Anacardic Acids from Amphipterygium adstringens Confer Cytoprotection against 5-Fluorouracil and Carboplatin Induced Blood Cell Toxicity While Increasing Antitumoral Activity and Survival in an Animal Model of Breast Cancer
Abstract
1. Introduction
2. Results
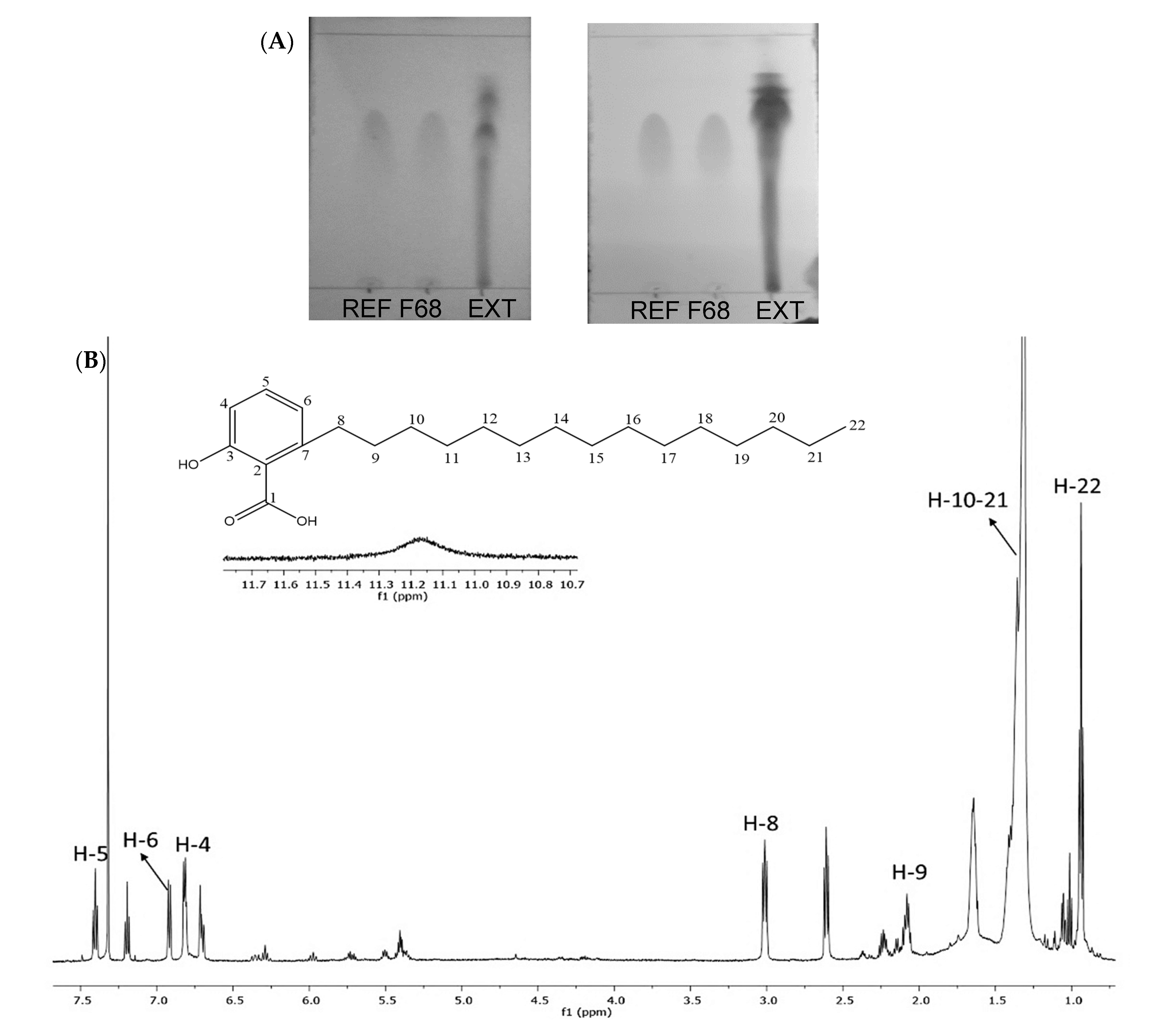
2.1. Analysis of AAs in a Hexane Extract from the Bark of A. adstringens
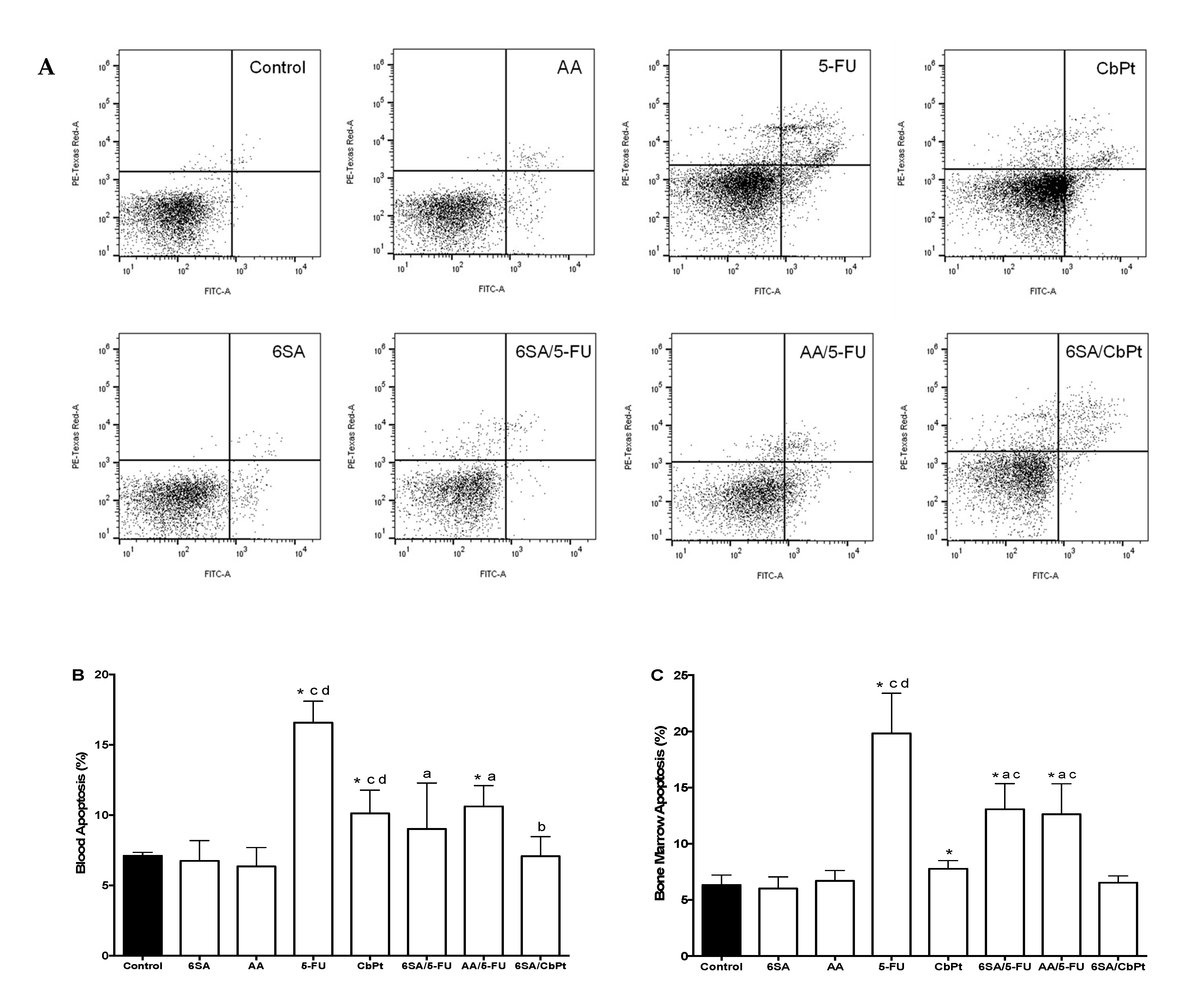
2.2. Myelotoxicity of Chemotherapeutic Agents and the Myeloprotective Effect of Anacardic Acids
2.3. Effect of Treatments on Animal Overall Systemic Health
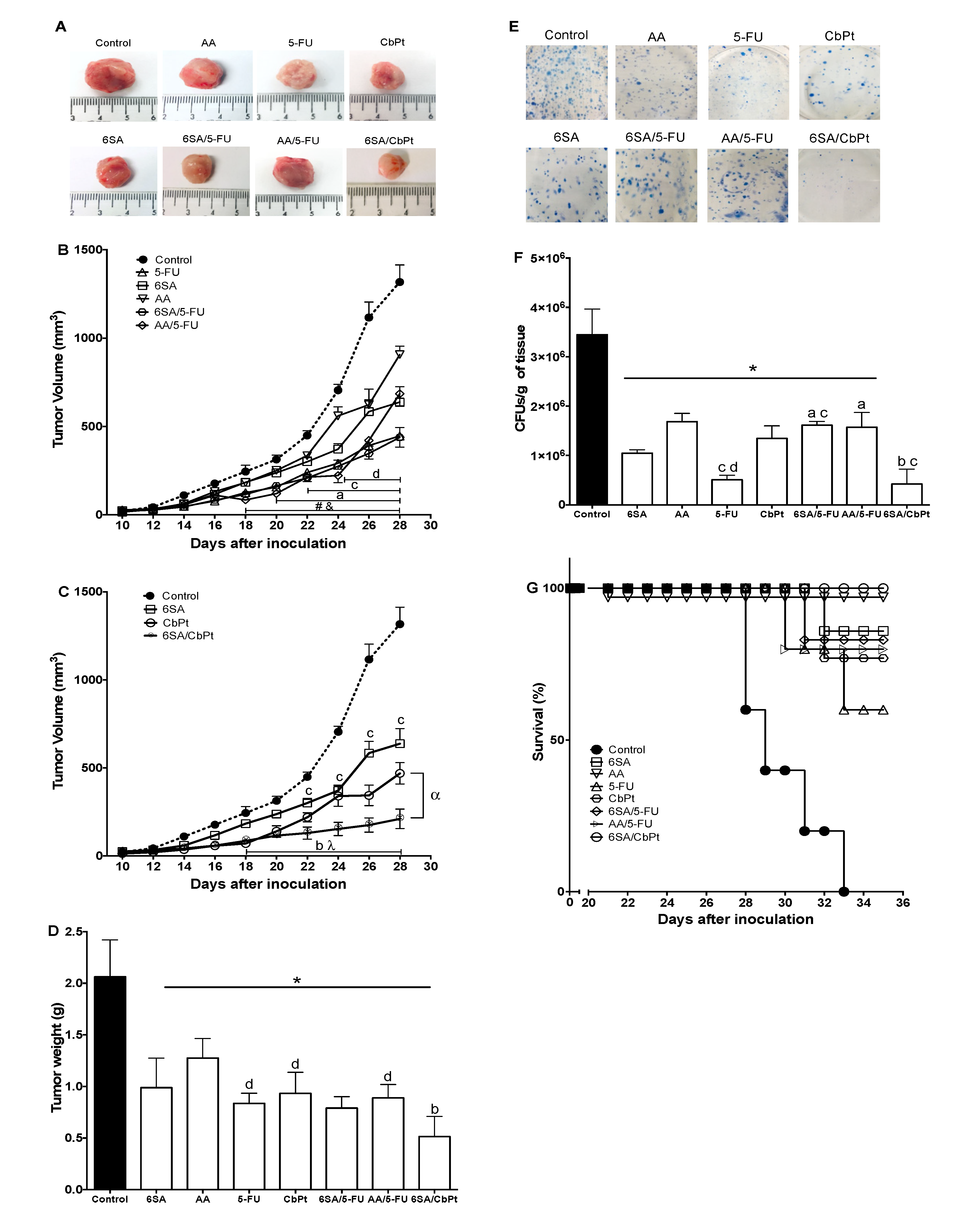
2.4. In Vivo Antitumoral, Antimetastatic, and Survival Effects of 6SA, Chemotherapeutics, and Co-Treatments
3. Discussion
4. Materials and Methods
4.1. Plant Material and Reagents
4.2. Extraction and Isolation of the AA Mixture from A. adstringens
4.3. Animals and Ethics
4.4. 4T1 Cell Line
4.5. Experimental Protocol
4.5.1. Tumor Development and Treatments
4.5.2. Tumor Growth
4.5.3. Determination of Lung Metastasis by Clonogenic Assay
4.5.4. Bone Marrow Collection
4.5.5. Total and Differential White Blood Cell (WBC) Counts in Peripheral Blood
4.5.6. Evaluation of Apoptosis in Leukocytes of Peripheral Blood and Bone Marrow Cells
4.5.7. Survival Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Chabner, B.A.; Roberts, T.G. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Carr, C.; Ng, J.; Tim, W. The side effects of chemotherapeutic agents. Curr. Anaesth. Crit. CARE 2008, 19, 70–79. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Fu, Z.; Zhu, B.; Wang, J.; Guan, S.; Hua, Z. Curcumin activates DNA repair pathway in bone marrow to improve carboplatin-induced myelosuppression. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, H.G.; Im, H.J.; Lee, J.S.; Lee, S.B.; Kim, W.Y.; Lee, H.W.; Lee, S.K.; Byun, C.K.; Son, C.G. Antiemetic and Myeloprotective Effects of Rhus verniciflua Stoke in a Cisplatin-Induced Rat Model. Evidence-based Complement. Altern. Med. 2017, 2017, 9830342. [Google Scholar] [CrossRef]
- Castillo-Juárez, I.; Rivero-Cruz, F.; Celis, H.; Romero, I. Anti-Helicobacter pylori activity of anacardic acids from Amphipterygium adstringens. J. Ethnopharmacol. 2007, 114, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Ortega, A.G.O.; Hernandez, M.S.; Vazquez, M.M.; Salgado, T.T.; Arenas, F.S. Phytochemical study of cuachalalate (Amphiptherygium adstringens, Schiede ex Schlecht). J. Ethnopharmacol. 1999, 68, 109–113. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Sebastin Santhosh, M.; Kemparaju, K.; Girish, K.S. Emerging roles of anacardic acid and its derivatives: A pharmacological overview. Basic Clin. Pharmacol. Toxicol. 2012, 110, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Mata, R.; Calzada, F.; Navarrete, A.; del Rio, F.; Delgado, G. Long-chain phenols from the bark of Amphypterygium adstringens. J. Ethnopharmacol. 1991, 34, 147–154. [Google Scholar] [CrossRef]
- Kubo, J.; Lee, J.R.; Kubo, I. Anti-Helicobacter pylori agents from the cashew apple. J. Agric. Food Chem. 1999, 47, 533–537. [Google Scholar] [CrossRef]
- Knauth, P.; Acevedo-Hernández, G.J.; Cano, M.E.; Gutiérrez-Lomelí, M.; López, Z. In Vitro Bioactivity of Methanolic Extracts from Amphipterygium adstringens (Schltdl.) Schiede ex Standl. Chenopodium ambrosioides L., Cirsium mexicanum DC., Eryngium carlinae F. Delaroche, and Pithecellobium dulce (Roxb.) Benth. Used in Traditional Medicine in Mexico. Evid. Based Complement. Alternat. Med. 2018, 2018, 3610364. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Cruz, B.E.; Esturau, N.; Sánchez-Nieto, S.; Romero, I.; Castillo-Juárez, I.; Rivero-Cruz, J.F. Isolation of the new anacardic acid 6-[16’Z-nonadecenyl]-salicylic acid and evaluation of its antimicrobial activity against Streptococcus mutans and Porphyromonas gingivalis. Nat. Prod. Res. 2011, 25, 1282–1287. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Peixoto, I.T.A.; Verde-Star, M.J.; De la Torre-Zavala, S.; Aviles-Arnaut, H.; Ruiz, A.L.T.G. In Vitro Antimicrobial and Antiproliferative Activity of Amphipterygium adstringens. Evid. Based Complement. Alternat. Med. 2015, 2015, 175497. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Juárez, I.; García-Contreras, R.; Velázquez-Guadarrama, N.; Soto-Hernández, M.; Martínez-Vázquezd, M. Amphypterygium adstringens Anacardic Acid Mixture Inhibits Quorum Sensing-controlled Virulence Factors of Chromobacterium violaceum and Pseudomonas aeruginosa. Arch. Med. Res. 2013, 44, 488–494. [Google Scholar] [CrossRef]
- Balasubramanyam, K.; Swaminathan, V.; Ranganathan, A.; Kundu, T.K. Small molecule modulators of histone acetyltransferase p300. J. Biol. Chem. 2003, 278, 19134–19140. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, E.J.; Shi, C.; Mou, P.K.; Zhang, B.; Wu, C.; Lyu, J.; Shim, J.S. Histone Acetyltransferase (HAT) P300/CBP Inhibitors Induce Synthetic Lethality in PTEN-Deficient Colorectal Cancer Cells through Destabilizing AKT. Int. J. Biol. Sci. 2020, 16, 1774–1784. [Google Scholar] [CrossRef] [PubMed]
- Alam-Escamilla, D.; Estrada-Muñiz, E.; Solís-Villegas, E.; Elizondo, G.; Vega, L. Genotoxic and cytostatic effects of 6-pentadecyl salicylic anacardic acid in transformed cell lines and peripheral blood mononuclear cells. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2015, 777, 43–53. [Google Scholar] [CrossRef]
- Ghizzoni, M.; Boltjes, A.; de Graaf, C.; Haisma, H.J.; Dekker, F.J. Improved inhibition of the histone acetyltransferase PCAF by an anacardic acid derivative. Bioorganic Med. Chem. 2010, 18, 5826–5834. [Google Scholar] [CrossRef]
- Huang, H.; Hua, X.; Liu, N.; Li, X.; Liu, S.; Chen, X.; Zhao, C.; Lan, X.; Yang, C.; Dou, Q.P.; et al. Anacardic acid induces cell apoptosis associated with induction of ATF4-dependent endoplasmic reticulum stress. Toxicol. Lett. 2014, 228, 170–178. [Google Scholar] [CrossRef]
- Kubo, I.; Industry, A.; De Janeiro, R. Antitumor Agents from the Cashew (Anacardium occidentale) apple juice. J. Agric. Food Chem. 1993, 8, 1012–1015. [Google Scholar] [CrossRef]
- Seong, Y.A.; Shin, P.G.; Yoon, J.S.; Yadunandam, A.K.; Kim, G.D. Induction of the Endoplasmic Reticulum Stress and Autophagy in Human Lung Carcinoma A549 Cells by Anacardic Acid. Cell Biochem. Biophys. 2014, 68, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Chen, B.; He, L.; Tang, Y.; Jiang, Z.; Yin, G.; Wang, J.; Jiang, X. Anacardic acid (6-pentadecylsalicylic acid) induces apoptosis of prostate cancer cells through inhibition of androgen receptor and activation of p53 signaling. Chin. J. Cancer Res. 2012, 24, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, H.R.; Rojas, M.D.; Arceo, S.D.B.; Hernández, M.S.; Vázquez, M.M.; Terrazas, T.; del Toro, G.V. Effect of 6-nonadecyl salicylic acid and its methyl ester on the induction of micronuclei in polychromatic erythrocytes in mouse peripheral blood. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2006, 609, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, L.; Zhang, L.; Chen, J.; Yi, Z.; Zhang, J.; Liu, M.; Pang, X. Anacardic Acid (6-Pentadecylsalicylic Acid) Inhibits Tumor Angiogenesis by Targeting Src/FAK/Rho GTPases Signaling Pathway. J. Pharmacol. Exp. Ther. 2011, 339, 403–411. [Google Scholar] [CrossRef]
- Gnanaprakasam, J.N.R.; Lopez-Bañuelos, L.; Vega, L. Anacardic 6-pentadecyl salicylic acid induces apoptosis in breast cancer tumor cells, immunomodulation in the host and decreases blood toxic effects of taxol in an animal model. Toxicol. Appl. Pharmacol. 2021, 410, 115359. [Google Scholar] [CrossRef] [PubMed]
- Gnanaprakasam, J.N.R.; Estrada-Muñiz, E.; Vega, L. The anacardic 6-pentadecyl salicylic acid induces macrophage activation via the phosphorylation of ERK1/2, JNK, P38 kinases and NF-κB. Int. Immunopharmacol. 2015, 29, 808–817. [Google Scholar] [CrossRef]
- Gnanaprakasam, J.N.R.; Estrada-Muñiz, E.; Vega, L. The antineoplastic agent anacardic 6-pentadecyl salicylic acid produces immunomodulation in vivo via the activation of MAPKs. Toxicol. Appl. Pharmacol. 2019, 376, 82–92. [Google Scholar] [CrossRef]
- Carvalho, A.L.N.; Annoni, R.; Torres, L.H.L.; Durão, A.C.C.S.; Shimada, A.L.B.; Almeida, F.M.; Hebeda, C.B.; Lopes, F.D.T.Q.S.; Dolhnikoff, M.; Martins, M.A.; et al. Anacardic acids from cashew nuts ameliorate lung damage induced by exposure to diesel exhaust particles in mice. Evid-Based Complement. Altern. Med. 2013, 2013, 549879. [Google Scholar] [CrossRef]
- Du Pre, S.A.; Hunter, K.W., Jr. Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: Association with tumor-derived growth factors. Exp.-Mol. Pathol. 2007, 82, 12–24. [Google Scholar] [CrossRef]
- Pulanski, B.A.; Ostrand-Rosenberg, S. Mouse 4T1 breast tumor model. Curr. Protoc. Immunol. 2001, 39, 20–22. [Google Scholar] [CrossRef]
- Washington State University Institutional Animal Care and Use Committee. Guideline #8: Tumor Burden. WSU IACUC Guidelines. Available online: https://iacuc.wsu.edu/documents/2017/12/tumor-burden-guidelines.pdf/ (accessed on 22 March 2021).
- Cui, L.; Miao, J.; Furuya, T.; Fan, Q.; Li, X.; Rathod, P.K.; Su, X.Z.; Cui, L. Histone acetyltransferase inhibitor anacardic acid causes changes in global gene expression during in vitro Plasmodium falciparum development. Eukaryot. Cell 2008, 7, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, X.; Chen, S.; Price, B.D. Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation. FEBS Lett. 2006, 580, 4353–4356. [Google Scholar] [CrossRef]
- Liu, W.; Cui, Y.; Ren, W.; Irudayaraj, J. Epigenetic biomarker screening by FLIM-FRET for combination therapy in ER+ breast cancer. Clin. Epigenetics. 2019, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Hemalswarya, S.; Doble, M. Potential synergism of natural products in the treatment of cancer. Phyther. Res. 2006, 20, 239–249. [Google Scholar] [CrossRef]
- Lewandowska, U.; Gorlach, S.; Owczarek, K.; Hrabec, E.; Szewczyk, K. Synergistic interactions between anticancer chemotherapeutics and phenolic compounds and anticancer synergy between polyphenols. Postepy Hig. Med. Dosw. 2014, 68, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yin, Y.; Xu, S.J.; Chen, W.S. 5-Fluorouracil: Mechanisms of resistance and reversal strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef]
- Seyfried, N.T.; Huysentruyt, C.L. On the Origin of Cancer Metastasis. Crit. Rev. Oncol. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Huang, D.; Peng, Y.; Yao, Y.; Zhao, Y.; Yang, Y.; Wang, H.; Cao, L.; Zhu, W.G.; Gu, J. 5-Fluorouracil targets histone acetyltransferases p300/CBP in the treatment of colorectal cancer. Cancer Lett. 2017, 400, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, X.; Hu, W.; Fang, Z.; Jin, Y.; Fang, X.; Miao, Q.R. Epigenetically Down-Regulated Acetyltransferase PCAF Increases the Resistance of Colorectal Cancer to 5-Fluorouracil. Neoplasia 2019, 21, 557–570. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, Z.; Huang, Z.; Wang, R.; Yang, A.; Liao, L.; Du, J. Antitumor and immunomodulatory effects of low-dose 5-FU on hepatoma 22 tumor-bearing mice. Oncol. Lett. 2014, 7, 1260–1264. [Google Scholar] [CrossRef]
- de Souza, C.M.; de Oliveira Gamba, C.; de Campos, C.B.; Lopes, M.T.; Ferreira, M.A.; Andrade, S.P.; Cassali, G.D. Carboplatin delays mammary cancer 4T1 growth in mice. Pathol. Res. Pract. 2013, 209, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Provencher-Bolliger, A.; Everds, N. Haematology of the mouse. In The Laboratory Mouse, 2nd, ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 235–369. [Google Scholar]

| Tissue | Control | 6SA | AA | 5-FU | CbPt | 6SA/5-FU | AA/5-FU | 6SA/CbPt |
|---|---|---|---|---|---|---|---|---|
| Blood cel/mL (106) | 7.7 ± 1.1 | 6.1 ± 2.0 | 7.9 ± 1.4 | 3.0 ± 1.0 *,c,d | 5.4 ± 0.7 *,c,d | 5.0 ± 1.4 *,a,c | 3.9 ± 1.1 *,d | 7.0 ± 1.0 *,b,c |
| Bone Marrow cel/mL (106) | 2.3 ± 0.3 | 1.8 ± 0.2 | 1.7 ± 0.2 | 0.6 ± 0.2 *,c,d | 1.0 ± 0.8 *,c,d | 1.1 ± 0.7 *,a c | 0.8 ± 0.2 *,d | 1.4 ± 0.0 *,b |
| WBC Type | Control | 6SA | AA | 5-FU | CbPt | 6SA/5-FU | AA/5-FU | 6SA/CbPt |
|---|---|---|---|---|---|---|---|---|
| Neutrophil | 43.3 ± 0.9 | 36.8 ± 3.0 | 39.3 ± 3.1 | 16.0 ± 4.1 *,c,d | 21.8 ± 8.2 *,c,d | 34.6 ± 9.0 *,a | 36.8 ± 1.5 a | 26.0 ± 3.6 *,c |
| Band | 20.5 ± 1.7 | 27.5 ± 3.1 * | 28.3 ± 4.0 * | 12.0 ± 3.6 *,c,d | 28.5 ± 4.7 *,a | 19.3 ± 4.2 c | 22.8 ± 2.6 a | 14.6 ± 1.5 *,b,c |
| Lymphocyte | 14.8 ± 2.1 | 13.5 ± 5.1 * | 12.3 ± 1.9 * | 24.3 ± 2.1 *,c,d | 15.0 ± 4.4 a | 24.0 ± 7.2 *,c | 23.7 ± 1.7 *,d | 47.0 ± 5.6 *,b,c |
| Monocyte | 3.5 ± 0.6 | 8.5 ± 0.6 * | 6.0 ± 1.8 * | 4.0 ± 0.8 c,d | 4.3 ± 1.7 | 8.0 ± 2.0 *,a | 8.5 ± 2.6 *,a | 6.3 ± 1.5 * |
| Basophil | 0.5 ± 1.0 | 1.8 ± 1.3 | 0.8 ± 1.3 | 0.0 ± 0.0 c | 1.3 ± 0.9 | 1.3 ± 1.1 a | 0.0 ± 0.0 | 0.0 ± 0.0 c |
| Eosinophil | 1.8 ± 1.3 | 1.8 ± 1.3 | 0.0 ± 0.0 * | 0.8 ± 0.5 d | 1.0 ± 0.8 d | 1.3 ± 1.1 | 0.3 ± 0.5 | 1.0 ± 0.0 |
| Immature | 14.5 ± 0.6 | 10.3 ± 4.5 | 14.0 ± 2.0 | 43.3 ± 5.9 *,c,d | 28.5 ± 4.7 *,c,d | 11.3 ± 3.1 a | 8.0 ± 1.4 *,a,d | 5.0 ± 1.0 *,b |
| Organ | Naive | Control | 6SA | AA | 5-FU | CbPt | 6SA/5-FU | AA/5-FU | 6SA/CbPt |
|---|---|---|---|---|---|---|---|---|---|
| Spleen & | 0.3 ± 0.0 | 2.7 ± 0.3 | 2.6 ± 0.1 | 2.0 ± 1.0 * | 2.7 ± 0.4 | 1.3 ± 0.1 *,c | 1.1 ± 0.1 *,a,c | 1.4 ± 0.5 *,a | 1.7 ± 0.1 *,c |
| Lung | 0.8 ± 0.0 | 2.5 ± 0.3 | 1.0 ± 0.1 * | 1.3 ± 0.2 * | 1.0 ± 1.1 * | 1.1 ± 0.1 * | 1.1 ± 0.2 * | 1.1 ± 0.3 * | 1.0 ± 0.1 * |
| Liver & | 5.1 ± 0.1 | 5.7 ± 0.1 | 5.3 ± 0.3 | 5.6 ± 0.6 | 6.1 ± 0.4 | 5.6 ± 0.5 | 6.4 ± 0.4 * | 6.9 ± 0.8 *,d | 7.2 ± 0.4 *,b,c |
| Kidney | 1.1 ± 0.0 | 1.2 ± 0.0 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.4 | 1.3 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 *,c |
| Body weight gain | 1.9 ± 0.9 | 1.7 ± 1.0 | 1.18 ± 0.5 | 1.03 ± 0.5 | −1.2 ± 0.7 *,c,d | −1.1 ± 0.7 * | −0.80 ± 0.3 *,c | −0.73 ± 0.7 *,d | 0.63 ± 0.4 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galot-Linaldi, J.; Hernández-Sánchez, K.M.; Estrada-Muñiz, E.; Vega, L. Anacardic Acids from Amphipterygium adstringens Confer Cytoprotection against 5-Fluorouracil and Carboplatin Induced Blood Cell Toxicity While Increasing Antitumoral Activity and Survival in an Animal Model of Breast Cancer. Molecules 2021, 26, 3241. https://doi.org/10.3390/molecules26113241
Galot-Linaldi J, Hernández-Sánchez KM, Estrada-Muñiz E, Vega L. Anacardic Acids from Amphipterygium adstringens Confer Cytoprotection against 5-Fluorouracil and Carboplatin Induced Blood Cell Toxicity While Increasing Antitumoral Activity and Survival in an Animal Model of Breast Cancer. Molecules. 2021; 26(11):3241. https://doi.org/10.3390/molecules26113241
Chicago/Turabian StyleGalot-Linaldi, Jairo, Karla M. Hernández-Sánchez, Elizabet Estrada-Muñiz, and Libia Vega. 2021. "Anacardic Acids from Amphipterygium adstringens Confer Cytoprotection against 5-Fluorouracil and Carboplatin Induced Blood Cell Toxicity While Increasing Antitumoral Activity and Survival in an Animal Model of Breast Cancer" Molecules 26, no. 11: 3241. https://doi.org/10.3390/molecules26113241
APA StyleGalot-Linaldi, J., Hernández-Sánchez, K. M., Estrada-Muñiz, E., & Vega, L. (2021). Anacardic Acids from Amphipterygium adstringens Confer Cytoprotection against 5-Fluorouracil and Carboplatin Induced Blood Cell Toxicity While Increasing Antitumoral Activity and Survival in an Animal Model of Breast Cancer. Molecules, 26(11), 3241. https://doi.org/10.3390/molecules26113241






