Preparation of New Sargassum fusiforme Polysaccharide Long-Chain Alkyl Group Nanomicelles and Their Antiviral Properties against ALV-J
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae and Reagents
2.2. Preparation of the SFP
2.3. Preparation and Characterization of SFP Long-Chain Alkyl Group Derivatives
2.4. Preparation of SFP Nanomicelles
2.5. Characterization of SFP Nanomicelles
2.5.1. Determination of Particle Size, Zeta Potential and Polydispersity Index
2.5.2. Detection of the Critical Micelle Concentration
2.5.3. Observation of Micelle Morphology
2.6. Cytotoxicity Test
2.7. Anti-ALV-J Activity In Vitro
2.7.1. Detection of the ALV p27 Antigen
2.7.2. Detection of the Relative ALV-J Gene Expression
2.8. Action Stage Assay
2.9. Giant Unilamellar Vesicle (GUV) Exposure Experiment
3. Results and Discussion
3.1. Characterization of the SFP
3.2. Characterization of SFP Long-Chain Alkyl Group Derivatives
3.3. Characterization of SFP Nanomicelles
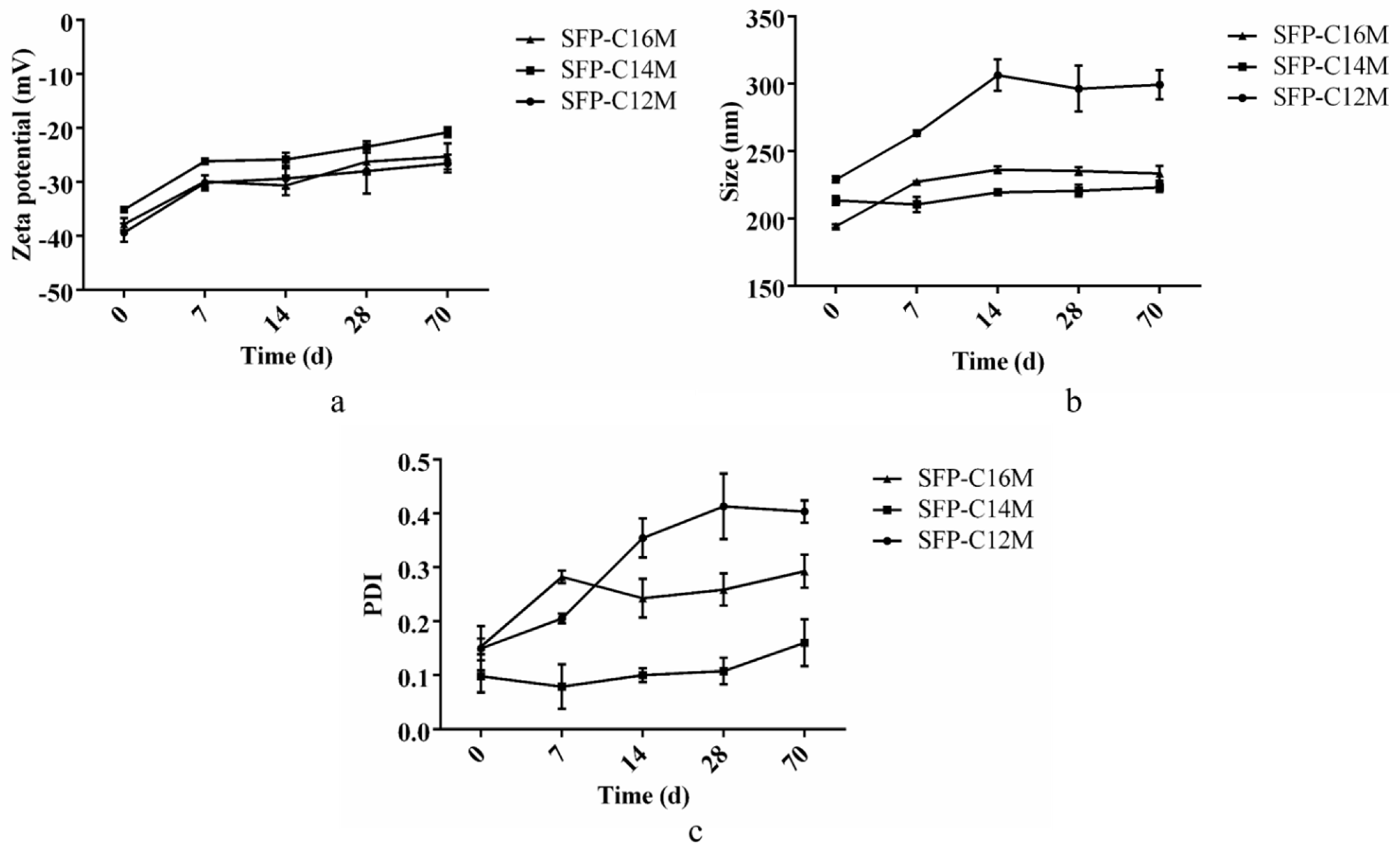
3.4. Detection of Micelle Stability
3.5. Cytotoxic Activity
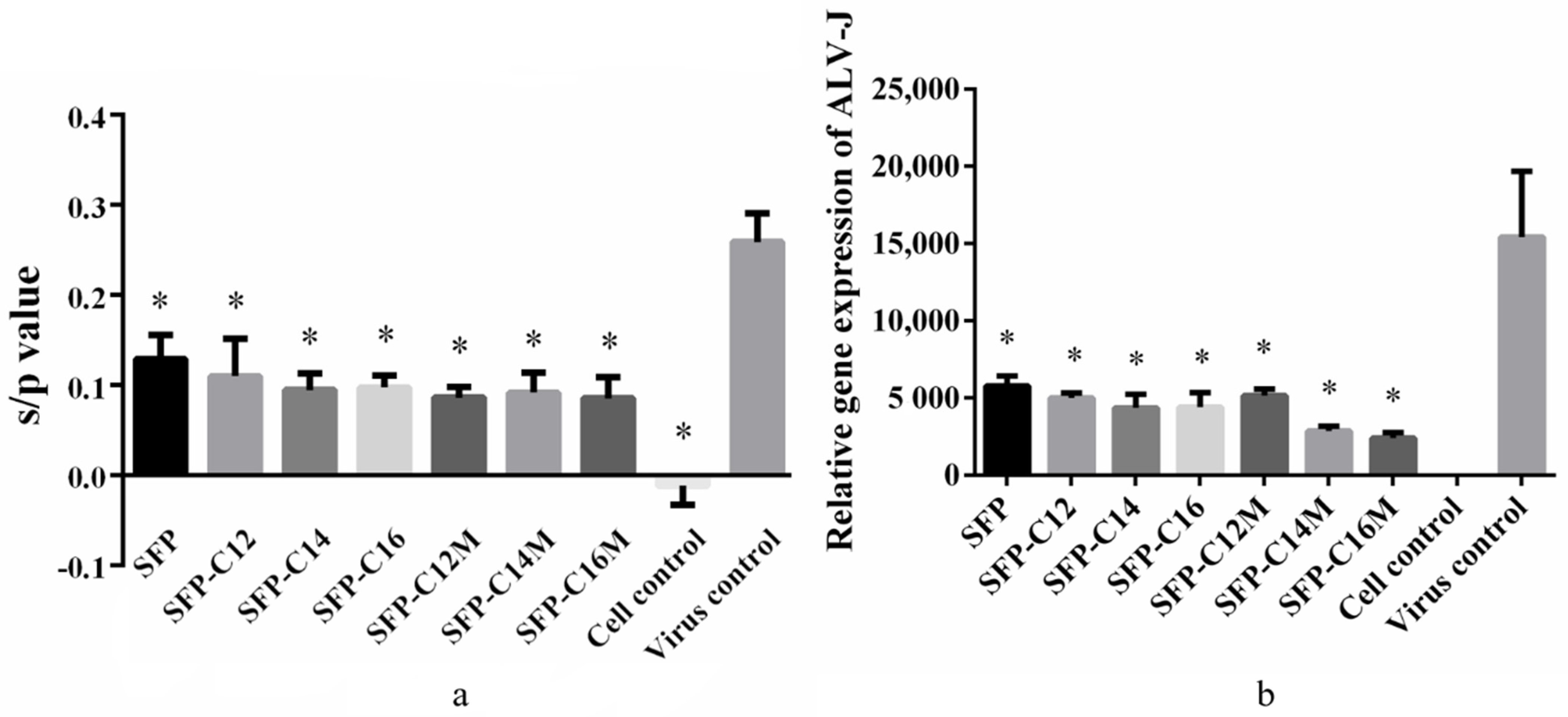
3.6. The Anti-ALV-J Activity of SFP Nanomicelles In Vitro
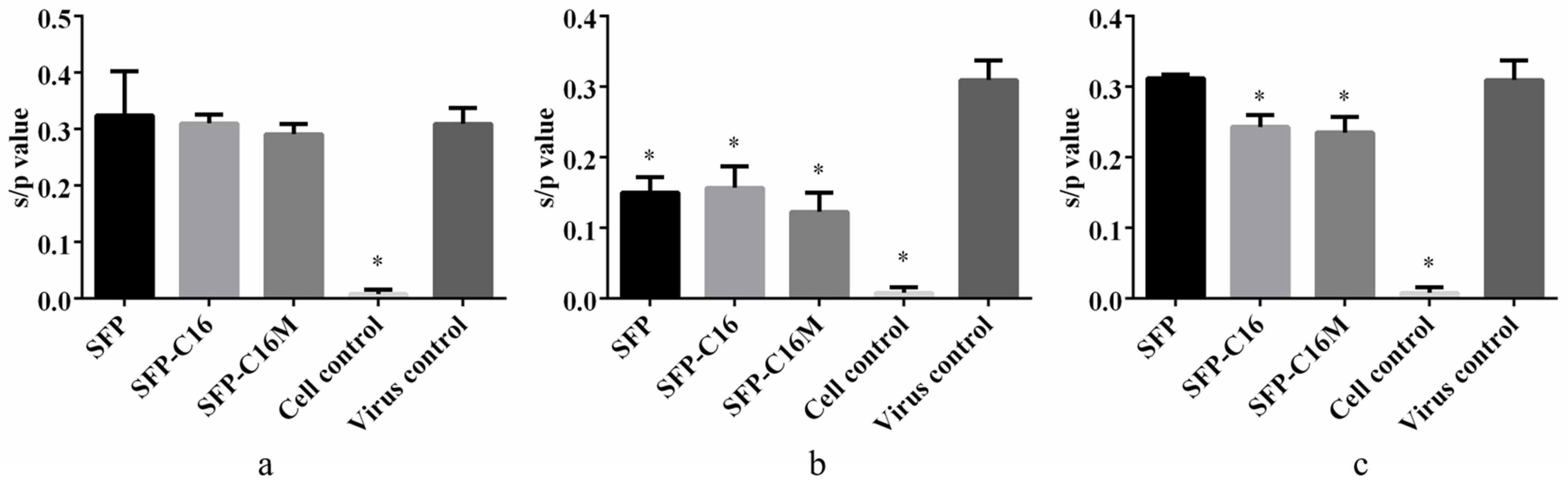
3.7. Action Stage Assay Results
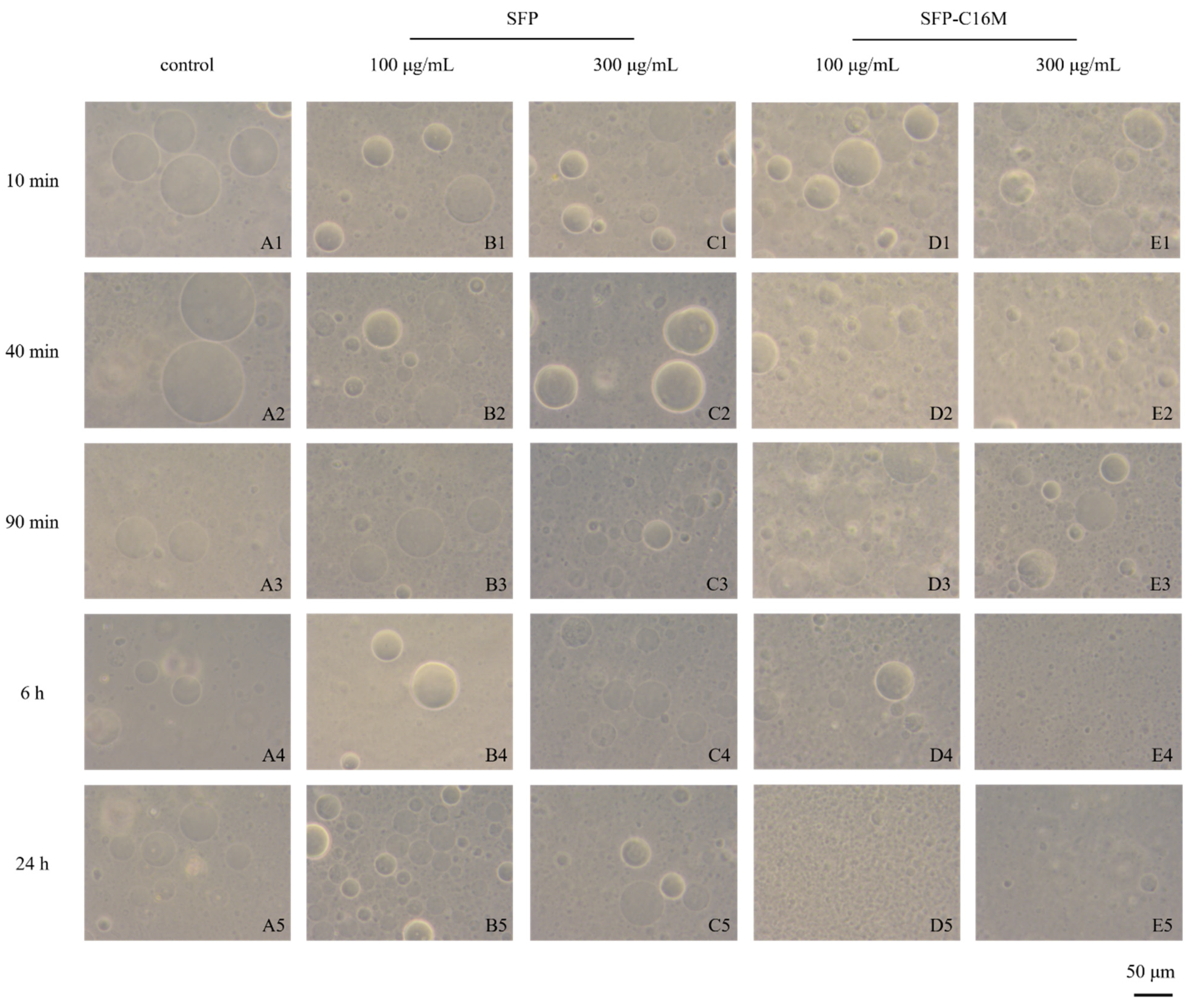
3.8. GUV Exposure Experiment Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, R.; Zhang, X.X.; Tang, Y.X.; Mao, J.L. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 14. [Google Scholar] [CrossRef]
- Li, J.Y.; Liu, Y.C.; Liu, Y.; Wang, Q.H.; Gao, X.; Gong, Q.L. Effects of temperature and salinity on the growth and biochemical composition of the brown alga Sargassum fusiforme (Fucales, Phaeophyceae). J. Appl. Phycol. 2019, 31, 3061–3068. [Google Scholar] [CrossRef]
- Li, Y.T.; Chen, B.J.; Wu, W.D.; Ge, K.; Wei, X.Y.; Kong, L.M.; Xie, Y.Y.; Gu, J.P.; Zhang, J.C.; Zhou, T. Antioxidant and antimicrobial evaluation of carboxymethylated and hydroxamated degraded polysaccharides from Sargassum fusiforme. Int. J. Biol. Macromol. 2018, 118, 1550–1557. [Google Scholar] [CrossRef]
- Jia, R.B.; Li, Z.R.; Wu, J.; Ou, Z.R.; Zhu, Q.Y.; Sun, B.G.; Lin, L.Z.; Zhao, M.M. Physicochemical properties of polysaccharide fractions from Sargassum fusiforme and their hypoglycemic and hypolipidemic activities in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 147, 428–438. [Google Scholar] [CrossRef]
- Ji, D.S.; You, L.J.; Ren, Y.L.; Wen, L.R.; Zheng, G.Q.; Li, C. Protective effect of polysaccharides from Sargassum fusiforme against UVB-induced oxidative stress in HaCaT human keratinocytes. J. Funct. Foods 2017, 36, 332–340. [Google Scholar] [CrossRef]
- Chen, X.M.; Yu, G.Q.; Fan, S.R.; Bian, M.M.; Ma, H.J.; Lu, J.X.; Jin, L.Q. Sargassum fusiforme polysaccharide activates nuclear factor kappa-B (NF-kappa B) and induces cytokine production via Toll-like receptors. Carbohydr. Polym. 2014, 105, 113–120. [Google Scholar] [CrossRef]
- Park, S.Y.; Hwang, E.; Shin, Y.K.; Lee, D.G.; Yang, J.E.; Park, J.H.; Yi, T.H. Immunostimulatory Effect of Enzyme-Modified Hizikia fusiforme in a Mouse Model In Vitro and Ex Vivo. Mar. Biotechnol. 2017, 19, 65–75. [Google Scholar] [CrossRef]
- Fan, S.R.; Zhang, J.F.; Nie, W.J.; Zhou, W.Y.; Jin, L.Q.; Chen, X.M.; Lu, J.X. Antitumor effects of polysaccharide from Sargassum fusiforme against human hepatocellular carcinoma HepG2 cells. Food Chem. Toxicol. 2017, 102, 53–62. [Google Scholar] [CrossRef]
- Hu, P.; Li, Z.X.; Chen, M.C.; Sun, Z.L.; Ling, Y.; Jiang, J.; Huang, C.G. Structural elucidation and protective role of a polysaccharide from Sargassum fusiforme on ameliorating learning and memory deficiencies in mice. Carbohydr. Polym. 2016, 139, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sibusiso, L.; Hou, L.F.; Jiang, H.J.; Chen, P.C.; Zhang, X.; Wu, M.J.; Tong, H.B. Sargassum fusiforme fucoidan modifies the gut microbiota during alleviation of streptozotocin-induced hyperglycemia in mice. Int. J. Biol. Macromol. 2019, 131, 1162–1170. [Google Scholar] [CrossRef]
- Sun, Y.H.; Chen, X.L.; Zhang, L.L.; Liu, H.; Liu, S.; Yu, H.H.; Wang, X.Q.; Qin, Y.K.; Li, P.C. The antiviral property of Sargassum fusiforme polysaccharide for avian leukosis virus subgroup J in vitro and in vivo. Int. J. Biol. Macromol. 2019, 138, 70–78. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part I): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef]
- Weissig, V.; Guzman-Villanueva, D. Nanopharmaceuticals (part 2): Products in the pipeline. Int. J. Nanomed. 2015, 10, 13. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Gong, J.; Chen, M.W.; Zheng, Y.; Wang, S.P.; Wang, Y.T. Polymeric micelles drug delivery system in oncology. J. Control. Release 2012, 159, 312–323. [Google Scholar] [CrossRef]
- Yang, H.; Khan, A.R.; Liu, M.; Fu, M.; Ji, J.; Chi, L.; Zhai, G. Stimuli-responsive polymeric micelles for the delivery of paclitaxel. J. Drug Deliv. Sci. Technol. 2020, 56, 101523. [Google Scholar] [CrossRef]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef]
- Torchilin, V.P. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1. [Google Scholar] [CrossRef]
- Lachowicz, D.; Karabasz, A.; Bzowska, M.; Szuwarzyński, M.; Karewicz, A.; Nowakowska, M. Blood-compatible, stable micelles of sodium alginate–Curcumin bioconjugate for anti-cancer applications. Eur. Polym. J. 2019, 113, 208–219. [Google Scholar] [CrossRef]
- Fang, X.; Zhao, X.; Yu, G.; Zhang, L.; Feng, Y.; Zhou, Y.; Liu, Y.; Li, J. Effect of molecular weight and pH on the self-assembly microstructural and emulsification of amphiphilic sodium alginate colloid particles. Food Hydrocoll. 2020, 103, 105593. [Google Scholar] [CrossRef]
- Yang, J.S.; Zhou, Q.Q.; He, W. Amphipathicity and self-assembly behavior of amphiphilic alginate esters. Carbohydr. Polym. 2013, 92, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Colinet, I.; Dulong, V.; Mocanu, G.; Picton, L.; Le Cerf, D. New amphiphilic and pH-sensitive hydrogel for controlled release of a model poorly water-soluble drug. Eur. J. Pharm. Biopharm. 2009, 73, 345–350. [Google Scholar] [CrossRef]
- Cong, Z.; Shi, Y.; Wang, Y.; Wang, Y.; Niu, J.; Chen, N.; Xue, H. A novel controlled drug delivery system based on alginate hydrogel/chitosan micelle composites. Int. J. Biol. Macromol. 2018, 107, 855–864. [Google Scholar] [CrossRef]
- Xie, P.; Liu, P. Core-shell-corona chitosan-based micelles for tumor intracellular pH-triggered drug delivery: Improving performance by grafting polycation. Int. J. Biol. Macromol. 2019, 141, 161–170. [Google Scholar] [CrossRef]
- Chen, T.; Tu, L.; Wang, G.; Qi, N.; Wu, W.; Zhang, W.; Feng, J. Multi-functional chitosan polymeric micelles as oral paclitaxel delivery systems for enhanced bioavailability and anti-tumor efficacy. Int. J. Pharm. 2020, 578, 119105. [Google Scholar] [CrossRef]
- Mei, L.; Liu, Y.Y.; Zhang, H.J.; Zhang, Z.R.; Gao, H.L.; He, Q. Antitumor and Antimetastasis Activities of Heparin-based Micelle Served as Both Carrier and Drug. ACS Appl. Mater. Interfaces 2016, 8, 9577–9589. [Google Scholar] [CrossRef]
- Wang, Y.; Xin, D.C.; Liu, K.J.; Zhu, M.Q.; Xiang, J.N. Heparin-Paclitaxel Conjugates as Drug Delivery System: Synthesis, Self-Assembly Property, Drug Release, and Antitumor Activity. Bioconjugate Chem. 2009, 20, 2214–2221. [Google Scholar] [CrossRef]
- Buchy, E.; Valetti, S.; Mura, S.; Mougin, J.; Troufflard, C.; Couvreur, P.; Desmaele, D. Synthesis and Cytotoxic Activity of Self-Assembling Squalene Conjugates of 3- (Pyrrol-2-yl)methylidene -2,3-dihydro-1H-indol-2-one Anticancer Agents. Eur. J. Org. Chem. 2015, 2015, 202–212. [Google Scholar] [CrossRef]
- Sun, Y.H.; Chen, X.L.; Liu, S.; Yu, H.H.; Li, R.F.; Wang, X.Q.; Qin, Y.K.; Li, P.C. Preparation of low molecular weight Sargassum fusiforme polysaccharide and its anticoagulant activity. J. Oceanol. Limnol. 2018, 36, 882–891. [Google Scholar] [CrossRef]
- Sun, Y.H.; Chen, X.L.; Cheng, Z.Q.; Liu, S.; Yu, H.H.; Wang, X.Q.; Li, P.C. Degradation of Polysaccharides from Grateloupia filicina and Their Antiviral Activity to Avian Leucosis Virus Subgroup J. Mar. Drugs 2017, 15, 345. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Kawai, Y.; Seno, N.; Anno, K. A modified method for chondrosulfatase assay. Anal. Biochem. 1969, 32, 314–321. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Wang, J.; Shi, X.; Zhang, Z. Analysis of the monosaccharide composition of fucoidan by precolumn derivation HPLC. Chin. J. Oceanol. Limnol. 2009, 27, 578–582. [Google Scholar] [CrossRef]
- Liu, C.G.; Desai, K.G.H.; Chen, X.G.; Park, H.J. Linolenic acid-modified chitosan for formation of self-assembled nanoparticles. J. Agric. Food Chem. 2005, 53, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Jiang, W.; Yu, J.; Ding, L.; Hu, J.; Jiang, G. Effects of SiO2 nanoparticles on phospholipid membrane integrity and fluidity. J. Hazard. Mater. 2015, 287, 217–224. [Google Scholar] [CrossRef]
- Bouhlal, R.; Haslin, C.; Chermann, J.-C.; Colliec-Jouault, S.; Sinquin, C.; Simon, G.; Cerantola, S.; Riadi, H.; Bourgougnon, N. Antiviral Activities of Sulfated Polysaccharides Isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales). Mar. Drugs 2011, 9, 1187–1209. [Google Scholar] [CrossRef]
- Camara, R.B.; Costa, L.S.; Fidelis, G.P.; Nobre, L.T.; Dantas-Santos, N.; Cordeiro, S.L.; Costa, M.S.; Alves, L.G.; Rocha, H.A. Heterofucans from the brown seaweed Canistrocarpus cervicornis with anticoagulant and antioxidant activities. Mar. Drugs 2011, 9, 124–138. [Google Scholar] [CrossRef]
- Chen, S.G.; Xue, C.H.; Yin, L.A.; Tang, Q.Q.; Yu, G.L.; Chai, W.G. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011, 83, 688–696. [Google Scholar] [CrossRef]
- Wang, S.C.; Bligh, S.W.; Shi, S.S.; Wang, Z.T.; Hu, Z.B.; Crowder, J.; Branford-White, C.; Vella, C. Structural features and anti-HIV-1 activity of novel polysaccharides from red algae Grateloupia longifolia and Grateloupia filicina. Int. J. Biol. Macromol. 2007, 41, 369–375. [Google Scholar] [CrossRef]
- Lim, S.J.; Aida, W.M.W.; Maskat, M.Y.; Latip, J.; Badri, K.H.; Hassan, O.; Yamin, B.M. Characterisation of fucoidan extracted from Malaysian Sargassum binderi. Food Chem. 2016, 209, 267–273. [Google Scholar] [CrossRef]
- Namazia, H.; Fathi, F.; Dadkhah, A. Hydrophobically modified starch using long-chain fatty acids for preparation of nanosized starch particles. Sci. Iran. 2011, 18, 439–445. [Google Scholar] [CrossRef][Green Version]
- Rogge, T.M.; Stevens, C.V. Facilitated synthesis of inulin esters by transesterification. Biomacromolecules 2004, 5, 1799–1803. [Google Scholar] [CrossRef]
- Engwayu, J.; Pawlik, M. Adsorption of anionic polymers on hematite-a study of zeta potential distributions. Miner. Eng. 2020, 148, 10. [Google Scholar] [CrossRef]
- Kumar, R.; Sirvi, A.; Kaur, S.; Samal, S.K.; Roy, S.; Sangamwar, A.T. Polymeric micelles based on amphiphilic oleic acid modified carboxymethyl chitosan for oral drug delivery of bcs class iv compound: Intestinal permeability and pharmacokinetic evaluation. Eur. J. Pharm. Sci. 2020, 153, 105466. [Google Scholar] [CrossRef]
- Li, S.P.; Zhao, W.; Liang, N.; Xu, Y.X.; Kawashima, Y.; Sun, S.P. Multifunctional micelles self-assembled from hyaluronic acid conjugate for enhancing anti-tumor effect of paclitaxel. React. Funct. Polym. 2020, 152, 10. [Google Scholar] [CrossRef]
- Shi, Y.J.; Yan, F.Y.; Jia, Q.Z.; Wang, Q. Norm descriptors for predicting the hydrophile-lipophile balance (HLB) and critical micelle concentration (CMC) of anionic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 7. [Google Scholar] [CrossRef]
- Čopíková, J.; Taubner, T.; Tůma, J.; Synytsya, A.; Dušková, D.; Marounek, M. Cholesterol and fat lowering with hydrophobic polysaccharide derivatives. Carbohydr. Polym. 2015, 116, 207–214. [Google Scholar] [CrossRef]
- Cagno, V.; Andreozzi, P.; D’Alicarnasso, M.; Silva, P.J.; Mueller, M.; Galloux, M.; Le Goffic, R.; Jones, S.T.; Vallino, M.; Hodek, J.; et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2018, 17, 195–203. [Google Scholar] [CrossRef]
- Katsuraya, K.; Ikushima, N.; Takahashi, N.; Shoji, T.; Nakashima, H.; Yamamoto, N.; Yoshida, T.; Uryu, T. Synthesis of sulfated alkyl malto- and laminara-oligosaccharides with potent inhibitory effects on AIDS virus infection. Carbohydr. Res. 1994, 260, 51–61. [Google Scholar] [CrossRef]
- Katsuraya, K.; Shoji, T.; Inazawa, K.; Nakashima, H.; Yamamoto, N.; Uryu, T. Synthesis of sulfated alkyl laminara-oligosaccharides having potent anti-HIV activity and the relationship between structure and biological activities. Macromolecules 1994, 27, 6695–6699. [Google Scholar] [CrossRef]
- Katsuraya, K.; Shibuya, T.; Inazawa, K.; Nakashima, H.; Yamamoto, N.; Uryu, T. Synthesis of Sulfated Alkyl Malto-oligosaccharides with Potent Inhibitory Effects on AIDS Virus Infection. Macromolecules 1995, 28, 6697–6700. [Google Scholar] [CrossRef]
- Bai, S.M.; Budragchaa, D.; Han, S.Q.; Kanamoto, T.; Nakashima, H.; Yoshida, T. Sulfated Alkyl Glucopyranans with Potent Antiviral Activity Synthesized by Ring-Opening Copolymerization of Anhydroglucose and Alkyl Anhydroglucose Monomers. Int. J. Polym. Sci. 2015, 2015, 317420. [Google Scholar] [CrossRef]
- Bai, M.X.; Bai, C.L.M.; Asai, D.; Takemura, H.; Miyazaki, K.; Yoshida, T. Role of a long-chain alkyl group in sulfated alkyl oligosaccharides with high anti-HIV activity revealed by SPR and DLS. Carbohydr. Polym. 2020, 245, 116518. [Google Scholar]
- Uryu, T.; Katsuraya, K.; Nakashima, H. Synthesis of sulfated alkyl oligosaccharides with potent anti-HIV activity. Macromol. Symp. 1997, 120, 147–158. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chan, Y.L.; Tsai, L.W.; Li, T.L.; Wu, C.J. Prevention of human enterovirus 71 infection by kappa carrageenan. Antivir. Res. 2012, 95, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, P.; Hao, C.; Zhang, X.E.; Cui, Z.Q.; Guan, H.S. In vitro inhibitory effect of carrageenan oligosaccharide on influenza A H1N1 virus. Antivir. Res. 2011, 92, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, P.; Yu, G.L.; Li, C.X.; Hao, C.; Qi, X.; Zhang, L.J.; Guan, H.S. Preparation and anti-influenza A virus activity of kappa-carrageenan oligosaccharide and its sulphated derivatives. Food Chem. 2012, 133, 880–888. [Google Scholar] [CrossRef]
- Klimyte, E.M.; Smith, S.E.; Oreste, P.; Lembo, D.; Dutch, R.E. Inhibition of Human Metapneumovirus Binding to Heparan Sulfate Blocks Infection in Human Lung Cells and Airway Tissues. J. Virol. 2016, 90, 9237–9250. [Google Scholar] [CrossRef]
- Chi, Y.Z.; Zhang, M.F.; Wang, X.; Fu, X.J.; Guan, H.S.; Wang, P. Ulvan lyase assisted structural characterization of ulvan from Ulva pertusa and its antiviral activity against vesicular stomatitis virus. Int. J. Biol. Macromol. 2020, 157, 75–82. [Google Scholar] [CrossRef]
- Sun, Y.H.; Chen, X.L.; Song, L.; Liu, S.; Yu, H.H.; Wang, X.Q.; Qin, Y.K.; Li, P.C. Antiviral Activity against Avian Leucosis Virus Subgroup J of Degraded Polysaccharides from Ulva pertusa. BioMed Res. Int. 2018, 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Battulga, T.; Tumurbaatar, O.; Ganzorig, O.; Ishimura, T.; Kanamoto, T.; Nakashima, H.; Miyazaki, K.; Yoshida, T. Analysis of interaction between sulfated polysaccharides and HIV oligopeptides by surface plasmon resonance. Int. J. Biol. Macromol. 2019, 125, 909–914. [Google Scholar] [CrossRef] [PubMed]
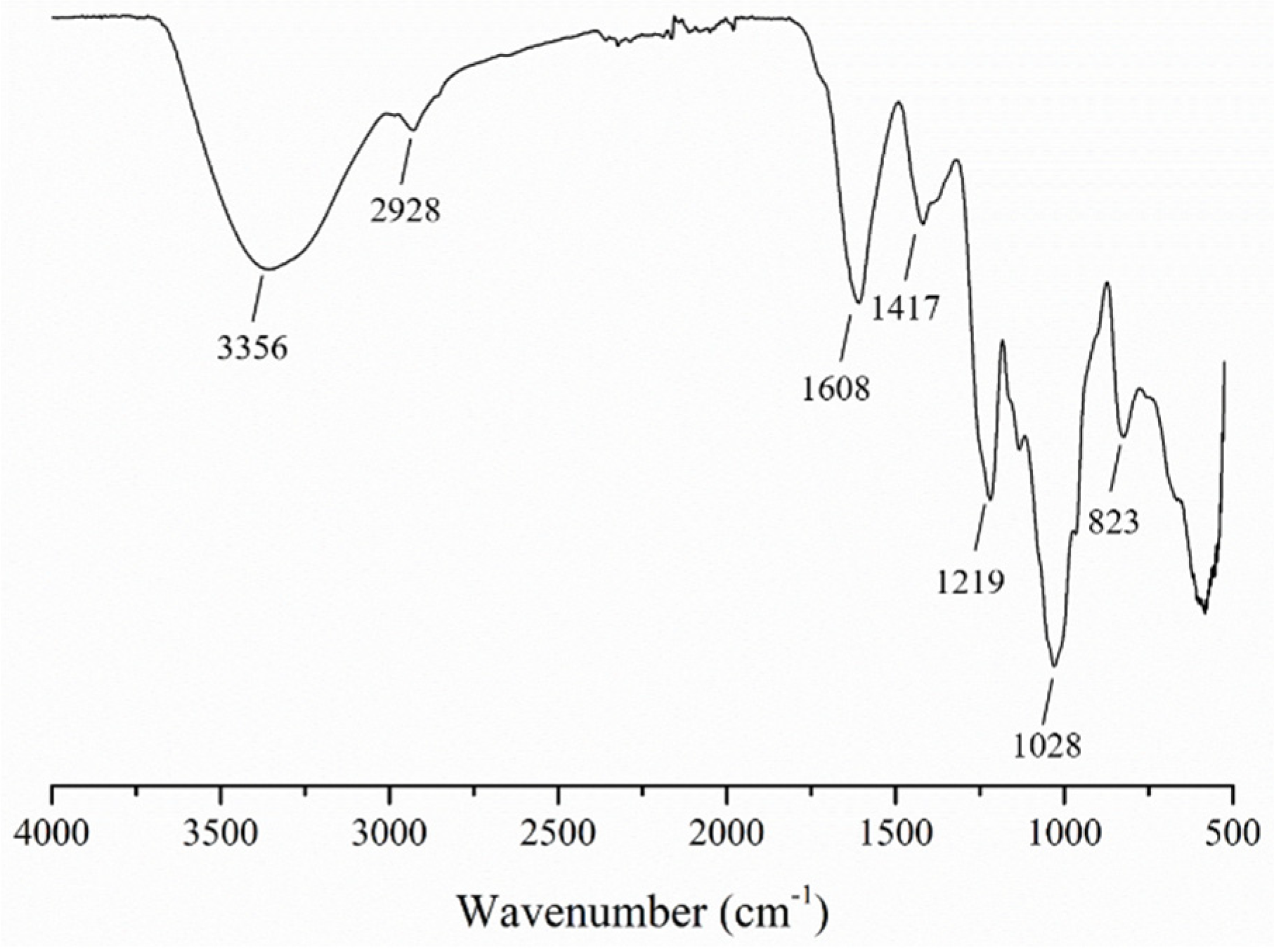
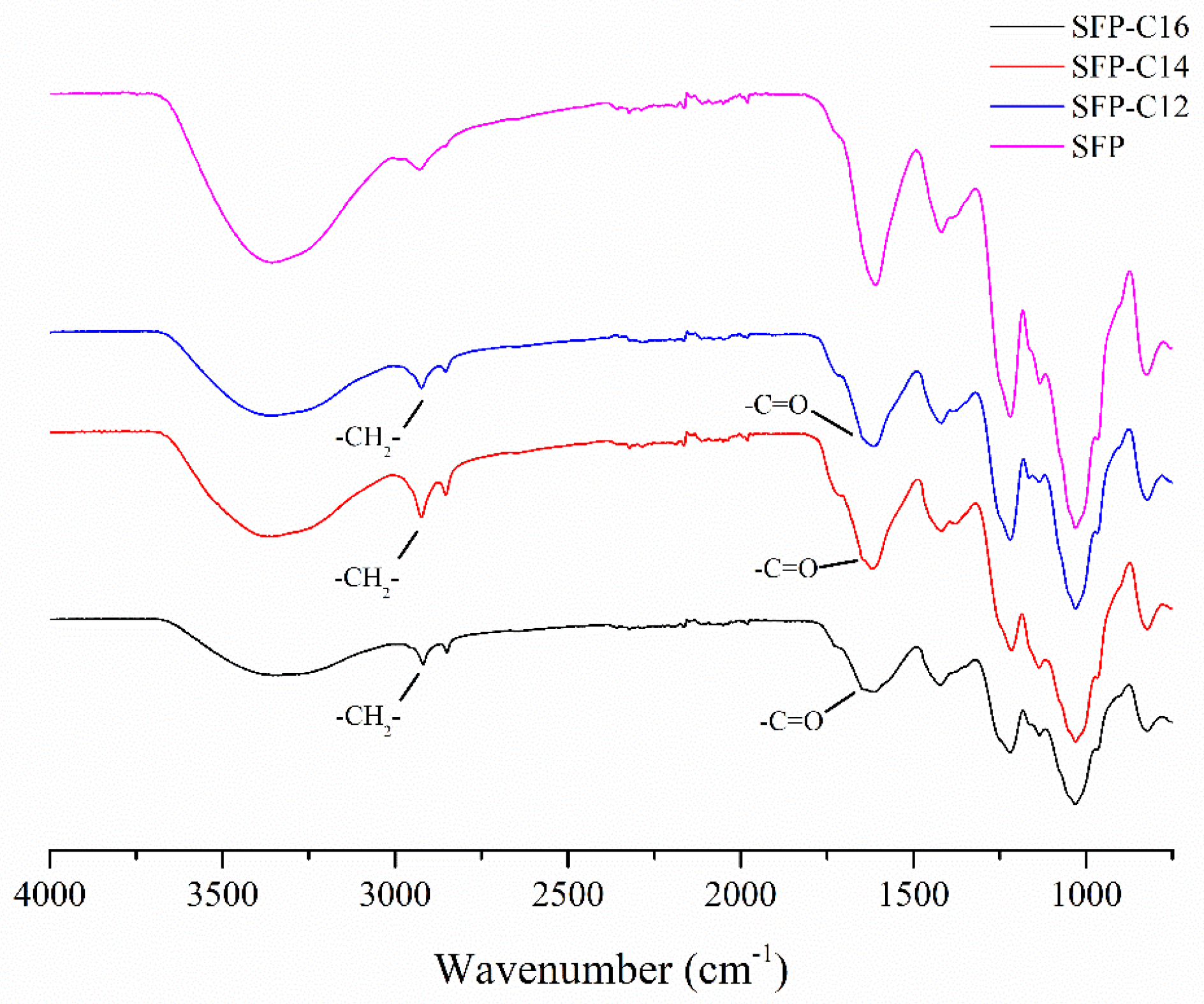
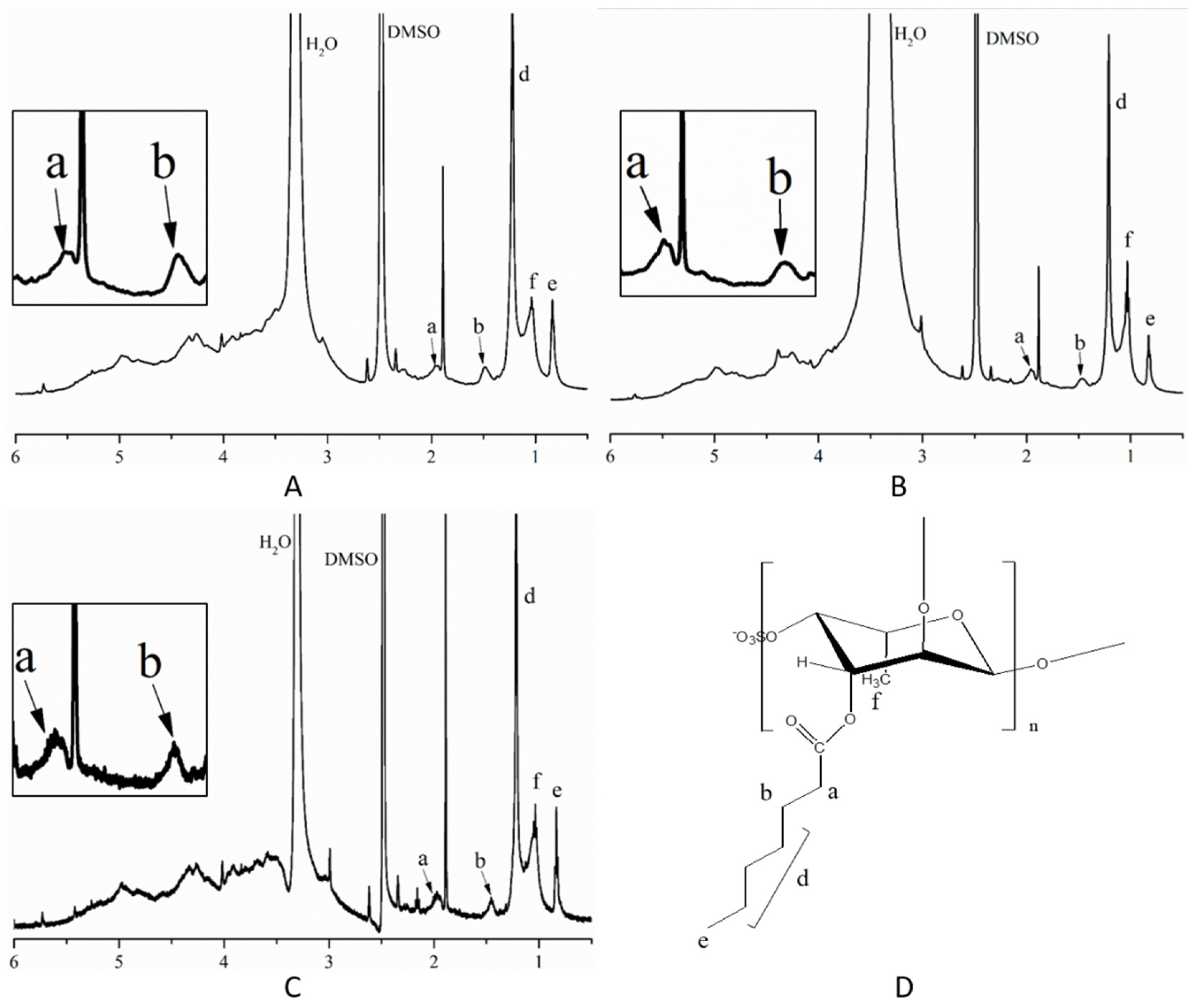
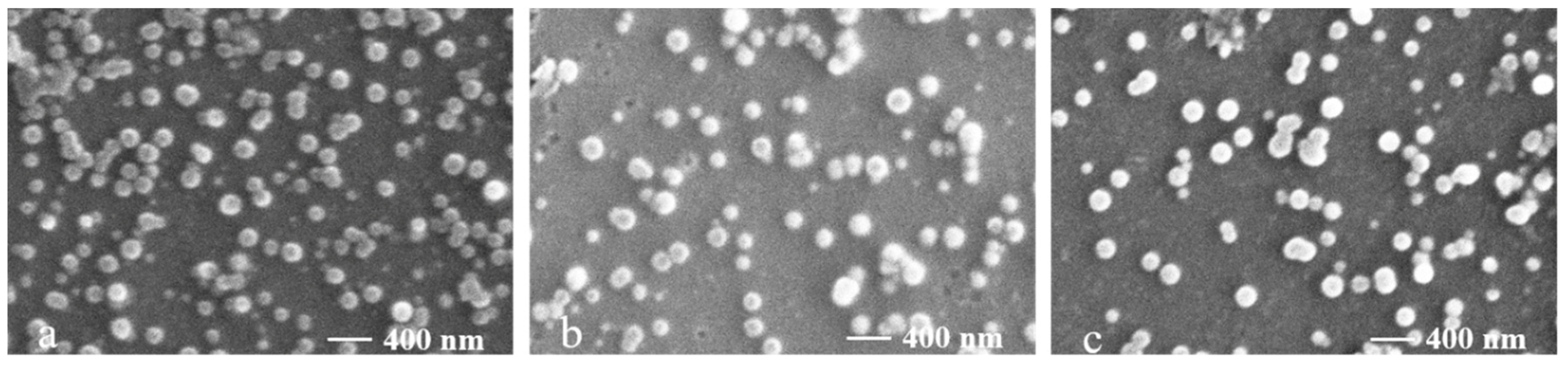
| Sample | Total Sugars (%) | Sulfate (%) | Monosaccharide Composition (Molar Ratio) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Man | Rha | Glc A | Glc | Gal | Xyl | Fuc | |||
| SFP | 54 ± 1 | 37 ± 1 | 0.34 | 0.15 | 0.18 | 0.01 | 0.72 | 0.19 | 1.00 |
| Sample | Yield (%) | Total Sugars (%) | Sulfate (%) |
|---|---|---|---|
| SFP | 54.13 | 36.92 | |
| SFP-C12 | 9.57 | 52.03 | 34.64 |
| SFP-C14 | 9.70 | 47.33 | 32.54 |
| SFP-C16 | 8.09 | 47.29 | 30.96 |
| Sample | Size (nm) | PDI | Zeta Potential (mV) | CMC (μg/mL) |
|---|---|---|---|---|
| SFP-C12M | 229 ± 2 a | 0.15 ± 0.04 a | −39.4 ± 1.7 a | 8.8 |
| SFP-C14M | 214 ± 3 b | 0.10 ± 0.03 a | −35.1 ± 0.4 b | 5.0 |
| SFP-C16M | 194 ± 1 c | 0.15 ± 0.02 a | −37.8 ± 1.1 ab | 12.1 |
| Concentration (mg/mL) | 2 | 1 | 0.5 | 0.25 | 0.125 | 0.0625 | 0.03125 |
|---|---|---|---|---|---|---|---|
| SFP-C12 | 0.59 | 0.8 | 0.95 | 0.94 | 0.97 | 0.99 | 1.11 |
| SFP-C14 | 0.73 | 0.85 | 0.96 | 1.11 | 1.04 | 1.02 | 1.04 |
| SFP-C16 | 0.86 | 0.99 | 0.97 | 1.03 | 1.00 | 0.96 | 1.11 |
| SFP-C12M | 0.75 | 0.87 | 1.15 | 0.98 | 1.02 | 0.93 | 0.99 |
| SFP-C14M | 0.51 | 0.76 | 1.03 | 0.97 | 1.01 | 1.00 | 1.11 |
| SFP-C16M | 0.61 | 0.87 | 0.95 | 0.94 | 0.96 | 0.97 | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Chen, X.; Liu, H.; Liu, S.; Yu, H.; Wang, X.; Qin, Y.; Li, P. Preparation of New Sargassum fusiforme Polysaccharide Long-Chain Alkyl Group Nanomicelles and Their Antiviral Properties against ALV-J. Molecules 2021, 26, 3265. https://doi.org/10.3390/molecules26113265
Sun Y, Chen X, Liu H, Liu S, Yu H, Wang X, Qin Y, Li P. Preparation of New Sargassum fusiforme Polysaccharide Long-Chain Alkyl Group Nanomicelles and Their Antiviral Properties against ALV-J. Molecules. 2021; 26(11):3265. https://doi.org/10.3390/molecules26113265
Chicago/Turabian StyleSun, Yuhao, Xiaolin Chen, Hong Liu, Song Liu, Huahua Yu, Xueqin Wang, Yukun Qin, and Pengcheng Li. 2021. "Preparation of New Sargassum fusiforme Polysaccharide Long-Chain Alkyl Group Nanomicelles and Their Antiviral Properties against ALV-J" Molecules 26, no. 11: 3265. https://doi.org/10.3390/molecules26113265
APA StyleSun, Y., Chen, X., Liu, H., Liu, S., Yu, H., Wang, X., Qin, Y., & Li, P. (2021). Preparation of New Sargassum fusiforme Polysaccharide Long-Chain Alkyl Group Nanomicelles and Their Antiviral Properties against ALV-J. Molecules, 26(11), 3265. https://doi.org/10.3390/molecules26113265








