183. [Google Scholar] [CrossRef]
- Hasaballah, A.I.; Shehata, A.Z.; Shehab, A.M. Antioxidant and anticancer activities of some maggots methanol extracts. Egypt. Acad. J. Biol. Sci. A Entomol. 2019, 12, 111–119. [Google Scholar] [CrossRef]
- Eldin, S.; Ziena, H.; Khair, S.; Rozan, M. Canola seed meal as a potential source of natural antioxidant. Alex. Sci. Exch. J. 2018, 39, 615–619. [Google Scholar]
- Azaam, M.M.; Kenawy, E.-R.; El-din, A.S.B.; Khamis, A.A.; El-Magd, M.A. Antioxidant and anticancer activities of α-aminophosphonates containing thiadiazole moiety. J. Saudi Chem. Soc. 2018, 22, 34–41. [Google Scholar] [CrossRef]
- Habib, E.S.; El-Bsoumy, E.; Ibrahim, A.K.; Helal, M.A.; El-Magd, M.A.; Ahmed, S.A. Anti-inflammatory effect of methoxyflavonoids from Chiliadenus montanus (Jasonia montana) growing in Egypt. Nat. Prod. Res. 2020, 1802272. [Google Scholar] [CrossRef]
- El-Magd, M.A.; Khalifa, S.F.; Alzahrani, F.A.A.; Badawy, A.A.; El-Shetry, E.S.; Dawood, L.M.; Alruwaili, M.M.; Alrawaili, H.A.; Risha, E.F.; El-Taweel, F.M.; et al. Incensole acetate prevents beta-amyloid-induced neurotoxicity in human olfactory bulb neural stem cells. Biomed. Pharmacother. 2018, 105, 813–823. [Google Scholar] [CrossRef]
- Badawy, A.A.; El-Magd, M.A.; AlSadrah, S.A. Therapeutic effect of camel milk and its exosomes on mcf7 cells in vitro and in vivo. Integr. Cancer Ther. 2018, 7, 1235–1246. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Higashijima, T.; Uzu, S.; Nakajima, T.; Ross, E.M. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating gtp-binding regulatory proteins (g proteins). J. Biol. Chem. 1988, 263, 6491–6494. [Google Scholar] [CrossRef]
- Danilenko, M.; Worland, P.; Carlson, B.; Sausville, E.A.; Sharoni, Y. Selective effects of mastoparan analogs: Separation of g-protein-directed and membrane-perturbing activities. Biochem. Biophys. Res. Commun. 1993, 196, 1296–1302. [Google Scholar] [CrossRef]
- Leite, N.B.; da Costa, L.C.; Dos Santos Alvares, D.; Dos Santos Cabrera, M.P.; de Souza, B.M.; Palma, M.S.; Ruggiero Neto, J. The effect of acidic residues and amphipathicity on the lytic activities of mastoparan peptides studied by fluorescence and cd spectroscopy. Amino Acids 2011, 40, 91–100. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Paydar, M.; Rouhollahi, E.; Karimian, H. Annona muricata leaves induced apoptosis in a549 cells through mitochondrial-mediated pathway and involvement of nf-kappab. BMC Complement. Altern. Med. 2014, 14, 299. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.Z.; Mehany, A.B.; El-Sheikh, T.M. Excretion/secretion of lucilia sericata and chrysomya albiceps (diptera: Calliphoridae) maggots as potential anticancer agent and kinases inhibitor. N. Y. Sci. J. 2016, 9, 95–101. [Google Scholar]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Elkeiy, M.; Khamis, A.; El-Gamal, M.; Abo Gazia, M.; Zalat, Z.; El-Magd, M. Chitosan nanoparticles from artemia salina inhibit progression of hepatocellular carcinoma in vitro and in vivo. Environ. Sci. Pollut. Res. Int. 2018, 27, 19016–19028. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; El-Magd, M.A.; Abdelfattah-Hassan, A.; Saleh, A.A.; Saadeldin, I.M.; El-Shetry, E.S.; Badawy, A.A.; Alkarim, S. Potential effect of exosomes derived from cancer stem cells and mscs on progression of den-induced hcc in rats. Stem Cells Int. 2018, 2018, 8058979. [Google Scholar] [CrossRef]
- Pritsos, C.A.; Ahmad, S.; Elliott, A.J.; Pardini, R.S. Antioxidant enzyme level response to prooxidant allelochemicals in larvae of the southern armyworm moth, spodoptera eridania. Free Radic. Res. Commun. 1990, 9, 127–133. [Google Scholar] [CrossRef]
- Wang, Y.; Oberley, L.W.; Murhammer, D.W. Antioxidant defense systems of two lipidopteran insect cell lines. Free Radic. Biol. Med. 2001, 30, 1254–1262. [Google Scholar] [CrossRef]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef]
- Suh, H.-J.; Kim, S.-R.; Lee, K.-S.; Park, S.; Kang, S.C. Antioxidant activity of various solvent extracts from Allomyrina dichotoma (arthropoda: Insecta) larvae. J. Photochem. Photobiol. B Biol. 2010, 99, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-C.; Jain, M.; Hsieh, C.-M.; Lee, W.-S.; Yoshizumi, M.; Patterson, C.; Perrella, M.A.; Cooke, C.; Wang, H.; Haber, E. Induction of apoptosis by pyrrolidinedithiocarbamate and n-acetylcysteine in vascular smooth muscle cells. J. Biol. Chem. 1996, 271, 3667–3670. [Google Scholar] [CrossRef]
- Mahdi, R.K.; Al-Hassnawi, A.T.S.; AL-Rubaei, H.M. Biochemical Investigation Effect of Vespa Orientalis Venom Therapy: Rat Rheumatoid Arthritis Model. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; p. 040004. [Google Scholar]
- Bonavita, E.; Galdiero, M.R.; Jaillon, S.; Mantovani, A. Phagocytes as corrupted policemen in cancer-related inflammation. Adv. Cancer Res. 2015, 128, 141–171. [Google Scholar] [PubMed]
- Elgazar, A.A.; Selim, N.M.; Abdel-Hamid, N.M.; El-Magd, M.A.; El Hefnawy, H.M. Isolates from alpinia officinarum hance attenuate lps induced inflammation in hepg2: Evidence from in silico and in vitro studies. Phytother. Res. 2018, 32, 1273–1288. [Google Scholar] [CrossRef]
- Han, J.; Bae, S.Y.; Oh, S.J.; Lee, J.; Lee, J.H.; Lee, H.C.; Lee, S.K.; Kil, W.H.; Kim, S.W.; Nam, S.J.; et al. Zerumbone suppresses il-1beta-induced cell migration and invasion by inhibiting il-8 and mmp-3 expression in human triple-negative breast cancer cells. Phytother. Res. 2014, 28, 1654–1660. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Lu, F.; Zahid, M.; Wang, C.; Saeed, M.; Cavalieri, E.L.; Rogan, E.G. Resveratrol prevents estrogen-DNA adduct formation and neoplastic transformation in mcf-10f cells. Cancer Prev. Res. 2008, 1, 135–145. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Wong, C.; John Bennett, D.; Wu, J.M. Regulation of p53 and cell proliferation by resveratrol and its derivatives in breast cancer cells: An in silico and biochemical approach targeting integrin αvβ3. Int. J. Cancer 2011, 129, 2732–2743. [Google Scholar] [CrossRef]
- Chang, H.L.; Chang, Y.M.; Lai, S.C.; Chen, K.M.; Wang, K.C.; Chiu, T.T.; Chang, F.H.; Hsu, L.S. Naringenin inhibits migration of lung cancer cells via the inhibition of matrix metalloproteinases-2 and -9. Exp. Ther. Med. 2017, 13, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Jin, L.; Lu, L.; Lu, X.; Zhang, C.; Zhang, F.; Liang, W. Naringenin reduces lung metastasis in a breast cancer resection model. Protein Cell 2011, 2, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Kee, J.Y.; Hong, S.H. Rosmarinic acid activates ampk to inhibit metastasis of colorectal cancer. Front. Pharm. 2018, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, Z.; Ji, G.; Liu, J. Inhibition of bone metastasis from breast carcinoma by rosmarinic acid. Planta Med. 2010, 76, 956–962. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharm. 2020, 11, 153. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Bax | GGACGAACTGGACAGTAACATGG | GCAAAGTAGAAAAGGGCGACAAC |
| Bcl2 | TTGATGGGATCGTTGCCTTATGC | CAGTCTACTTCCTCTGTGATGTTG |
| Cas3 | GAAGCGAATCAATGGACTCTGG | GACCGAGATGTCATTCCAGTGC |
| p53 | TAACAGTTCCTGCATGGGCGGC | AGGACAGGCACAAACACGCACC |
| NrF2 | CAGCGACGGAAAGAGTATG | TGGGCAACCTGGGAGTAG |
| HO-1 | CGGGCCAGCAACAAAGTG | AGTGTAAGGACCCATCGGAGAA |
| MMP9 | GCCACTACTGTGCCTTTGAGTC | CCCTCAGAGAATCGCCAGTACT |
| TIMP1 | GGGCTTCACCAAGACCTACA | TGCAGGGGATGGATAAACAG |
| NFκB | ATGGCTTCTATGAGGCTGAG | GTTGTTGTTGGTCTGGATGC |
| IL8 | ACTGAGAGTGATTGAGAGTGGAC | AACCCTCTGCACCCAGTTTTC |
| GAPDH | GGTGAAGGTCGGAGTCAACG | TGAAGGGGTCATTGATGGCAAC |
| Compounds | Aqueous Larval Extract | Alcoholic Larval Extract | ||
|---|---|---|---|---|
| Retention Time (min) | Amount (mg/kg) | Retention Time (min) | Amount (mg/kg) | |
| Catechol | 5.40 | - | 5.40 | - |
| p-Hydroxy benzoic acid | 7.68 | 0.019 | 7.70 | - |
| Catechin | 8.92 | 0.011 | 8.88 | 0.873 |
| Chlorogenic | 9.30 | - | 9.30 | - |
| Vanillic acid | 9.88 | 0.114 | 9.70 | 0.679 |
| Caffeic acid | 10.15 | 0.020 | 9.93 | 0.675 |
| Syringic acid | 10.37 | 0.025 | 10.50 | - |
| p-Coumaric acid | 13.45 | - | 13.45 | - |
| Benzoic acid | 14.27 | 0.032 | 14.30 | - |
| Ferulic acid | 15.42 | 0.004 | 15.69 | 0.337 |
| Rutin | 16.79 | 0.375 | 16.70 | - |
| Ellagic | 16.90 | - | 17.08 | 0.920 |
| o-Coumaric acid | 17.43 | 0.029 | 17.40 | - |
| Resveratrol | 19.74 | 1.580 | 19.80 | - |
| Cinnamic acid | 20.20 | - | 20.20 | - |
| Quercetin | 21.20 | 0.350 | 21.60 | - |
| Rosemarinic | 21.81 | 0.679 | 21.83 | 34.031 |
| Naringenin | 22.56 | 0.819 | 22.40 | - |
| Myricetin | 23.58 | 0.396 | 23.48 | - |
| Kampherol | 24.70 | - | 24.70 | - |
| Total | 4.452 | 45.460 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedan, A.M.G.; Sakran, M.I.; Bahattab, O.; Hawsawi, Y.M.; Al-Amer, O.; Oyouni, A.A.A.; Nasr Eldeen, S.K.; El-Magd, M.A. Oriental Hornet (Vespa orientalis) Larval Extracts Induce Antiproliferative, Antioxidant, Anti-Inflammatory, and Anti-Migratory Effects on MCF7 Cells. Molecules 2021, 26, 3303. https://doi.org/10.3390/molecules26113303
Zedan AMG, Sakran MI, Bahattab O, Hawsawi YM, Al-Amer O, Oyouni AAA, Nasr Eldeen SK, El-Magd MA. Oriental Hornet (Vespa orientalis) Larval Extracts Induce Antiproliferative, Antioxidant, Anti-Inflammatory, and Anti-Migratory Effects on MCF7 Cells. Molecules. 2021; 26(11):3303. https://doi.org/10.3390/molecules26113303
Chicago/Turabian StyleZedan, Amina M. G., Mohamed I. Sakran, Omar Bahattab, Yousef M. Hawsawi, Osama Al-Amer, Atif A. A. Oyouni, Samah K. Nasr Eldeen, and Mohammed A. El-Magd. 2021. "Oriental Hornet (Vespa orientalis) Larval Extracts Induce Antiproliferative, Antioxidant, Anti-Inflammatory, and Anti-Migratory Effects on MCF7 Cells" Molecules 26, no. 11: 3303. https://doi.org/10.3390/molecules26113303
APA StyleZedan, A. M. G., Sakran, M. I., Bahattab, O., Hawsawi, Y. M., Al-Amer, O., Oyouni, A. A. A., Nasr Eldeen, S. K., & El-Magd, M. A. (2021). Oriental Hornet (Vespa orientalis) Larval Extracts Induce Antiproliferative, Antioxidant, Anti-Inflammatory, and Anti-Migratory Effects on MCF7 Cells. Molecules, 26(11), 3303. https://doi.org/10.3390/molecules26113303








