Adsorptive Removal of Cd, Cu, Ni and Mn from Environmental Samples Using Fe3O4-Zro2@APS Nanocomposite: Kinetic and Equilibrium Isotherm Studies
Abstract
1. Introduction
2. Results and Discussion
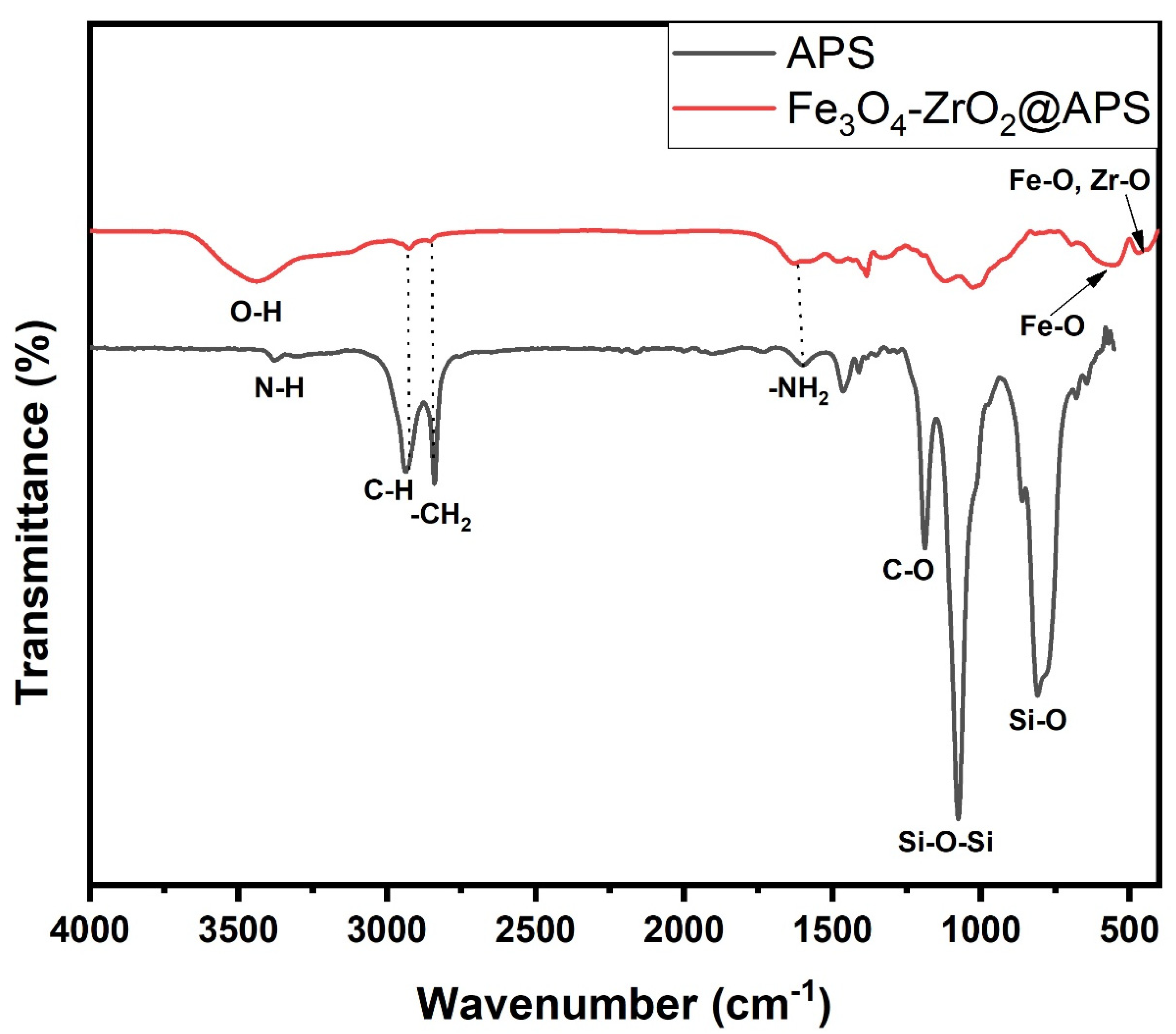
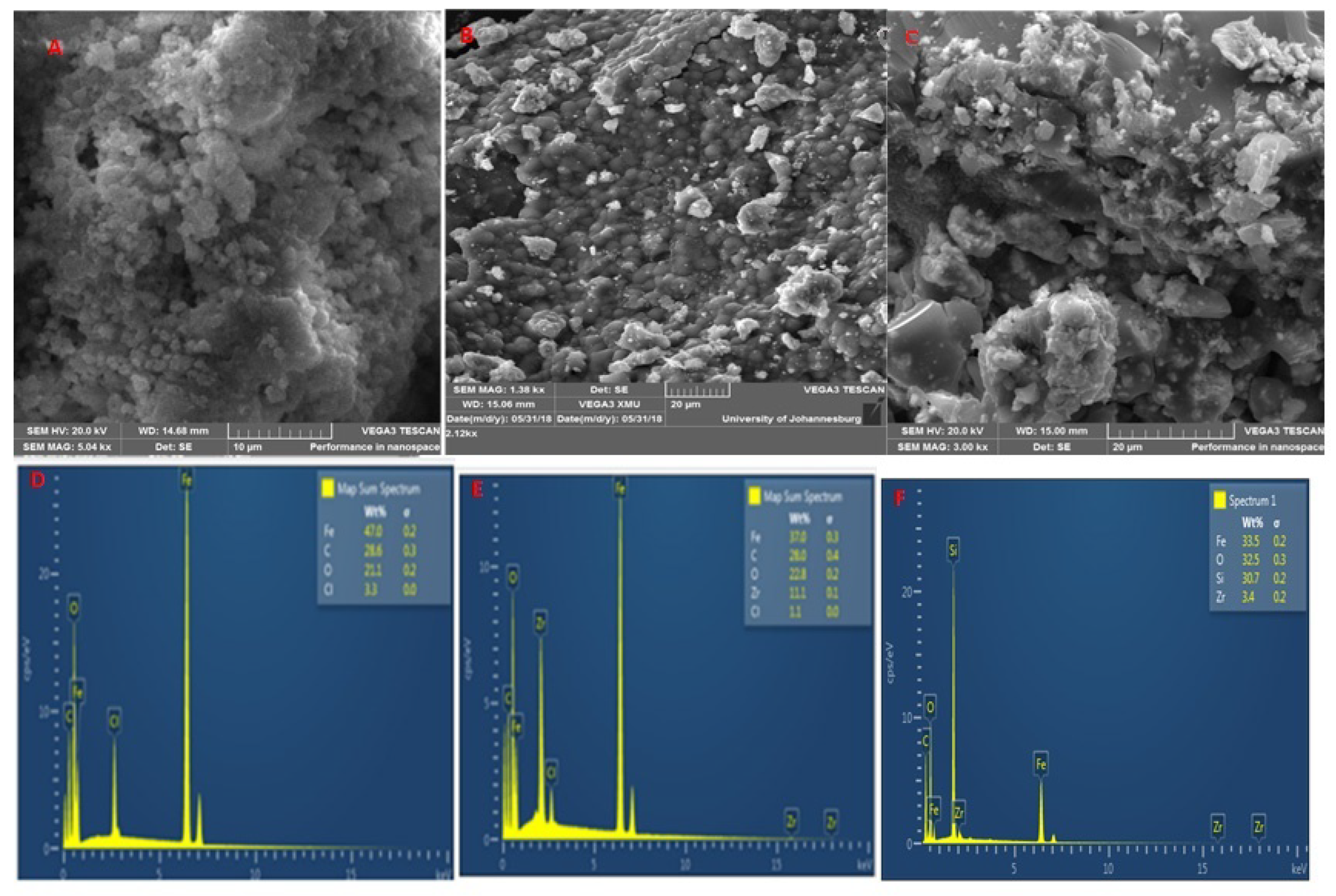
2.1. Characterization of the Adsorbent
2.2. Preliminary Studies
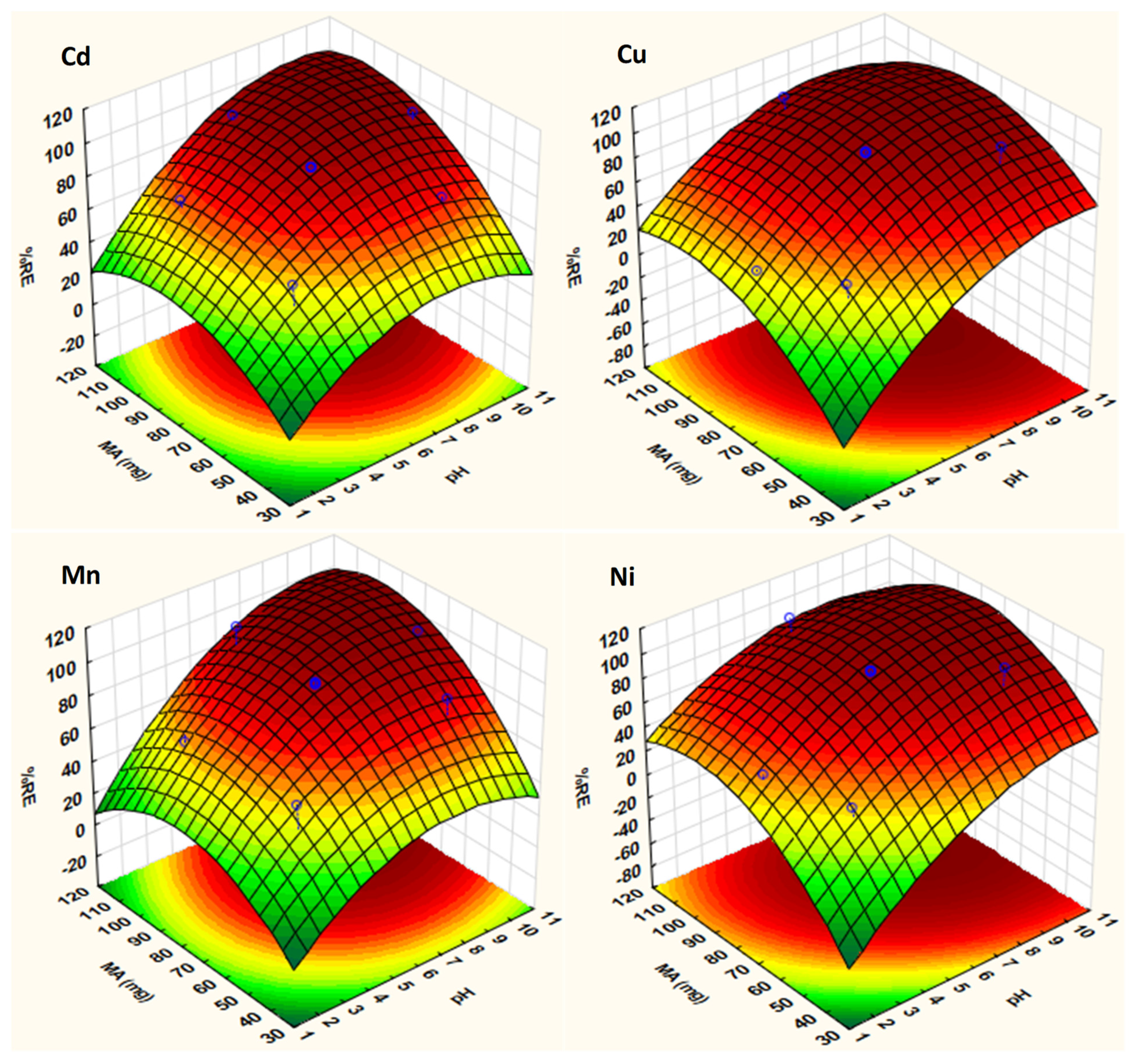
2.3. Optimization Strategy
Validation of the Optimized Conditions
2.4. Adsorption Isotherms
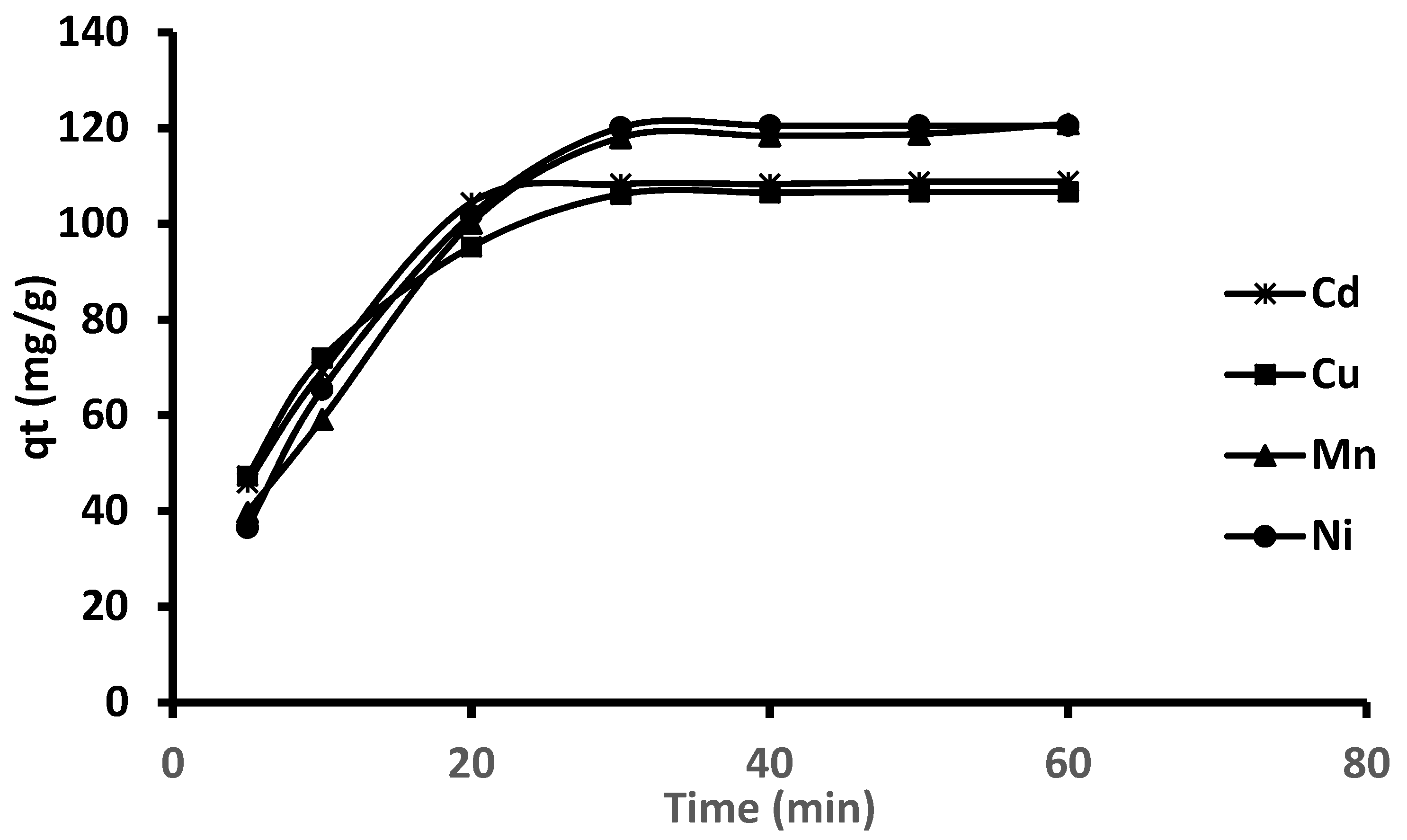
2.5. Kinetics Studies
2.6. Adsorption Mechanism
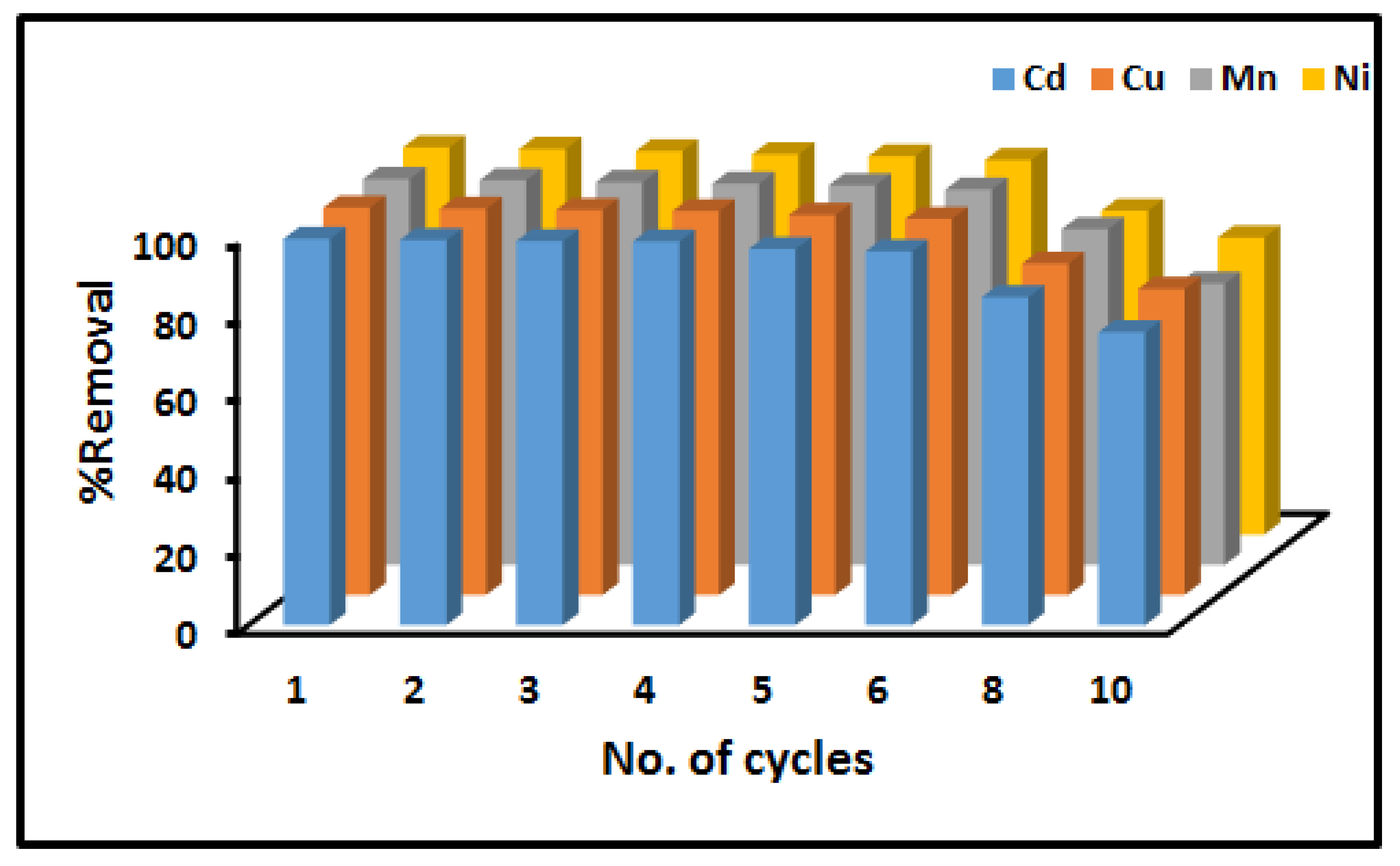
2.7. Stability and Reusability
2.8. Application to Real Samples
3. Materials and Methods
3.1. Materials and Reagents
3.2. Instrumentation
3.3. Sampling and Storage
3.4. Synthesis of the Adsorbent
3.4.1. Preparation of the Fe3O4 Nanoparticles
3.4.2. Preparation of Fe3O4-ZrO2 Nanocomposites
3.4.3. Functionalization of the Adsorbent
3.5. Experimental Procedure
3.6. Optimization Procedure
3.7. Regeneration Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy Metal Contamination: An Alarming Threat to Environment and Human Health. In Principles and Applications of Environmental Biotechnology for a Sustainable Future; Springer Science and Business Media LLC: Berlin, Germany, 2019; pp. 103–125. [Google Scholar]
- Agoro, M.A.; Adeniji, A.O.; Adefisoye, M.A.; Okoh, O.O. Heavy Metals in Wastewater and Sewage Sludge from Selected Municipal Treatment Plants in Eastern Cape Province, South Africa. Water 2020, 12, 2746. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, 04691. [Google Scholar] [CrossRef]
- Munonde, T.S.; Nomngongo, P.N. Nanocomposites for Electrochemical Sensors and Their Applications on the Detection of Trace Metals in Environmental Water Samples. Sensors 2020, 21, 131. [Google Scholar] [CrossRef] [PubMed]
- Jakavula, S.; Biata, N.R.; Dimpe, K.M.; Pakade, V.E.; Nomngongo, P.N. A Critical Review on the Synthesis and Application of Ion-Imprinted Polymers for Selective Preconcentration, Speciation, Removal and Determination of Trace and Essential Metals from Different Matrices. Crit. Rev. Anal. Chem. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tanong, K.; Tran, L.-H.; Mercier, G.; Blais, J.-F. Recovery of Zn (II), Mn (II), Cd (II) and Ni (II) from the unsorted spent batteries using solvent extraction, electrodeposition and precipitation methods. J. Clean. Prod. 2017, 148, 233–244. [Google Scholar] [CrossRef]
- Mazumder, M.A.J.; Raja, P.H.; Isloor, A.M.; Usman, M.; Chowdhury, S.H.; Ali, S.A.; Inamuddin; Al-Ahmed, A. Assessment of sulfonated homo and co-polyimides incorporated polysulfone ultrafiltration blend membranes for effective removal of heavy metals and proteins. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Nqombolo, A.; Mpupa, A.; Gugushe, A.S.; Moutloali, R.M.; Nomngongo, P.N. Adsorptive removal of lead from acid mine drainage using cobalt-methylimidazolate framework as an adsorbent: Kinetics, isotherm, and regeneration. Environ. Sci. Pollut. Res. 2018, 26, 3330–3339. [Google Scholar] [CrossRef]
- Ravi, T.; Sundararaman, S. Synthesis and characterization of chicken eggshell powder coated magnetic nano adsorbent by an ultrasonic bath assisted co-precipitation for Cr (VI) removal from its aqueous mixture. J. Environ. Chem. Eng. 2020, 8, 103877. [Google Scholar]
- Shehzad, K.; Ahmad, M.; He, J.; Liu, T.; Xu, W.; Liu, J. Synthesis of ultra-large ZrO2 nanosheets as novel adsorbents for fast and efficient removal of As(III) from aqueous solutions. J. Colloid Interface Sci. 2019, 533, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.E.; Abdou, A.E.; Sobhy, M.E.; Fekry, N.A. Solid–solid crosslinking of carboxymethyl cellulose nanolayer on titanium oxide nanoparticles as a novel biocomposite for efficient removal of toxic heavy metals from water. Int. J. Biol. Macromol. 2017, 105, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Mahdavian, L. Efficiency evaluating of modified fullerene nanofiltration on removal of heavy metals from aqueous media. J. Res. Many-Body Syst. 2020, 10, 85–95. [Google Scholar]
- Ullah, M.; Nazir, R.; Khan, M.; Khan, W.; Shah, M.; Afridi, S.G.; Zada, A. The effective removal of heavy metals from water by activated carbon adsorbents of Albizia lebbeck and Melia azedarach seed shells. Soil Water Res. 2019, 15, 30–37. [Google Scholar] [CrossRef]
- Dimpe, K.M.; Nyaba, L.; Magoda, C.; Ngila, J.; Nomngongo, P.N. Synthesis, modification, characterization and application of AC@Fe2O3@MnO2 composite for ultrasound assisted dispersive solid phase microextraction of refractory metals in environmental samples. Chem. Eng. J. 2017, 308, 169–176. [Google Scholar] [CrossRef]
- Xin, Y.; Li, C.; Liu, J.; Liu, J.; Liu, Y.; He, W.; Gao, Y. Adsorption of heavy metal with modified eggshell membrane and the in situ synthesis of Cu–Ag/modified eggshell membrane composites. R. Soc. Open Sci. 2018, 5, 180532. [Google Scholar] [CrossRef]
- Ramasamy, D.L.; Khan, S.; Repo, E.; Sillanpää, M. Synthesis of mesoporous and microporous amine and non-amine functionalized silica gels for the application of rare earth elements (REE) recovery from the waste water-understanding the role of pH, temperature, calcination and mechanism in Light REE and Hea. Chem. Eng. J. 2017, 322, 56–65. [Google Scholar] [CrossRef]
- Chauhan, A.; Islam, A.; Javed, H.; Kumar, S. Facile fabrication of Amberlite XAD-16 with dipicolylamine for remediation of industrial wastewater containing lead and copper: Isotherm, kinetics, thermodynamics and selectivity studies. Microchem. J. 2019, 146, 606–613. [Google Scholar] [CrossRef]
- Wołowiec, M.; Komorowska-Kaufman, M.; Pruss, A.; Rzepa, G.; Bajda, T. Removal of Heavy Metals and Metalloids from Water Using Drinking Water Treatment Residuals as Adsorbents: A Review. Minerals 2019, 9, 487. [Google Scholar] [CrossRef]
- Sarma, G.K.; Gupta, S.S.; Bhattacharyya, K.G. Nanomaterials as versatile adsorbents for heavy metal ions in water: A review. Environ. Sci. Pollut. Res. 2019, 26, 6245–6278. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.E. Nanoadsorbents for water and wastewater remediation. Sci. Total. Environ. 2020, 739, 139903. [Google Scholar] [CrossRef]
- Zhang, W.; An, Y.; Li, S.; Liu, Z.; Chen, Z.; Ren, Y.; Wang, S.; Zhang, X.; Wang, X. Enhanced heavy metal removal from an aqueous environment using an eco-friendly and sustainable adsorbent. Sci. Rep. 2020, 10, 1–19. [Google Scholar] [CrossRef]
- Munonde, T.S.; Maxakato, N.W.; Nomngongo, P.N. Preconcentration and speciation of chromium species using ICP-OES after ultrasound-assisted magnetic solid phase extraction with an amino-modified magnetic nanocomposite prepared from Fe3O4, MnO2 and Al2O3. Microchim. Acta 2017, 184, 1223–1232. [Google Scholar] [CrossRef]
- Nyaba, L.; Munonde, T.S.; Mpupa, A.; Nomngongo, P.N. Magnetic Fe3O4@Mg/Al-layered double hydroxide adsorbent for preconcentration of trace metals in water matrices. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gugushe, A.S.; Mpupa, A.; Nomngongo, P.N. Ultrasound-assisted magnetic solid phase extraction of lead and thallium in complex environmental samples using magnetic multi-walled carbon nanotubes/zeolite nanocomposite. Microchem. J. 2019, 149, 103960. [Google Scholar] [CrossRef]
- Tamjidi, S.; Esmaeili, H.; Moghadas, B.K. Application of magnetic adsorbents for removal of heavy metals from wastewater: A review study. Mater. Res. Express 2019, 6, 102004. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.A.E.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Babakhani, P. The impact of nanoparticle aggregation on their size exclusion during transport in porous media: One- and three-dimensional modelling investigations. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef]
- Shen, J.; Shafiq, M.; Ma, M.; Chen, H. Synthesis and Surface Engineering of Inorganic Nanomaterials Based on Microfluidic Technology. Nanomaterials 2020, 10, 1177. [Google Scholar] [CrossRef]
- Nyaba, L.; Matong, J.M.; Nomngongo, P.N. Nanoparticles consisting of magnetite and Al2O3 for ligandless ultrasound-assisted dispersive solid phase microextraction of Sb, Mo and V prior to their determination by ICP-OES. Microchim. Acta 2016, 183, 1289–1297. [Google Scholar] [CrossRef]
- Manoharan, D.; Loganathan, A.; Kurapati, V.; Nesamony, V.J. Unique sharp photoluminescence of size-controlled sonochemically synthesized zirconia nanoparticles. Ultrason. Sonochem. 2015, 23, 174–184. [Google Scholar] [CrossRef]
- Rauta, P.; Manivasakan, P.; Rajendran, V.; Sahu, B.; Panda, B.; Mohapatra, P. Phase transformation of ZrO2nanoparticles produced from zircon. Phase Transit. 2012, 85, 13–26. [Google Scholar] [CrossRef]
- Sun, T.; Zhao, Z.; Liang, Z.; Liu, J.; Shi, W.; Cui, F. Efficient removal of arsenite through photocatalytic oxidation and adsorption by ZrO2-Fe3O4 magnetic nanoparticles. Appl. Surf. Sci. 2017, 416, 656–665. [Google Scholar] [CrossRef]
- Khan, I.; Zada, N.; Khan, I.; Sadiq, M.; Saeed, K. Enhancement of photocatalytic potential and recoverability of Fe3O4 nanoparticles by decorating over monoclinic zirconia. J. Environ. Heal. Sci. Eng. 2020, 18, 1473–1489. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, N.; He, M.; Chen, B.; Hu, B. Simultaneous speciation analysis of inorganic arsenic, chromium and selenium in environmental waters by 3-(2-aminoethylamino) propyltrimethoxysilane modified multi-wall carbon nanotubes packed microcolumn solid phase extraction and ICP-MS. Talanta 2015, 131, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.-Z.; Mei, B.; Zhou, S.-X.; Shi, Z.-G.; Feng, Y.-Q.; Wu, J.-Y.; Yan, G.-P.; Li, L. Synthesis, characterization and application of magnetic-zirconia nanocomposites. J. Non-Crystalline Solids 2009, 355, 922–925. [Google Scholar] [CrossRef]
- Tran, H.V.; Bui, L.T.; Dinh, T.T.; Le, D.H.; Huynh, C.D.; Trinh, A.X. Graphene oxide/Fe3O4/chitosan nanocomposite: A recoverable and recyclable adsorbent for organic dyes removal. Application to methylene blue. Mater. Res. Express 2017, 4, 035701. [Google Scholar] [CrossRef]
- Noormohamadi, A.; Homayoonfal, M.; Mehrnia, M.R.; Davar, F. Synergistic effect of concurrent presence of zirconium oxide and iron oxide in the form of core-shell nanoparticles on the performance of Fe3O4@ZrO2 /PAN nanocomposite membrane. Ceram. Int. 2017, 43, 17174–17185. [Google Scholar] [CrossRef]
- Shah, B.A.; Shah, A.V.; Singh, R.R. Sorption isotherms and kinetics of chromium uptake from wastewater using natural sorbent material. Int. J. Environ. Sci. Technol. 2008, 6, 77–90. [Google Scholar] [CrossRef]
- Covelo, E.F.; Couce, M.L.A.; Vega, F.A. Competitive adsorption and desorption of cadmium, chromium, copper, nickel, lead, and zinc by humic umbrisols. Commun. Soil Sci. Plant Anal. 2004, 35, 2709–2729. [Google Scholar] [CrossRef]
- Zou, J.; Liu, X.; Zhang, D.; Yuan, X. Adsorption of three bivalent metals by four chemical distinct microplastics. Chemosphere 2020, 248, 126064. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.; Dai, C.; Hu, Y.; Kubicki, J.D.; Kabengi, N. Adsorption Study of Al3+, Cr3+, and Mn2+onto Quartz and Corundum using Flow Microcalorimetry, Quartz Crystal Microbalance, and Density Functional Theory. ACS Earth Space Chem. 2019, 3, 432–441. [Google Scholar] [CrossRef]
- Chaba, J.M.; Nomngongo, P.N. Preparation of V2O5-ZnO coated carbon nanofibers: Application for removal of selected antibiotics in environmental matrices. J. Water Process. Eng. 2018, 23, 50–60. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Loganathan, P.; Nguyen, T.V.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies. Chem. Eng. J. 2015, 270, 393–404. [Google Scholar] [CrossRef]
- Adeli, M.; Yamini, Y.; Faraji, M. Removal of copper, nickel and zinc by sodium dodecyl sulphate coated magnetite nanoparticles from water and wastewater samples. Arab. J. Chem. 2017, 10, S514–S521. [Google Scholar] [CrossRef]
- Wang, X.; Huang, K.; Chen, Y.; Liu, J.; Chen, S.; Cao, J.; Mei, S.; Zhou, Y.; Jing, T. Preparation of dumbbell manganese dioxide/gelatin composites and their application in the removal of lead and cadmium ions. J. Hazard. Mater. 2018, 350, 46–54. [Google Scholar] [CrossRef]
- Ates, A.; Akgül, G. Modification of natural zeolite with NaOH for removal of manganese in drinking water. Powder Technol. 2016, 287, 285–291. [Google Scholar] [CrossRef]
- Najafi, F.; Moradi, O.; Rajabi, M.; Asif, M.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Thermodynamics of the adsorption of nickel ions from aqueous phase using graphene oxide and glycine functionalized graphene oxide. J. Mol. Liq. 2015, 208, 106–113. [Google Scholar] [CrossRef]
- Ihsanullah; Al-Khaldi, F.A.; Abusharkh, B.; Khaled, M.; Atieh, M.A.; Nasser, M.; Laoui, T.; Saleh, T.; Agarwal, S.; Tyagi, I.; et al. Adsorptive removal of cadmium(II) ions from liquid phase using acid modified carbon-based adsorbents. J. Mol. Liq. 2015, 204, 255–263. [Google Scholar] [CrossRef]
- Hallajiqomi, M.; Eisazadeh, H. Adsorption of manganese ion using polyaniline and it’s nanocomposite: Kinetics and isotherm studies. J. Ind. Eng. Chem. 2017, 55, 191–197. [Google Scholar] [CrossRef]
- Madala, S.; Nadavala, S.K.; Vudagandla, S.; Boddu, V.M.; Abburi, K. Equilibrium, kinetics and thermodynamics of Cadmium (II) biosorption on to composite chitosan biosorbent. Arab. J. Chem. 2017, 10, S1883–S1893. [Google Scholar] [CrossRef]
- Brdar, M.; Sciban, M.; Kukic, D.; Dosenovic, T. Kinetic model for the sorption of copper ions onto sugar beet shreds. Chem. Ind. 2014, 68, 793–799. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Kooh, M.R.R.; Lim, L.B.L.; Dahri, M.K.; Lim, L.H.; Bandara, J.M.R.S. Azolla pinnata: An Efficient Low Cost Material for Removal of Methyl Violet 2B by Using Adsorption Method. Waste Biomass Valoriz. 2015, 6, 547–559. [Google Scholar] [CrossRef]
- Biata, N.R.; Jakavula, S.; Mashile, G.P.; Nqombolo, A.; Moutloali, R.M.; Nomngongo, P.N. Recovery of gold(III) and iridium(IV) using magnetic layered double hydroxide (Fe3O4/Mg-Al-LDH) nanocomposite: Equilibrium studies and application to real samples. Hydrometallurgy 2020, 197, 105447. [Google Scholar] [CrossRef]
- Riahi, K.; Chaabane, S.; Thayer, B. Ben A kinetic modeling study of phosphate adsorption onto Phoenix dactylifera L. date palm fibers in batch mode. J. Saudi Chem. Soc. 2017, 21, S143–S152. [Google Scholar] [CrossRef]
- Mpupa, A.; Nqombolo, A.; Mizaikoff, B.; Nomngongo, P.N. Enhanced Adsorptive Removal of β-Estradiol from Aqueous and Wastewater Samples by Magnetic Nano-Akaganeite: Adsorption Isotherms, Kinetics, and Mechanism. Processesdc 2020, 8, 1197. [Google Scholar] [CrossRef]
- Robati, D. Pseudo-second-order kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J. Nanostruct. Chem. 2013, 3, 55. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Zhang, J.; Liu, J.-F.; Chen, L.; Deng, Z.-L.; Han, M.-X.; Wei, X.-S.; Yu, A.-M.; Zhang, H.-L. Fe3O4@ZrO2 nanoparticles magnetic solid phase extraction coupled with flame atomic absorption spectrometry for chromium(III) speciation in environmental and biological samples. Appl. Surf. Sci. 2012, 258, 6772–6776. [Google Scholar] [CrossRef]





| Adsorption Capacity (mg g−1) | ||
|---|---|---|
| With Sonication | Without Sonication | |
| Cd | 8.97 ± 0.01 | 0.878 ± 0.005 |
| Cu | 9.40 ± 0.05 | 0.579 ± 0.003 |
| Mn | 9.19 ± 0.03 | 0.777 ± 0.005 |
| Ni | 9.87 ± 0.04 | 1.55 ± 0.02 |
| Langmuir | Freundlich | |
|---|---|---|
| Cadmium(Cd) | Qmax = 114 | KF = 79.7 |
| KL = 5.18 | n = 3 | |
| RL = 0.004–0.03 | ||
| R2 = 0.09984 | R2 = 0.9336 | |
| Cu | Qmax = 111 | KF = 78.3 |
| KL = 5.63 | n = 3 | |
| RL = 0.004–0.04 | ||
| R2 = 0.9994 | R2 = 0.9131 | |
| Ni | Qmax = 128 | KF = 75.6 |
| KL = 2.17 | n = 3 | |
| RL = 0.009−0.08 | ||
| R2 = 0.9961 | R2 = 0.9851 | |
| Mn | Qmax = 123 | KF = 93.1 |
| KL = 7.36 | n = 3 | |
| RL = 0.002−0.03 | ||
| R2 = 0.9993 | R2 = 0.8091 |
| Analytes | Adsorbent | Adsorption Capacity (mg/g) | pH | Reference |
|---|---|---|---|---|
| Cd and Cu | Iron-Coated Australian Zeolite | 3.7–7.6 | 6.5 | [45] |
| Cu and Ni | Sodium Dodecyl Sulphate Coated Magnetite Nanoparticles | 24.3 and 41.2 | 6 | [46] |
| Cd | Mno2/Gelatin Composites | 89.2 | 6 | [47] |
| Mn | Natural Zeolite (1), Natural Zeolite (1.5) | 66, 51.5 | 6 | [48] |
| Ni | Glycine Functionalized Graphene Oxide | 38.61 | 6 | [49] |
| Cd | acid modified carbon-based adsorbents | 1.22 to 2.02 | 7 | [50] |
| Mn | Polyaniline (PAB) Nanocomposite | 50.31 | 10 | [51] |
| Cd, Cu, Ni and Mn | Fe3O4-ZrO2@APS | 114, 111, 128 and 123 | 7 | Current Study |
| Equations | Parameters | Cd | Cu | Mn | Ni | |
|---|---|---|---|---|---|---|
| qe exp | 111 | 110 | 122 | 122 | ||
| Pseudo-first order | k1 (min−1) | 0.0629 | 0.0563 | 0.0778 | 0.0820 | |
| qe (mg g−1) | 46.4 | 52.7 | 101 | 89.5 | ||
| R2 | 0.7854 | 0.8214 | 0.9267 | 0.8314 | ||
| k2 (g mg−1 min−1) | 0.436 | 0.786 | 0.650 | 0.105 | ||
| Pseudo-second order | qe (mg g−1) | 110 | 108 | 121 | 123 | |
| R2 | 0.9912 | 0.9955 | 0.9832 | 0.9822 | ||
| kid1 (mg g−1 min1/2) | 26.2 | 21.2 | 25.2 | 26.0 | ||
| C1 (mg g−1) | 13.1 | 18.6 | 17.3 | 18.2 | ||
| Intraparticle diffusion | R12 | 0.9996 | 0.9867 | 9894 | 9898 | |
| kid2 (mg g−1 min1/2) | 0.263 | 0.286 | 1.17 | 0.0190 | ||
| C2 (mg g−1) | 107 | 105 | 118 | 120 | ||
| R22 | 0.8690 | 0.8896 | 0.8319 | 0.7631 | ||
| Slope | 0.134 | 0.110 | 0.122 | 0.150 | ||
| Boyd model | Intercept | 0.671 | 0.547 | 0.923 | 1.16 | |
| R2 | 0.9768 | 0.9921 | 0.9679 | 0.9455 |
| Analytes | Parameter | Samples | ||||
|---|---|---|---|---|---|---|
| AMDE1 | AMDE1 | RW | WW1 | WW2 | ||
| Cd | Initial concentration (µg/L) | 0.61 ± 0.02 | ND | ND | 0.29 ± 0.04 | 0.23 ± 0.01 |
| Concentration after adsorption(µg/L) | ND | ND | ND | ND | ND | |
| %RE | 100 | 100 | 100 | |||
| Cu | Initial concentration (mg/L) | 4.82 ± 0.06 | 3.45 ± 0.04 | 1.73 ± 0.03 | 2.07 ± 0.03 | 4.79 ± 0.05 |
| Concentration after adsorption(mg/L) | ND | ND | ND | ND | ND | |
| %RE | 100 | 100 | 100 | 100 | 100 | |
| Mn | Initial concentration (mg/L) | 33.2 ± 1.3 | 22.9 ± 1.2 | 1.27 ± 0.09 | 7.28 ± 0.11 | 15.8 ± 0.9 |
| Concentration after adsorption (mg/L) | 2.17 ± 0.02 | 1.61 ± 0.03 | ND | ND | 0.16 ± 0.02 | |
| %RE | 93.4 ± 2.1 | 92.9 ± 1.6 | 100 | 100 | 98.9 ± 1.7 | |
| Ni | Initial concentration (µg/L) | 72.6 ± 0.3 | 83.4 ± 0.1 | 8.92 ± 0.05 | 4.52 ± 0.06 | 91.3 ± 0.7 |
| Concentration after adsorption (µg/L) | ND | ND | ND | ND | ND | |
| %RE | 100 | 100 | 100 | 100 | 100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugushe, A.S.; Mpupa, A.; Munonde, T.S.; Nyaba, L.; Nomngongo, P.N. Adsorptive Removal of Cd, Cu, Ni and Mn from Environmental Samples Using Fe3O4-Zro2@APS Nanocomposite: Kinetic and Equilibrium Isotherm Studies. Molecules 2021, 26, 3209. https://doi.org/10.3390/molecules26113209
Gugushe AS, Mpupa A, Munonde TS, Nyaba L, Nomngongo PN. Adsorptive Removal of Cd, Cu, Ni and Mn from Environmental Samples Using Fe3O4-Zro2@APS Nanocomposite: Kinetic and Equilibrium Isotherm Studies. Molecules. 2021; 26(11):3209. https://doi.org/10.3390/molecules26113209
Chicago/Turabian StyleGugushe, Aphiwe Siyasanga, Anele Mpupa, Tshimangadzo Saddam Munonde, Luthando Nyaba, and Philiswa Nosizo Nomngongo. 2021. "Adsorptive Removal of Cd, Cu, Ni and Mn from Environmental Samples Using Fe3O4-Zro2@APS Nanocomposite: Kinetic and Equilibrium Isotherm Studies" Molecules 26, no. 11: 3209. https://doi.org/10.3390/molecules26113209
APA StyleGugushe, A. S., Mpupa, A., Munonde, T. S., Nyaba, L., & Nomngongo, P. N. (2021). Adsorptive Removal of Cd, Cu, Ni and Mn from Environmental Samples Using Fe3O4-Zro2@APS Nanocomposite: Kinetic and Equilibrium Isotherm Studies. Molecules, 26(11), 3209. https://doi.org/10.3390/molecules26113209







