Abstract
The synthesis of naproxen-containing diaryliodonium salts has been realized from naproxen methyl ester and ArI(OH)OTs activated by trimethylsilyl trifluoromethanesulfonate (TMSOTf) in a solvent mixture comprising dichloromethane and 2,2,2-trifluoroethanol (TFE). Those iodonium salts have been successfully used in the functionalization of an aromatic ring of naproxen methyl ester, including fluorination, iodination, alkynylation, arylation, thiophenolation, and amination and esterification reactions. Moreover, further hydrolysis of the obtained 5-iodo-naproxen methyl ester afforded 5-iodo-naproxen.
1. Introduction
Naproxen I, 2-(6-methoxynaphthalen-2-yl) propionic acid, is a widely prescribed nonsteroidal anti-inflammatory drug that relieves pain, fever, swelling, and stiffness. Naproxen is known to exert its protective effects as an anticancer agent [1]. Recently, naproxen has also been discovered to exhibit antiviral activity, reducing viral load in cells infected with influenza A (H1N1, H3N2) [2,3]. Industrial production of naproxen is currently about ten thousand tons per year, which creates a good basis for conducting research on its structural modification. Significant efforts have been made in the structural modification of naproxen [4,5], including the introduction of fluorine atom into the side chain of this molecule or an esterification process of the carboxylic acid with alcohols (Figure 1A, Ia) [6,7]. There have also been a few scattered methods for the functionalization of the aromatic ring of naproxen, including the introduction of the halogen atom into the aromatic ring (Figure 1B, Ib–Id) [8,9,10]. Some special functionalization has also been applied to the aromatic ring, including hydroxylation and the incorporation of the SCF3 group (Figure 1B, Ie, If) [11,12]. However, these separated methods and monotonous modifications are not conducive to the systematic expansion of naproxen. Therefore, there is an urgent and long-term need for more efficient and systematic methods to modify the aromatic ring of naproxen, particularly in the process of screening and discovering new drugs in medicinal chemistry. In this communication, we envision that the incorporation of the naproxen moiety with iodonium salts will open a new door to the generation of naproxen-based active molecules. Those naproxen-containing iodonium salts could serve as a versatile intermediate for library construction in medicinal chemistry.
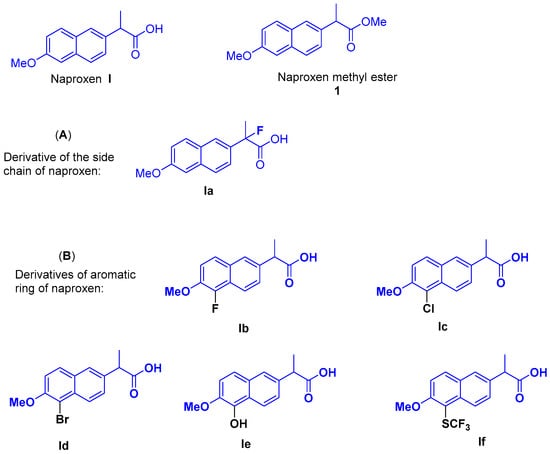
Figure 1.
The structure of Naproxen and its typical derivatives: (A) the introduction of fluorine atom into the side chain; (B) the functionalization of the aromatic ring of naproxen.
In the past several decades, hypervalent iodine chemistry has witnessed prosperous development in organic synthesis [13,14,15]. Diaryliodonium salts, as one kind of the best-known iodine (III) compounds, are widely used as arylating agents and offer an alternative approach to realizing aryl group transformations under mild conditions. Stable iodonium salts have been found to have numerous practical applications, and a summary of the biological properties of iodonium salts is provided in review [14]. In a specific example, a study of the in vitro activities of several iodonium salts against oral and dental anaerobes has demonstrated that their activities are comparable to that of chlorhexidine and these compounds may be suitable for incorporation into an oral mouthwash [16]. Since the pioneering contributions of Kita et al., λ3-iodanes have been known to substitute electron-rich arenes with certain nucleophiles (e.g., N3, CN, OAc) [17,18,19,20,21,22,23]. As part of our long-term interest in the synthesis and application of hypervalent iodine compounds [24,25,26,27,28,29], we envision that diaryliodonium salts may serve the purpose of the modification of naproxen backbone. In this communication, we would like to report an effective approach to the synthesis of naproxen-containing diaryliodonium salts, i.e., naproxen methyl ester diaryliodonium salts, and the modification of the aromatic ring by substitution from formed naproxen methyl ester diaryliodonium salts (Scheme 1).
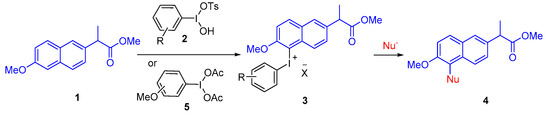
Scheme 1.
Preparation and synthetic application of naproxen methyl ester diaryliodonium salts.
2. Results and Discussion
2.1. Selective Synthesis of Naproxen Methyl Ester Diaryliodonium Salts
At the outset of the study, we attempted to synthesize naproxen-containing iodonium salts according to the literature as shown in Scheme 2A [30,31]. Unfortunately, we did not get the desired iodonium salts by these methods. We speculated that the carboxylic acid on the side chain may interfere with the formation of diaryliodonium salts. Therefore, we decided to complete methylation of the carboxylic acid group. The methylation of naproxen with methanol and sulfuric acid was carried out at 70 °C. Koser’s reagent and its derivatives were prepared according to the reported literature [32]. The initial reactivity assay employed a simple Koser’s reagent 2a as the model substrate to optimize the reaction parameters. Initially, the reaction conditions were optimized for naproxen methyl ester diaryliodonium salts with Koser’s reagent in dichloromethane [33]. However, the low solubility of Koser’s reagent in dichloromethane might prevent the formation of the diaryliodonium salt. As known, Koser’s reagent could be dissolved with TFE as a co-solvent. In this case, diaryliodonium salts were observed by TLC when one equivalent of TMSOTf was added, but it could not be crystalized from ether. To our surprise, when 0.9 equivalent of TMSOTf was added, naproxen methyl ester diaryliodonium salt was formed as a white solid from ether (Scheme 2B). Herein, TMSOTf was employed to activate the Koser’s reagent and simultaneously deliver the triflate anion to the salts. Furthermore, the structure of 3a was undoubtedly confirmed by X-ray analysis (Figure 2; for details see Table S1 in the Supporting Information).
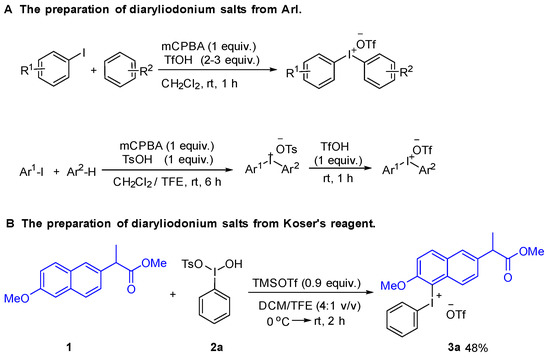
Scheme 2.
Some known methods for the preparation of diaryliodonium salts: (A) The preparation of diaryliodonium salts from ArI with mCPBA; (B) The preparation of diaryliodonium salts from the Koser’s reagent.
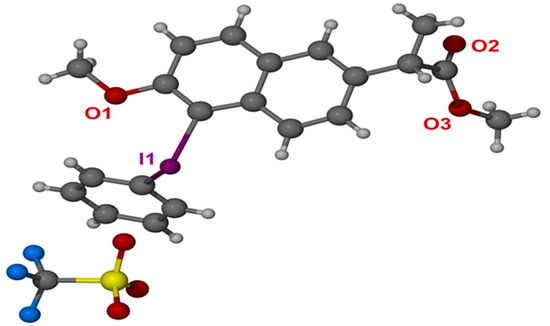
Figure 2.
X-ray crystal structure of 3a (CCDC no. 2050698).
Analogously, 4-methylphenyl and mesityl Koser’s reagents were successful in giving corresponding diaryliodonium salts by this approach (Scheme 3). However, in sharp contrast to the above method, the newly prepared 4-methoxy Koser’s reagent was quite unstable and decomposed violently during the isolation process [34]. After an extensive screening and optimization of reaction conditions, the corresponding 4-methoxyphenyl naproxen methyl ester diaryliodonium salt 3d was prepared with 1-(diacetoxyiodo)-4-methoxybenzene activated by TMSOTf in a solvent of hexafluoroisopropanol at room temperature for 1 h in only 3% yield [35]. When TMSCl was used instead of TMSOTf, iodonium salt 3d was obtained in 6% yield. The corresponding naproxen methyl ester diaryliodonium salt with 3-methoxyphenyl was prepared by the same method in 26% yield. Interestingly, TMSOTf was successfully used to prepare the corresponding naproxen methyl ester diaryliodonium salt with 2-methoxyphenyl in 39% yield (Scheme 4).
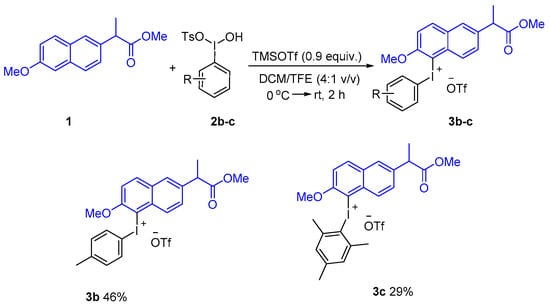
Scheme 3.
Preparation of naproxen methyl ester diaryliodonium salts from Koser’s reagent.
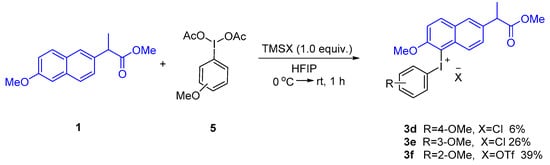
Scheme 4.
Preparation of naproxen methyl ester diaryliodonium salts from MeO-PhI(OAc)2.
2.2. The Application of Naproxen Methyl Ester Diaryliodonium Salts
Next, we attempted to investigate the synthetic application of these new naproxen methyl ester diaryliodonium salts. The mesityl naproxen methyl ester diaryliodonium salts 3c reacted smoothly with CsF, 4-ethynyltoluene, 4-methylbenzeneboronic acid, sodium thiophenolate, and aniline to give substituted naproxen methyl ester in moderate to good yields (Figure 3, 4a, 4c–4f) [36,37,38]. However, when 3c was treated with 4-methylbenzoic acid, the desired product was obtained in a low yield of 7% because of the preference to transfer the mesityl moiety. According to the general regular pattern and encouraged by Olofsson’s method, the methoxyphenyl group was then chosen as the dummy aromatic group of naproxen methyl ester diaryliodonium salts to improve selectivity [39,40]. As expected, the 4-methoxyphenyl naproxen methyl ester diaryliodonium salt 3d reacted with 4-methylbenzoic acid to give aryl ester 4g with good selectivity and yield (Figure 3, 4g) and 2-methoxypheny iodonium salt 3f showed the same good selectivity and yield as 3d. In addition, iodonium salt 3a reacted smoothly with KI to realize the iodination of naproxen methyl ester and further hydrolysis of the obtained 5-iodo naproxen methyl ester 4b afforded 5-iodo-naproxen 6 in 97% yield (Figure 3, 4b and 6).
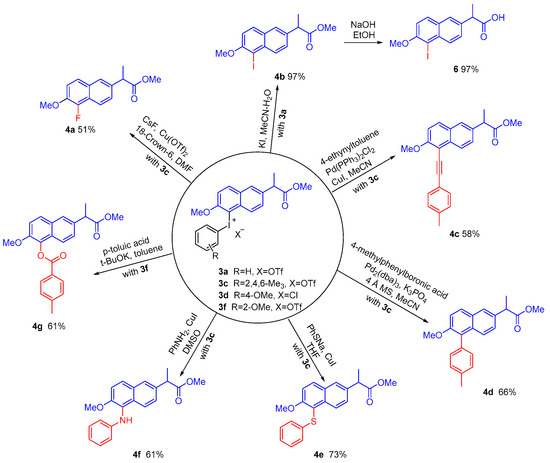
Figure 3.
The modification of the aromatic ring of naproxen methyl ester from iodonium salts 3.
3. Materials and Methods
3.1. General Information
All reactions were carried out using a pre-dried screw capped tube with a Teflon-lined septum under nitrogen, unless otherwise noted. None of the solvents were dried before use, unless otherwise noted. The methylation of naproxen with methanol catalyzed by 10 mol% sulfuric acid was carried out at 70 °C. Koser’s reagent and its derivatives were prepared according to the reported literature [32], 1-(diacetoxyiodo)-4-methoxybenzene was prepared according to the reported literature [35], and other starting materials were commercially available and used without further purification. Column chromatography was performed using silica gel (particle size 10–40 μm, Ocean Chemical Factory of Qingdao, China). 1H-NMR, 13C-NMR, and 19F-NMR spectra were recorded on a JNM-ECA600, JNM-ECS400, or JNM-ECZ400S spectrometer (supplied by JEOL, Tokyo, Japan) at ambient temperature with CDCl3 or DMSO-d6 as the solvent. Chemical shifts (δ) were given in ppm, referenced to the residual proton resonance of CDCl3 (7.26) or DMSO-d6 (2.54), to the carbon resonance of CDCl3 (77.16) or DMSO-d6 (40.45). Coupling constants (J) were given in Hertz (Hz). The terms m, q, t, d, and s refer to multiplet, quartet, triplet, doublet, and singlet, respectively. High-resolution mass spectra were acquired on LCMS-IT/TOF (Shimadzu, Kyoto, Japan) in an 80% acetonitrile−20% water mixture.
3.2. The Synthesis of Naproxen Methyl Ester Diaryliodonium Salts
Method A for 3a–3c: Naproxen methyl ester (1.22 g, 5.0 mmol) was added to a solution of ArI(OH)OTs (5.0 mmol) in DCM (15 mL) and 2,2,2-trifluoroethanol(TFE) (3.5 mL) in a 100 mL round-bottomed flask equipped with a stirring bar. The stirring solution was cooled to 0 °C and trimethylsilyl trifluoromethanesulfonate (TMSOTf) (815 µL, 4.5 mmol) was added dropwisely. The solution was warmed to room temperature and stirred for 2 h. The solvent was evaporated under reduced pressure, then ether (30 mL) was added. A white precipitate was separated, filtered with suction, and washed with ether (20 mL) to obtain the product as a white solid. If the diaryliodonium salt was not precipitated, ether was evaporated under reduced pressure and dried under vacuum for 1 h. Then, ether (30 mL) was added again and stirred vigorously. The white precipitate was separated, filtered with suction, and washed with ether (20 mL) to obtain the product as a white solid.
Method B for 3d-3f: Naproxen methyl ester (1.22 g, 5.0 mmol) was added to a solution of MeO-PhI(OAc)2 (5.0 mmol) in hexafluoroisopropanol (HFIP) (15 mL) in a 50 mL sealed tube. The stirring solution was cooled to 0 °C and trimethyl chlorosilane (TMSCl) (634 µL, 5.0 mmol) was added dropwisely. The solution was warmed to room temperature and stirred for 1 h. The solvent was evaporated under reduced pressure, then ether (30 mL) was added. A white precipitate was separated, filtered with suction, and washed with ether (5 mL) to obtain the product as a white solid.
Naproxen methyl ester (phenyl) iodonium trifluoromethanesulfonate (3a): White solid 1.43 g (2.4 mmol, 48%). M.p.: 126–127 °C.
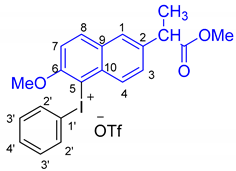
1H-NMR (600 MHz, CDCl3): δ 8.09 (d, J = 9.1 Hz, 1H, 8-H), 7.92 (d, J = 8.7 Hz, 1H, 4-H), 7.84 (d, J = 8.1 Hz, 2H, 2′-H), 7.74 (d, J = 1.3 Hz, 1H, 1-H), 7.60 (dd, J = 8.8, 1.6 Hz, 1H, 3-H), 7.45 (t, J = 7.4 Hz, 1H, 4′-H), 7.41 (d, J = 9.1 Hz, 1H, 7-H), 7.30 (t, J = 7.9 Hz, 2H, 3′-H), 4.05 (s, 3H, 6-OMe), 3.87 (q, J = 7.2 Hz, 1H, CH), 3.63 (s, 3H, COOMe), 1.54 (d, J = 7.2 Hz, 3H, CH3).
13C-NMR (151 MHz, CDCl3): δ 174.5(C=O), 157.6 (C-6), 138.1 (C-2), 137.1 (C-8), 134.3 (C-2′), 132.6 (C-10), 132.2 (C-4′), 132.1 (C-3′), 130.8 (C-3), 130.2 (C-9), 127.6 (C-1), 127.5 (C-4), 113.3 (C-7), 113.2 (C-1′), 101.2 (C-5), 58.0 (6-OMe), 52.3 (COOMe), 45.0 (CH), 18.4 (CH3).
1H,1H-COSY (600 MHz, CDCl3): δ 1H/1H = 8.09/7.41 (8-H/7-H), 7.92/7.60 (4-H/3-H), 7.84/7.30 (2′-H/3′-H), 7.30/7.45 (2′-H/3′-H), 3.87/1.54 (CH/CH3).
1H,13C-HMQC (600 MHz/151 MHz, CDCl3): δ 1H/13C = 8.09/137.1 (C-8), 7.92/127.5 (C-4), 7.84/134.3 (C-2′), 7.74/127.6 (C-1), 7.60/130.8 (C-3), 7.45/132.2 (C-4′), 7.41/113.3 (C-7), 7.30/132.1 (C-3′), 4.05/58.0 (6-OMe), 3.87/45.0 (CH), 3.63/52.3 (COOMe), 1.54/18.4 (CH3).
HRMS (ESI) calculated for [C21H20IO3]+ 447.0452 found: 447.0451. Element analysis: calculated for C22H20F3IO6S: C: 44.31%, H:3.38%; found: C: 44.36%, H: 3.34%.
The crystal suitable for XRD was obtained by the slow difussion of Et2O into its DCM solution. The crystal data are available via CCDC no. 2050698.
Naproxen methyl ester (4-methylphenyl) iodonium trifluoromethanesulfonate (3b): White solid 1.40 g (2.3 mmol, 46%). M.p.: 130–131 °C. 1H-NMR (400 MHz, CDCl3): δ 8.09 (d, J = 9.0 Hz, 1H), 7.95 (d, J = 8.7 Hz, 1H), 7.80–7.71 (m, 3H), 7.64 (d, J = 7.2 Hz, 1H), 7.39 (d, J = 9.1 Hz, 1H), 7.13 (d, J = 8.0 Hz, 2H), 4.09 (s, 3H), 3.90 (q, J = 7.2 Hz, 1H), 3.67 (s, 3H), 2.31 (s, 3H), 1.58 (d, J = 7.2 Hz, 3H). 13C-NMR (101 MHz, CDCl3): δ 174.5, 157.5, 143.3, 138.0, 137.0, 134.4, 132.9, 132.5, 130.8, 130.2, 127.6, 127.5, 113.3, 109.3, 101.2, 58.0, 52.3, 44.9, 21.3, 18.4. HR-MS (ESI) calculated for [C22H22IO3]+ 461.0608 found: 461.0609.
Naproxen methyl ester (mesityl) iodonium trifluoromethanesulfonate (3c): White solid 925 mg (1.45 mmol, 29%). M.p.: 160–161 °C.1H-NMR (400 MHz, CDCl3): δ 8.04 (d, J = 9.0 Hz, 1H), 7.97 (d, J = 8.7 Hz, 1H), 7.75 (s, 1H), 7.66 (d, J = 8.7 Hz, 1H), 7.29 (d, J = 9.0 Hz, 1H), 6.98 (s, 2H), 3.94 (s, 3H), 3.90 (q, J = 7.2 Hz, 1H), 3.68 (s, 2H), 2.57 (s, 6H), 2.27 (s, 3H), 1.58 (d, J = 7.2 Hz, 3H). 13C-NMR (101 MHz, CDCl3): δ 174.5, 157.6, 143.7, 142.5, 138.1, 136.5, 132.7, 130.8, 130.4, 130.2, 127.6, 127.2, 120.0, 113.4, 98.4, 57.6, 52.4, 45.0, 26.9, 21.0, 18.5. HR-MS (ESI) calculated for [C24H26IO3]+ 489.0921 found: 489.0924.
Naproxen methyl ester (4-methoxyphenyl) iodonium chloride (3d): White solid 153 mg (0.3 mmol, 6%). M.p.: 191–192 °C.1H-NMR (400 MHz, CDCl3): δ 8.09 (d, J = 9.0 Hz, 1H), 7.95 (d, J = 8.7 Hz, 1H), 7.82–7.70 (m, 3H), 7.64 (d, J = 8.3 Hz, 1H), 7.39 (d, J = 9.0 Hz, 1H), 7.14 (d, J = 8.3 Hz, 2H), 4.10 (s, 3H), 3.90 (q, J = 7.2 Hz, 1H), 3.67 (s, 3H), 2.31 (s, 3H), 1.58 (d, J = 7.2 Hz, 3H). 13C-NMR (101 MHz, CDCl3): δ 174.7, 161.3, 156.4, 137.3, 135.9, 134.9, 132.5, 130.1, 129.9, 128.6, 127.0, 116.8, 113.4, 110.9, 110.3, 57.9, 55.5, 52.3, 45.1, 18.6. HR-MS (ESI) calculated for [C22H22IO4]+ 477.0557 found: 477.0559.
Naproxen methyl ester (3-methoxyphenyl) iodonium chloride (3e): White solid 665 mg (1.3 mmol, 26%). M.p.: 164–165 °C. 1H-NMR (400 MHz, CDCl3): δ 8.15 (d, J = 8.7 Hz, 1H), 7.89 (d, J = 8.9 Hz, 1H), 7.63 (s, 2H), 7.54 (d, J = 8.8 Hz, 1H), 7.25 (dd, J = 8.5, 2.6 Hz, 2H), 7.00 (t, J = 8.1 Hz, 1H), 6.78 (dd, J = 8.2, 1.9 Hz, 1H), 3.97 (s, 3H), 3.82 (q, J = 7.0 Hz, 1H), 3.64 (s, 3H), 3.61 (s, 3H), 1.51 (d, J = 7.1 Hz, 3H). 13C-NMR (101 MHz, CDCl3): δ 174.6, 160.6, 156.8, 137.3, 135.1, 132.9, 131.3, 130.1, 129.9, 128.8, 126.9, 125.2, 119.9, 118.9, 116.7, 113.2, 109.4, 57.7, 55.7, 52.2, 45.0, 18.5. HR-MS (ESI) calculated for [C22H22IO4]+ 477.0557 found: 477.0558.
Naproxen methyl ester (2-methoxyphenyl) iodonium trifluoromethanesulfonate (3f): White solid 1.22 g (1.95 mmol, 39%). M.p.: 150–151 °C. 1H-NMR (400 MHz, CDCl3): δ 8.12 (d, J = 9.1 Hz, 1H), 7.91 (d, J = 8.8 Hz, 1H), 7.76 (d, J = 1.2 Hz, 1H), 7.62 (dd, J = 8.8, 1.6 Hz, 1H), 7.49–7.45 (m, 1H), 7.43 (d, J = 9.1 Hz, 1H), 7.29 (dd, J = 8.2, 1.2 Hz, 1H), 7.03 (dd, J = 8.3, 0.9 Hz, 1H), 6.91–6.87 (m, 1H), 4.06 (s, 3H), 3.91 (s, 4H), 3.67 (s, 3H), 1.58 (d, J = 7.2 Hz, 3H).13C-NMR (101 MHz, CDCl3): δ 174.6, 158.1, 156.3, 138.1, 137.2, 134.2, 133.5, 132.8, 130.6, 130.3, 128.1, 127.4, 124.2, 113.3, 112.8, 101.4, 98.7, 58.1, 57.1, 52.3, 45.0, 18.5. HR-MS (ESI) calculated for [C22H22IO4]+477.0557 found: 477.0558.
3.3. The Application of Naproxen Methyl Ester Diaryliodonium Salts
Methyl 2-(5-fluoro-6-methoxynaphthalen-2-yl)propanoate (4a)
Cu(OTf)2 (72 mg, 0.2 mmol), anhydrous CSF (91 mg, 0.6 mmol), and 18-crown-6 (26 mg, 0.1 mmol, 0.5 equivalent) were added to naproxen methyl ester (mesityl) iodonium trifluoromethanesulfonate 3c (128 mg, 0.2 mmol) in a sealed tube. The reaction sealed tube was evacuated and backfilled with nitrogen 3 times, and anhydrous DMF (2 mL) was added via a syringe. The reaction mixture was stirred at 85 °C for 12 h. After cooling to room temperature, the reaction was quenched with saturated aqueous NaHCO3 (4 mL), and the mixture was diluted with DCM (2 × 10 mL) and washed with water (3 × 10 mL) and brine (2 × 10 mL). The organic phase was dried with anhydrous Na2SO4 and concentrated in vacuo. The crude material was purified by column chromatography on silica gel (PE/EtOAc = 15/1) to give 4a (26 mg, 51% yield) as a white solid. M.p.: 53–54 °C. 1H-NMR (400 MHz, CDCl3): δ 8.10 (d, J = 8.7 Hz, 1H), 7.79 (d, J = 8.9 Hz, 1H), 7.64 (s, 1H), 7.49 (d, J = 7.6 Hz, 1H), 7.20 (d, J = 8.9 Hz, 1H), 4.01 (s, 3H), 3.89 (q, J = 7.2 Hz, 1H), 3.67 (s, 3H), 1.58 (d, J = 7.6, Hz, 3H). 13C-NMR (101 MHz, CDCl3): δ 175.0, 148.1, 145.6, 143.0, 142.9, 136.9, 126.7, 125.7, 123.9, 123.8, 120.3, 120.2, 57.8, 52.3, 45.5, 18.6. 19F-NMR (376 MHz, CDCl3) δ −146.96 (d, J = 8.1 Hz, 1F). HR-MS (ESI) calculated for C15H15FO3 [M + H]+ 263.1083 found: 263.1085.
Methyl 2-(5-iodo-6-methoxynaphthalen-2-yl)propanoate (4b)
Naproxen methyl ester (phenyl) iodonium trifluoromethanesulfonate3a (119 mg, 0.2 mmol) and KI (166 mg, 1 mmol) were combined with MeCN (1.5 mL) and H2O (0.5 mL) in a 25 mL sealed tube. The reaction mixture was stirred at 100 °C for 12 h. After cooling to room temperature, the mixture was diluted with DCM (2 × 10 mL) and washed with water (10 mL) and brine (10 mL). The organic phase was dried with anhydrous Na2SO4 and concentrated in vacuo. The crude material was purified by column chromatography on silica gel (PE/EtOAc = 15/1) to give 4b (71 mg, 97% yield) as a light yellow solid. M.p.: 57–58 °C. 1H-NMR (400 MHz, CDCl3): δ 8.11 (d, J = 8.8 Hz, 1H), 7.78 (d, J = 8.9 Hz, 1H), 7.65 (s, 1H), 7.49 (d, J = 8.8 Hz, 1H), 7.18 (d, J = 9.0 Hz, 1H), 4.00 (s, 3H), 3.89 (q, J = 7.2 Hz, 1H), 3.67 (s, 3H), 1.59 (d, J = 7.2 Hz, 3H). 13C-NMR (101 MHz, CDCl3): δ 175.0, 156.7, 136.6, 134.9, 131.8, 130.3, 129.9, 128.1, 126.4, 113.3, 87.5, 57.3, 52.2, 45.1, 18.6. HRMS (ESI) calculated for C15H15IO3 [M + H]+ 371.0144 found: 371.0141.
Methyl 2-(6-methoxy-5-(p-tolylethynyl)naphthalen-2-yl)propanoate (4c)
Pd(PPh3)2Cl2 (7 mg, 0.01 mol), CuI (7.6 mg, 0.04 mol), and Et3N (56 μL, 0.4 mmol) were added to naproxen methyl ester (mesityl) iodonium trifluoromethanesulfonate 3c (128 mg, 0.2 mmol) in a sealed tube. The reaction sealed tube was evacuated and backfilled with nitrogen three times, and a solution of 4-Methylphenylacetylene (50 μL, 0.4 mmol) in anhydrous MeCN (2 mL) was added via a syringe. The reaction mixture was stirred at 50 °C under nitrogen atmosphere for 2 h. The reaction mixture was then diluted with DCM (2 × 10 mL), washed with water (10 mL) and brine (10 mL), dried over anhydrous Na2SO4, and concentrated under vacuo. The crude material was purified by column chromatography on silica gel (PE/EtOAc = 15/1) to give 4c (41 mg, 58% yield) as a brownish yellow solid. M.p.: 50–51 °C.1H-NMR (400 MHz, DMSO-d6): δ 8.23 (d, J = 8.7 Hz, 1H), 8.02 (d, J = 9.1 Hz,1H), 7.85 (s,1H), 7.70–7.41 (m, 4H), 7.31 (d, J = 8.0 Hz, 2H), 4.04 (s, 3H), 3.99 (q, J = 7.2 Hz, 1H), 3.64 (s, 3H), 3.40 (s, 3H), 2.40 (s, 3H), 1.53 (d, J = 7.1 Hz, 3H). 13C-NMR (101 MHz, DMSO-d6): δ 175.2, 159.6, 139.3, 137.4, 133.7, 132.1, 131.3, 130.4, 129.0, 128.5, 127.3, 125.9, 120.9, 114.5, 105.7, 99.5, 84.4, 57.4, 52.8, 45.2, 22.0, 19.3. HR-MS (ESI) calculated for C24H22O3 [M + H]+ 359.1647 found: 359.1644.
Methyl 2-(6-methoxy-5-(p-tolyl)naphthalen-2-yl)propanoate (4d)
Pd2(dba)3 (18 mg, 0.02 mol), 4Å MS (100 mg), K3PO4(127 mg, 0.6 mmol), and 4-methylphenyl boronic acid (54 mg, 0.4 mmol) were added to naproxen methyl ester (mesityl) iodonium trifluoromethanesulfonate 3c (128 mg, 0.2 mmol) in a sealed tube. The reaction sealed tube was evacuated and backfilled with nitrogen 3 times, anhydrous MeCN (2 mL) was added via a syringe. The reaction mixture was stirred at room temperature under nitrogen atmosphere for 12 h. The reaction mixture was then diluted with DCM (10 mL × 2), washed with water (10 mL) and brine (10 mL), dried over anhydrous Na2SO4, and concentrated under vacuum. The crude material was purified by column chromatography on silica gel (PE/EtOAc = 15/1) to give 4d (44 mg, 66% yield) as a light-yellow oil. 1H-NMR (400 MHz, CDCl3): δ 7.83 (d, J = 9.0 Hz, 1H), 7.71 (d, J = 1.7 Hz, 1H), 7.49 (d, J = 8.8 Hz, 1H), 7.36 (d, J = 9.0 Hz, 1H), 7.31–7.22 (m, 5H), 3.86 (q, J = 7.2 Hz, 1H), 3.83 (s, 3H), 3.65 (s, 3H), 2.45 (s, 3H), 1.56 (d, J = 7.1 Hz, 3H). 13C-NMR (101 MHz, CDCl3): δ 175.2, 153.9, 136.8, 135.6, 133.3, 133.0, 130.8, 129.1, 129.0, 128.9, 126.2, 126.1, 126.0, 125.4, 114.2, 56.9, 52.2, 45.3, 21.5, 18.6. HR-MS (ESI) calculated for C22H22O3 [M + H]+ 335.1647 found: 335.1645.
Methyl 2-(6-methoxy-5-(phenylthio)naphthalen-2-yl)propanoate (4e)
Sodium thiophenolate (48 mg, 0.36 mol) and CuI (3 mg, 0.016 mmol) were added to naproxen methyl ester (mesityl) iodonium trifluoromethanesulfonate 3c (77 mg, 0.12 mmol) in a sealed tube. The reaction sealed tube was evacuated and backfilled with nitrogen 3 times, anhydrous THF (1 mL) was added via a syringe. The reaction mixture was stirred at 40 °C under nitrogen atmosphere for 3 h. The reaction mixture was then diluted with DCM (10 mL × 2), washed with water (5 mL) and brine (5 mL), dried over anhydrous Na2SO4, and concentrated under vacuum. The crude material was purified by column chromatography on silica gel (PE/EtOAc = 25/1) to give 4f (31 mg, 73% yield) as a colorless oil. 1H-NMR (400 MHz, CDCl3): δ 8.42 (d, J = 8.8 Hz, 1H), 7.94 (d, J = 9.1 Hz, 1H), 7.72 (d, J = 1.6 Hz, 1H), 7.45 (dd, J = 8.9, 1.9 Hz, 1H), 7.36 (d, J = 9.1 Hz, 1H), 7.15–7.11 (m, 2H), 7.06–7.01(m, 3H), 3.95 (s, 3H), 3.87 (q, J = 7.2 Hz, 1H), 3.67 (s, 3H), 1.58 (d, J = 7.1 Hz, 3H). 13C-NMR (101 MHz, CDCl3): δ 175.1, 159.4, 138.2, 136.4, 135.7, 132.1, 129.7, 128.8, 127.9, 126.5, 126.5, 126.1, 124.9, 113.9, 113.1, 57.1, 52.3, 45.3, 18.7. HR-MS (ESI) calculated for C21H20O3S [M + H]+ 353.1211 found: 353.1214.
Methyl 2-(6-methoxy-5-(phenylamino)naphthalen-2-yl)propanoate (4f)
A flame-dried sealed tube was charged with CuI (3 mg, 0.015 mmol) and naproxen methyl ester (mesityl) iodonium trifluoromethanesulfonate 3c (77 mg, 0.12 mmol). The sealed tube was kept under a positive pressure of nitrogen. Anhydrous dimethyl sulfoxide (1 mL) and aniline (33 µL, 0.36 mol) were introduced via syringe. The reaction mixture was stirred at 140 °C under nitrogen atmosphere for 1 h. The reaction mixture was then diluted with DCM (10 mL × 2), washed with water (3 × 10 mL) and brine (10 mL), dried over anhydrous Na2SO4, and concentrated under vacuum. The crude material was purified by column chromatography on silica gel (PE/EtOAc = 30/1) to give 4g (25 mg, 61% yield) as a colorless oil. 1H-NMR (400 MHz, CDCl3): δ 7.80 (d, J = 8.8 Hz, 1H), 7.71–7.68 (m, 2H), 7.33 (d, J = 9.0 Hz, 2H), 7.15 (dd, J = 8.2, 7.6 Hz, 2H), 6.81 (t, J = 7.3 Hz, 1H), 6.67 (d, J = 8.3 Hz, 2H), 3.91 (s, 3H), 3.86 (q, J = 7.2 Hz, 1H), 3.68 (s, 3H), 1.58 (d, J = 7.2 Hz, 3H). 13C-NMR (101 MHz, CDCl3): δ 175.2, 150.9, 146.9, 136.0, 129.9, 129.6, 129.1, 126.3, 126.1, 124.3, 124.3, 119.6, 115.7, 114.0, 56.8, 52.2, 45.4, 18.7. HR-MS (ESI) calculated for C21H21NO3 [M + H]+ 336.1600 found: 336.1603.
2-Methoxy-6-(1-methoxy-1-oxopropan-2-yl)naphthalen-1-yl 4-methylbenzoate (4g)
A flame-dried sealed tube was charged with a magnetic stir bar and tBuOK (31 mg, 0.28 mmol). The sealed tube was kept under a positive pressure of nitrogen. Anhydrous toluene (4 mL) was introduced via syringe, and 4-methylbenzoic acid (38 mg, 0.28 mmol) and naproxen methyl ester (4-methoxyphenyl) iodonium chloride 3d (143 mg, 0.25 mmol) were added sequentially as solids. The sealed tube was then heated to reflux for 3 h. The reaction mixture was then diluted with DCM (10 mL × 2), washed with water (10 mL) and brine (10 mL), dried over anhydrous Na2SO4, and concentrated under vacuum. The crude material was purified by column chromatography on silica gel (PE/EtOAc = 15/1) to give 4g (60 mg, 64% yield) as a colorless liquid. When 4-methylbenzoic acid was treated with naproxen methyl ester (2-methoxyphenyl) iodonium trifluoromethanesulfonate 3f, the yield of 4g was 61%. 1H-NMR (400 MHz, CDCl3): δ 8.24 (d, J = 8.2 Hz, 2H), 7.82–7.73 (m, 3H), 7.43–7.36 (m, 4H), 3.93 (s, 3H), 3.87 (q, J = 7.1 Hz, 1H), 3.66 (s, 1H), 2.49 (s, 3H), 1.58 (d, J = 7.2 Hz, 3H). 13C-NMR (101 MHz, CDCl3): δ 175.1, 164.8, 148.1, 144.6, 136.4, 133.6, 130.6, 129.5, 129.4, 127.6, 127.0, 126.8, 126.6, 126.2, 121.0, 114.7, 57.0, 52.2, 45.4, 22.0, 18.6. HR-MS (ESI) calculated for C23H22O5 [M + H]+ 379.1545 found: 379.1546.
2-(5-Iodo-6-methoxynaphthalen-2-yl)propanoic acid (6)
A 25 mL sealed tube was charged with 5-iodo naproxen methyl ester 4b (56 mg, 0.15 mmol) and ethanol (0.5 mL). Then, a solution of sodium hydroxide (12 mg, 0.3 mmol) in ethanol (0.6 mL) was added slowly over 1 min. The mixture was stirred at reflux for about 3 h. After cooling the mixture to 0 °C, the pH value was adjusted to 2 by adding 1 N hydrogen dchloride. A standard extractive workup with ethyl acetate (3 × 5 mL) gave the product 6 (52 mg, 97% yield) as a white solid. M.p.: 177–178 °C. 1H-NMR (400 MHz, DMSO-d6): δ 12.43 (s, 1H), 8.02–7.99 (m, 2H), 7.81 (s, 1H), 7.57 (d, J = 7.2 Hz, 1H), 7.44 (d, J = 9.0 Hz, 1H), 4.00 (s, 3H), 3.89 (q, J = 7.1 Hz, 1H), 1.50 (d, J = 7.1 Hz, 3H). 13C-NMR (101 MHz, DMSO-d6): δ 176.3, 157.3, 138.0, 134.9, 131.5, 131.3, 130.3, 129.4, 127.3, 114.7, 87.6, 58.1, 45.3, 19.3. HR-MS (ESI) calculated for C14H13IO3 [M + H]+ 356.9988 found: 356.9987.
1H-NMR, 13C-NMR and 19F-NMR spectrum of compounds 3, 4 and 6 are presented in Supplementary Material (Figures S2–S32).
4. Conclusions
In summary, naproxen-cantaining diaryliodonium salts were successfully synthesized from naproxen methyl ester and ArI(OH)OTs in dichloromethane and 2,2,2-trifluoroethanol. The structure of the iodonium salt 3a was confirmed by X-ray diffraction. Moreover, those iodonium salts were successfully used in further derivatization, including fluorination, iodination, alkynylation, arylation, thiophenolation, and amination and esterification reactions. This strategy offered a new source for the generation of naproxen-based active molecules.
Supplementary Materials
Supplementary Materials are available online. 1H-NMR,13C-NMR, and 19F-NMR spectroscopic data for all new compounds are presented in Figures S2–S32. Figure S1. X-Ray crystallographic structure for compound 3a, Table S1. Details of data collection, processing and structure refinement for compound 3a.
Author Contributions
J.Z. and Z.B. performed the experimental work and analyzed the results. P.W. and C.C. designed the experiments, discussed and provided advice on the work. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key Research and Development Program of China (2016YFB0401400), the National Natural Science Foundation of China (21871158, 91645203, and 21672120), the Fok Ying Tong Education Foundation of China (Grant No. 151014), the Department of Education ofGuangdong Province (No. 2016KCXTD005), and the Youth Foundation of Wuyi University (No. 2017td01). The APC was funded by the Youth Foundation of Wuyi University (No. 2017td01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the communication.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2016YFB0401400), the National Natural Science Foundation of China (22071129, 21871158 and 21672120), and the Fok Ying Tong Education Foundation of China (Grant No. 151014) and the Youth Foundation of Wuyi University (No. 2017td01).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Mohammed, A.; Yarla, N.S.; Madka, V.; Rao, C.V. Clinically relevant anti-inflammatory agents for chemoprevention of colorectal cancer: New perspectives. Int. J. Mol. Sci. 2018, 19, 2332. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.S.; Lee, N.; Chan, P.K.; Beigel, J.H. The role of adjuvant immunomodulatory agents for treatment of severe influenza. Antivir. Res. 2018, 150, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Lejal, N.; Tarus, B.; Bouguyon, E.; Chenavas, S.; Bertho, N.; Delmas, B.; Ruigrok, R.W.H.; Primo, C.D.; Slama-Schwok, A. Structure-based discovery of the novel antiviral properties of naproxen against the nucleoprotein of influenza a virus. Antimicrob. Agents Chemother. 2013, 57, 2231–2242. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Chen, C.C.; Yang, H.S.; Ng, I.S.; Chen, T.L. Implication of substrate-assisted catalysis on improving lipase activity or enantioselectivity in organic solvents. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2006, 1764, 1424–1428. [Google Scholar] [CrossRef]
- Adeniji, A.; Uddin, M.J.; Zang, T.; Tamae, D.; Wangtrakuldee, P.; Marnett, L.J.; Penning, T.M. Discovery of (R)-2-(6-methoxynaphthalen-2-yl)butanoic acid as a potent and selective aldo-keto reductase 1C3 inhibitor. J. Med. Chem. 2016, 59, 7431–7444. [Google Scholar] [CrossRef] [PubMed]
- Goj, O.; Kotila, S.; Haufe, G. Convenient routes to 2-aryl-2-fluoropropionic acids: Synthesis of monofluorinated analogues of (±)-ibuprofen, (±)-naproxen and related compounds. Tetrahedron 1996, 39, 12761–12774. [Google Scholar] [CrossRef]
- Chatterjee, A.; König, B. Birch-type photoreduction of arenes and heteroarenes by sensitized electron transfer. Angew. Chem. Int. Ed. 2019, 58, 14289–14294. [Google Scholar] [CrossRef] [PubMed]
- Borodkina, G.I.; Elanova, I.R.; Gatilova, Y.V.; Shubin, V.G. Direct electrophilic fluorination of naproxen with NF-reagents. J. Fluo. Chem. 2019, 228, 109412. [Google Scholar] [CrossRef]
- Song, S.; Li, X.Y.; Wei, J.L.; Wang, W.J.; Zhang, Y.Q.; Ai, L.S.; Zhu, Y.C.; Shi, X.M.; Zhang, X.H.; Jiao, N. DMSO-catalysed late-stage chlorination of (hetero)arenes. Nat. Catal. 2020, 3, 107–115. [Google Scholar] [CrossRef]
- Satkar, Y.; Ramadoss, V.; Nahide, P.D.; García-Medina, E.; Juárez-Ornelas, K.A.; Alonso-Castro, A.J.; Chávez-Rivera, R.; Jiménez-Halla, J.O.C.; Solorio-Alvarado, C.R. Practical, mild and efficient electrophilic bromination of phenols by a new I(iii)-based reagent: The PIDA–AlBr3system. RSC Adv. 2018, 8, 17806–17812. [Google Scholar] [CrossRef]
- Song, Z.Q.; Wang, D.H. Palladium-catalyzed hydroxylation of aryl halides with boric acid. Org. Lett. 2020, 22, 8470–8474. [Google Scholar] [CrossRef]
- Nalbandian, C.J.; Brown, Z.E.; Alvarez, E.; Gustafson, J.L. Lewis base/Bronsted acid dual-catalytic C-H sulfenylation of aromatics. Org. Lett. 2018, 20, 3211–3214. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Zhdankin, V.V. Advances in synthetic applications of hypervalent iodine compounds. Chem. Rev. 2016, 116, 3328–3435. [Google Scholar] [CrossRef] [PubMed]
- Stang, P.J.; Zhdankin, V.V. Organic polyvalent Iodine compounds. Chem. Rev. 1996, 96, 1123–1178. [Google Scholar] [CrossRef] [PubMed]
- Yusubov, M.S.; Maskaev, A.V.; Zhdankin, V.V. Iodonium salts in organic synthesis. Arkivoc 2011, 2011, 370–409. [Google Scholar] [CrossRef]
- Goldstein, E.J.C.; Citron, D.M.; Warren, Y.; Merriam, C.V.; Tyrrell, K.; Fernandez, H.; Radhakrishnan, U.; Stang, P.J.; Conrads, G. In vitro activities of iodonium salts against oral and dental anaerobes. Antimicrob. Agents Chemother. 2004, 48, 2766–2770. [Google Scholar] [CrossRef][Green Version]
- Kita, Y.; Tohma, H.; Hatanaka, K.; Takada, T.; Fujita, S.; Mitoh, S.; Sakurai, H.; Oka, S. Hypervalent iodine-induced nucleophilic substitution of para-substituted phenol ethers. Generation of cation radicals as reactive intermediates. J. Am. Chem. Soc. 1994, 116, 3684–3691. [Google Scholar] [CrossRef]
- Dohi, T.; Ito, M.; Morimoto, K.; Iwata, M.; Kita, Y. Oxidative cross-coupling of arenes induced by single-electron transfer leading to biaryls by use of organoiodine(III) oxidants. Angew. Chem. Int. Ed. 2008, 47, 1301–1304. [Google Scholar] [CrossRef]
- Kita, Y.; Egi, M.; Okajima, A.; Ohtsubo, M.; Takada, T.; Tohma, H. Hypervalent iodine(III) induced intramolecular cyclization of substituted phenol ethers bearing an alkyl azido sidechain–a novel synthesis of quinone imine ketals. Chem. Commun. 1996, 13, 1491–1492. [Google Scholar] [CrossRef]
- Magnus, P.; Lacour, J.; Evans, P.A.; Roe, M.B.; Hulme, C. Hypervalent iodine chemistry: New oxidation reactions using the iodosylbenzene-trimethylsilyl azide reagent combination. Direct α- and β-Azido functionalization of triisopropylsilyl enol ethers. J. Am. Chem. Soc. 1996, 118, 3406–3418. [Google Scholar] [CrossRef]
- Evans, P.A.; Brandt, T.A. Hypervalent iodine chemistry: Mechanistic investigation of the novel haloacetoxylation, halogenation, and acetoxylation reactions of 1,4-dimethoxynaphthalenes. J. Org. Chem. 1997, 62, 5321–5326. [Google Scholar] [CrossRef]
- Akula, R.; Galligan, M.; Ibrahim, H. Umpolung of halide reactivity: Efficient (diacetoxyiodo)benzene-mediated electrophilic a-halogenation of 1,3-dicarbonyl compounds. Chem. Commun. 2009, 45, 6991–6993. [Google Scholar] [CrossRef]
- Fosu, S.C.; Hambira, C.M.; Chen, A.D.; Fuchs, J.R.; Nagib, D.A. Site-selective C–H functionalization of (hetero)Arenes via transient, non-symmetric lodanes. Chemical 2019, 5, 417–428. [Google Scholar] [CrossRef]
- Peng, J.; Chen, C.; Wang, Y.; Lou, Z.; Li, M.; Xi, C.; Chen, H. Direct Vicinal Disubstitution of Diaryliodonium Salts by Pyridine N-oxides and N-amidates by a 1,3-Radical Rearrangement. Angew. Chem. Int. Ed. 2013, 52, 7574–7578. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Peng, J.; Li, M. Copper(II)-Catalyzed Three-Component Cascade Annulation of Diaryliodoniums, Nitriles, and Alkynes: A Regioselective Synthesis of Multiply Substituted Quinolines. Angew. Chem. Int. Ed. 2013, 52, 5323–5327. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, C.; Chen, J.; Su, X.; Xi, C.; Chen, H. Cu-catalyzed arylcarbocyclization of alkynes with diaryliodonium salts through C–C bond formation on inert C(sp3)–H bond. Org. Lett. 2014, 16, 3776–3779. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, C.; Xi, C. β-Arylation of oxime ethers using diaryliodonium salts through activation of inert C(sp3)–H bonds using a palladium catalyst. Chem. Sci. 2016, 7, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Wang, Y.; Su, X.; He, R.; Chen, C. Copper-Catalyzed [2+2+2] Modular Synthesis of Multisubstituted Pyridines: Alkenylation of Nitriles with Vinyliodonium Salts. Angew. Chem. Int. Ed. 2017, 56, 4824–4828. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, C.; Zhou, J.; Hu, H.-S.; Li, J.; Wu, P.; Chen, C. Wet carbonate-promoted radical arylation of vinyl pinacolboronates with diaryliodonium salts yields substituted olefins. Commun. Chem. 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Bielawski, M.; Olofsson, B. High-yielding one-pot synthesis of diaryliodonium triflates from arenes and iodine or aryl iodides. Chem. Commun. 2007, 24, 2521–2523. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Jalalian, N.; Olofsson, B. One-pot synthesis of diaryliodonium salts using toluenesulfonic acid: A fast entry to electron-rich diaryliodonium tosylates and triflates. Synlett 2008, 4, 592–596. [Google Scholar] [CrossRef]
- Merritt, E.A.; Carneiro, V.M.T.; Silva, L.F.; Olofsson, B. Facile synthesis of Koser’s reagent and derivatives from iodine or aryl iodides. J. Org. Chem. 2010, 75, 7416–7741. [Google Scholar] [CrossRef]
- McCammant, M.S.; Thompson, S.; Brooks, A.F.; Krska, S.W.; Scott, P.J.H.; Sanford, M.S. Cu-mediated C–H 18F-fluorination of electron-rich (hetero)arenes. Org. Lett. 2017, 19, 3939–3942. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, I.; Spyroudis, S.; Varvoglis, A.; Raptopoulou, C.P. Aryliodonium derivatives of 2-amino-1,4-quinones: Preparation and reactivity. Tetrahedron 1997, 53, 6097–6112. [Google Scholar] [CrossRef]
- Wang, B.; Qin, L.; Neumann, K.D.; Uppaluri, S.; Cerny, R.L.; DiMagno, S.G. Improved arene fluorination methodology for I(III) salts. Org. Lett. 2010, 12, 3352–3355. [Google Scholar] [CrossRef][Green Version]
- Ichiishi, N.; Canty, A.J.; Yates, B.F.; Sanford, M.S. Cu-catalyzed fluorination of diaryliodonium salts with KF. Org. Lett. 2013, 15, 5134–5137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhou, Z.S.; Chen, R. A novel Pd/Ag-catalyzed Sonogashira coupling reaction of terminal alkynes with hypervalent iodonium salts. Synthesis 2008, 17, 2680–2682. [Google Scholar] [CrossRef]
- Yang, J.; Han, Q.Y.; Zhao, C.L.; Dong, T.; Hou, Z.Y.; Hua-Li Qin, H.L.; Zhang, C.P. Pd-catalyzed divergent trifluoroethylation and arylation of arylboronic acids by aryl(2,2,2-trifluoroethyl)iodonium triflates. Org. Biomol. Chem. 2016, 14, 7654–7658. [Google Scholar] [CrossRef] [PubMed]
- Lancer, K.M.; Wiegand, G.H. The ortho effect in the pyrolysis of iodonium halides. A case for a sterically controlled nucleophilic aromatic (SN) substitution reaction. J. Org. Chem. 1976, 41, 3360–3364. [Google Scholar] [CrossRef]
- Jalalian, N.; Petersen, T.B.; Olofsson, B. Metal-free arylation of oxygen nucleophiles with diaryliodonium salts. Chem. Eur. J. 2012, 18, 14140–14149. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).