Isolation of a Novel Polyketide from Neodidymelliopsis sp.
Abstract
1. Introduction
2. Results and Discussion
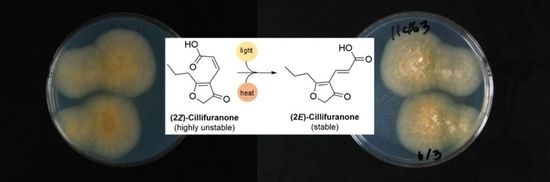
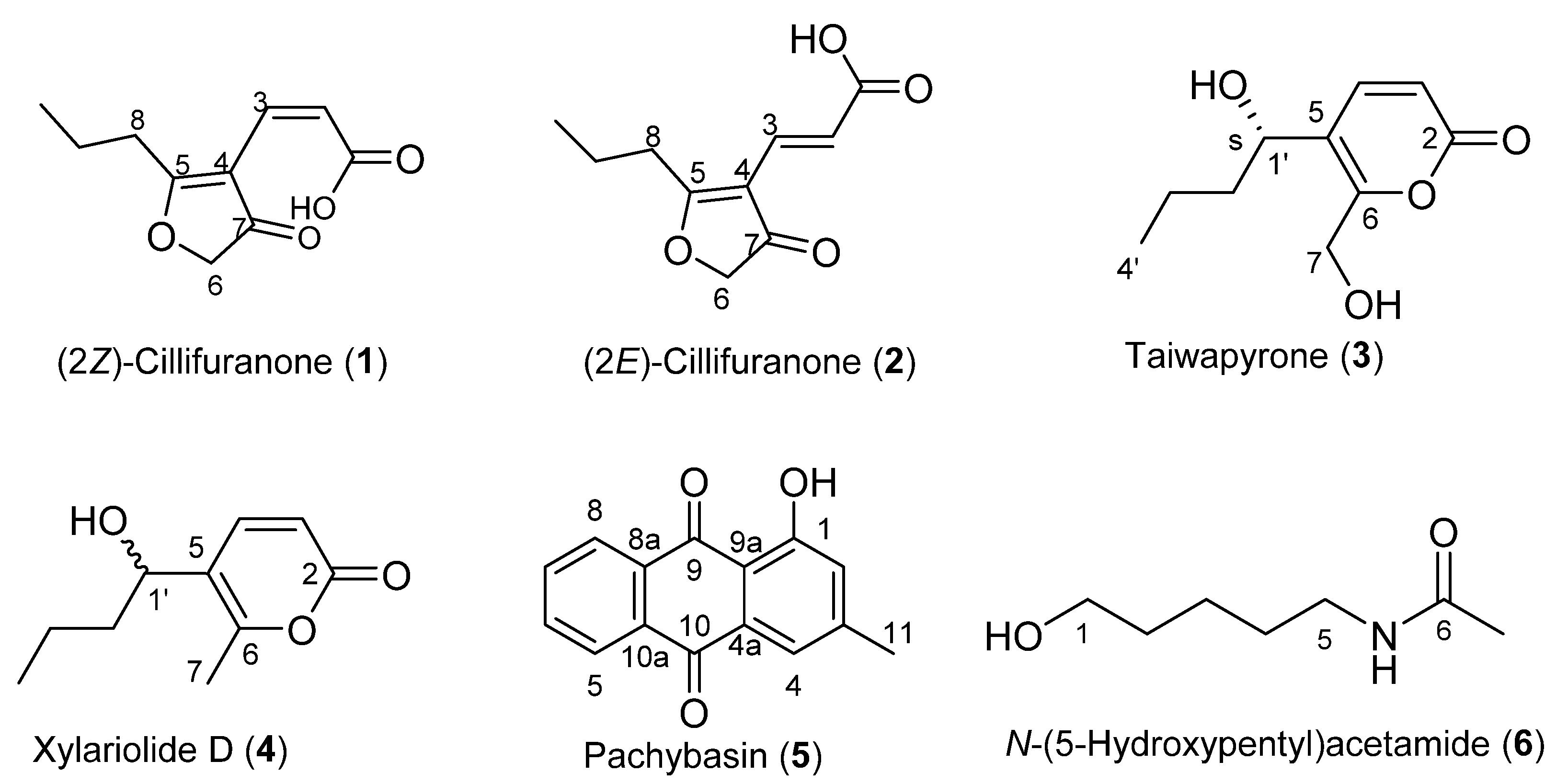
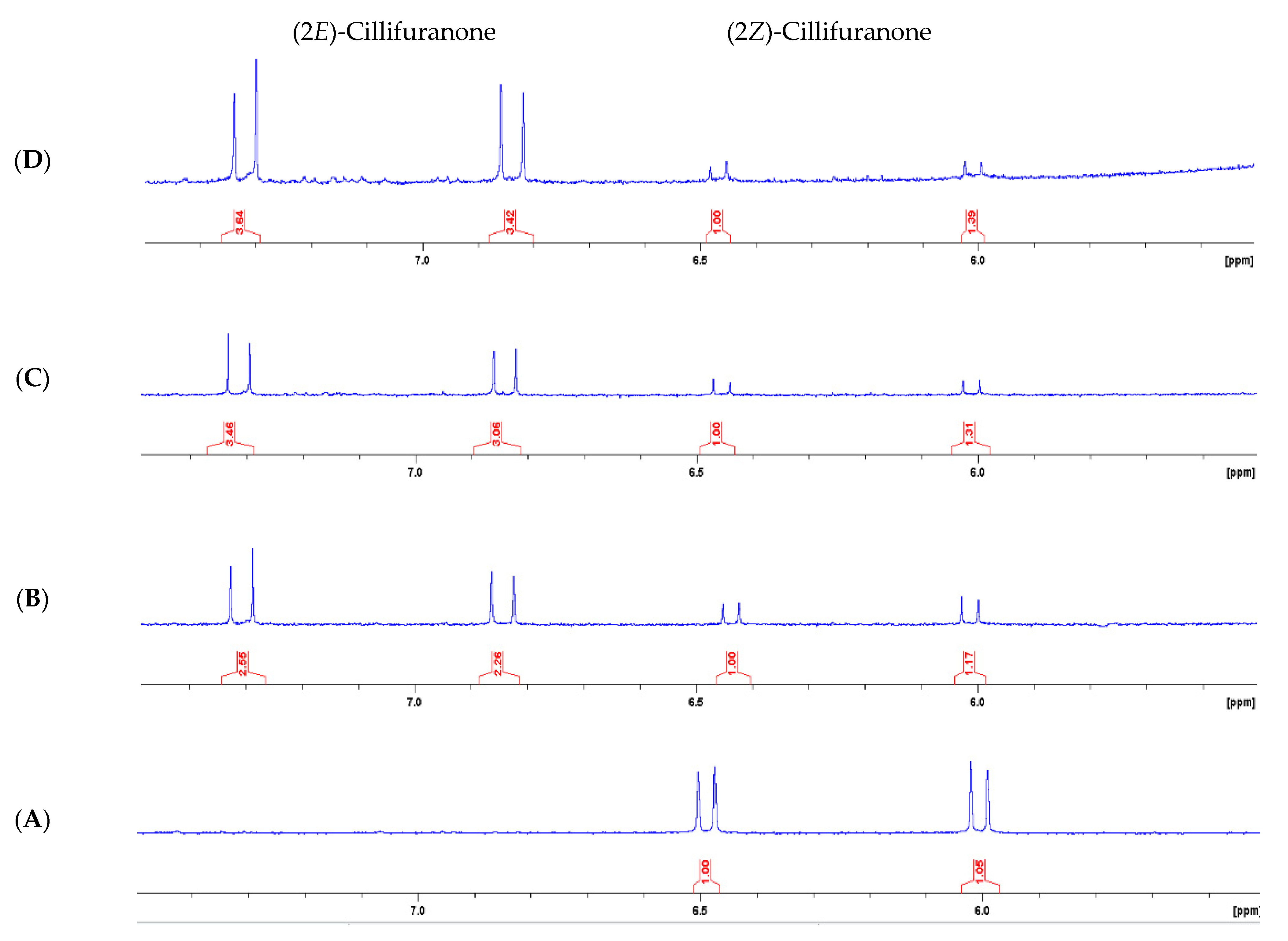
2.1. Isolation and Structure Elucidation
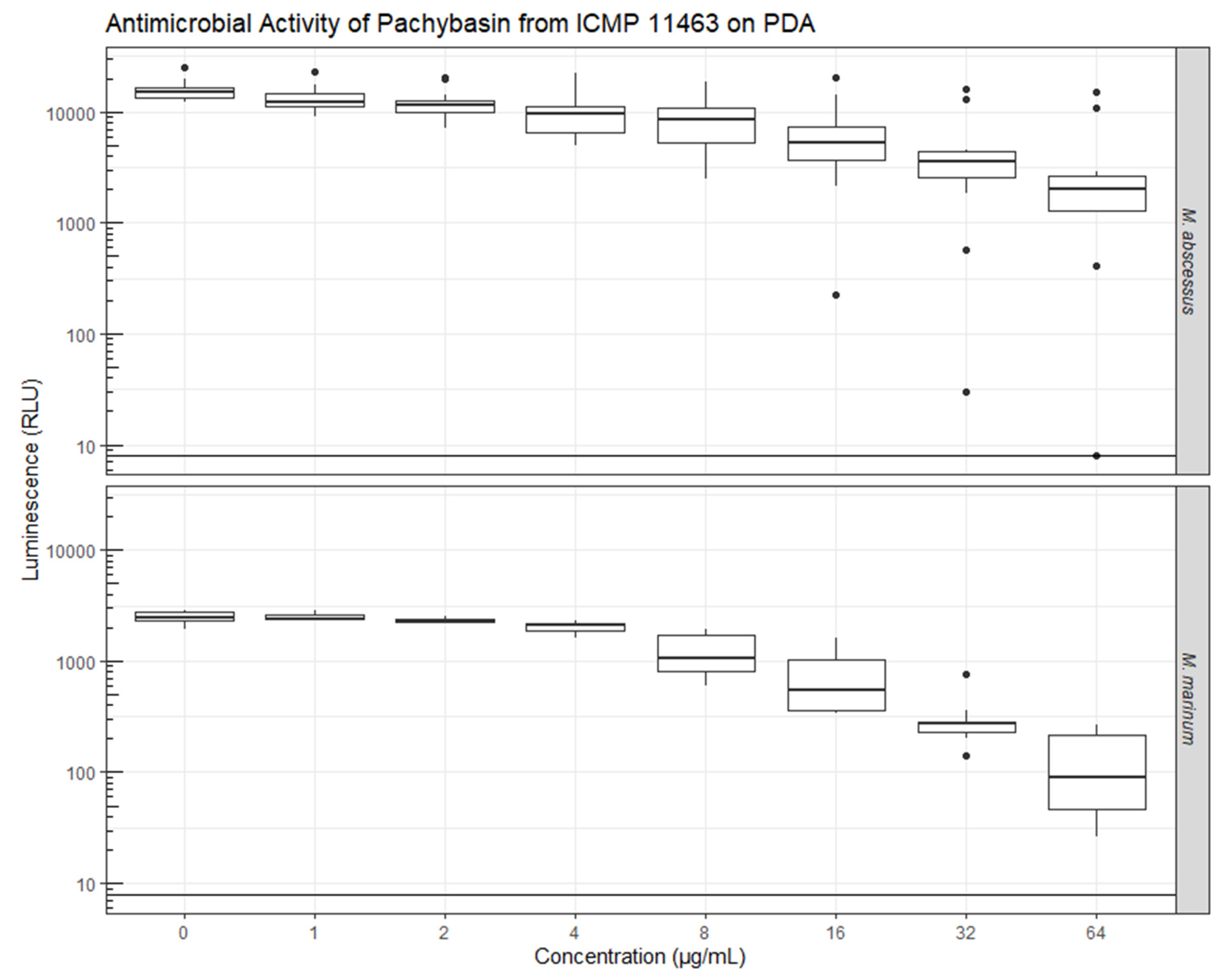
2.2. Evaluation of Bioactivity
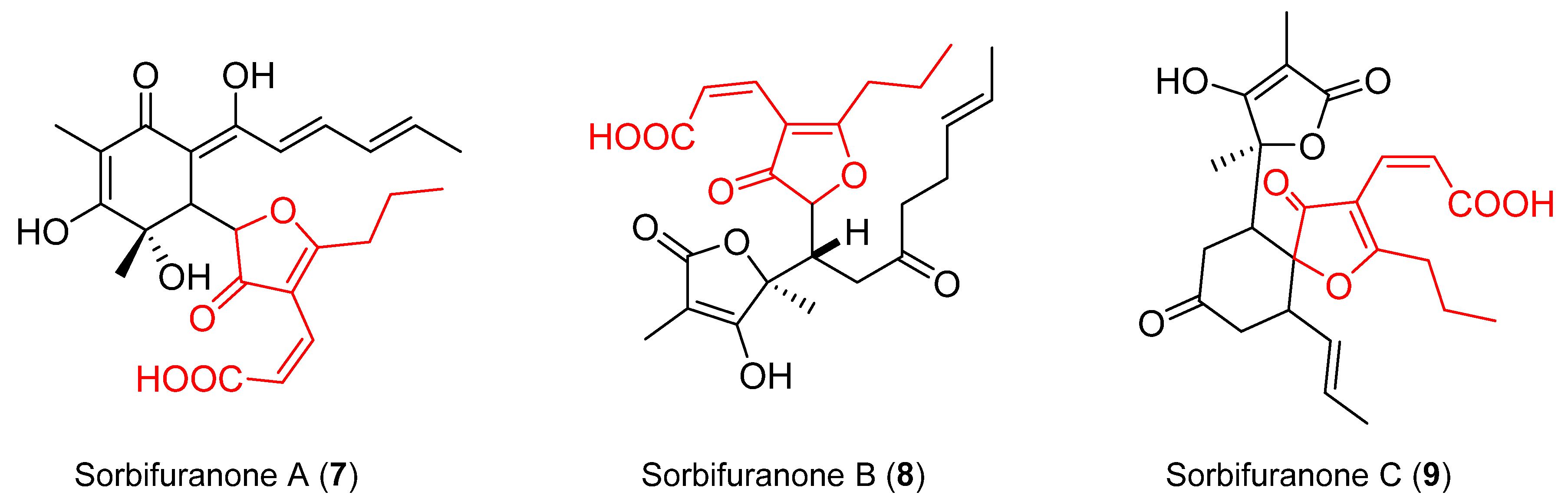
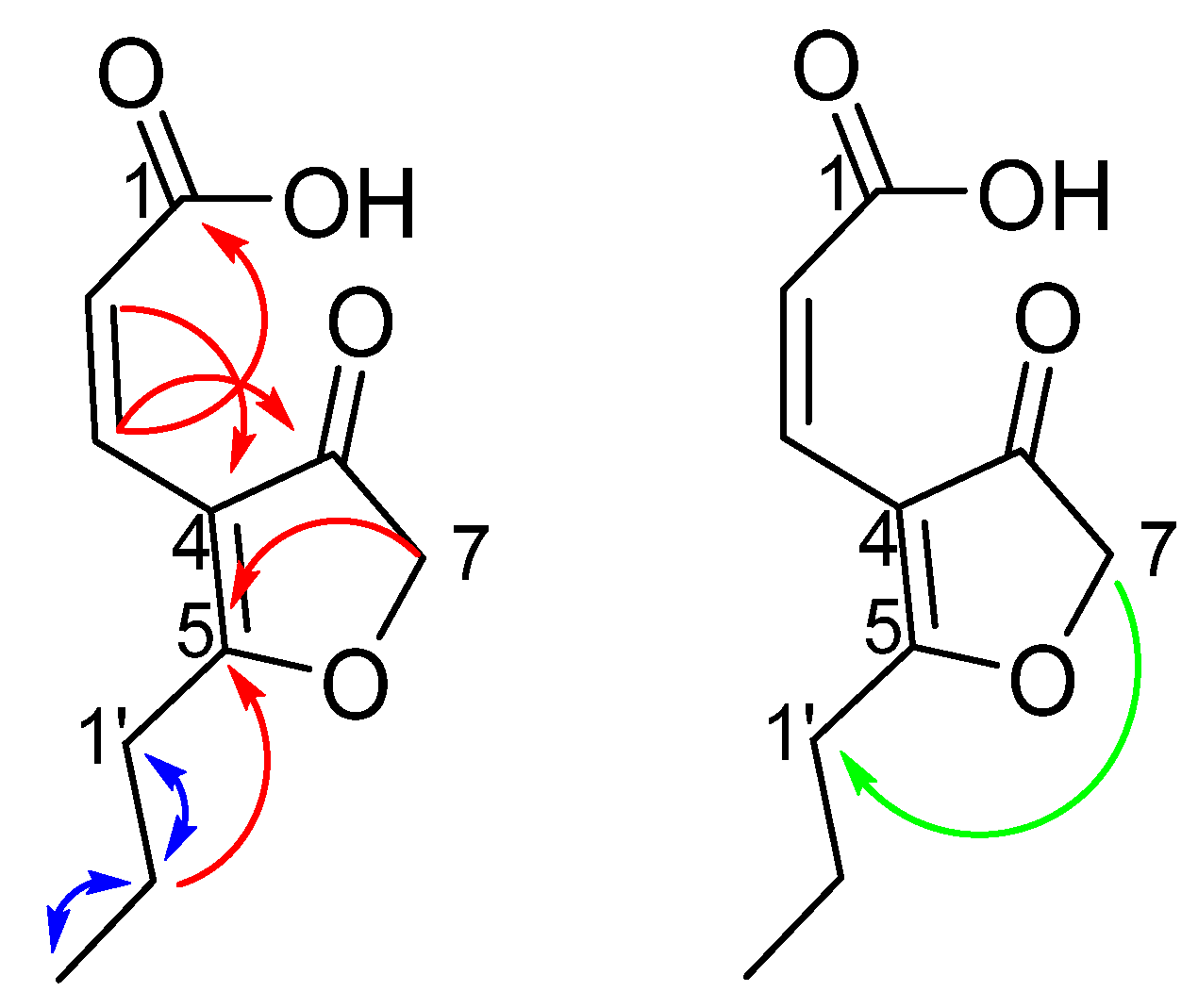
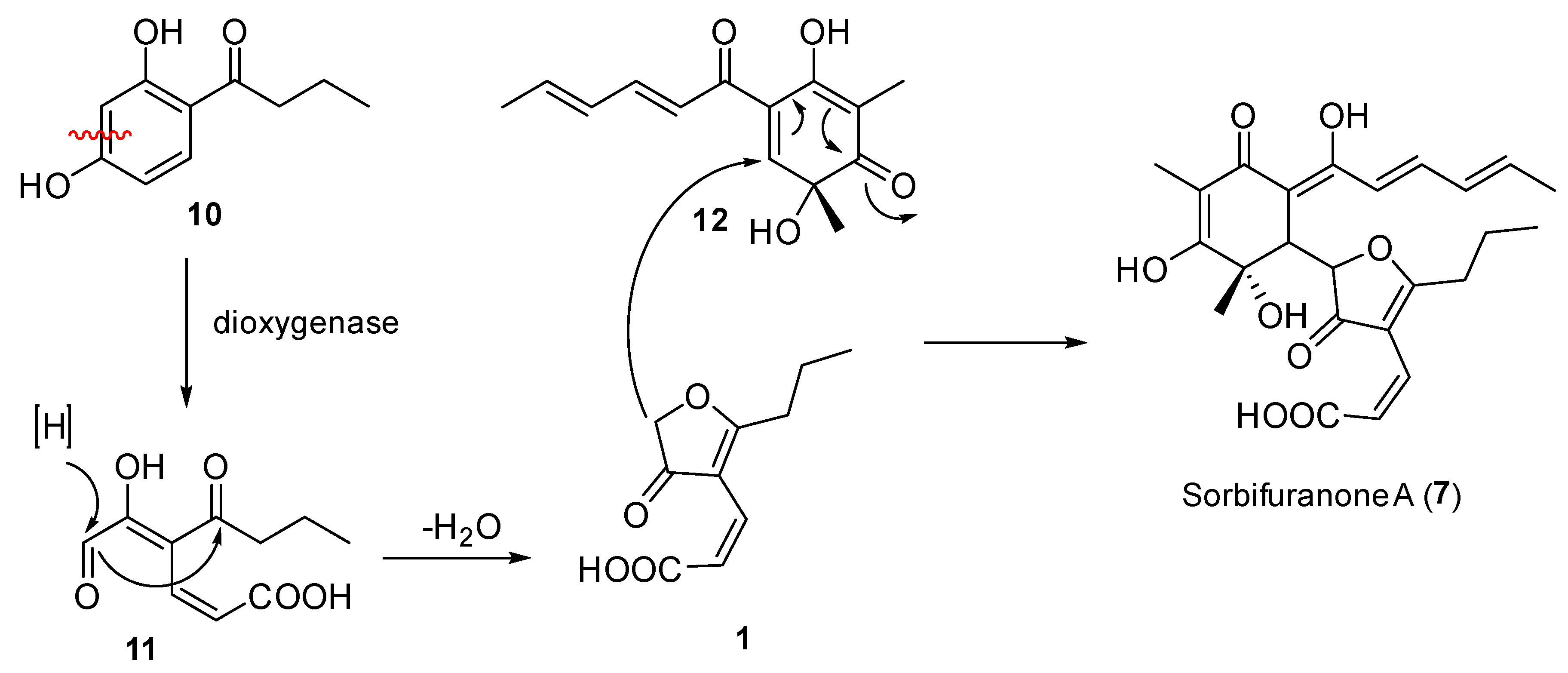
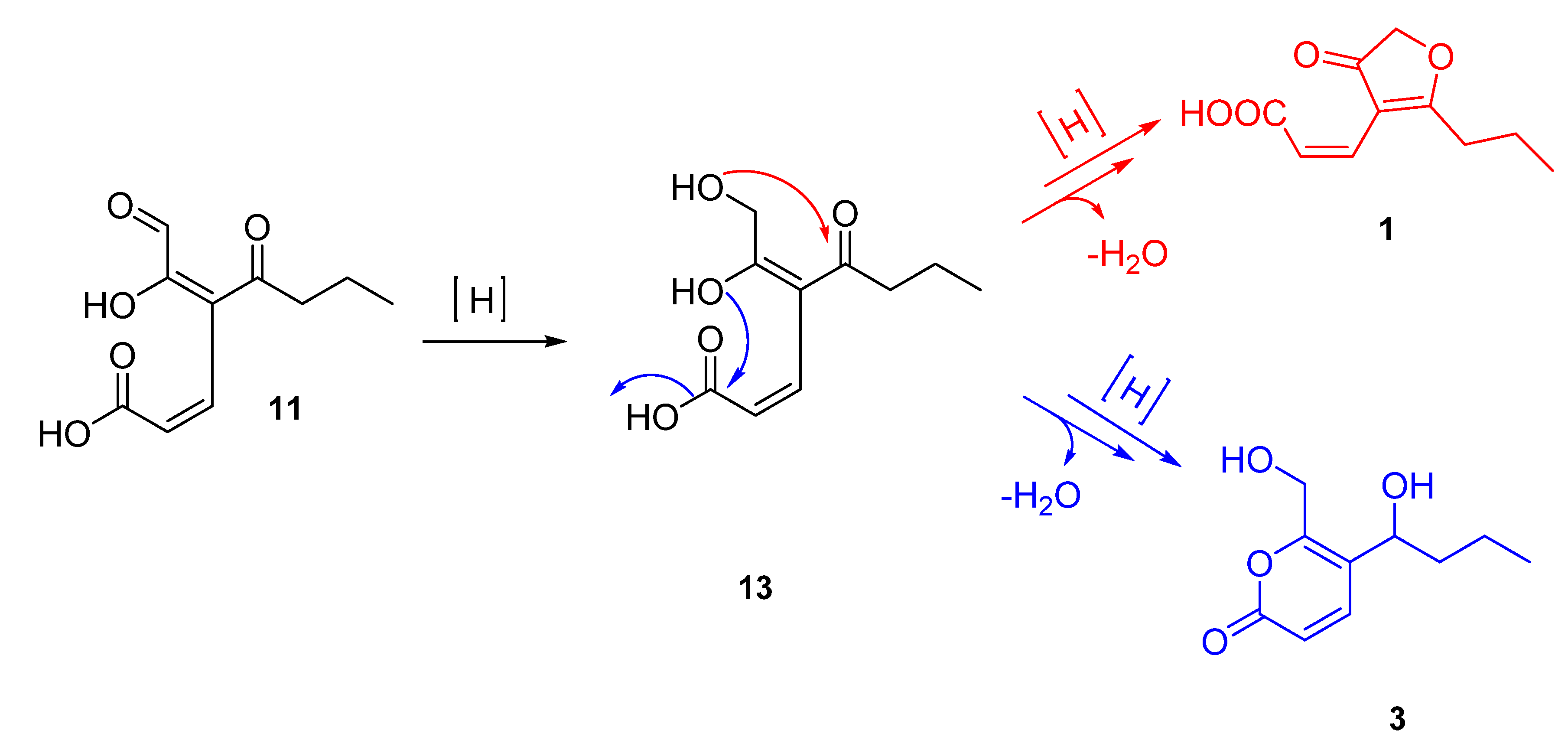
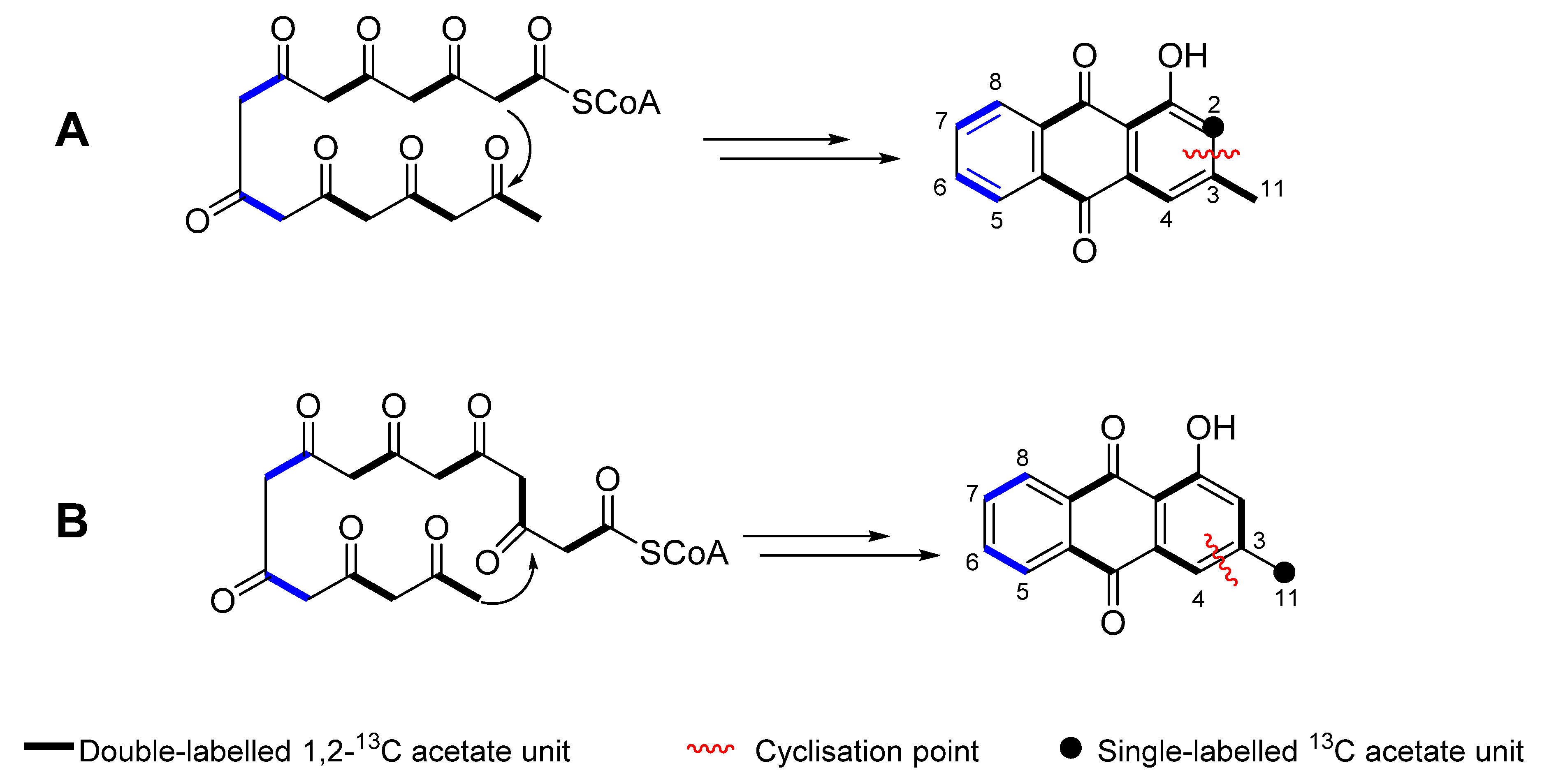
2.3. Proposed Biosynthesis
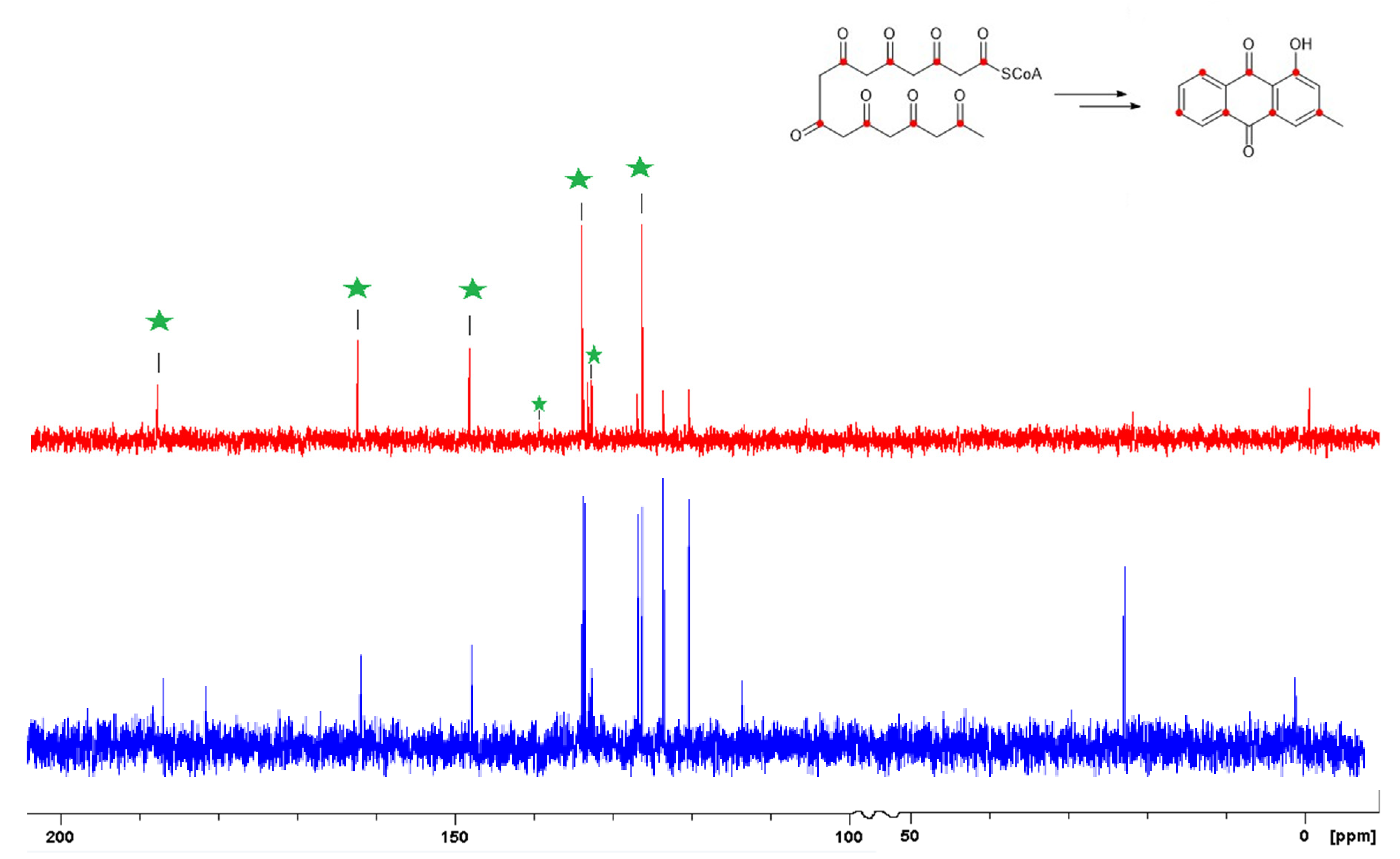
2.4. Sodium [1-13C] Acetate Incorporation Study
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction and Isolation
3.3.1. (2Z)-Cillifuranone (1)
3.3.2. (2E)-Cillifuranone (2)
3.3.3. Taiwapyrone (3)
3.3.4. Xylariolide D (4)
3.3.5. Pachybasin (5)
3.3.6. N-(5-Hydroxypentyl)acetamide (6)
3.4. Sodium [1-13C] Acetate Incroporated Acetate Fermentation of Neodidymelliopsis sp. ICMP 11463
3.4.1. Fungal Material
3.4.2. Extraction and Isolation
3.5. Antimicrobial Assays of Pure Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Blackwell, M. The Fungi: 1, 2, 3 … 5.1 Million Species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Cadelis, M.M.; Geese, S.; Gris, L.; Weir, B.S.; Copp, B.R.; Wiles, S. A Revised Structure and Assigned Absolute Configuration of Theissenolactone A. Molecules 2020, 25, 4823. [Google Scholar] [CrossRef] [PubMed]
- Camarda, L.; Merlini, L.; Nasini, G. Metabolites of Cercospora. Taiwapyrone, an α-Pyrone of Unusual Structure from Cercospora Taiwanensis. Phytochemistry 1976, 15, 537–539. [Google Scholar] [CrossRef]
- Wiese, J.; Ohlendorf, B.; Blümel, M.; Schmaljohann, R.; Imhoff, J.F. Phylogenetic Identification of Fungi Isolated from the Marine Sponge Tethya aurantium and Identification of Their Secondary Metabolites. Mar. Drugs 2011, 9, 561–585. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-Y.; Li, Y.-Y.; Lu, C.-H.; Lin, T.; Hu, P.; Shen, Y.-M. Seven Novel Linear Polyketides from Xylaria Sp. NCY2. Helv. Chim. Acta 2010, 93, 925–933. [Google Scholar] [CrossRef]
- Shibata, S.; Takido, M. Metabolic Products of Fungi, III. The Coloring Matters of Pachybasium candidum Saccardo. Pharm. Bull. 1955, 3, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Green, K.A.; Berry, D.; Feussner, K.; Eaton, C.J.; Ram, A.; Mesarich, C.H.; Solomon, P.; Feussner, I.; Scott, B. Lolium perenne Apoplast Metabolomics for Identification of Novel Metabolites Produced by the Symbiotic Fungus Epichloë festucae. New Phytol. 2020, 227, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Lang, G.; Bruhn, T.; Schäffler, K.; Steffens, S.; Schmaljohann, R.; Wiese, J.; Imhoff, J.F. Sorbifuranones A–C, Sorbicillinoid Metabolites from Penicillium Strains Isolated from Mediterranean Sponges. Tetrahedron 2010, 66, 9894–9901. [Google Scholar] [CrossRef]
- Wu, C.-J.; Qiao, H.; Cui, C.-B.; Yang, Y.; Xu, R.; Sun, C.-X.; Li, D.-H.; Li, C.-W. Two New Polyketides Isolated from a Diethyl Sulphate Mutant of Marine-Derived Penicillium purpurogenum G59. Nat. Prod. Res. 2019, 33, 2977–2981. [Google Scholar] [CrossRef] [PubMed]
- Wulansari, D.; Jamal, Y.; Praptiwi; Agusta, A. Pachybasin, a Major Metabolite from Culture Broth of Endophytic Coelomyceteous AFKR-18 Fungus Isolated from a Yellow Moonsheed Plant, Arcangelisia flava (L.) Merr. HAYATI J. Biosci. 2014, 21, 95–100. [Google Scholar] [CrossRef]
- Bringmann, G.; Gulder, T.A.M.; Hamm, A.; Goodfellow, M.; Fiedler, H.-P. Multiple Convergence in Polyketide Biosynthesis: A Third Folding Mode to the Anthraquinone Chrysophanol. Chem. Commun. 2009, 6810. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Y.; Lo, C.-T.; Chen, C.; Liu, M.-Y.; Chen, J.-H.; Peng, K.-C. Efficient Isolation of Anthraquinone-Derivatives from Trichoderma harzianum ETS 323. J. Biochem. Biophys. Methods 2007, 70, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Noll, T.F.; Gulder, T.A.M.; Grüne, M.; Dreyer, M.; Wilde, C.; Pankewitz, F.; Hilker, M.; Payne, G.D.; Jones, A.L.; et al. Different Polyketide Folding Modes Converge to an Identical Molecular Architecture. Nat. Chem. Biol. 2006, 2, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Specimen Details. Available online: https://scd.landcareresearch.co.nz/Specimen/ICMP%2011463?collection=ICMP&searchCollection=ICMP&query=11463¤tDisplayTab=list&pageNumber=0&sortField=relevance (accessed on 20 November 2020).
- Dalton, J.P.; Uy, B.; Okuda, K.S.; Hall, C.J.; Denny, W.A.; Crosier, P.S.; Swift, S.; Wiles, S. Screening of Anti-Mycobacterial Compounds in a Naturally Infected Zebrafish Larvae Model. J. Antimicrob. Chemother. 2017, 72, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Andreu, N.; Zelmer, A.; Fletcher, T.; Elkington, P.T.; Ward, T.H.; Ripoll, J.; Parish, T.; Bancroft, G.J.; Schaible, U.; Robertson, B.D.; et al. Optimisation of Bioluminescent Reporters for Use with Mycobacteria. PLoS ONE 2010, 5, e10777. [Google Scholar] [CrossRef] [PubMed]
- Andreu, N.; Fletcher, T.; Krishnan, N.; Wiles, S.; Robertson, B.D. Rapid Measurement of Antituberculosis Drug Activity in vitro and in Macrophages Using Bioluminescence. J. Antimicrob. Chemother. 2012, 67, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.P.; Uy, B.; Phummarin, N.; Copp, B.R.; Denny, W.A.; Swift, S.; Wiles, S. Effect of Common and Experimental Anti-Tuberculosis Treatments on Mycobacterium tuberculosis Growing as Biofilms. PeerJ 2016, 4, e2717. [Google Scholar] [CrossRef] [PubMed]
- Andreu, N.; Zelmer, A.; Sampson, S.L.; Ikeh, M.; Bancroft, G.J.; Schaible, U.E.; Wiles, S.; Robertson, B.D. Rapid in vivo Assessment of Drug Efficacy against Mycobacterium tuberculosis Using an Improved Firefly Luciferase. J. Antimicrob. Chemother. 2013, 68, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Wiles, S.; Grey, A. Bioluminescence-Based Minimum Inhibitory Concentration (MIC) Testing of Pure Compounds Isolated from Fungi against Mycobacterium abscessus. 2021. Available online: https://aperta.ulakbim.gov.tr/record/5637#.YKt7GqgzaUk (accessed on 24 May 2021).
- Wiles, S.; Grey, A. Bioluminescence-Based Minimum Inhibitory Concentration (MIC) Testing of Pure Compounds Isolated from Fungi against Mycobacterium marinum. 2021. Available online: https://aperta.ulakbim.gov.tr/record/5637#.YKt7UKgzaUk (accessed on 24 May 2021).
- Blaskovich, M.A.T.; Zuegg, J.; Elliott, A.G.; Cooper, M.A. Helping Chemists Discover New Antibiotics. ACS Infect. Dis. 2015, 1, 285–287. [Google Scholar] [CrossRef] [PubMed]
| Position | δH (m, J in Hz) a | δC b |
|---|---|---|
| 1 | – | 169.5 |
| 2 | 6.01 (d, 11.6) | 124.4 |
| 3 | 6.49 (d, 11.6) | 130.0 |
| 4 | – | 114.9 |
| 5 | – | 193.4 |
| 7 | 4.62 (s) | 76.1 |
| 8 | – | 201.2 |
| 1′ | 2.60 (t, 7.0) | 32.9 |
| 2′ | 1.75–1.70 (m) | 20.3 |
| 3′ | 0.99 (t, 7.0) | 14.0 |
| Compound | Percentage Growth Inhibition a | ||||||
|---|---|---|---|---|---|---|---|
| S. a. b | P. a. c | E. c. d | K. p. e | A. b. f | C. a. g | C. n. h | |
| 2 | 12.28 | 4.76 | −0.69 | 8.93 | 1.4 | 0.03 | −5.11 |
| 3 | 5.32 | 15.56 | 15.94 | 19.17 | 13.89 | 8.13 | −2.28 |
| 5 | 53.02 | 5.27 | 3.32 | 11.9 | 22.71 | 5.75 | −13.06 |
| Position | δC | Relative Intensity a | δC b |
|---|---|---|---|
| 1 | 162.9 | 5.5 | 162.7 |
| 2 | 124.3 | 1.2 | 124.1 |
| 3 | 148.8 | 7.4 | 114.0 |
| 4 | 121.0 | 1.2 | 120.7 |
| 4a | 133.3 | 6.1 | 148.6 |
| 5 | 127.5 | 1.3 | 127.3 |
| 6 | 134.6 | 6.1 | 134.4 |
| 7 | 134.3 | 1.2 | 134.0 |
| 8 | 127.0 | 6.2 | 126.1 |
| 8a | 133.5 | 1.0 | 133.5 |
| 9 | 188.3 | 6.4 | 187.9 |
| 9a | 114.3 | 1.0 | 133.1 |
| 10 | 182.9 | 1.0 | 182.6 |
| 10a | 133.8 | 5.0 | 133.2 |
| 11 | 22.4 | 1.0 | 22.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadelis, M.M.; Gordon, H.; Grey, A.; Geese, S.; Mulholland, D.R.; Weir, B.S.; Copp, B.R.; Wiles, S. Isolation of a Novel Polyketide from Neodidymelliopsis sp. Molecules 2021, 26, 3235. https://doi.org/10.3390/molecules26113235
Cadelis MM, Gordon H, Grey A, Geese S, Mulholland DR, Weir BS, Copp BR, Wiles S. Isolation of a Novel Polyketide from Neodidymelliopsis sp. Molecules. 2021; 26(11):3235. https://doi.org/10.3390/molecules26113235
Chicago/Turabian StyleCadelis, Melissa M., Hugo Gordon, Alex Grey, Soeren Geese, Daniel R. Mulholland, Bevan S. Weir, Brent R. Copp, and Siouxsie Wiles. 2021. "Isolation of a Novel Polyketide from Neodidymelliopsis sp." Molecules 26, no. 11: 3235. https://doi.org/10.3390/molecules26113235
APA StyleCadelis, M. M., Gordon, H., Grey, A., Geese, S., Mulholland, D. R., Weir, B. S., Copp, B. R., & Wiles, S. (2021). Isolation of a Novel Polyketide from Neodidymelliopsis sp. Molecules, 26(11), 3235. https://doi.org/10.3390/molecules26113235