Progress in PET Imaging of Neuroinflammation Targeting COX-2 Enzyme
Abstract
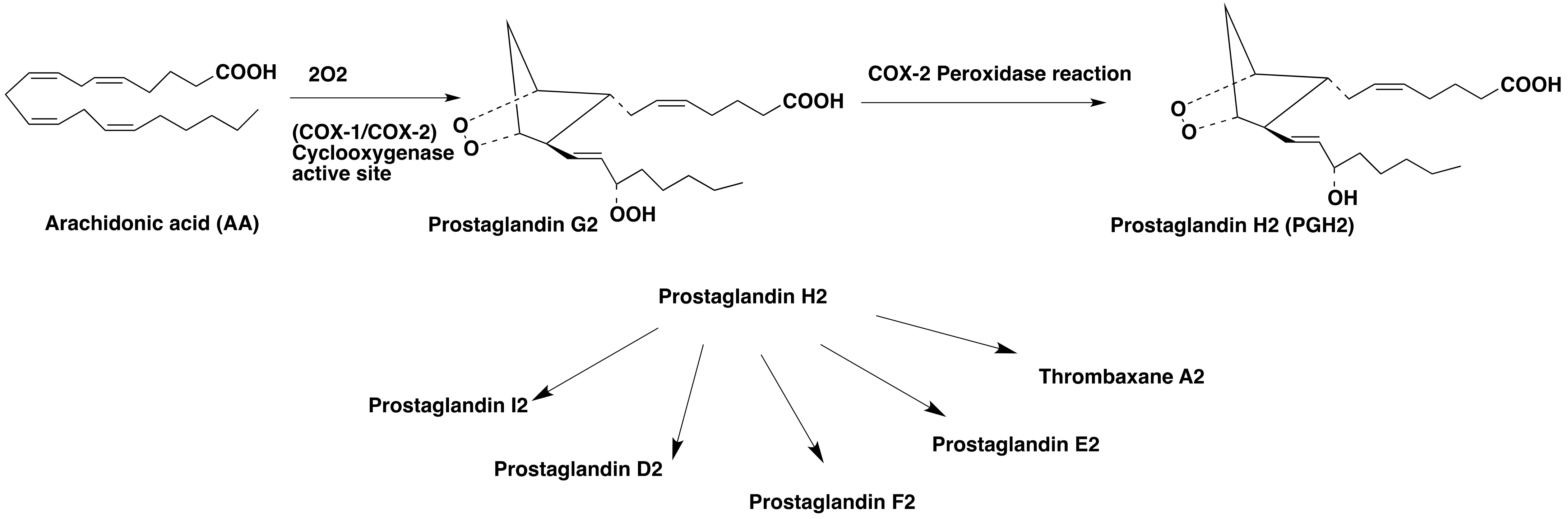
1. Introduction
1.1. Role of COX-2 in the Central Nervous System (CNS)
1.2. COX-2 Induction in the Pathognesis of CNS Diseases
1.3. Clinical Trials Using Coxibs in Neurological Disorders
2. In Vivo Measurement of Neuroinflammation
2.1. Animal Models of Neuroinflammation
2.2. PET Radioligands Developed for COX-2 Imaging in the Brain
2.3. PET Studies of [11C]Celecoxib and [11C]Rofecoxib in Rodents:
2.4. PET Studies of [11C]Celecoxib in Baboon
2.5. [18.F]-Labled Analogues of Celecoxib
2.6. PET Studies of [18F]Celecoxib in Baboons
2.7. PET Studies Using [11C]MOV
2.8. PET Studies of [11C]TMI
2.9. PET Studies of [11C]MC1
2.10. PET Studies of [18F]FMTP
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Significance Statement
References
- Valesini, G. Selective Cyclooxygenase-2 Inhibition: Biological and Clinical Effects. Isr. Med. Assoc. J. 2000, 2, 841–847. [Google Scholar]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, Cellular, and Molecular Biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Berl, T.; McDonald, K.M.; Schrier, R.W. Prostaglandins: Effects on Blood Pressure, Renal Blood Flow, Sodium and Water Excretion. Kidney Int. 1976, 10, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Adegboyega, P.A.; Ololade, O. Immunohistochemical Expression of Cyclooxygenase-2 in Normal Kidneys. Appl. Immunohistochem. Mol. Morphol. 2004, 12, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, N.V.; Dai, H.; Roos, K.L.T.; Evanson, N.K.; Tomsik, J.; Elton, T.S.; Simmons, D.L. COX-3, a Cyclooxygenase-1 Variant Inhibited by Acetaminophen and Other Analgesic/Antipyretic Drugs: Cloning, Structure, and Expression. Proc. Natl. Acad. Sci. USA 2002, 99, 13926–13931. [Google Scholar] [CrossRef]
- Norregaard, R.; Kwon, T.-H.; Frokiaer, J. Physiology and pathophysiology of cyclooxygenase-2 and prostaglandin E2 in the kidney. Kidney Res. Clin. Pract. 2015, 34, 194–200. [Google Scholar] [CrossRef]
- Blobaum, A.L.; Marnett, L.J. Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 2007, 50, 1425–1441. [Google Scholar] [CrossRef]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; Van De Putte, L.B.; Lipsky, P.E. Cyclooxygenase in Biology and Disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [CrossRef]
- Willoughby, D.A.; Moore, A.R.; Colville-Nash, P.R. COX-1, COX-2, and COX-3 and the Future Treatment of Chronic Inflammatory Disease. Lancet 2000, 355, 646–648. [Google Scholar] [CrossRef]
- Roughead, E.E.; Ramsay, E.; Pratt, N.; Gilbert, A.L. Nsaid use in individuals at risk of renal adverse events: An observational study to investigate trends in Australian veterans. Drug Saf. 2008, 31, 997–1003. [Google Scholar] [CrossRef]
- Luo, C.; He, M.-L.; Bohlin, L. Is COX-2 a perpetrator or a protector? Selective COX-2 inhibitors remain controversial. Acta Pharmacol. 2005, 26, 926–933. [Google Scholar] [CrossRef]
- Minghetti, L. Cyclooxygenase-2 in inflammatory and Degenerative Brain Diseases. J. Neuropathol. Exp. Neurol. 2004, 63, 901–910. [Google Scholar] [CrossRef]
- Crofford, L.J.; Lipsky, P.E.; Brooks, P.; Abramson, S.B.; Simon, L.S.; van de Putte, L.B. Basic biology and clinical application of specific cyclooxygenase-2 inhibitors. Arthritis Rheum. 2000, 43, 4–13. [Google Scholar] [CrossRef]
- Pratico, D.; Dogne, J.-M. Selective cyclooxygenase-2 Inhibitors. Dev. Cardiovasc. Med. 2005, 112, 1073–1079. [Google Scholar]
- Zhang, J.; Ding, E.L.; Song, Y. Adverse Effects of Cyclooxygenase 2 Inhibitors on Renal and Arrhythmia Events. J. Am. Med. Assoc. 2006, 296, 1619–1632. [Google Scholar] [CrossRef]
- Davies, N.M.; Jamali, F. COX-2 Selective Inhibitors Cardiac Toxicity: Getting to the Heart of the Matter. J. Pharm. Pharm. Sci. 2004, 7, 332–336. [Google Scholar]
- Bridget, M.; Kuehn, M.S.J. FDA approves first celecoxib generic. JAMA 2014, 311, 2470. [Google Scholar]
- Hoffman, C. COX-2 in brain and spinal cord implications for therapeutic use. Curr. Med. Chem. 2000, 7, 1113–1120. [Google Scholar] [CrossRef]
- Yang, H.; Chen, C. Cyclooxygenase-2 in synaptic signaling. Curr. Pharm. Des. 2008, 14, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Rouzer, C.A.; Marnett, L.J. Cyclooxygenase: Structural and functional insights. J. Lipid. Res. 2009, 50, S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Whigham, K.B.; Burns, T.G.; Lageman, S.K. National Institute of Neurological Disorders and Stroke. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Pal, S. Incidence and Prevalence of Major Neurologic Disorders. Neurology 2018, 43, 24. [Google Scholar]
- Vlad, S.C.; Miller, D.R.; Kowall, N.W.; Felson, D.T. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 2008, 70, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Hernán, M.A.; Logroscino, G.; Rodríguez, L.A.G. Nonsteroidal anti-inflammatory drugs and the incidence of Parkinson disease. Neurology 2006, 66, 1097–1099. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Ho, L.; Yemul, S.; Zhao, Z.; Pompl, P.; Kelley, K.; Dang, A.; Quing, W.; Teplow, D.; Pasinetti, G.M. Cyclooxygenase-2 promotes Amyloid plaque deposition in a mouse model of Alzheimer’s disease neuropathology. Gene Expr. 2002, 10, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Hoozemans, J.J.M.; Rozemuller, J.M.; van Haastert, E.S.; Veerhuis, R.; Eikelenboom, P. Cyclooxygenase-1 and-2 in the different stages of Alzheimer’s disease pathology. Curr. Pharm. Des. 2008, 14, 1419–1427. [Google Scholar] [CrossRef]
- Ho, L.; Purohit, D.; Haroutunian, V.; Luterman, J.D.; Willis, F.; Naslund, J.; Buxbaum, J.D.; Mohs, R.C.; Aisen, P.S.; Pasinetti, G.M. Neuronal cyclooxygenase 2 expression in the hippocampal formation as a function of the clinical progression of Alzheimer disease. Arch. Neurol. 2001, 58, 487–492. [Google Scholar] [CrossRef]
- Bartels, A.L.; Leenders, K.L. Cyclooxygenase and Neuroinflammatin in Parkinson’s Disease Neurodegeneration. Curr. Neuropharmacol. 2010, 8, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Mirjany, M.; Ho, L.; Pasinetti, G.M. Role of cyclooxy- genase-2 in neuronal cell cycle activity and glutamate-mediated excitotoxicity. J. Pharmacol. Exp. Ther. 2002, 301, 494–500. [Google Scholar] [CrossRef]
- Consilvio, C.; Feldman, E.L. Neuro- inflammation, COX-2, and ALS—A dual role? Exp. Neurol. 2004, 187, 1–10. [Google Scholar] [CrossRef]
- Dembo, G.; Park, S.B.; Kharasch, E.D. Central Nervous system concentrations of Cyclooxygenase-2 inhibitors in humans. Anesthesiology 2005, 102, 409–415. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, Y.; Kishioka, S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr. Opin. Pharmacol. 2012, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.; Quan, N.; Godbout, J.P. Neuroinflammation: The Devil is in the Details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef]
- Rodriguez-Vieitez, E.; Nordberg, A. Imaging Neuroinflammation: Quantificaitoin of Astrocytes in a Multitracer PET Approach. Methods Mol. Biol. 2018, 1750, 231–251. [Google Scholar]
- Werry, E.L.; Bright, F.M.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kril, J.J.; Kassiou, M. Recent Developments in TSPO PET imaging as a Biomarker of Neuroinflammation in Neurodegenerative Disorders. Int. J. Mol. Sci. 2019, 20, 3161. [Google Scholar] [CrossRef]
- Zanderigo, F.; Kang, Y.; Kumar, D.; Nikolopoulou, A.; Mozley, P.D.; Kothari, P.J.; He, B.; Schlyer, D.; Rapoport, S.I.; Oquendo, M.A.; et al. [11 C]arachidonic acid incorporation measurement in human brain: Optimization for clinical use. Synapse 2018, 72. [Google Scholar] [CrossRef]
- Largeau, B.; Dupont, A.-C.; Guilloteau, D.; Santiago-Ribeiro, M.-J.; Arlicot, N. TSPO PET imaging: From Microglial Activation to Peripheral Sterile Inflammatory Diseases? Contrast Media Mol. Imaging 2017, 2017, 6592139. [Google Scholar] [CrossRef] [PubMed]
- Janks, L.; Sharma, C.V.R.; Egan, T.M. A central role for P2X7 receptors in human microglia. J. Neuroinflammation 2018, 15, 325. [Google Scholar] [CrossRef] [PubMed]
- Bie, B.; Wu, J.; Foss, J.F.; Naguib, M. An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr. Opin. Anaesthesiol. 2018, 31, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Ishikawa, T.O.; Spigelman, I.; Herschman, H.R. COX-2 expression and function in the hyperalgesic response to paw inflammation in mice. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 183–190. [Google Scholar] [CrossRef]
- Eliopoulos, A.G.; Dumitru, C.D.; Wang, C.C.; Cho, J.; Tsichlis, P.N. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO J. 2002, 21, 4831–4840. [Google Scholar] [CrossRef]
- Banks, W.A.; Gray, A.M.; Erickson, M.A.; Salameh, T.S.; Damodarasamy, M.; Sheibani, N.; Meabon, J.S.; Wing, E.E.; Morofuji, Y.; Cook, D.G.; et al. Lipopolysaccharide-induced blood-brain barrier disruption: Roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J. Neuroinflammation 2015, 12, 223. [Google Scholar] [CrossRef]
- Haggerty, D.L.; Grecco, G.G.; Reeves, K.C.; Atwood, B. Adeno-Associated Viral Vectors in Neuroscience Research. Mol. Ther. Methods Clin. Dev. 2019, 17, 69–82. [Google Scholar] [CrossRef]
- Xiang, Z.; Ho, L.; Valdellon, J.; Borchelt, D.; Kelley, K.; Spielman, L.; Aisen, P.S.; Pasinetti, G.M. Cyclooxygenase (COX)-2 and cell cycle activity in a transgenic mouse model of Alzheimer’s disease neuropathology. Neurobiol. Aging. 2002, 23, 327–334. [Google Scholar] [CrossRef]
- McCluskey, S.P.; Plisson, C.; Rabiner, E.A.; Howes, O. Advances in CNS PET: The state-of-the-art for new imaging targets for pathophysiology and drug development. Eur. J. Nucl Med. Mol. Imaging 2020, 47, 451–489. [Google Scholar] [CrossRef]
- Channing, M.A.; Simpson, N. Radiosynthesis of 11Cplyhomoallylic fatty acids. J. Label. Compd. Radiopharm. 1993, 33, 541–546. [Google Scholar] [CrossRef]
- Laube, M.; Kniess, T. Pietzsch Radiolabeled COX-2 inhibitors for non-invasive visualization of COX-2 expression and activity-A critical update. Molecules 2013, 18, 6311–6355. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Toyohara, J.; Ishiwata, K.; Sano, K.; Yamamoto, F.; Mukai, T.; Maeda, M. 11C-labeled analogs of indomethacin esters and amides for brain cyclooxygenase-2 imaging: Radiosynthesis, in vitro evaluation and in vivo characteristics in mice. Chem. Pharm. Bull. 2011, 59, 938–946. [Google Scholar] [CrossRef]
- De Vries, E.F. Imaging of cyclooxygenase-2 (COX-2) expression: Potential use in diagnosis and drug evaluation. Curr. Pharm. Des. 2006, 12, 3847–3856. [Google Scholar] [CrossRef]
- Ji, B.; Kumata, K.; Onoe, H.; Kaneko, H.; Zhang, M.R.; Seki, C.; Ono, M.; Shukuri, M.; Tokunaga, M.; Minamihisamatsu, T.; et al. Assessment of radioligands for PET imaging of cyclooxygenase-2 in an ischemic neuronal injury model. Brain Res. 2013, 1533, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, J.; Majo, V.J.; Simpson, N.R.; Van Heertum, R.L.; Mann, J.J.; Kumar, J.S.D. Synthesis of [11C]celecoxib: A potential PET probe for imaging COX-2 expression. J. Label. Compd. Radiopharm. 2005, 48, 887–895. [Google Scholar] [CrossRef]
- Kumar, J.S.D.; Bai, B.; Zanderigo, F.; DeLorenzo, C.; Prabhakaran, J.; Parsey, R.V.; Mann, J.J. In Vivo Brain Imaging, Biodistribution, and Radiation Dosimetry Estimation of [11C]Celecoxib, a COX-2 PET Ligand, in Nonhuman Primates. Molecules 2018, 23, 1929. [Google Scholar] [CrossRef]
- Lebedev, A.; Jiao, J.; Lee, J.; Yang, F.; Allison, N.; Herschman, H.; Sadeghi, S. Radiochemistry on electrodes: Synthesis of an 18F-labelled and in vivo stable COX-2 inhibitor. PLoS ONE 2017, 12, e0176606. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Tietz, O.; Bhardwaj, A.; Marshall, A.; Way, J.; Wuest, M.; Wuest, F. Design, Synthesis, and Evaluation of an (18)F-Labeled Radiotracer Based on Celecoxib-NBD for Positron Emission Tomography (PET) Imaging of Cyclooxygenase-2 (COX-2). Chem. Med. Chem. 2015, 10, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, J.; Underwood, M.D.; Parsey, R.V.; Arango, V.; Majo, V.J.; Simpson, N.R.; Van Heertum, R.; Mann, J.J.; Kumar, J.S. Synthesis and in vivo evaluation of [18F]-4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide as a PET imaging probe for COX-2 expression. Bioorg. Med. Chem. 2007, 15, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, J.; Underwood, M.; Zanderigo, F.; Simpson, N.R.; Cooper, A.R.; Matthew, J.; Rubin-Falcone, H.; Parsey, R.V.; Mann, J.J.; Kumar, J.S.D. Radiosynthesis and in vivo evaluation of [11C]MOV as a PET imaging agent for COX-2. Bioorg. Med. Chem. Lett. 2018, 28, 2432–2435. [Google Scholar] [CrossRef]
- Kumar, J.S.D.; Zanderigo, F.; Prabhakaran, J.; Rubin-Falcone, H.; Parsey, R.V.; Mann, J.J. In vivo evaluation of [11C]TMI, a COX-2 selective PET tracer, in baboons. Bioorg. Med. Chem. Lett. 2018, 28, 3592–3595. [Google Scholar] [CrossRef]
- Mann, J.J.; Kumar, J.S.D. Radiolabeled arylsulfonyl compounds and uses thereof. WO200512 0584A3, 2005.
- Habeeb, A.G.; Rao, P.N.P.; Knaus, E.E. Design and synthesis of Diarylisoxazoles: Novel inhibitors of cyclooxygenase-2 (COX-2) with analgesic-antiinflammatory activity. Drug Dev. Res. 2000, 51, 273–286. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, M.J.; Eldridge, M.; Lehmann, M.L.; Frankland, M.; Liow, J.S.; Yu, Z.X.; Cortes-Salva, M.; Telu, S.; Henter, I.D.; et al. PET measurement of cyclooxygenase-2 using a novel radioligand: Upregulation in primate neuroinflammation and first-in-human study. J. Neuroinflammation 2020, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Cortes, M.; Singh, P.; Morse, C.; Shrestha, S.; Jenko, K.; Kowalski, A.; Zoghbi, S.; Fujita, M.; Innis, R.; Pike, W. Synthesis of PET radioligands as potential probes for imaging COX-2 in neuroinflammation. J. Nucl. Med. 2015, 56, 1092. [Google Scholar]
- Kumar, J.S.D.; Prabhakaran, J.; Molotkov, A.; Sattiraju, A.; Kim, J.; Doubrovin, M.; Mann, J.J.; Mintz, A. Radiosynthesis and evaluation of [18F]FMTP, a COX-2 PET ligand. Pharmacol. Rep. 2020, 72, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
| Entry | PET Ligand | COX-2 IC50 | COX-1/COX-2 | LogP | In Vivo Binding in Brain |
|---|---|---|---|---|---|
| 1 | 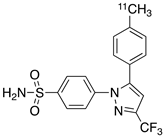 [11C]celecoxib [11C]celecoxib | 40 nM | 30 | 3.6 | PET imaging did not show binding in rodent brain. However, low binding was found in healthy baboon brain, indicating BBB permeability. PET studies in inflammation model are not reported [49,50,51,52]. |
| 2 | 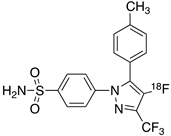 [18F]celecoxib analogue 1 [18F]celecoxib analogue 1 | 1.7 nM | 224 | 3.3 | No in vivo binding is reported in rodent brain. PET imaging in other species or neuroinflammation model are not reported [53]. |
| 3 | 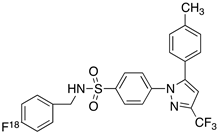 [18F]celecoxib analogue 2 [18F]celecoxib analogue 2 | 360 nM | 278 | 5.8 | In vivo binding was not reported in rodent brain. PET imaging in other species or in neuroinflammation model are not reported [54]. |
| 4 |  [18F]celecoxib (4) [18F]celecoxib (4) | 40 nM | 30 | 3.7 | Significant bone uptake was detected in rodent PET imaging. Minor binding was observed in normal baboon brain, which indicates BBB penetration. We detected less skeletal uptake in baboon compared to rodents [55]. |
| 5 | 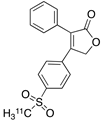 [11C]rofecoxib (5) [11C]rofecoxib (5) | 19 nM | 272 | 1.8 | In vivo binding of the tracer is not reported in rodent brain. PET imaging in other species or in neuroinflammation models are also not reported [47,49]. |
| 6 | 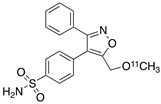 [11C]MOV (6) [11C]MOV (6) | 35 nM | 342 | 1.6 | There was no detectable binding in rat brain. Binding in healthy baboon brain was also not significant. Therefore, no neuroinflammation model was tested [56]. |
| 7 | 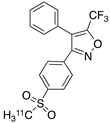 [11C]TMI (7) [11C]TMI (7) | 1 nM | 500,000 | 2.7 | Moderate binding was detected in brain by PET imaging in normal baboon. The binding was blocked using meloxicam [57,58,59]. |
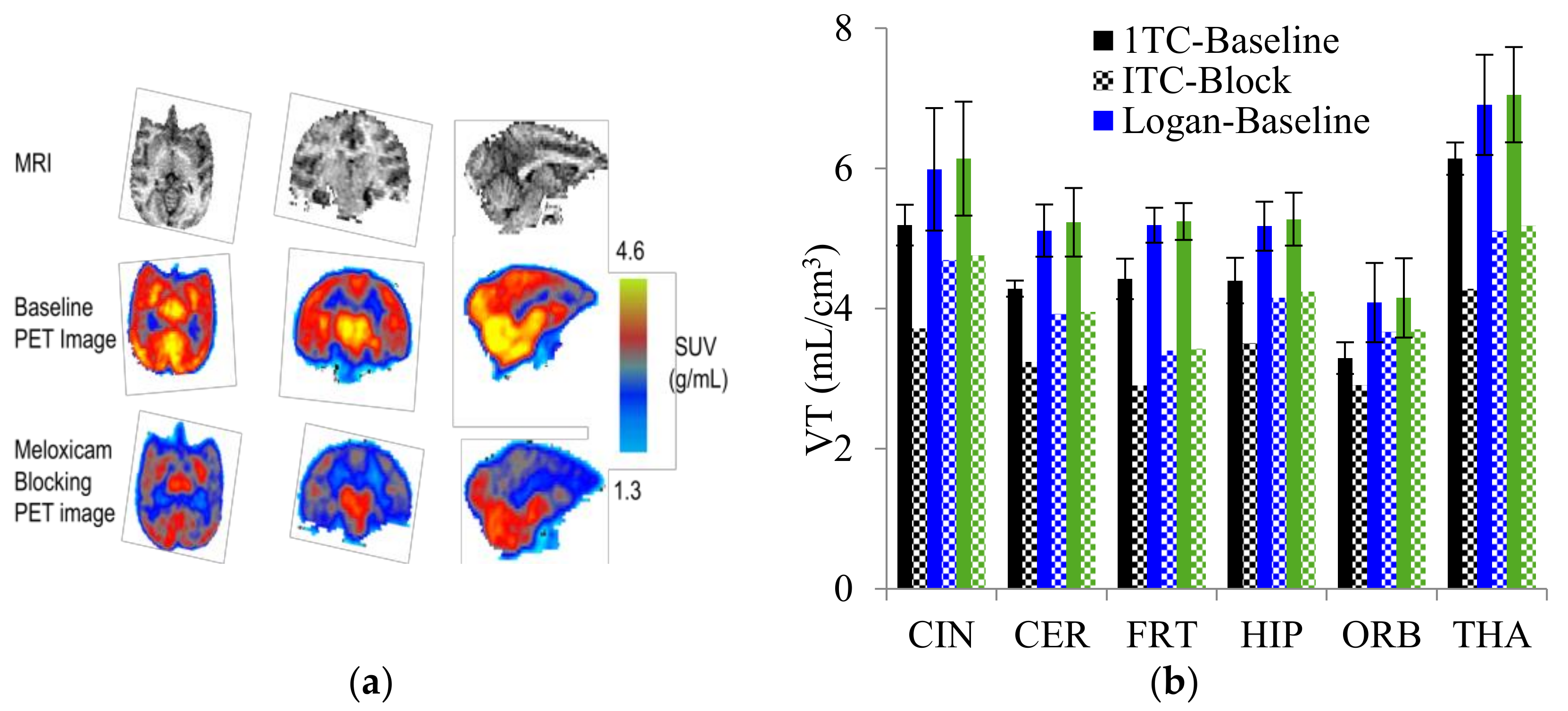
| 8 | 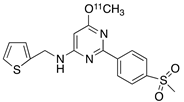 [11C]MC1 (8) [11C]MC1 (8) | 3 nM | 100,000 | 2.6 | The VT value was increased by 32–42% after LPS injection on to the right putamen of monkeys compared to healthy monkeys [60,61]. |
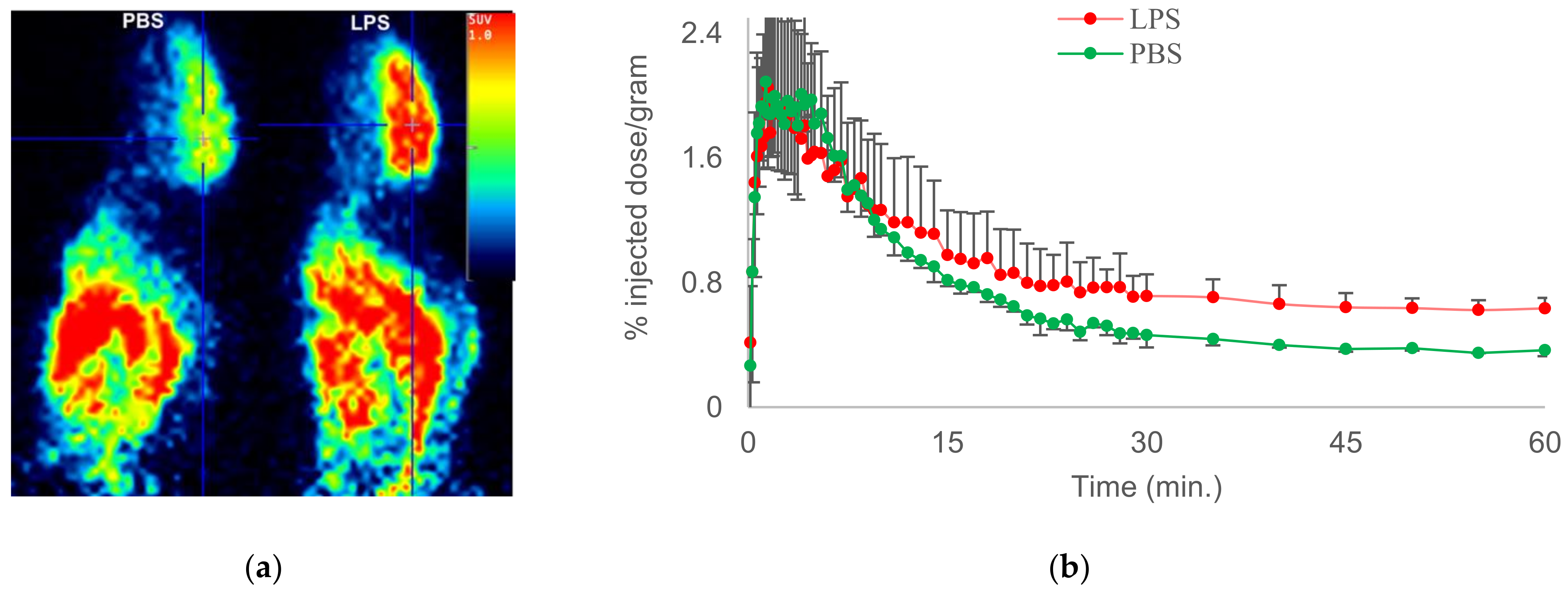
| 9 |  [18F]MTP (9) [18F]MTP (9) | 2.3 nM | 52,000 | 2.6 | Although the baseline binding in normal rodent brain was low, a higher binding was observed in LPS administered mice brain. No bone uptake was detected [62]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabhakaran, J.; Molotkov, A.; Mintz, A.; Mann, J.J. Progress in PET Imaging of Neuroinflammation Targeting COX-2 Enzyme. Molecules 2021, 26, 3208. https://doi.org/10.3390/molecules26113208
Prabhakaran J, Molotkov A, Mintz A, Mann JJ. Progress in PET Imaging of Neuroinflammation Targeting COX-2 Enzyme. Molecules. 2021; 26(11):3208. https://doi.org/10.3390/molecules26113208
Chicago/Turabian StylePrabhakaran, Jaya, Andrei Molotkov, Akiva Mintz, and J. John Mann. 2021. "Progress in PET Imaging of Neuroinflammation Targeting COX-2 Enzyme" Molecules 26, no. 11: 3208. https://doi.org/10.3390/molecules26113208
APA StylePrabhakaran, J., Molotkov, A., Mintz, A., & Mann, J. J. (2021). Progress in PET Imaging of Neuroinflammation Targeting COX-2 Enzyme. Molecules, 26(11), 3208. https://doi.org/10.3390/molecules26113208






