Comparison of Nonheme Manganese- and Iron-Containing Flavone Synthase Mimics
Abstract
1. Introduction
2. Results and Discussion
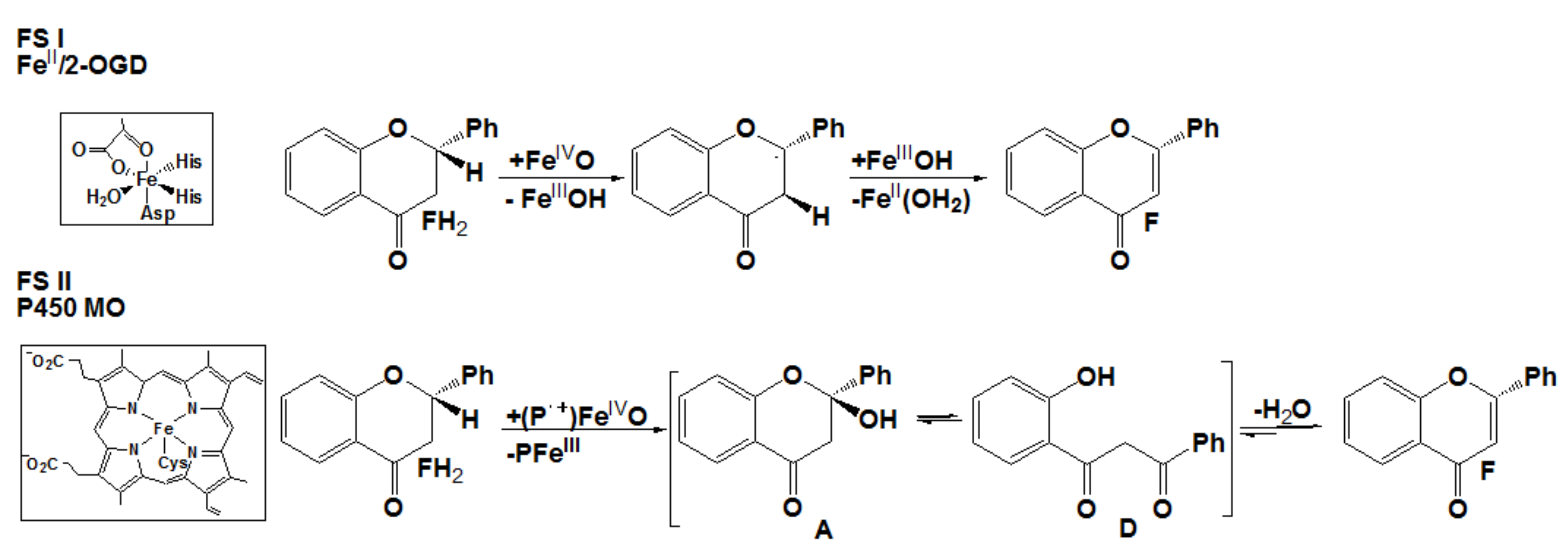
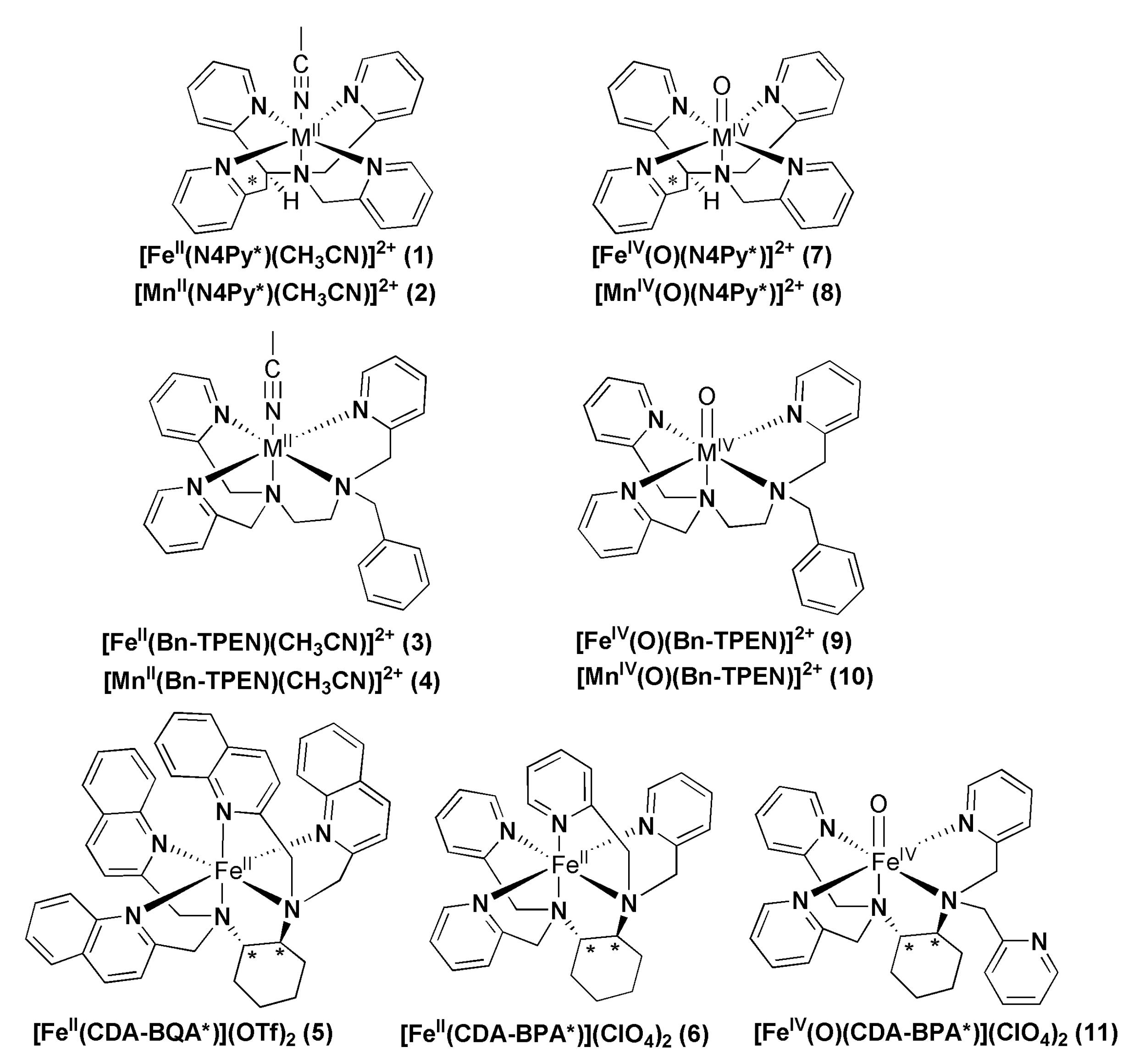
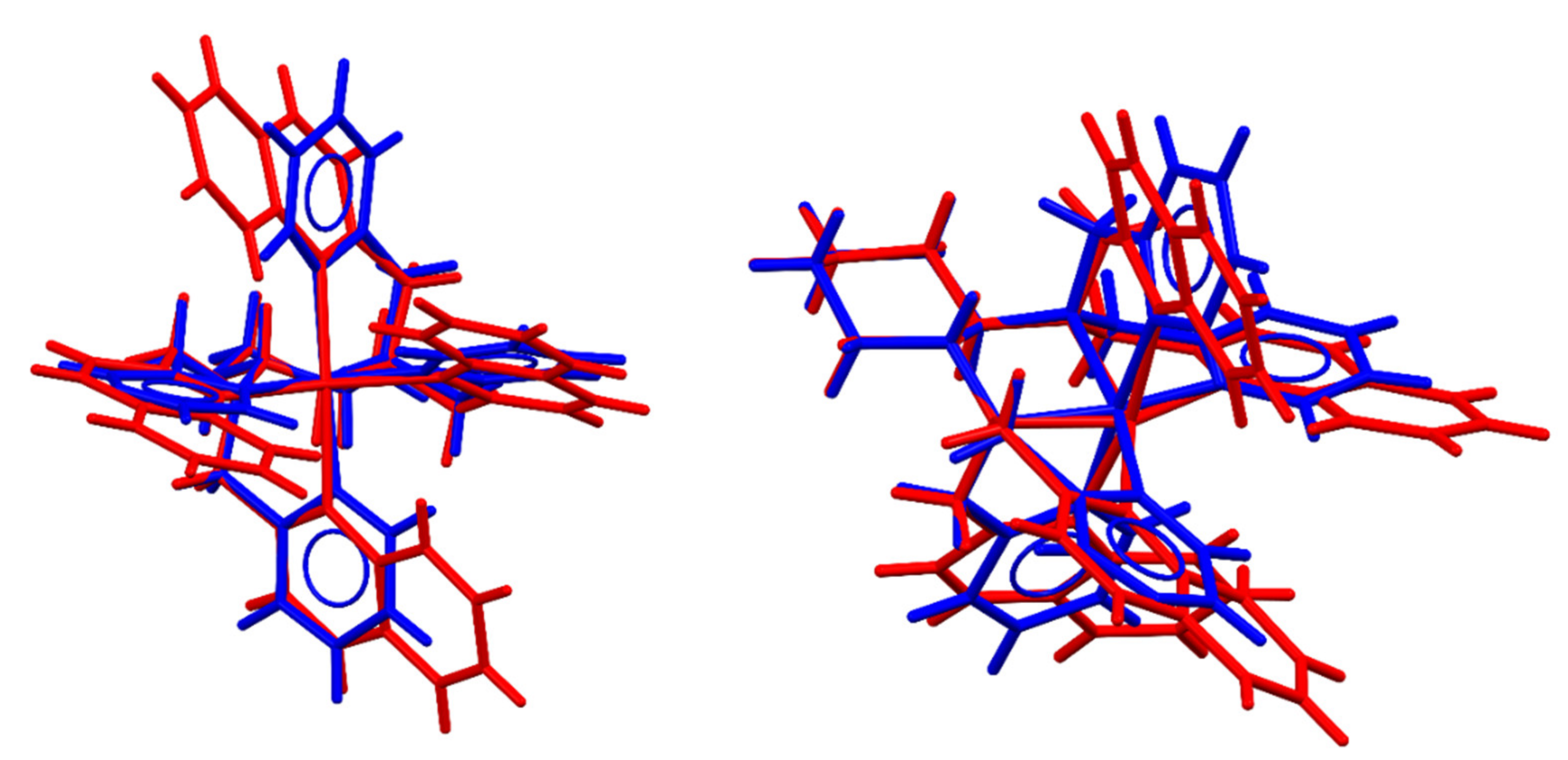
2.1. Nonheme Iron and Manganese-Containing Biomimics of the Flavone Synthase Enzyme
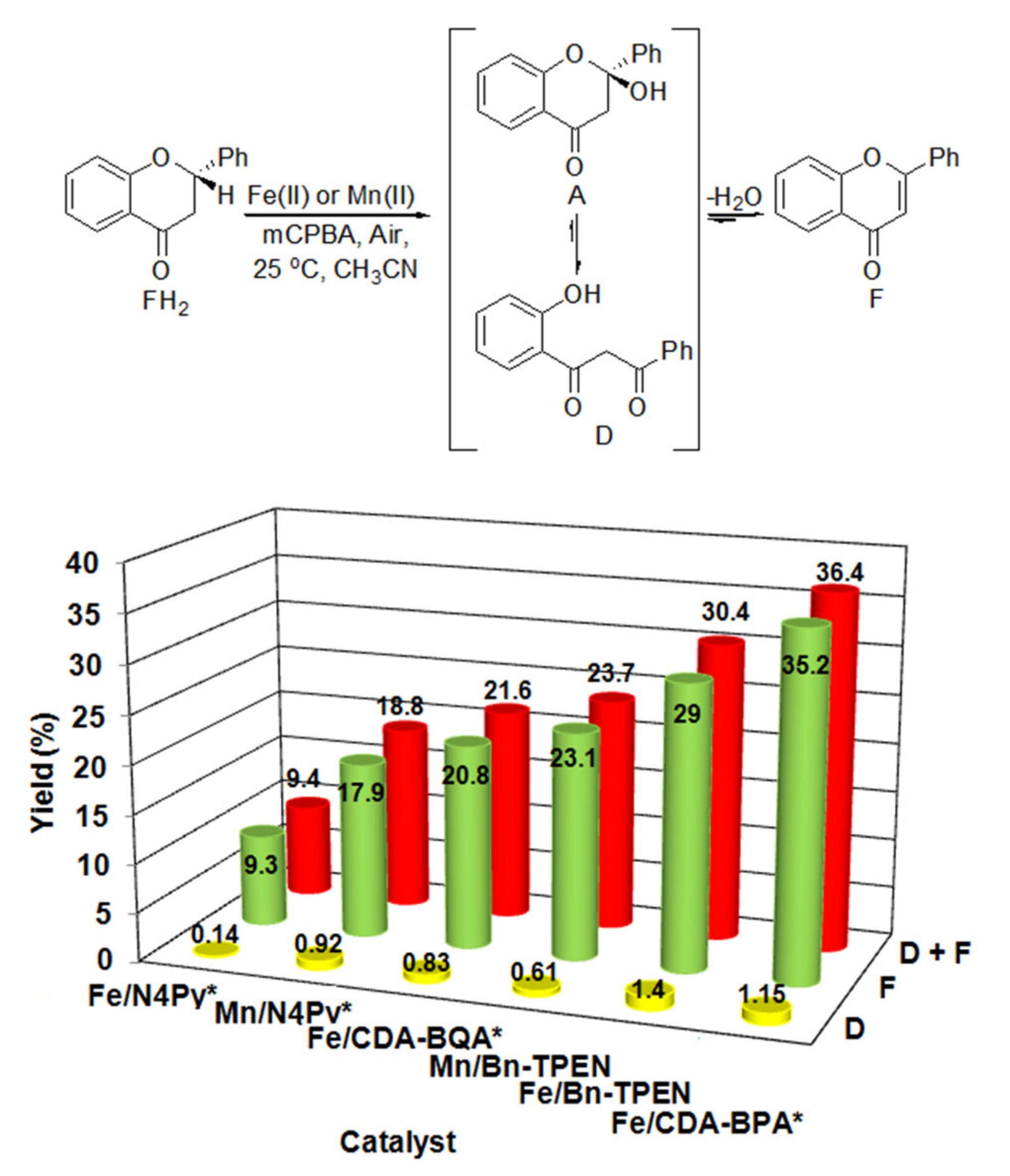
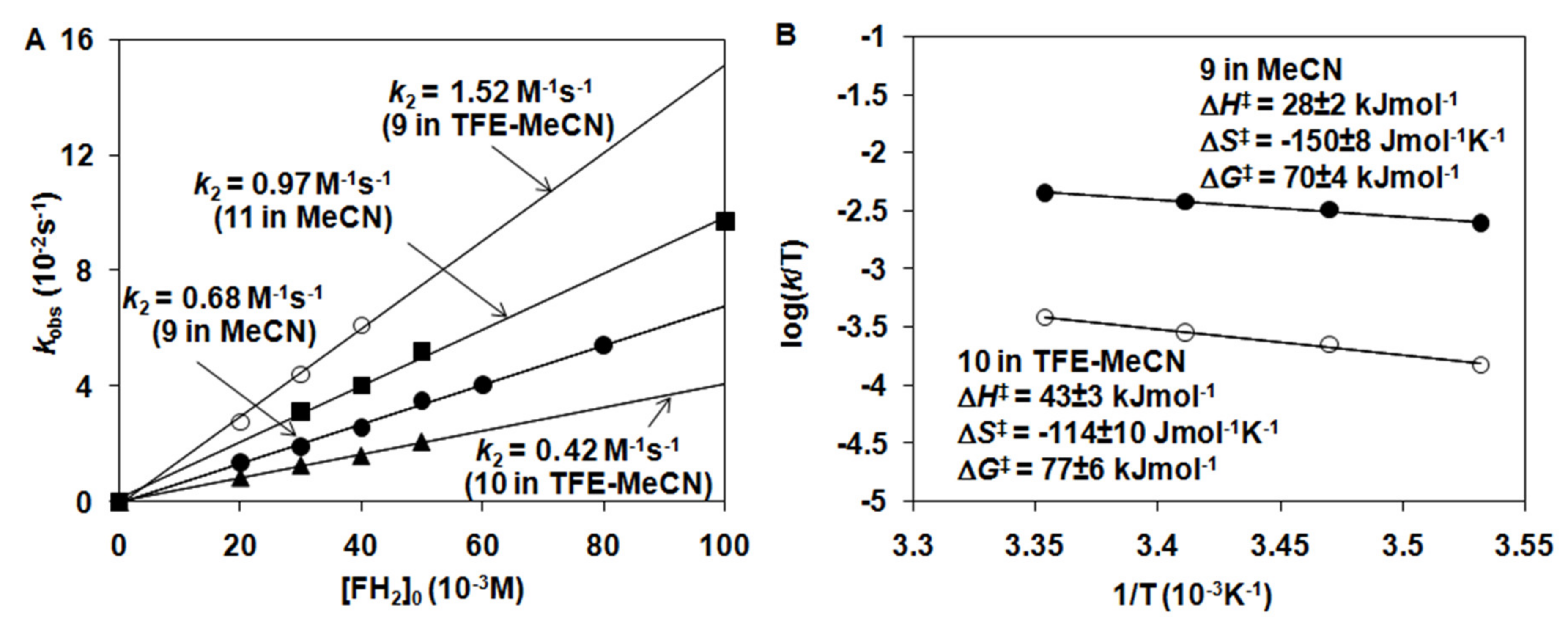
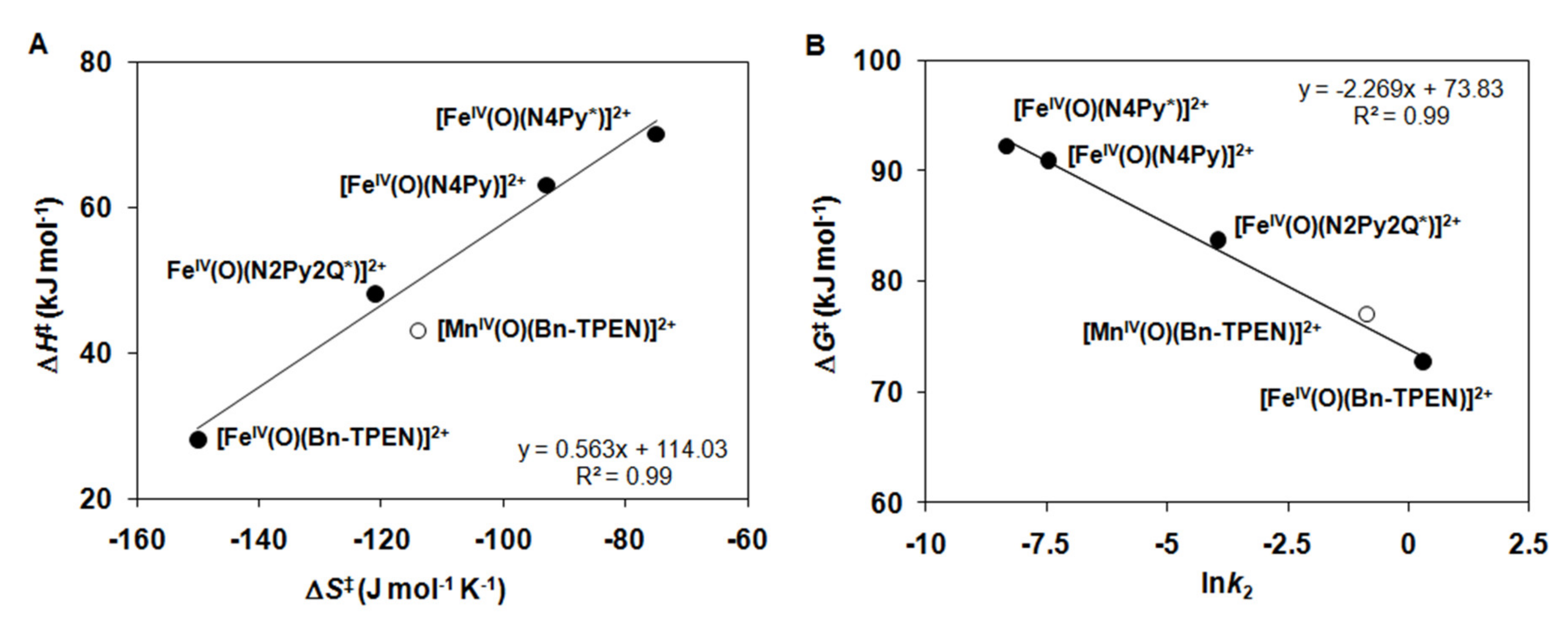
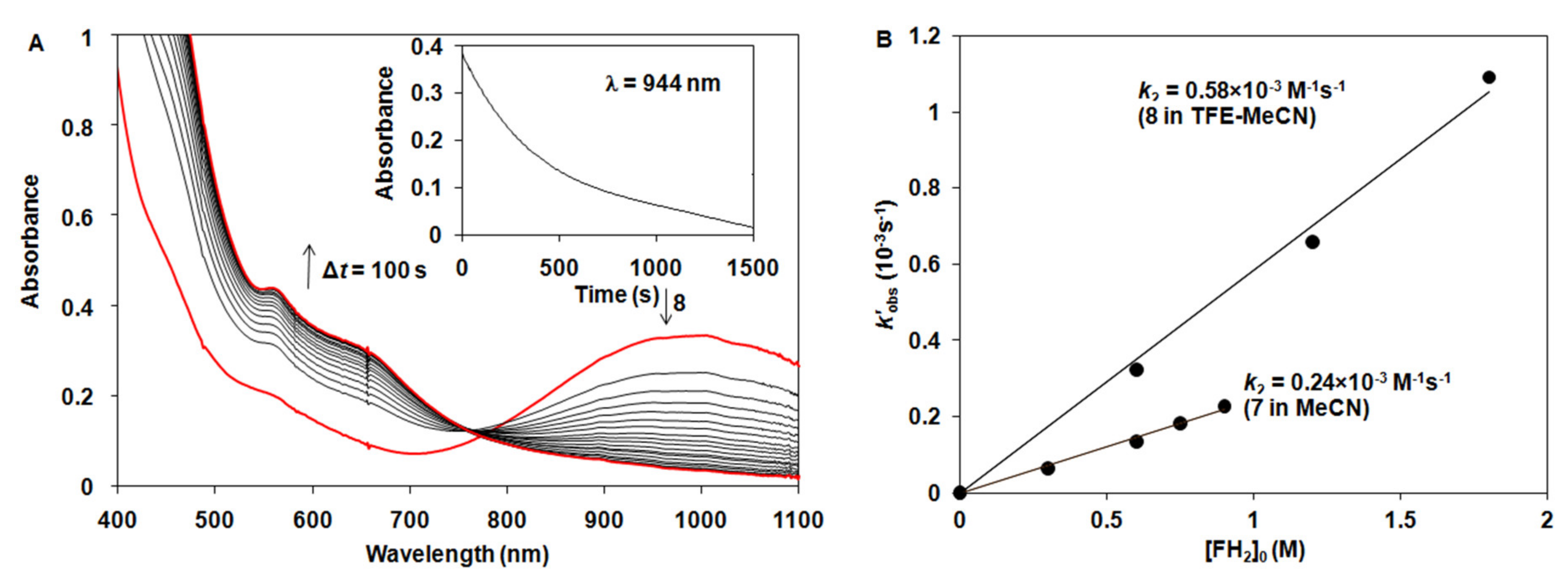
2.2. Catalytic Oxidation Reactions
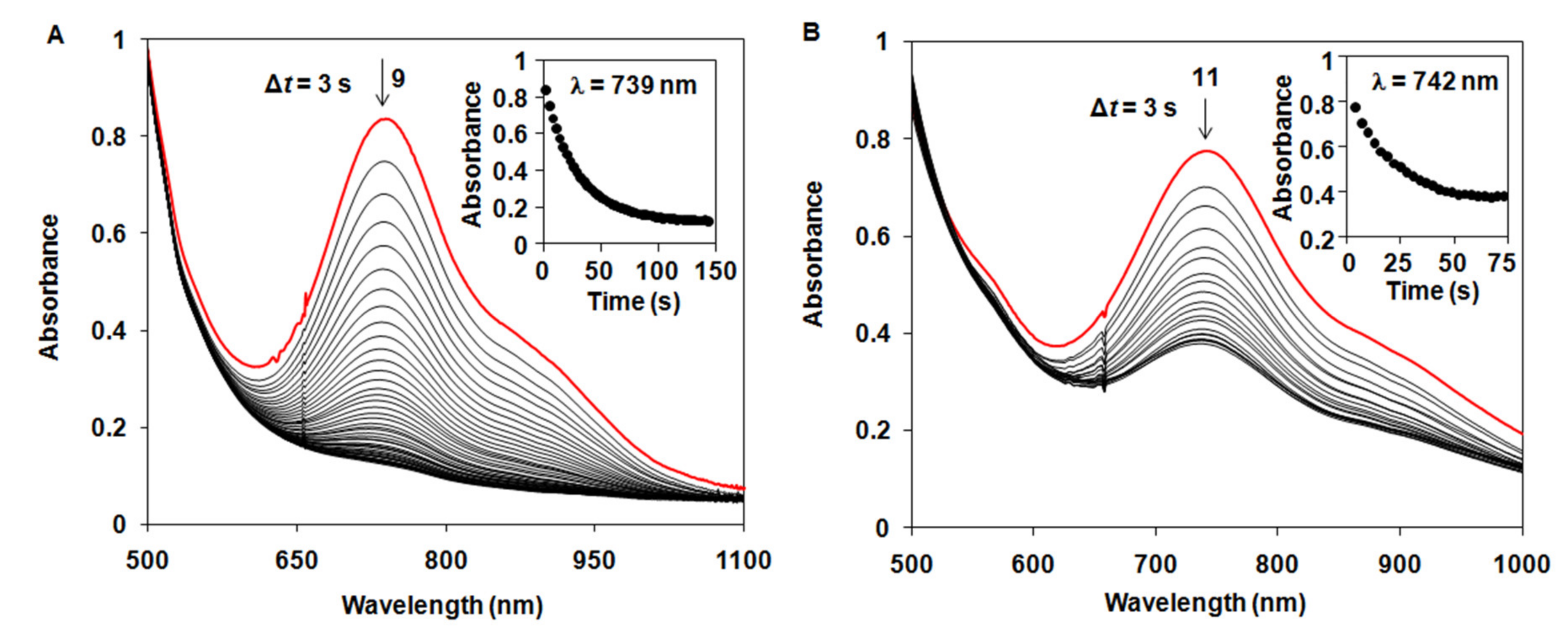
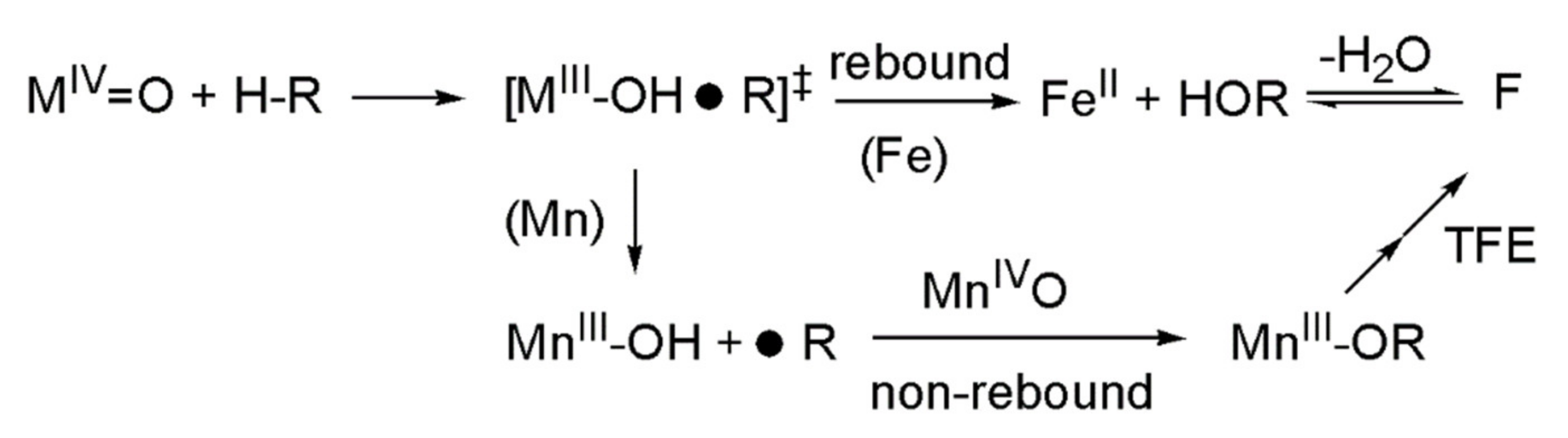
2.3. Stoichiometric Oxidation Reactions
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Kandaswami, C., Jr.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Kromhout, D. Diet and cardiovascular diseases. J. Nutr. Health Aging 2001, 5, 144–149. [Google Scholar] [PubMed]
- Tabak, C.; Arts, I.C.; Smit, H.A.; Heedrik, D.; Kromhout, D. Chronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones: The MORGAN Study. Am. J. Respir. Crit. Care Med. 2001, 164, 61–64. [Google Scholar] [CrossRef]
- Ross, J.A.; Kansum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2003, 16, 77–84. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317–325. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Doshi, A.G.; Soni, P.A.B.; Ghiya, J. Oxidation of 2′-hydroxychalcones. Indian J. Chem. 1986, 25B, 759. [Google Scholar]
- Mahal, H.S.; Rai, H.S.; Venkataraman, K. Synthetical experiments in the chromone group. Part XVI. Chalkones and flavanones and their oxidation to flavones by means of selenium dioxide. J. Chem. Soc. 1935, 866–868. [Google Scholar] [CrossRef]
- Shanker, C.G.; Mallaiah, B.V.; Srimannarayana, G. Dehydrogenation of chromanones and flavanones by 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ)- A facile method for the synthesis of chromanones and flavones. Synthesis 1983, 4, 310–311. [Google Scholar] [CrossRef]
- Singh, O.V.; Kapoor, R.P. Dehydrogenation of flavanones to flavones using thallium(III) acetate (TTA). Tetrahedron Lett. 1990, 31, 1459–1462. [Google Scholar] [CrossRef]
- Singh, O.V.; Muthukrishnan, M.; Gopan, R. Manganese(III) acetate mediated oxidation of flavanones: A facile synthesis of flavones. Synth. Commun. 2005, 35, 2723–2728. [Google Scholar] [CrossRef]
- Ko, T.P.; Day, J.; Malkin, A.J.; McPherson, A. Structure of orthorhombic crystals of beef liver catalase. Acta Crystallogr. 1999, D55, 1383–1394. [Google Scholar] [CrossRef]
- Ivancich, A.; Jouve, H.M.; Sartor, B.; Gaillard, J. EPR investigation of compound I in Proteus mirabilis and bovin liver catalases: Formation of porphyrin and tyrosyl radical intermediates. Biochemistry 1997, 36, 9356–9364. [Google Scholar] [CrossRef]
- Martens, S.; Forkmann, G. Genetic control of flavone synthase II activity in flowers of gerbera hybrids. Phytochemistry 1998, 49, 1953–1958. [Google Scholar] [CrossRef]
- Akashi, T.; Aoki, T.; Ayabe, S.I. Cloning and functional expression of a cytochrome P450 cDNA encoding 2-hydroxyisoflavanone synthase involved in biosynthesis of the isoflavonoid skeleton in licorice. Plant Physiol. 1999, 121, 821–828. [Google Scholar] [CrossRef]
- Akashi, T.; Aoki, T.; Ayabe, S.I. Identification of a cytochrome P450 cDNA encoding (2S)-flavanone 2-hydroxylase of licorice (Glycyrrhiza echinata L.; Fabaceae) which represents licodione synthase and flavone synthase II. FEBS Lett. 1998, 431, 287–290. [Google Scholar] [CrossRef]
- Zhang, J.; Subramanian, S.; Zhang, Y.; Yu, O. Flavone synthases from Medicago truncatula are flavanone-2-hydroxylases and are important for nodulation. Plant Physiol. 2007, 144, 741–751. [Google Scholar] [CrossRef]
- Du, Y.; Chu, H.; Wang, M.; Chu, I.K.; Lo, C. Identification of flavone phytoalexins and a pathogen-inducible flavone synthase II gene (SbFNSII) in sorghum. J. Exp. Bot. 2010, 61, 983–994. [Google Scholar] [CrossRef]
- Kappock, T.J.; Caradonna, J.P. Pterin-dependent amino acid hydroxylases. Chem. Rev. 1996, 96, 2659–2756. [Google Scholar] [CrossRef] [PubMed]
- Elkins, J.M.; Ryle, M.J.; Clifton, I.J.; Dunning Hottop, J.C.; Lloyd, J.S.; Burzlaff, N.I.; Baldwin, J.E.; Hausinger, R.P.; Roach, P.L. X-ray Crystal structure of escherichia coli taurine/α-ketoglutarate dioxygenase complexed to ferrous iron and substrates. Biochemistry 2002, 41, 5185–5192. [Google Scholar] [CrossRef] [PubMed]
- Costas, M.; Mehn, M.P.; Jensen, M.P.; Que, L., Jr. Dioxygen Activation at mononuclear nonheme iron active sites: Enzymes, models, and intermediates. Chem. Rev. 2004, 104, 939–986. [Google Scholar] [CrossRef] [PubMed]
- Nam, W. Dioxygen activation by metalloenzymes and models. Acc. Chem. Res. 2007, 40, 465. [Google Scholar] [CrossRef]
- Gebhardt, Y.; Witte, S.; Forkmann, G.; Lukacˇin, R.; Matern, U.; Martens, S. Molecular evolution of flavonoid dioxygenases in the family Apiaceae. Phytochemistry 2005, 66, 1273–1284. [Google Scholar] [CrossRef]
- Gebhardt, Y.H.; Witte, S.; Steuber, H.; Matern, U.; Martens, S. Evolution of flavone synthase I from parsley flavanone 3β-hydroxylase by sitedirected mutagenesis. Plant Physiol. 2007, 144, 1442–1454. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, J.H.; Kim, B.G.; Lim, Y.; Ahn, J.H. Characterization of flavone synthase I from rice. BMB Rep. 2008, 41, 68–71. [Google Scholar] [CrossRef]
- Britsch, L. Purification and characterization of flavone synthase I, 2- oxoglutarate-dependent desaturase. Arch. Biochem. Biophys. 1990, 282, 152–160. [Google Scholar] [CrossRef]
- Fliegmann, J.; Furtwangler, K.; Malterer, G.; Cantarello, C.; Schüler, G.; Ebel, J.; Mithöfer, A. Flavone synthase II (CYP93B16) from soybean (Glycine max L.). Phytochemistry 2010, 71, 508–514. [Google Scholar] [CrossRef]
- Martens, S.; Forkmann, G.; Britsch, L.; Wellmann, F.; Matern, U.; Lukačin, R. Divergent evolution of flavonoid 2-oxoglutarate-dependent dioxygenases in parsley. FEBS Lett. 2003, 544, 93–98. [Google Scholar] [CrossRef]
- Du, J.; Zhang, J.; Zhu, J.; Xia, C.; Sun, W. Synthesis, characterization, and reactivity of a chiral Fe(IV)-oxo complex bearing an L-proline derived aminopyridine ligand. New J. Chem. 2018, 42, 8315–8319. [Google Scholar] [CrossRef]
- Duboc-Toia, C.; Ménage, S.; Ho, R.Y.N.; Que, L., Jr.; Lambeaux, C.; Fontecave, M. Enantioselective sulfoxidation as a probe for a metal-based mechanism in H2O2-dependent oxidations catalyzed by a diiron complex. Inorg. Chem. 1999, 38, 1261–1268. [Google Scholar] [CrossRef]
- Lakk-Bogáth, D.; Csonka, R.; Speier, G.; Reglier, M.; Simaan, A.J.; Naubron, J.V.; Giorgi, M.; Lázár, K.; Kaizer, J. Formation, characterization, and reactivity of a nonheme oxoiron(IV) complex derived from the chiral pentadentate ligand asN4Py. Inorg. Chem. 2016, 55, 10090–10093. [Google Scholar] [CrossRef]
- Meena, B.I.; Lakk-Bogáth, D.; Kripli, B.; Speir, G.; Kaizer, J. Kinetics and mechanism of epoxidation of olefins by chiral tetrapyridyl oxoiron(IV) complex. Polyhedron 2018, 151, 141–145. [Google Scholar] [CrossRef]
- Turcas, R.; Lakk-Bogáth, D.; Speier, G.; Kaizer, J. Kinetics and enantioselectivity of the Baeyer-Villiger oxidation of cyclohexanones by chiral tetrapyridyl oxoiron(IV) complex. Inorg. Chem. Commun. 2018, 92, 141–144. [Google Scholar] [CrossRef]
- Lakk-Bogáth, D.; Kripli, B.; Meena, B.I.; Speier, G.; Kaizer, J. Catalytic and stoichiometric C-H oxidation of benzylalcohols and hydrocarbons mediated by nonheme oxoiron(IV) complex with chiral tetrapyridyl ligand. Inorg. Chem. Commun. 2019, 104, 165–170. [Google Scholar] [CrossRef]
- Kaizer, J.; Klinker, E.J.; Oh, N.Y.; Rohde, J.-U.; Song, W.J.; Stubna, A.; Kim, J.; Münck, E.; Nam, W.; Que, L., Jr. Nonheme FeIVO complexes that can oxidize th C-H bonds of cyclohexane at room temperature. J. Am. Chem. Soc. 2004, 126, 472–473. [Google Scholar] [CrossRef]
- Klinker, E.J.; Kaizer, J.; Brennessel, W.B.; Woodrum, N.L.; Cramer, C.J.; Que, L., Jr. Structures of nonheme oxoiron(IV) complexes from X-ray crystallography, NMR spectroscopy, and DFT calculations. Angew. Chem. 2005, 117, 3756–3760. [Google Scholar] [CrossRef]
- Kripli, B.; Garda, Z.; Sólyom, B.; Tircsó, G.; Kaizer, J. Formation, stability and catalase-like activity of mononuclear manganese(II) and oxomanganese complexes in protic and aprotic solvents. New J. Chem. 2020, 44, 5545–5555. [Google Scholar] [CrossRef]
- Wu, X.; Seo, M.S.; Davis, K.M.; Lee, Y.-M.; Chen, J.; Cho, K.-B.; Pushkar, Y.N.; Nam, W. A highly reactive mononuclear non-heme manganese(IV)-oxo complex that can activate the strong C-H bonds of alkanes. J. Am. Chem. Soc. 2011, 133, 20088–20091. [Google Scholar] [CrossRef]
- McCuster, J.K.; Toftlund, H.; Rheingold, A.L.; Hendrickson, D.N. Ligand conformational changes affecting 5T2-fwdarw. 1A1 intersystem crossing in a ferrous complex. J. Am. Chem. Soc. 1993, 115, 1797–1804. [Google Scholar] [CrossRef]
- Kaizer, J.; Costas, M.; Que, L., Jr. A dramatic push effect on the homolysis of FeIII(OOR) intermediates to form non-heme FeIV=O vomplexes. Angew. Chem. 2003, 115, 3799–3801. [Google Scholar] [CrossRef]
- McDonald, A.R.; Que, L., Jr. High-valent nonheme iron-oxo complexes: Synthesis, structure, and spectroscopy. Coord. Chem. Rev. 2013, 257, 414–428. [Google Scholar] [CrossRef]
- Krebs, C.; Fujimori, D.G.; Walsh, C.T.; Bollinger, J.M., Jr. Non-heme Fe(IV)-oxo intermediates. Acc. Chem. Res. 2007, 40, 484–492. [Google Scholar] [CrossRef]
- Que, L., Jr. The Road to Non-heme oxoferryls and beyond. Acc. Chem. Res. 2007, 40, 493–500. [Google Scholar] [CrossRef]
- Turcas, R.; Kripli, B.; Attia, A.A.A.; Lakk-Bogáth, D.; Speier, G.; Giorgi, M.; Silaghi-Dumitrescu, R.; Kaizer, J. Catalytic and stoichiometric flavanone oxidation by nonheme oxoiron(IV) complexes as flavone synthase mimics: Kinetic, mechanistic and computational studies. Dalton Trans. 2018, 47, 14416–14420. [Google Scholar] [CrossRef]
- Mikata, Y.; Sato, Y.; Takeuchi, S.; Kuroda, Y.; Konno, H.; Iwatsuki, S. Quinoline-based fluorescent zinc sensors with enhanced fluorescence intensity, Zn/Cd selectivity and metal binding affinity by conformational restriction. Dalton Trans. 2013, 42, 9688–9698. [Google Scholar] [CrossRef]
- Mikata, Y.; Wakamatsu, M.; Kawamura, A.; Yamanaka, N.; Yano, S.; Odani, A.; Morihiro, K.; Tamotsu, S. Methoxy-substituted TQEN family of fluorescent zinc sensors. Inorg. Chem. 2006, 45, 9262–9268. [Google Scholar] [CrossRef]
- Tamura, M.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Hirobe, M.; Nagano, T. Superoxide dismutase activity of iron(II)TPEN complex and its derivatives. Chem. Pharm. Bull. 2000, 48, 1514–1518. [Google Scholar] [CrossRef][Green Version]
- Balland, V.; Banse, F.; Anxolabéhère-Mallart, E.; Nierlich, M.; Girerd, J.-J. Iron complexes containing the ligand N,N′-bis(6-methyl-2-pyridylmethyl)-N,N′-bis(2-pyridylmethyl)ethane-1,2-diamine: Structural, spectroscopic, and electrochemical studies, reactivity with hydrogen peroxide and the formation of a low-spin Fe−OOH complex. Eur. J. Inorg. Chem. 2003, 2529–2535. [Google Scholar] [CrossRef]
- Sénéchal-David, K.; Buron, C.; Ségaud, N.; Rebilly, J.-N.; Dos Santos, A.; Farjon, J.; Guillot, R.; Herrero, C.; Inceoglu, T.; Banse, F. Non-heme Fe(II) diastereomeric complexes bearing a hexadentate ligand:unexpected consequences on the spin state and oxidation catalytic properties. Chem. A Eur. J. 2019, 25, 12405–12411. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Espinosa-Perez, G.; Zentella-Dehesa, A.; Silaghi-Dumitrescu, I.; Lara-Ochoa, F. (Tetrakis(2-pyridylmethyl)ethylenediamine)iron(II) perchlorate. Study of density functional methods. Inorg. Chem. 2000, 39, 3440–3448. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Cavallaro, G.; Lorenzini, S. FindGeo: A tool for determining metal coordination geometry. Bioinformatics 2012, 28, 1658–1660. [Google Scholar] [CrossRef] [PubMed]
- Paine, T.K.; Costas, M.; Kaizer, J.; Que, L., Jr. Oxoiron(IV) complexes of the tris(2-pyridylmethyl)amine ligand family: Effect of pyridine a-substituents. J. Biol. Inorg. Chem. 2006, 11, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Lakk-Bogáth, D.; Speier, G.; Kaizer, J. Comparison of the stability and reactivity of achiral versus chiral nonheme oxoiron(IV) complexes supported by pentadentate N5 ligands in oxygen-atom and hydrogen-atom transfer reactions. Inorg. Chem. Commun. 2019, 107, 107446. [Google Scholar] [CrossRef]
- Cho, K.-B.; Hirao, H.; Shaik, S.; Nam, W. To rebound or dissociate? This is the mechanistic question in C-H hydroxylation by heme and nonheme metal-oxo complexes. Chem. Soc. Rev. 2016, 45, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.S. Flavone. Org. Synth. 1952, 32, 72–76. [Google Scholar] [CrossRef]
| Fe-N1/Å | Fe-N2/Å | Fe-N3/Å | Geometry/RMSD/Å | |
|---|---|---|---|---|
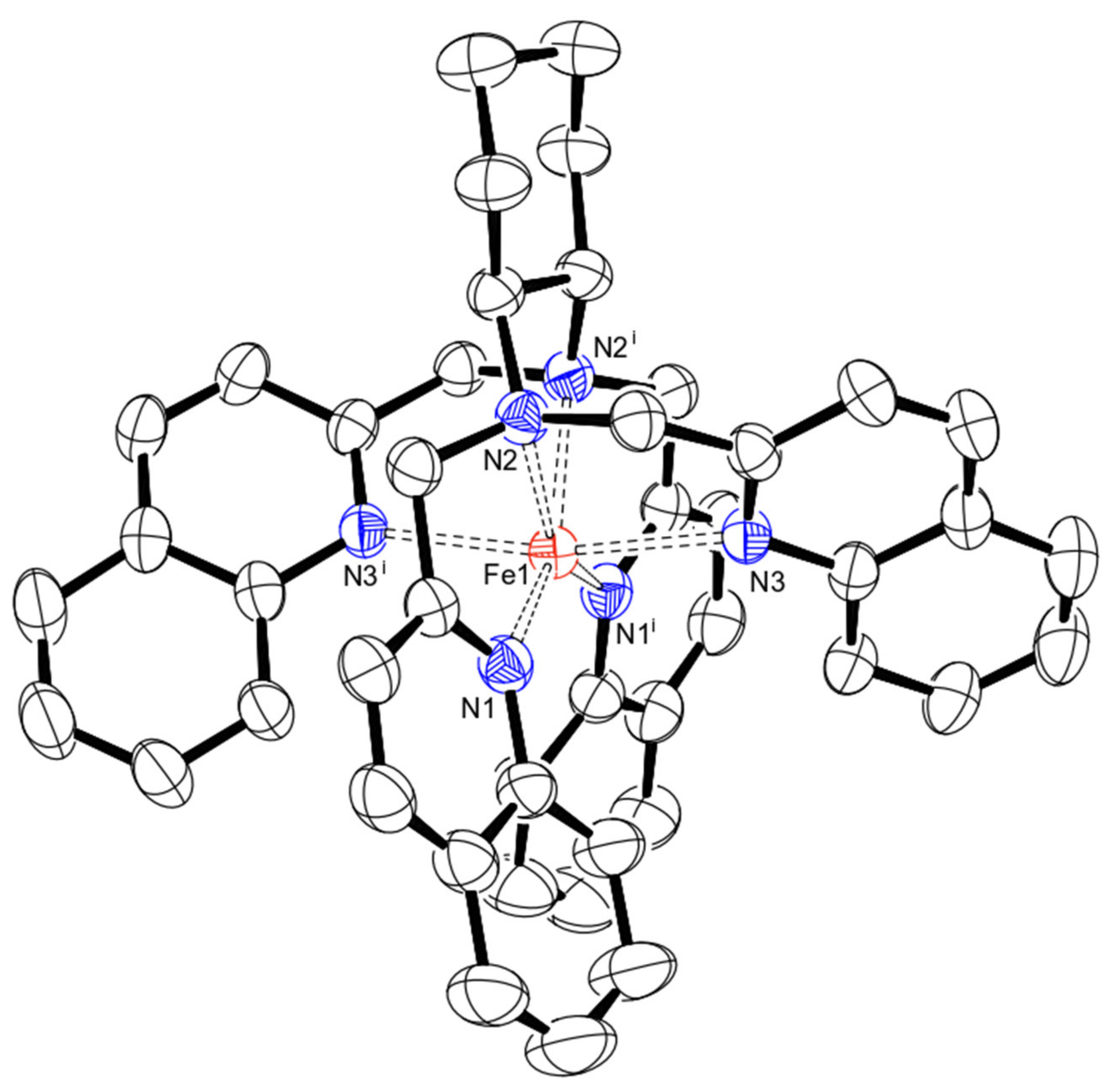
| [FeII(±CDA-BQA*)](CF3SO3)2 (5) | 2.194 | 2.238 | 2.252 | Pentagonal bipyramid with a vacancy (equatorial) (regular) 0.271 |
| [FeII(CDA-BPA*)](ClO4)2 (6) [41] | 1.993 2.003 | 2.012 2.038 | 1.994 1.993 | Octahedron (regular) 0.369 |
| MII | [H2O] /mM | [MII] /mM | [FH2] /mM | [mCPBA] /mM | Yield (F) /% | TON | Yield (D) c /% | TON | Yieldt /% |
|---|---|---|---|---|---|---|---|---|---|
| [FeII(CDA-BPA*)]2+ (6) | 44 | 5 | 100 | 500 | 35.2 | 7.04 | 1.15 | 0.23 | 36.35 |
| [FeII(Bn-TPEN)]2+ (3) | 44 | 5 | 100 | 500 | 29 | 5.8 | 1.4 | 0.28 | 30.4 |
| [FeII(CDA-BQA*)]2+ (5) | 44 | 5 | 100 | 500 | 20.8 | 4.16 | 0.83 | 0.17 | 21.63 |
| [MnII(N4Py*)]2+ (2) | 44 | 5 | 100 | 500 | 17.9 | 3.58 | 0.92 | 0.19 | 18.8 |
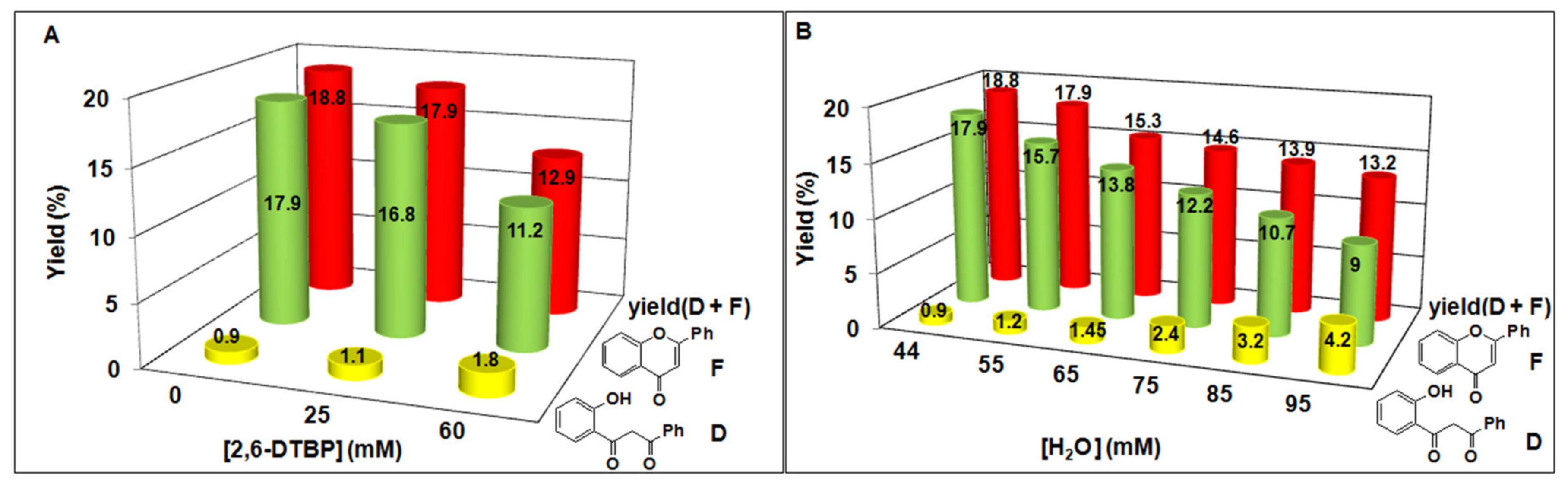
| [MnII(N4Py*)]2+ (2) | 55 | 5 | 100 | 500 | 15.7 | 3.14 | 1.2 | 0.23 | 16.9 |
| [MnII(N4Py*)]2+ (2) | 65 | 5 | 100 | 500 | 13.8 | 2.76 | 1.45 | 0.3 | 15.25 |
| [MnII(N4Py*)]2+ (2) | 75 | 5 | 100 | 500 | 12.2 | 2.44 | 2.4 | 0.48 | 14.64 |
| [MnII(N4Py*)]2+ (2) | 85 | 5 | 100 | 500 | 10.7 | 2.14 | 3.2 | 0.64 | 13.9 |
| [MnII(N4Py*)]2+ (2) | 95 | 5 | 100 | 500 | 9 | 1.88 | 4.2 | 0.92 | 13.2 |
| [MnII(N4Py*)]2+ (2) | 44 | 5 | 100 a | 500 | 16.8 a | 3.36 | 1.1 | 0.2 | 17.9 |
| [MnII(N4Py*)]2+ (2) | 44 | 5 | 100 b | 500 | 11.2 b | 2.24 | 1.8 | 0.36 | 12.95 |
| [MnII(Bn-TPEN)]2+ (4) | 44 | 5 | 100 | 500 | 23.1 | 4.62 | 0.61 | 0.12 | 23.7 |
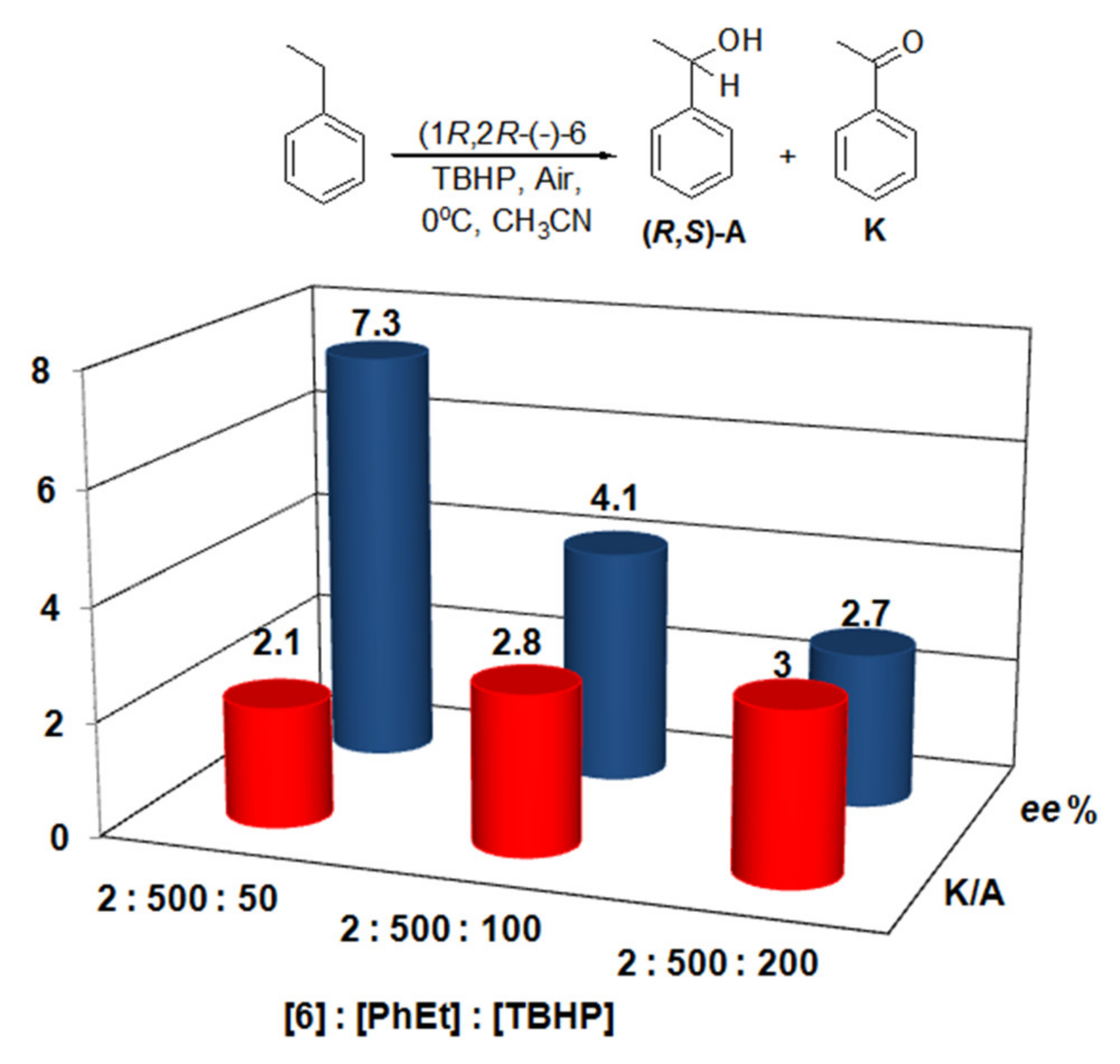
| [6]:[PhEt]:[Co-oxidant] | TON (A) b | TON (K) c | Yield /% d | K/A | ee /% |
|---|---|---|---|---|---|
| 2:500:50 (TBHP) | 1.38 | 2.83 | 16.84 | 2.05 | 7.25 (R) |
| 2:500:100 (TBHP) | 1.82 | 5.20 | 14.04 | 2.86 | 4.13 (R) |
| 2:500:200 (TBHP) | 2.06 | 6.17 | 8.23 | 3.00 | 2.68 (R) |
| 2:1500:16 (PhIO) | 0.96 | 2.14 | 38.75 | 2.23 | 12.21 (R) |
| [5]:[PhEt]:[Co-oxidant] | TON (A b) | TON (K c) | Yield /% d | K/A | ee /% |
| 2:500:100 (TBHP) | - | 10.08 | 20.16 | - | - |
| Complex | 103k2 /s−1 M−1 | ΔH‡ /kJ mol−1 | ΔS‡ /J mol−1 K−1 | ΔG‡ /kJ mol−1 | Solvent /T | Refs. |
|---|---|---|---|---|---|---|
| [FeIV(O)(N4Py*)]2+ (7) | 0.24 ± 0.01 | 70 ± 3 | −75 ± 9 | 92.2 | CH3CN (25 °C) | [46] |
| [FeIV(O)(N4Py)]2+ | 0.57 ± 0.03 | 63 ± 4 | −93 ± 13 | 90.9 | CH3CN (25 °C) | [46] |
| [FeIV(O)(N2Py2Q*)]2+ | 19.2 ± 1.1 | 48 ± 2 | −121 ± 8 | 83.7 | CH3CN (25 °C) | [46] |
| [FeIV(O)(N2Py2Q*)]2+ | 6.50 ± 0.32 | CH3CN (10 °C) | [46] | |||
| [FeIV(O)(Bn-TPEN)]2+ (9) | 1340 ± 67 | 28 ± 2 | −150 ± 8 | 72.7 | CH3CN (25 °C) | This work |
| [FeIV(O)(Bn-TPEN)]2+ (9) | 680 ± 27 | CH3CN (10 °C) | This work | |||
| [FeIV(O)(Bn-TPEN)]2+ (9) | 1520 ± 90 | CH3CN/TFE (10 °C) | This work | |||
| [FeIV(O)(CDA-BPA*)]2+ (11) | 970 ± 40 | CH3CN (10 °C) | This work | |||
| [MnIV(O)(N4Py*)]2+ (8) | 0.58 ± 0.03 | CH3CN/TFE (25 °C) | This work | |||
| [MnIV(O)(Bn-TPEN)]2+ (10) | 420 ± 15 | 43 ± 3 | −114 ± 10 | 76.7 | CH3CN/TFE (10 °C) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakk-Bogáth, D.; Juraj, N.P.; Meena, B.I.; Perić, B.; Kirin, S.I.; Kaizer, J. Comparison of Nonheme Manganese- and Iron-Containing Flavone Synthase Mimics. Molecules 2021, 26, 3220. https://doi.org/10.3390/molecules26113220
Lakk-Bogáth D, Juraj NP, Meena BI, Perić B, Kirin SI, Kaizer J. Comparison of Nonheme Manganese- and Iron-Containing Flavone Synthase Mimics. Molecules. 2021; 26(11):3220. https://doi.org/10.3390/molecules26113220
Chicago/Turabian StyleLakk-Bogáth, Dóra, Natalija Pantalon Juraj, Bashdar I. Meena, Berislav Perić, Srećko I. Kirin, and József Kaizer. 2021. "Comparison of Nonheme Manganese- and Iron-Containing Flavone Synthase Mimics" Molecules 26, no. 11: 3220. https://doi.org/10.3390/molecules26113220
APA StyleLakk-Bogáth, D., Juraj, N. P., Meena, B. I., Perić, B., Kirin, S. I., & Kaizer, J. (2021). Comparison of Nonheme Manganese- and Iron-Containing Flavone Synthase Mimics. Molecules, 26(11), 3220. https://doi.org/10.3390/molecules26113220







