Advanced Materials Based on Nanosized Hydroxyapatite
Abstract
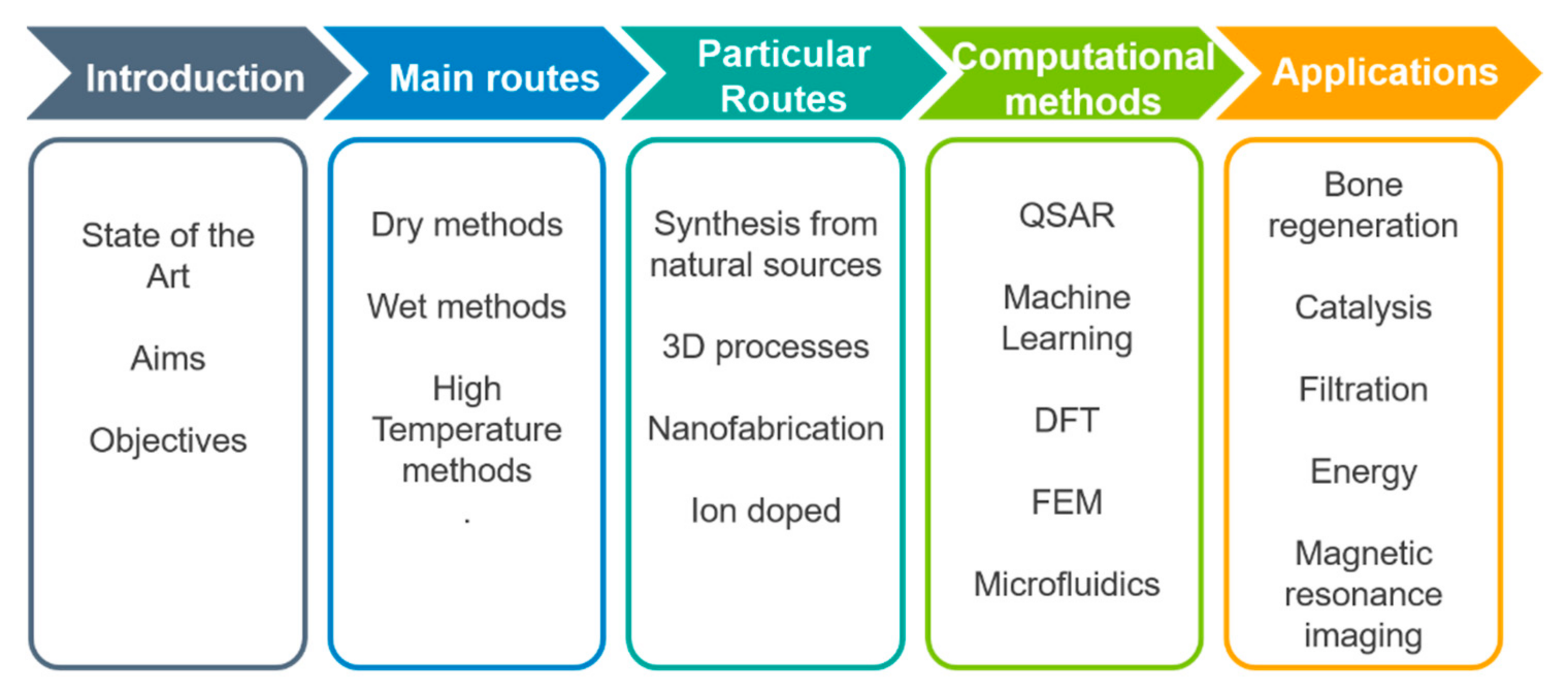
1. Introduction
2. Main Routes of Hydroxyapatite Production
3. Particular Routes of Hydroxyapatite Synthesis
4. Application of Modern Computational Methods in HAP Based Materials
5. Applications of Smart Hydroxyapatite-Based Materials
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Hecht, E. An historico-critical account of potential energy: Is PE really real? Phys. Teach. 2003, 41, 486–493. [Google Scholar] [CrossRef]
- Fratzl, P. Biomimetic materials research: What can we really learn from nature’s structural materials? J. R. Soc. Interface 2007, 4, 637–642. [Google Scholar] [CrossRef]
- McShea, D.W. The hierarchical structure of organisms: A scale and documentation of a trend in the maximum. Paleobiology 2001, 27, 405–423. [Google Scholar] [CrossRef]
- Vallet-Regi, M. Bio-Ceramics with Clinical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Colilla, M.; Manzano, M.; Vallet-Regí, M. Recent advances in ceramic implants as drug delivery systems for biomedical applications. Int. J. Nanomed. 2008, 3, 403–414. [Google Scholar] [CrossRef]
- Hidouri, M.; Dorozhkin, S.V.; Albeladi, N. Thermal behavior, sintering and mechanical characterization of multiple ion-substituted hydroxyapatite bioceramics. J. Inorg. Organomet. Polym. Mater. 2019, 29, 87–100. [Google Scholar] [CrossRef]
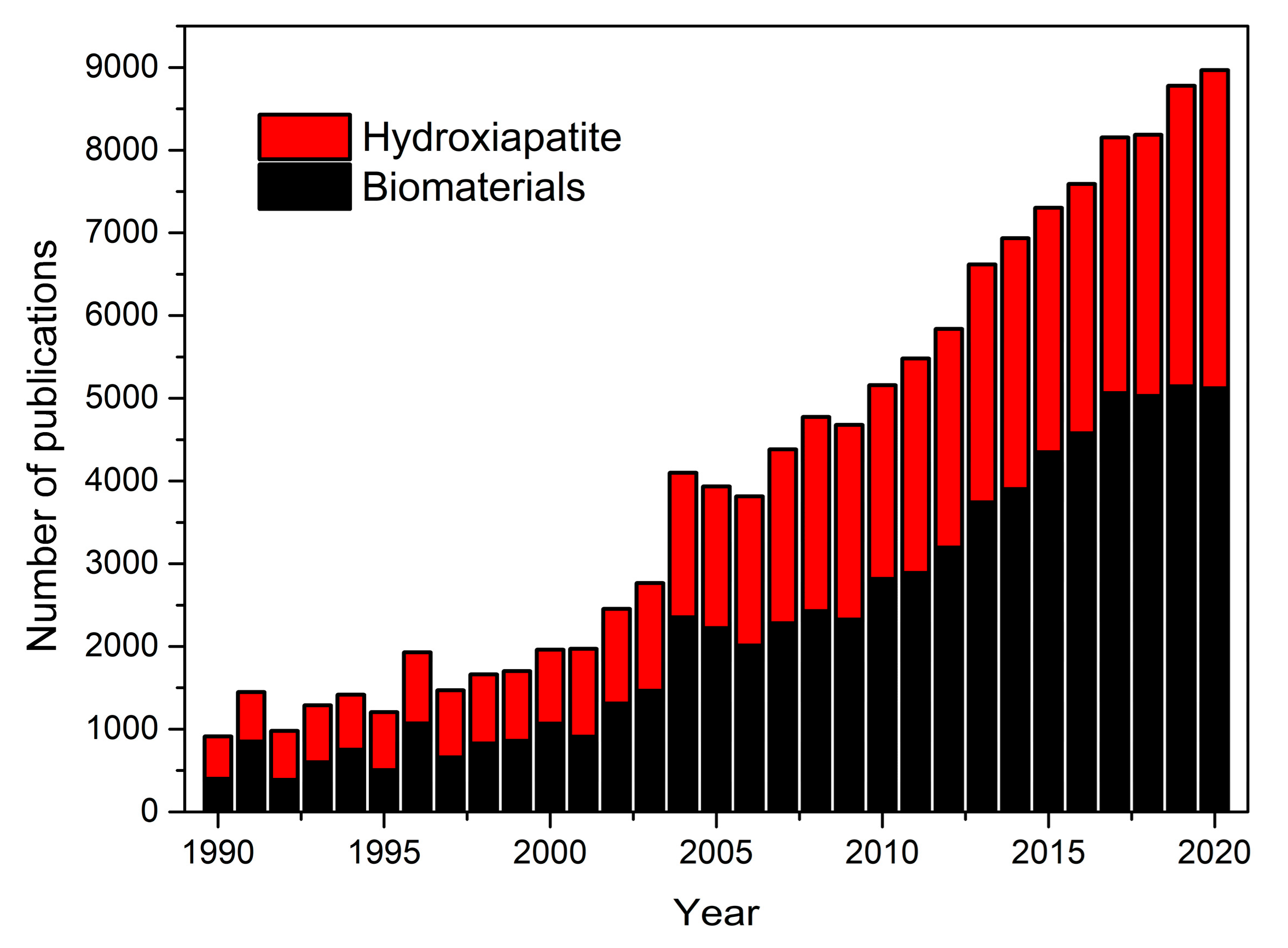
- Larsen, P.; Von Ins, M. The rate of growth in scientific publication and the decline in coverage provided by Science Citation Index. Scientometrics 2010, 84, 575–603. [Google Scholar] [CrossRef]
- Boom time for biomaterials. Nat. Mater. 2009, 8, 439. [CrossRef] [PubMed]
- Ripamonti, U. Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials 1996, 17, 31–35. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Dummer, P.M.H. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int. Endod. J. 2011, 44, 697–730. [Google Scholar] [CrossRef] [PubMed]
- Meleshko, A.A.; Tolstoy, V.P.; Afinogenov, G.E.; Levshakova, A.S.; Afinogenova, A.G.; Muldiyarov, V.P.; Vissarionov, S.V.; Linnik, S.A. Prospects of hydroxyapatite-based nanomaterials application synthesized by layer-by-layer method for pediatric traumatology and orthopedics. Probl. Endocrinol. 2020, 8, 217–230. [Google Scholar] [CrossRef]
- Sobti, M.M.; Shams, F.; Jawaheer, L.; Cauchi, P.; Chadha, V. Unwrapped hydroxyapatite orbital implants: Our experience in 347 cases. Eye 2020, 34, 675–682. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Fabrication, Properties and Applications of Dense Hydroxyapatite: A Review. J. Funct. Biomater. 2015, 6, 1099–1140. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Zhan, Y.; Zheng, C.; Wang, G. A simple route to hydroxyapatite nanofibers. Mater. Lett. 2002, 56, 496–501. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Zhu, Y.-J.; Wu, J.; Shao, Y.-T.; Cai, A.-Y.; Dong, L.-Y. Ultralong Hydroxyapatite Nanowire-Based Filter Paper for High-Performance Water Purification. ACS Appl. Mater. Interfaces 2019, 11, 4288–4301. [Google Scholar] [CrossRef] [PubMed]
- Orlovskii, V.; Komlev, V.; Barinov, S. Hydroxyapatite and hydroxyapatite-based ceramics. Inorg. Mater. 2002, 38, 973–984. [Google Scholar] [CrossRef]
- Zhao, Z.; Espanol, M.; Guillem-Marti, J.; Kempf, D.; Diez-Escudero, A.; Ginebra, M.-P. Ion-doping as a strategy to modulate hydroxyapatite nanoparticle internalization. Nanoscale 2016, 8, 1595–1607. [Google Scholar] [CrossRef]
- Zeitz, C.; Faidt, T.; Grandthyll, S.; Hähl, H.; Thewes, N.; Spengler, C.; Schmauch, J.R.; Deckarm, M.J.; Gachot, C.; Natter, H. Synthesis of hydroxyapatite substrates: Bridging the gap between model surfaces and enamel. ACS Appl. Mater. Interfaces 2016, 8, 25848–25855. [Google Scholar] [CrossRef]
- Ramesh, S.; Tan, C.; Tolouei, R.; Amiriyan, M.; Purbolaksono, J.; Sopyan, I.; Teng, W. Sintering behavior of hydroxyapatite prepared from different routes. Mater. Des. 2012, 34, 148–154. [Google Scholar] [CrossRef]
- Liu, D.-M.; Troczynski, T.; Tseng, W.J. Water-based sol–gel synthesis of hydroxyapatite: Process development. Biomaterials 2001, 22, 1721–1730. [Google Scholar] [CrossRef]
- Mahabole, M.; Aiyer, R.; Ramakrishna, C.; Sreedhar, B.; Khairnar, R. Synthesis, characterization and gas sensing property of hydroxyapatite ceramic. Bull. Mater. Sci. 2005, 28, 535–545. [Google Scholar] [CrossRef]
- Pu’ad, N.M.; Haq, R.A.; Noh, H.M.; Abdullah, H.; Idris, M.; Lee, T. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar]
- Cox, S.C.; Walton, R.I.; Mallick, K.K. Comparison of techniques for the synthesis of hydroxyapatite. Bioinspired Biomim. Nanobiomater. 2015, 4, 37–47. [Google Scholar] [CrossRef]
- Rhee, S.-H. Synthesis of hydroxyapatite via mechanochemical treatment. Biomaterials 2002, 23, 1147–1152. [Google Scholar] [CrossRef]
- Yeong, K.; Wang, J.; Ng, S. Mechanochemical synthesis of nanocrystalline hydroxyapatite from CaO and CaHPO4. Biomaterials 2001, 22, 2705–2712. [Google Scholar] [CrossRef]
- Liu, D.-M.; Yang, Q.; Troczynski, T.; Tseng, W.J. Structural evolution of sol–gel-derived hydroxyapatite. Biomaterials 2002, 23, 1679–1687. [Google Scholar] [CrossRef]
- Hsieh, M.-F.; Chin, T.-S.; Perng, L.-H.; Perng, H.-G. Gel-to-Ceramic Conversion during Hydroxyapatite Synthesis. J. Am. Ceram. Soc. 2001, 84, 2123–2125. [Google Scholar] [CrossRef]
- Seckler, M.; Danese, M.; Derenzo, S.; Valarelli, J.; Giulietti, M.; Rodríguez-Clemente, R. Influence of process conditions on hydroxyapatite crystallinity obtained by direct crystallization. Mater. Res. 1999, 2, 59–62. [Google Scholar] [CrossRef]
- Weng, W.; Baptista, J. A new synthesis of hydroxyapatite. J. Eur. Ceram. Soc. 1997, 17, 1151–1156. [Google Scholar] [CrossRef]
- Velayudhan, S.; Ramesh, P.; Sunny, M.; Varma, H. Extrusion of hydroxyapatite to clinically significant shapes. Mater. Lett. 2000, 46, 142–146. [Google Scholar] [CrossRef]
- Rodríguez-Lugo, V.; Karthik, T.; Mendoza-Anaya, D.; Rubio-Rosas, E.; Villaseñor Cerón, L.; Reyes-Valderrama, M.; Salinas-Rodríguez, E. Wet chemical synthesis of nanocrystalline hydroxyapatite flakes: Effect of pH and sintering temperature on structural and morphological properties. R. Soc. Open Sci. 2018, 5, 180962. [Google Scholar] [CrossRef]
- Yelten-Yilmaz, A.; Yilmaz, S. Wet chemical precipitation synthesis of hydroxyapatite (HA) powders. Ceram. Int. 2018, 44, 9703–9710. [Google Scholar] [CrossRef]
- Ramesh, S.; Adzila, S.; Tan, C. Properties of hydroxyapatite synthesize by wet chemical method. J. Ceram. Process. Res. 2013, 14, 448–452. [Google Scholar]
- Chen, F.; Zhu, Y.-J. Large-Scale Automated Production of Highly Ordered Ultralong Hydroxyapatite Nanowires and Construction of Various Fire-Resistant Flexible Ordered Architectures. ACS Nano 2016, 10, 11483–11495. [Google Scholar] [CrossRef]
- Gomes, D.; Santos, A.; Neves, G.; Menezes, R. A brief review on hydroxyapatite production and use in biomedicine. Cerâmica 2019, 65, 282–302. [Google Scholar] [CrossRef]
- Majumdar, S.; Genant, H.; Grampp, S.; Newitt, D.; Truong, V.H.; Lin, J.; Mathur, A. Correlation of trabecular bone structure with age, bone mineral density, and osteoporotic status: In vivo studies in the distal radius using high resolution magnetic resonance imaging. J. Bone Mineral. Res. 1997, 12, 111–118. [Google Scholar] [CrossRef]
- Vogler, J., 3rd; Murphy, W. Bone marrow imaging. Radiology 1988, 168, 679–693. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.-Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
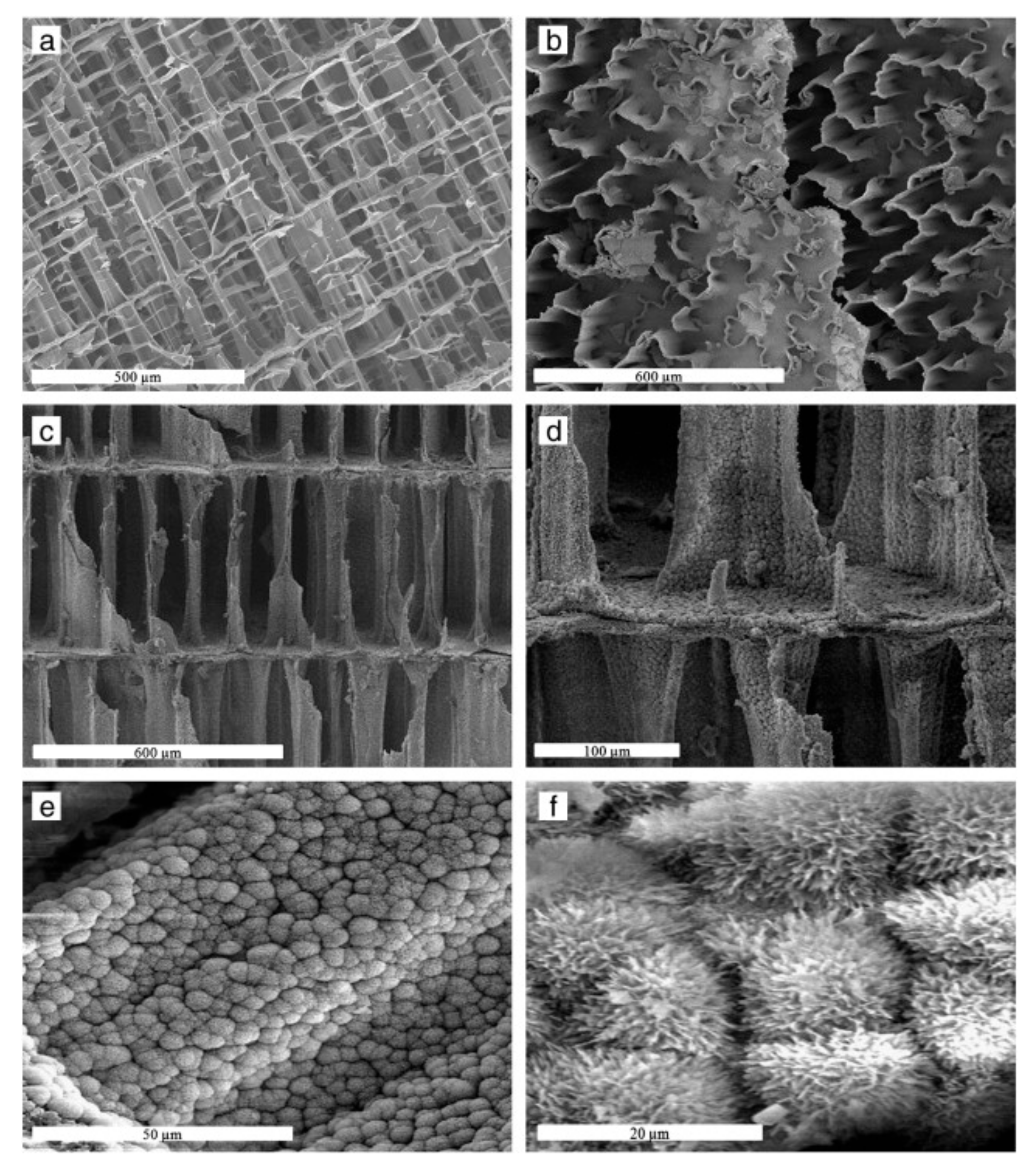
- Tadic, D.; Beckmann, F.; Schwarz, K.; Epple, M. A novel method to produce hydroxyapatite objects with interconnecting porosity that avoids sintering. Biomaterials 2004, 25, 3335–3340. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Myoui, A. Bone tissue engineering with porous hydroxyapatite ceramics. J. Artif. Organs 2005, 8, 131–136. [Google Scholar] [CrossRef]
- Sopyan, I.; Mel, M.; Ramesh, S.; Khalid, K. Porous hydroxyapatite for artificial bone applications. Sci. Technol. Adv. Mater. 2007, 8, 116. [Google Scholar] [CrossRef]
- Nie, W.; Peng, C.; Zhou, X.; Chen, L.; Wang, W.; Zhang, Y.; Ma, P.X.; He, C. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon 2017, 116, 325–337. [Google Scholar] [CrossRef]
- Türk, S.; Altınsoy, I.; Efe, G.Ç.; İpek, M.; Özacar, M.; Bindal, C. 3D porous collagen/functionalized multiwalled carbon nanotube/chitosan/hydroxyapatite composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2018, 92, 757–768. [Google Scholar] [CrossRef]
- Le Huec, J.; Schaeverbeke, T.; Clement, D.; Faber, J.; Le Rebeller, A. Influence of porosity on the mechanical resistance of hydroxyapatite ceramics under compressive stress. Biomaterials 1995, 16, 113–118. [Google Scholar] [CrossRef]
- Chang, B.-S.; Hong, K.-S.; Youn, H.-J.; Ryu, H.-S.; Chung, S.-S.; Park, K.-W. Osteoconduction at porous hydroxyapatite with various pore configurations. Biomaterials 2000, 21, 1291–1298. [Google Scholar] [CrossRef]
- Itoh, S.; Nakamura, S.; Nakamura, M.; Shinomiya, K.; Yamashita, K. Enhanced bone ingrowth into hydroxyapatite with interconnected pores by Electrical Polarization. Biomaterials 2006, 27, 5572–5579. [Google Scholar] [CrossRef] [PubMed]
- Korkusuz, F.; Karamete, K.; Irfanoğlu, B.; Yetkin, H.; Hastings, G.W.; Akkas, N. Do porous calcium hydroxyapatite ceramics cause porosis in bone? A bone densitometry and biomechanical study on cortical bones of rabbits. Biomaterials 1995, 16, 537–543. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Arita, I.H.; Wilkinson, D.S.; Mondragón, M.A.; Castaño, V.M. Chemistry and sintering behaviour of thin hydroxyapatite ceramics with controlled porosity. Biomaterials 1995, 16, 403–408. [Google Scholar] [CrossRef]
- Klein, C.; de Groot, K.; Chen, W.; Li, Y.; Zhang, X. Osseous substance formation induced in porous calcium phosphate ceramics in soft tissues. Biomaterials 1994, 15, 31–34. [Google Scholar] [CrossRef]
- Ozgür Engin, N.; Tas, A.C. Manufacture of macroporous calcium hydroxyapatite bioceramics. J. Eur. Ceram. Soc. 1999, 19, 2569–2572. [Google Scholar] [CrossRef]
- Milovac, D.; Gallego Ferrer, G.; Ivankovic, M.; Ivankovic, H. PCL-coated hydroxyapatite scaffold derived from cuttlefish bone: Morphology, mechanical properties and bioactivity. Mater. Sci. Eng. C 2014, 34, 437–445. [Google Scholar] [CrossRef]
- Pal, A.; Maity, S.; Chabri, S.; Bera, S.; Chowdhury, A.R.; Das, M.; Sinha, A. Mechanochemical synthesis of nanocrystalline hydroxyapatite from Mercenaria clam shells and phosphoric acid. Biomed. Phys. Eng. Express 2017, 3, 015010. [Google Scholar] [CrossRef]
- Goloshchapov, D.L.; Kashkarov, V.M.; Rumyantseva, N.A.; Seredin, P.V.; Lenshin, A.S.; Agapov, B.L.; Domashevskaya, E.P. Synthesis of nanocrystalline hydroxyapatite by precipitation using hen’s eggshell. Ceram. Int. 2013, 39, 4539–4549. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Salvatore, L.; Gervaso, F.; Catalano, M.; Taurino, A.; Sannino, A.; Licciulli, A. Synthesis and characterization of collagen scaffolds reinforced by eggshell derived hydroxyapatite for tissue engineering. J. Nanosci. Nanotechnol. 2015, 15, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Ji, X.; Banks, C.E.; Song, J. Flower-like agglomerates of hydroxyapatite crystals formed on an egg-shell membrane. Colloids Surf. B Biointerfaces 2011, 82, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Heidari, F.; Bahrololoom, M.E.; Vashaee, D.; Tayebi, L. In situ preparation of iron oxide nanoparticles in natural hydroxyapatite/chitosan matrix for bone tissue engineering application. Ceram. Int. 2015, 41, 3094–3100. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Aghaie, E.; Nadernezhad, A.; Zargarzadeh, M.; Khakzad, A.; Shakeri, M.; Khosrowshahi, Y.B.; Siadati, M. Influence of Fe3O4 nanoparticles in hydroxyapatite scaffolds on proliferation of primary human fibroblast cells. J. Mater. Eng. Perform. 2016, 25, 2331–2339. [Google Scholar] [CrossRef]
- Ding, G.-J.; Zhu, Y.-J.; Qi, C.; Lu, B.-Q.; Chen, F.; Wu, J. Porous hollow microspheres of amorphous calcium phosphate: Soybean lecithin templated microwave-assisted hydrothermal synthesis and application in drug delivery. J. Mater. Chem. B 2015, 3, 1823–1830. [Google Scholar] [CrossRef]
- Gopi, D.; Kanimozhi, K.; Kavitha, L. Opuntia ficus indica peel derived pectin mediated hydroxyapatite nanoparticles: Synthesis, spectral characterization, biological and antimicrobial activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 141, 135–143. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Zhao, W.; Sahai, N. A potential mechanism for amino acid-controlled crystal growth of hydroxyapatite. J. Mater. Chem. B 2015, 3, 9157–9167. [Google Scholar] [CrossRef]
- Jiang, S.; Cao, Y.; Li, S.; Pang, Y.; Sun, Z. Dual function of poly(acrylic acid) on controlling amorphous mediated hydroxyapatite crystallization. J. Cryst. Growth 2021, 557, 125991. [Google Scholar] [CrossRef]
- Fernández-Leyes, M.; Verdinelli, V.; Hassan, N.; Ruso, J.M.; Pieroni, O.; Schulz, P.C.; Messina, P. Biomimetic formation of crystalline bone-like apatite layers on spongy materials templated by bile salts aggregates. J. Mater. Sci. 2012, 47, 2837–2844. [Google Scholar] [CrossRef]
- Nie, H.; Wang, C.-H. Fabrication and characterization of PLGA/HAp composite scaffolds for delivery of BMP-2 plasmid DNA. J. Control. Release 2007, 120, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Li, X.; Chen, J.; Zhou, S.; Weng, J. In Situ Growth Kinetics of Hydroxyapatite on Electrospun Poly(dl-lactide) Fibers with Gelatin Grafted. Cryst. Growth Des. 2008, 8, 4576–4582. [Google Scholar] [CrossRef]
- Yao, Y.; Qin, W.; Xing, B.; Sha, N.; Jiao, T.; Zhao, Z. High performance hydroxyapatite ceramics and a triply periodic minimum surface structure fabricated by digital light processing 3D printing. J. Adv. Ceram. 2021, 10, 39–48. [Google Scholar] [CrossRef]
- Li, S.H.; de Wijn, J.R.; Layrolle, P.; de Groot, K. Novel method to manufacture porous hydroxyapatite by dual-phase mixing. J. Am. Ceram. Soc. 2003, 86, 65–72. [Google Scholar] [CrossRef]
- Lakrat, M.; Jabri, M.; Alves, M.; Fernandes, M.H.; Ansari, L.L.; Santos, C.; Mejdoubi, E.M. Three-dimensional nano-hydroxyapatite sodium silicate glass composite scaffold for bone tissue engineering-A new fabrication process at a near-room temperature. Mater. Chem. Phys. 2021, 260, 124185. [Google Scholar] [CrossRef]
- Kermani, F.; Gharavian, A.; Mollazadeh, S.; Kargozar, S.; Youssefi, A.; Khaki, J.V. Silicon-doped calcium phosphates; the critical effect of synthesis routes on the biological performance. Mater. Sci. Eng. C 2020, 111, 110828. [Google Scholar] [CrossRef]
- Lim, G.; Wang, J.; Ng, S.; Chew, C.; Gan, L. Processing of hydroxyapatite via microemulsion and emulsion routes. Biomaterials 1997, 18, 1433–1439. [Google Scholar] [CrossRef]
- Rathje, W. Zur Kenntnis der Phosphate I: Über Hydroxylapatit. Bodenkd. Pflanz. 1939, 12, 121–128. [Google Scholar] [CrossRef]
- Miculescu, F.; Mocanu, A.-C.; Dascălu, C.A.; Maidaniuc, A.; Batalu, D.; Berbecaru, A.; Voicu, S.I.; Miculescu, M.; Thakur, V.K.; Ciocan, L.T. Facile synthesis and characterization of hydroxyapatite particles for high value nanocomposites and biomaterials. Vacuum 2017, 146, 614–622. [Google Scholar] [CrossRef]
- Mitran, V.; Ion, R.; Miculescu, F.; Necula, M.G.; Mocanu, A.-C.; Stan, G.E.; Antoniac, I.V.; Cimpean, A. Osteoblast Cell Response to Naturally Derived Calcium Phosphate-Based Materials. Materials 2018, 11, 1097. [Google Scholar] [CrossRef]
- Ucar, S.; Bjørnøy, S.H.; Bassett, D.C.; Strand, B.L.; Sikorski, P.; Andreassen, J.-P. Formation of hydroxyapatite via transformation of amorphous calcium phosphate in the presence of alginate additives. Cryst. Growth Des. 2019, 19, 7077–7087. [Google Scholar] [CrossRef]
- Sans, J.; Sanz, V.; Puiggalí, J.; Turon, P.; Alemán, C. Controlled Anisotropic Growth of Hydroxyapatite by Additive-Free Hydrothermal Synthesis. Cryst. Growth Des. 2020, 2, 748–756. [Google Scholar]
- González-Díaz, H.; Arrasate, S.; Sotomayor, N.; Lete, E.; R Munteanu, C.; Pazos, A.; Besada-Porto, L.; M Ruso, J. MIANN models in medicinal, physical and organic chemistry. Curr. Top. Med. Chem. 2013, 13, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Diaz, H.; Arrasate, S.; Gomez-San Juan, A.; Sotomayor, N.; Lete, E.; Speck-Planche, A.; M Ruso, J.; Luan, F.; Natalia Dias Soeiro Cordeiro, M. Matrix trace operators: From spectral moments of molecular graphs and complex networks to perturbations in synthetic reactions, micelle nanoparticles, and drug ADME processes. Curr. Drug Metab. 2014, 15, 470–488. [Google Scholar] [CrossRef]
- Messina, P.V.; Besada-Porto, J.M.; González-Díaz, H.; Ruso, J.M. Self-assembled binary nanoscale systems: Multioutput model with lfer-covariance perturbation theory and an experimental–computational study of nagdc-ddab micelles. Langmuir 2015, 31, 12009–12018. [Google Scholar] [CrossRef]
- Sabín, J.; Prieto, G.; González-Pérez, A.; Ruso, J.M.; Sarmiento, F. Effects of Fluorinated and Hydrogenated Surfactants on Human Serum Albumin at Different pHs. Biomacromolecules 2006, 7, 176–182. [Google Scholar] [CrossRef] [PubMed]
- González-Durruthy, M.; Scanavachi, G.; Rial, R.; Liu, Z.; Cordeiro, M.N.D.; Itri, R.; Ruso, J.M. Mapping the underlying mechanisms of fibrinogen benzothiazole drug interactions using computational and experimental approaches. Int. J. Biol. Macromol. 2020, 163, 730–744. [Google Scholar] [CrossRef]
- Ruso, J.M.; Taboada, P.; Varela, L.M.; Attwood, D.; Mosquera, V.C. Adsorption of an amphiphilic penicillin onto human serum albumin: Characterisation of the complex. Biophys. Chem. 2001, 92, 141–153. [Google Scholar] [CrossRef]
- González-Durruthy, M.; Rial, R.; Cordeiro, M.N.D.S.; Liu, Z.; Ruso, J.M. Exploring the conformational binding mechanism of fibrinogen induced by interactions with penicillin β-lactam antibiotic drugs. J. Mol. Liq. 2021, 324, 114667. [Google Scholar] [CrossRef]
- Shang, S.; Zhao, Q.; Zhang, D.; Sun, R.; Tang, Y. Molecular dynamics simulation of the adsorption behavior of two different drugs on hydroxyapatite and Zn-doped hydroxyapatite. Mater. Sci. Eng. C 2019, 105, 110017. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Lam, J.S.L.; Savalani, M. A new computational intelligence approach in formulation of functional relationship of open porosity of the additive manufacturing process. Int. J. Adv. Manuf. Technol. 2015, 80, 555–565. [Google Scholar] [CrossRef]
- Okafor, E.; Obada, D.O.; Dodoo-Arhin, D. Ensemble learning prediction of transmittance at different wavenumbers in natural hydroxyapatite. Sci. Afr. 2020, 9, e00516. [Google Scholar]
- Yu, J.; Wang, Y.; Dai, Z.; Yang, F.; Fallahpour, A.; Nasiri-Tabrizi, B. Structural features modeling of substituted hydroxyapatite nanopowders as bone fillers via machine learning. Ceram. Int. 2021, 47, 9034–9047. [Google Scholar] [CrossRef]
- Hou, Y.; Morrison, C.J.; Cramer, S.M. Classification of protein binding in hydroxyapatite chromatography: Synergistic interactions on the molecular scale. Anal. Chem. 2011, 83, 3709–3716. [Google Scholar] [CrossRef]
- Avakyan, L.A.; Paramonova, E.V.; Coutinho, J.; Öberg, S.; Bystrov, V.S.; Bugaev, L.A. Optoelectronics and defect levels in hydroxyapatite by first-principles. J. Chem. Phys. 2018, 148, 154706. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, P. Surface energetics of hydroxyapatite: A DFT study. Chem. Phys. Lett. 2004, 396, 38–42. [Google Scholar] [CrossRef]
- Zilm, M.; Chen, L.; Sharma, V.; McDannald, A.; Jain, M.; Ramprasad, R.; Wei, M. Hydroxyapatite substituted by transition metals: Experiment and theory. Phys. Chem. Chem. Phys. 2016, 18, 16457–16465. [Google Scholar] [CrossRef]
- Gafurov, M.; Biktagirov, T.; Mamin, G.; Klimashina, E.; Putlayev, V.; Kuznetsova, L.; Orlinskii, S. The Interplay of manganese and nitrate in hydroxyapatite nanoparticles as revealed by pulsed EPR and DFT. Phys. Chem. Chem. Phys. 2015, 17, 20331–20337. [Google Scholar] [CrossRef]
- Kebiroglu, M.H.; Orek, C.; Bulut, N.; Kaygili, O.; Keser, S.; Ates, T. Temperature dependent structural and vibrational properties of hydroxyapatite: A theoretical and experimental study. Ceram. Int. 2017, 43, 15899–15904. [Google Scholar] [CrossRef]
- Palazzo, B.; Sidoti, M.C.; Roveri, N.; Tampieri, A.; Sandri, M.; Bertolazzi, L.; Galbusera, F.; Dubini, G.; Vena, P.; Contro, R. Controlled drug delivery from porous hydroxyapatite grafts: An experimental and theoretical approach. Mater. Sci. Eng. C 2005, 25, 207–213. [Google Scholar] [CrossRef]
- Iafisco, M.; Palazzo, B.; Marchetti, M.; Margiotta, N.; Ostuni, R.; Natile, G.; Morpurgo, M.; Gandin, V.; Marzano, C.; Roveri, N. Smart delivery of antitumoral platinum complexes from biomimetic hydroxyapatite nanocrystals. J. Mater. Chem. 2009, 19, 8385–8392. [Google Scholar] [CrossRef]
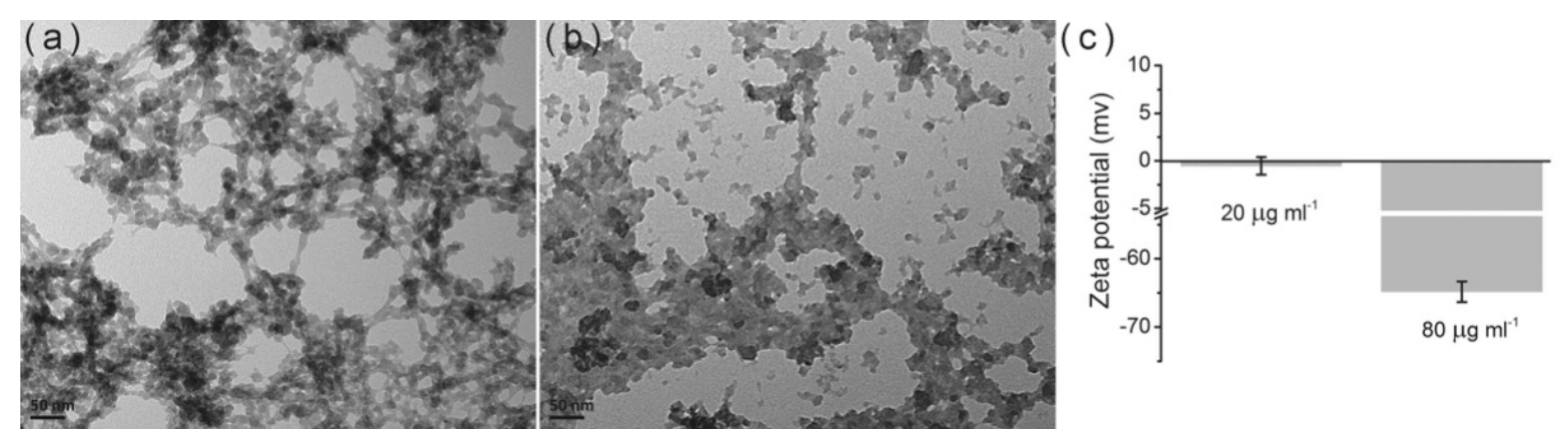
- Rial, R.; Tahoces, P.G.; Hassan, N.; Cordero, M.; Liu, Z.; Ruso, J.M. Noble microfluidic system for bioceramic nanoparticles engineering. Mater. Sci. Eng. C 2019, 102, 221–227. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, J.-X.; Shao, L.; Wang, Q.-A.; Guo, F.; Chen, J.-F.; Gu, L.; An, Y.-T. High throughput methodology for continuous preparation of hydroxyapatite nanoparticles in a microporous tube-in-tube microchannel reactor. Ind. Eng. Chem. Res. 2010, 49, 140–147. [Google Scholar] [CrossRef]
- Ahn, J.; Lim, J.; Jusoh, N.; Lee, J.; Park, T.-E.; Kim, Y.; Kim, J.; Jeon, N.L. 3D microfluidic bone tumor microenvironment comprised of hydroxyapatite/fibrin composite. Front. Bioeng. Biotechnol. 2019, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- LiáJeon, N. Microfluidic vascularized bone tissue model with hydroxyapatite-incorporated extracellular matrix. Lab. A Chip 2015, 15, 3984–3988. [Google Scholar]
- Boken, J.; Soni, S.K.; Kumar, D. Microfluidic synthesis of nanoparticles and their biosensing applications. Crit. Rev. Anal. Chem. 2016, 46, 538–561. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.; Ma, Y.; Sun, J. Microfluidic methods for fabrication and engineering of nanoparticle drug delivery systems. ACS Appl. Bio Mater. 2019, 3, 107–120. [Google Scholar] [CrossRef]
- Mendiratta, S.; Ali, A.A.A.; Hejazi, S.H.; Gates, I. Dual Stimuli-Responsive Pickering Emulsions from Novel Magnetic Hydroxyapatite Nanoparticles and Their Characterization Using a Microfluidic Platform. Langmuir 2021, 37, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Hasani-Sadrabadi, M.M.; Hajrezaei, S.P.; Emami, S.H.; Bahlakeh, G.; Daneshmandi, L.; Dashtimoghadam, E.; Seyedjafari, E.; Jacob, K.I.; Tayebi, L. Enhanced osteogenic differentiation of stem cells via microfluidics synthesized nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Wang, Z.; Wang, X.; Yang, W.; Wang, S.; Li, C.; Dai, H.; Tao, S. Sr2+ adsorbents produced by microfluidics. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126072. [Google Scholar] [CrossRef]
- Cheng, J.; Jun, Y.; Qin, J.; Lee, S.-H. Electrospinning versus microfluidic spinning of functional fibers for biomedical applications. Biomaterials 2017, 114, 121–143. [Google Scholar] [CrossRef]
- Castro, F.; Kuhn, S.; Jensen, K.; Ferreira, A.; Rocha, F.; Vicente, A.; Teixeira, J.A. Continuous-flow precipitation of hydroxyapatite in ultrasonic microsystems. Chem. Eng. J. 2013, 215, 979–987. [Google Scholar] [CrossRef]
- Moradikhah, F.; Doosti-Telgerd, M.; Shabani, I.; Soheili, S.; Dolatyar, B.; Seyedjafari, E. Microfluidic fabrication of alendronate-loaded chitosan nanoparticles for enhanced osteogenic differentiation of stem cells. Life Sci. 2020, 254, 117768. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Kuhn, S.; Jensen, K.; Ferreira, A.; Rocha, F.; Vicente, A.; Teixeira, J.A. Process intensification and optimization for hydroxyapatite nanoparticles production. Chem. Eng. Sci. 2013, 100, 352–359. [Google Scholar] [CrossRef]
- Wilson, O.C.; Hull, J.R. Surface modification of nanophase hydroxyapatite with chitosan. Mater. Sci. Eng. C 2008, 28, 434–437. [Google Scholar] [CrossRef]
- Palazzo, B.; Iafisco, M.; Laforgia, M.; Margiotta, N.; Natile, G.; Bianchi, C.L.; Walsh, D.; Mann, S.; Roveri, N. Biomimetic Hydroxyapatite–Drug Nanocrystals as Potential Bone Substitutes with Antitumor Drug Delivery Properties. Adv. Funct. Mater. 2007, 17, 2180–2188. [Google Scholar] [CrossRef]
- Laurencin, C.; Attawia, M.; Lu, L.; Borden, M.; Lu, H.; Gorum, W.; Lieberman, J. Poly (lactide-co-glycolide)/hydroxyapatite delivery of BMP-2-producing cells: A regional gene therapy approach to bone regeneration. Biomaterials 2001, 22, 1271–1277. [Google Scholar] [CrossRef]
- Ono, I.; Yamashita, T.; Jin, H.-Y.; Ito, Y.; Hamada, H.; Akasaka, Y.; Nakasu, M.; Ogawa, T.; Jimbow, K. Combination of porous hydroxyapatite and cationic liposomes as a vector for BMP-2 gene therapy. Biomaterials 2004, 25, 4709–4718. [Google Scholar] [CrossRef]
- D’Elía, N.L.; Gravina, A.N.; Ruso, J.M.; Laiuppa, J.A.; Santillán, G.E.; Messina, P.V. Manipulating the bioactivity of hydroxyapatite nano-rods structured networks: Effects on mineral coating morphology and growth kinetic. Biochim. Biophys. Acta (Bba) Gen. Subj. 2013, 1830, 5014–5026. [Google Scholar] [CrossRef]
- Sartuqui, J.; Gravina, A.N.; Rial, R.; Benedini, L.A.; Ruso, J.M.; Messina, P.V. Biomimetic fiber mesh scaffolds based on gelatin and hydroxyapatite nano-rods: Designing intrinsic skills to attain bone reparation abilities. Colloids Surf. B Biointerfaces 2016, 145, 382–391. [Google Scholar] [CrossRef]
- Uskoković, V. Ion-doped hydroxyapatite: An impasse or the road to follow? Ceram. Int. 2020, 46, 11443–11465. [Google Scholar] [CrossRef]
- Andrés, N.C.; D’Elía, N.L.; Ruso, J.M.; Campelo, A.N.E.; Massheimer, V.L.; Messina, P.V. Manipulation of Mg2+–Ca2+ switch on the development of bone mimetic hydroxyapatite. ACS Appl. Mater. Interfaces 2017, 9, 15698–15710. [Google Scholar] [CrossRef] [PubMed]
- Placente, D.; Ruso, J.M.; Baldini, M.; Laiuppa, J.A.; Sieben, J.M.; Santillán, G.E.; Messina, P.V. Self-fluorescent antibiotic MoOx–hydroxyapatite: A nano-theranostic platform for bone infection therapies. Nanoscale 2019, 11, 17277–17292. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, T.; Wang, C.; Wang, Z.; Yang, Y.; Li, P.; Cai, R.; Sun, M.; Yuan, H.; Nie, L. Synthesis and characterization of silver nanoparticles-doped hydroxyapatite/alginate microparticles with promising cytocompatibility and antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124081. [Google Scholar] [CrossRef]
- Xing, Q.; Zhang, X.; Wu, D.; Han, Y.; Wickramaratne, M.N.; Dai, H.; Wang, X. Ultrasound-assisted synthesis and characterization of heparin-coated Eu3+ doped hydroxyapatite luminescent nanoparticles. Colloid Interface Sci. Commun. 2019, 29, 17–25. [Google Scholar] [CrossRef]
- Rial, R.n.; Costa, R.R.; Reis, R.L.; Liu, Z.; Pashkuleva, I.; Ruso, J.M. Mineralization of layer-by-layer ultrathin films containing microfluidic-produced hydroxyapatite nanorods. Cryst. Growth Des. 2019, 19, 6351–6359. [Google Scholar] [CrossRef]
- D’Elía, N.L.; Silva, R.R.; Sartuqui, J.; Ercoli, D.; Ruso, J.; Messina, P.; Mestres, G. Development and characterisation of bilayered periosteum-inspired composite membranes based on sodium alginate-hydroxyapatite nanoparticles. J. Colloid Interface Sci. 2020, 572, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Ruso, J.M.; Deo, N.; Somasundaran, P. Complexation between dodecyl sulfate surfactant and zein protein in solution. Langmuir 2004, 20, 8988–8991. [Google Scholar] [CrossRef]
- Hassan, N.; Barbosa, L.R.; Itri, R.; Ruso, J.M. Fibrinogen stability under surfactant interaction. J. Colloid Interface Sci. 2011, 362, 118–126. [Google Scholar] [CrossRef]
- D’Elia, N.L.; Gravina, N.; Ruso, J.M.; Marco-Brown, J.L.; Sieben, J.M.; Messina, P.V. Albumin-mediated deposition of bone-like apatite onto nano-sized surfaces: Effect of surface reactivity and interfacial hydration. J. Colloid Interface Sci. 2017, 494, 345–354. [Google Scholar] [CrossRef]
- Rial, R.N.; Tichnell, B.; Latimer, B.; Liu, Z.; Messina, P.V.; Ruso, J.M. Structural and kinetic visualization of the protein corona on bioceramic nanoparticles. Langmuir 2018, 34, 2471–2480. [Google Scholar] [CrossRef] [PubMed]
- Rial, R.; Soltero, J.A.; Verdes, P.V.; Liu, Z.; Ruso, J.M. Mechanical properties of composite hydrogels for tissue engineering. Curr. Top. Med. Chem. 2018, 18, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Rial, R.; Liu, Z.; Ruso, J.M. Soft actuated hybrid hydrogel with bioinspired complexity to control mechanical flexure behavior for tissue engineering. Nanomaterials 2020, 10, 1302. [Google Scholar] [CrossRef] [PubMed]
- Taboada, P.; Attwood, D.; Ruso, J.M.; García, M.; Sarmiento, F.; Mosquera, V. Influence of Molecular Structure on the Ideality of Mixing in Micelles Formed in Binary Mixtures of Surface-Active Drugs. J. Colloid Interface Sci. 1999, 216, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Taboada, P.; Mosquera, V.; Ruso, J.M.; Sarmiento, F.; Jones, M.N. Interaction between Penicillins and Human Serum Albumin: A ζ-Potential Study. Langmuir 2000, 16, 6795–6800. [Google Scholar] [CrossRef]
- Taboada, P.; Mosquera, V.; Ruso, J.M.; Sarmiento, F.; Jones, M.N. Interaction between Penicillins and Human Serum Albumin: A Thermodynamic Study of Micellar-like Clusters on a Protein. Langmuir 2000, 16, 934–938. [Google Scholar] [CrossRef]
- Mondal, S.; Dorozhkin, S.V.; Pal, U. Recent progress on fabrication and drug delivery applications of nanostructured hydroxyapatite. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1504. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Pal, U. 3D hydroxyapatite scaffold for bone regeneration and local drug delivery applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101131. [Google Scholar] [CrossRef]
- Benedini, L.; Placente, D.; Ruso, J.; Messina, P. Adsorption/desorption study of antibiotic and anti-inflammatory drugs onto bioactive hydroxyapatite nano-rods. Mater. Sci. Eng. C 2019, 99, 180–190. [Google Scholar] [CrossRef]
- Wuyts, S.; De Vos, D.E.; Verpoort, F.; Depla, D.; De Gryse, R.; Jacobs, P.A. A heterogeneous Ru–hydroxyapatite catalyst for mild racemization of alcohols. J. Catal. 2003, 219, 417–424. [Google Scholar] [CrossRef]
- Tsuchida, T.; Yoshioka, T.; Sakuma, S.; Takeguchi, T.; Ueda, W. Synthesis of biogasoline from ethanol over hydroxyapatite catalyst. Ind. Eng. Chem. Res. 2008, 47, 1443–1452. [Google Scholar] [CrossRef]
- Tounsi, H.; Djemal, S.; Petitto, C.; Delahay, G. Copper loaded hydroxyapatite catalyst for selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B Environ. 2011, 107, 158–163. [Google Scholar] [CrossRef]
- Guo, J.; Yu, H.; Dong, F.; Zhu, B.; Huang, W.; Zhang, S. High efficiency and stability of Au–Cu/hydroxyapatite catalyst for the oxidation of carbon monoxide. RSC Adv. 2017, 7, 45420–45431. [Google Scholar] [CrossRef]
- Webler, G.D.; Rodrigues, W.C.; Silva, A.E.S.; Silva, A.O.S.; Fonseca, E.J.S.; Degenhardt, M.F.S.; Oliveira, C.L.P.; Otubo, L.; Barros Filho, D.A. Use of micrometric latex beads to improve the porosity of hydroxyapatite obtained by chemical coprecipitation method. Appl. Surf. Sci. 2018, 436, 141–151. [Google Scholar] [CrossRef]
- Chen, G.; Shan, R.; Shi, J.; Liu, C.; Yan, B. Biodiesel production from palm oil using active and stable K doped hydroxyapatite catalysts. Energy Convers. Manag. 2015, 98, 463–469. [Google Scholar] [CrossRef]
- Reichert, J.; Binner, J. An evaluation of hydroxyapatite-based filters for removal of heavy metal ions from aqueous solutions. J. Mater. Sci. 1996, 31, 1231–1241. [Google Scholar] [CrossRef]
- Inoue, K.; Sassa, K.; Yokogawa, Y.; Sakka, Y.; Okido, M.; Asai, S. Control of crystal orientation of hydroxyapatite by imposition of a high magnetic field. Mater. Trans. 2003, 44, 1133–1137. [Google Scholar] [CrossRef]
- Zhang, C.; Uchikoshi, T.; Liu, L.; Kikuchi, M.; Ichinose, I. Effect of Surface Modification with TiO2 Coating on Improving Filtration Efficiency of Whisker-Hydroxyapatite (HAp) Membrane. Coatings 2020, 10, 670. [Google Scholar] [CrossRef]
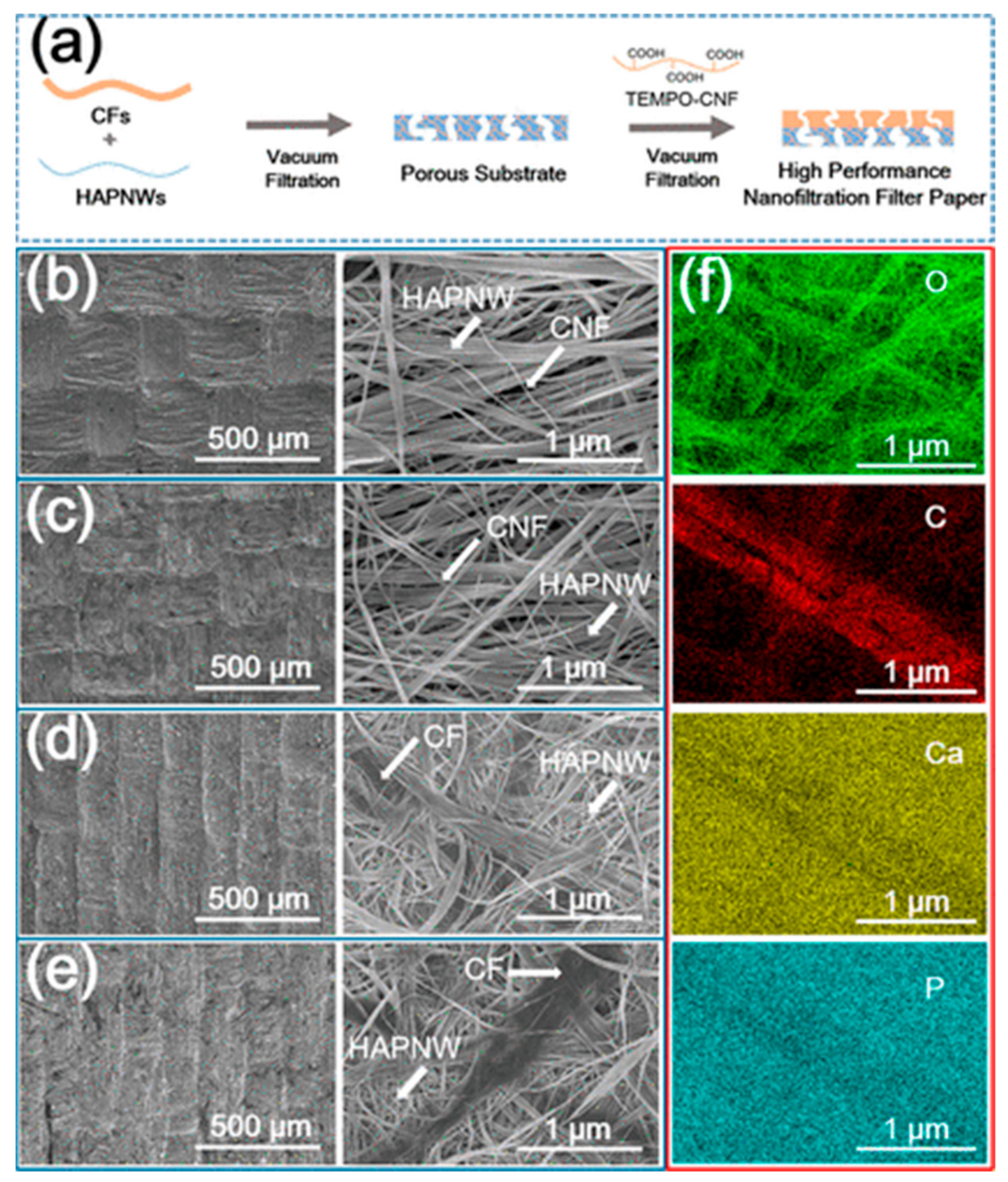
- Zhang, Q.-Q.; Zhu, Y.-J.; Wu, J.; Dong, L.-Y. Nanofiltration Filter Paper Based on Ultralong Hydroxyapatite Nanowires and Cellulose Fibers/Nanofibers. ACS Sustain. Chem. Eng. 2019, 7, 17198–17209. [Google Scholar] [CrossRef]
- Yang, L.; Ning, X.; Chen, K.; Zhou, H. Preparation and properties of hydroxyapatite filters for microbial filtration. Ceram. Int. 2007, 33, 483–489. [Google Scholar] [CrossRef]
- Kuiper, M.; Sanches, R.M.; Walford, J.A.; Slater, N.K.H. Purification of a functional gene therapy vector derived from Moloney murine leukaemia virus using membrane filtration and ceramic hydroxyapatite chromatography. Biotechnol. Bioeng. 2002, 80, 445–453. [Google Scholar] [CrossRef]
- Chang, H.; Park, N.; Jang, Y.; Lim, H.; Kim, W. Application of the hydroxyapatite crystallization-filtration process to recover phosphorus from wastewater effluents. Water Sci. Technol. 2020, 81, 2300–2310. [Google Scholar] [CrossRef]
- Xiong, Z.-C.; Yang, R.-L.; Zhu, Y.-J.; Chen, F.-F.; Dong, L.-Y. Flexible hydroxyapatite ultralong nanowire-based paper for highly efficient and multifunctional air filtration. J. Mater. Chem. A 2017, 5, 17482–17491. [Google Scholar] [CrossRef]
- Ibrahim, M.; Labaki, M.; Giraudon, J.-M.; Lamonier, J.-F. Hydroxyapatite, a multifunctional material for air, water and soil pollution control: A review. J. Hazard. Mater. 2020, 383, 121139. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Pineiro-Redondo, Y.; De Santis, R.; Gloria, A.; Ambrosio, L.; Tampieri, A.; Dediu, V.; Rivas, J. Poly (caprolactone) based magnetic scaffolds for bone tissue engineering. J. Appl. Phys. 2011, 109, 07B313. [Google Scholar] [CrossRef]
- Foroughi, F.; Hassanzadeh-Tabrizi, S.A.; Amighian, J. Microemulsion synthesis and magnetic properties of hydroxyapatite-encapsulated nano CoFe2O4. J. Magn. Magn. Mater. 2015, 382, 182–187. [Google Scholar] [CrossRef]
- Petchsang, N.; Pon-On, W.; Hodak, J.H.; Tang, I.M. Magnetic properties of Co-ferrite-doped hydroxyapatite nanoparticles having a core/shell structure. J. Magn. Magn. Mater. 2009, 321, 1990–1995. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, H.; Li, L.; Zhang, C. Temperature dependence of magnetic property and photocatalytic activity of Fe3O4/hydroxyapatite nanoparticles. Mater. Res. Bull. 2010, 45, 2036–2039. [Google Scholar] [CrossRef]
- Inukai, A.; Sakamoto, N.; Aono, H.; Sakurai, O.; Shinozaki, K.; Suzuki, H.; Wakiya, N. Synthesis and hyperthermia property of hydroxyapatite–ferrite hybrid particles by ultrasonic spray pyrolysis. J. Magn. Magn. Mater. 2011, 323, 965–969. [Google Scholar] [CrossRef]
- Bock, N.; Riminucci, A.; Dionigi, C.; Russo, A.; Tampieri, A.; Landi, E.; Goranov, V.A.; Marcacci, M.; Dediu, V. A novel route in bone tissue engineering: Magnetic biomimetic scaffolds. Acta Biomater. 2010, 6, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Tampieri, A.; D’Alessandro, T.; Sandri, M.; Sprio, S.; Landi, E.; Bertinetti, L.; Panseri, S.; Pepponi, G.; Goettlicher, J.; Bañobre-López, M.; et al. Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite. Acta Biomater. 2012, 8, 843–851. [Google Scholar] [CrossRef]
- Iafisco, M.; Sandri, M.; Panseri, S.; Delgado-López, J.M.; Gómez-Morales, J.; Tampieri, A. Magnetic Bioactive and Biodegradable Hollow Fe-Doped Hydroxyapatite Coated Poly(l-lactic) Acid Micro-nanospheres. Chem. Mater. 2013, 25, 2610–2617. [Google Scholar] [CrossRef]
- Russo, T.; D’Amora, U.; Gloria, A.; Tunesi, M.; Sandri, M.; Rodilossi, S.; Albani, D.; Forloni, G.; Giordano, C.; Cigada, A. Systematic analysis of injectable materials and 3D rapid prototyped magnetic scaffolds: From CNS applications to soft and hard tissue repair/regeneration. Procedia Eng. 2013, 59, 233–239. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Pineiro-Redondo, Y.; Sandri, M.; Tampieri, A.; De Santis, R.; Dediu, V.A.; Rivas, J. Hyperthermia induced in magnetic scaffolds for bone tissue engineering. IEEE Trans. Magn. 2014, 50, 1–7. [Google Scholar] [CrossRef]
- Sprio, S.; Campodoni, E.; Sandri, M.; Preti, L.; Keppler, T.; Müller, F.A.; Pugno, N.M.; Tampieri, A. A graded multifunctional hybrid scaffold with superparamagnetic ability for periodontal regeneration. Int. J. Mol. Sci. 2018, 19, 3604. [Google Scholar] [CrossRef]
- Sarda, S.; Iafisco, M.; Pascaud-Mathieu, P.; Adamiano, A.; Montesi, M.; Panseri, S.; Marsan, O.; Thouron, C.; Dupret-Bories, A.; Tampieri, A. Interaction of folic acid with nanocrystalline apatites and extension to methotrexate (antifolate) in view of anticancer applications. Langmuir 2018, 34, 12036–12048. [Google Scholar] [CrossRef] [PubMed]
- Marrella, A.; Iafisco, M.; Adamiano, A.; Rossi, S.; Aiello, M.; Barandalla-Sobrados, M.; Carullo, P.; Miragoli, M.; Tampieri, A.; Scaglione, S. A combined low-frequency electromagnetic and fluidic stimulation for a controlled drug release from superparamagnetic calcium phosphate nanoparticles: Potential application for cardiovascular diseases. J. R. Soc. Interface 2018, 15, 20180236. [Google Scholar] [CrossRef] [PubMed]
- Adamiano, A.; Wu, V.M.; Carella, F.; Lamura, G.; Canepa, F.; Tampieri, A.; Iafisco, M.; Uskoković, V. Magnetic calcium phosphates nanocomposites for the intracellular hyperthermia of cancers of bone and brain. Nanomedicine 2019, 14, 1267–1289. [Google Scholar] [CrossRef]
- Patrício, T.M.F.; Mumcuoglu, D.; Montesi, M.; Panseri, S.; Witte-Bouma, J.; Garcia, S.F.; Sandri, M.; Tampieri, A.; Farrell, E.; Sprio, S. Bio-inspired polymeric iron-doped hydroxyapatite microspheres as a tunable carrier of rhBMP-2. Mater. Sci. Eng. C 2021, 119, 111410. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, B.; Devarajan, N.; Jobanputra, R.; Gowd, G.S.; Anna, I.M.; Ashokan, A.; Nair, S.; Koyakutty, M. nCP: Fe Nanocontrast Agent for Magnetic Resonance Imaging-Based Early Detection of Liver Cirrhosis and Hepatocellular Carcinoma. Acs Appl. Bio Mater. 2021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rial, R.; González-Durruthy, M.; Liu, Z.; Ruso, J.M. Advanced Materials Based on Nanosized Hydroxyapatite. Molecules 2021, 26, 3190. https://doi.org/10.3390/molecules26113190
Rial R, González-Durruthy M, Liu Z, Ruso JM. Advanced Materials Based on Nanosized Hydroxyapatite. Molecules. 2021; 26(11):3190. https://doi.org/10.3390/molecules26113190
Chicago/Turabian StyleRial, Ramón, Michael González-Durruthy, Zhen Liu, and Juan M. Ruso. 2021. "Advanced Materials Based on Nanosized Hydroxyapatite" Molecules 26, no. 11: 3190. https://doi.org/10.3390/molecules26113190
APA StyleRial, R., González-Durruthy, M., Liu, Z., & Ruso, J. M. (2021). Advanced Materials Based on Nanosized Hydroxyapatite. Molecules, 26(11), 3190. https://doi.org/10.3390/molecules26113190





