Potential Chemicals from Plastic Wastes
Abstract
1. Introduction
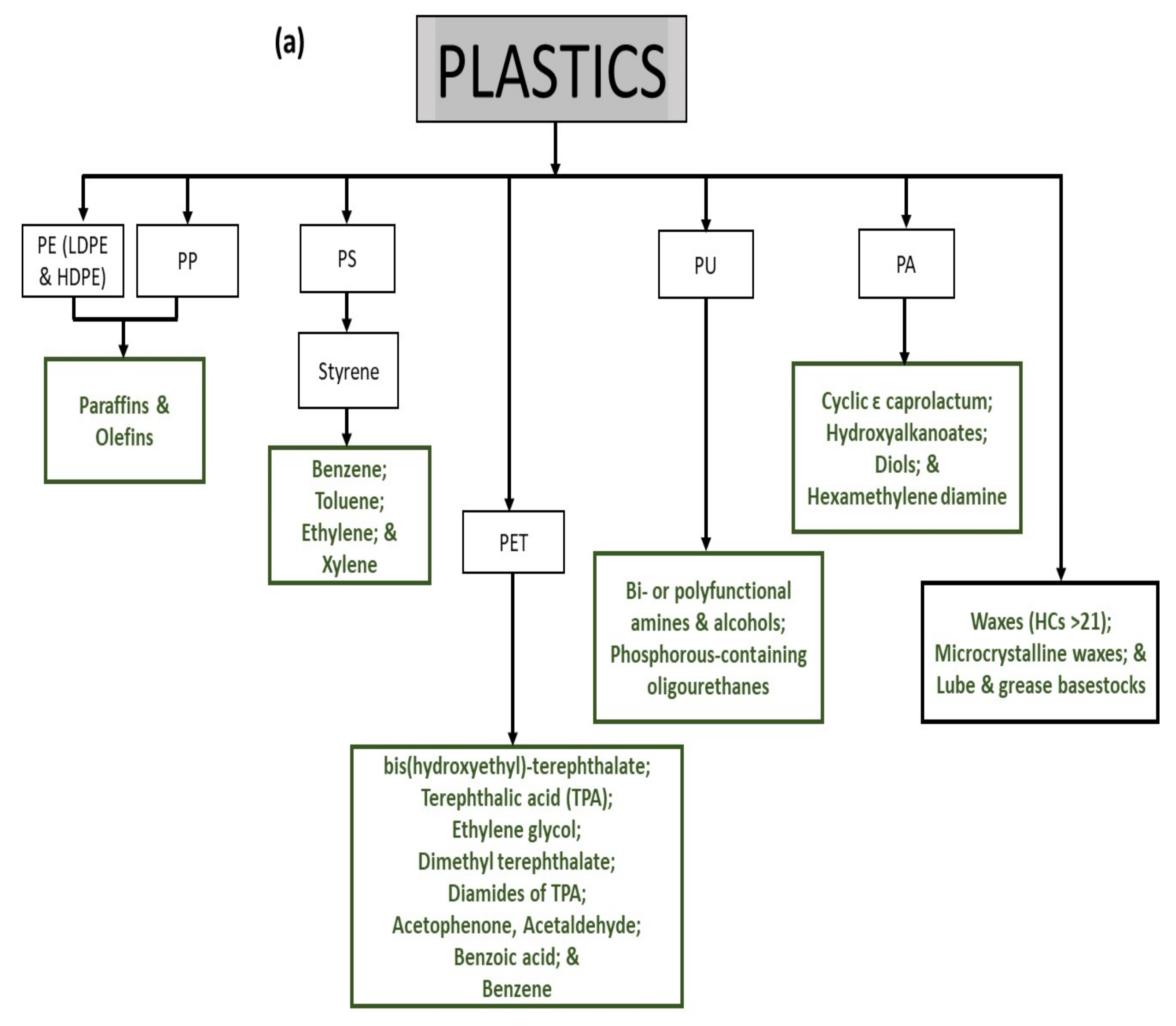
2. Production of Chemicals from Plastic Wastes
2.1. Polyethylene (PE) and Polypropylene (PP)
2.2. Polystyrene (PS)
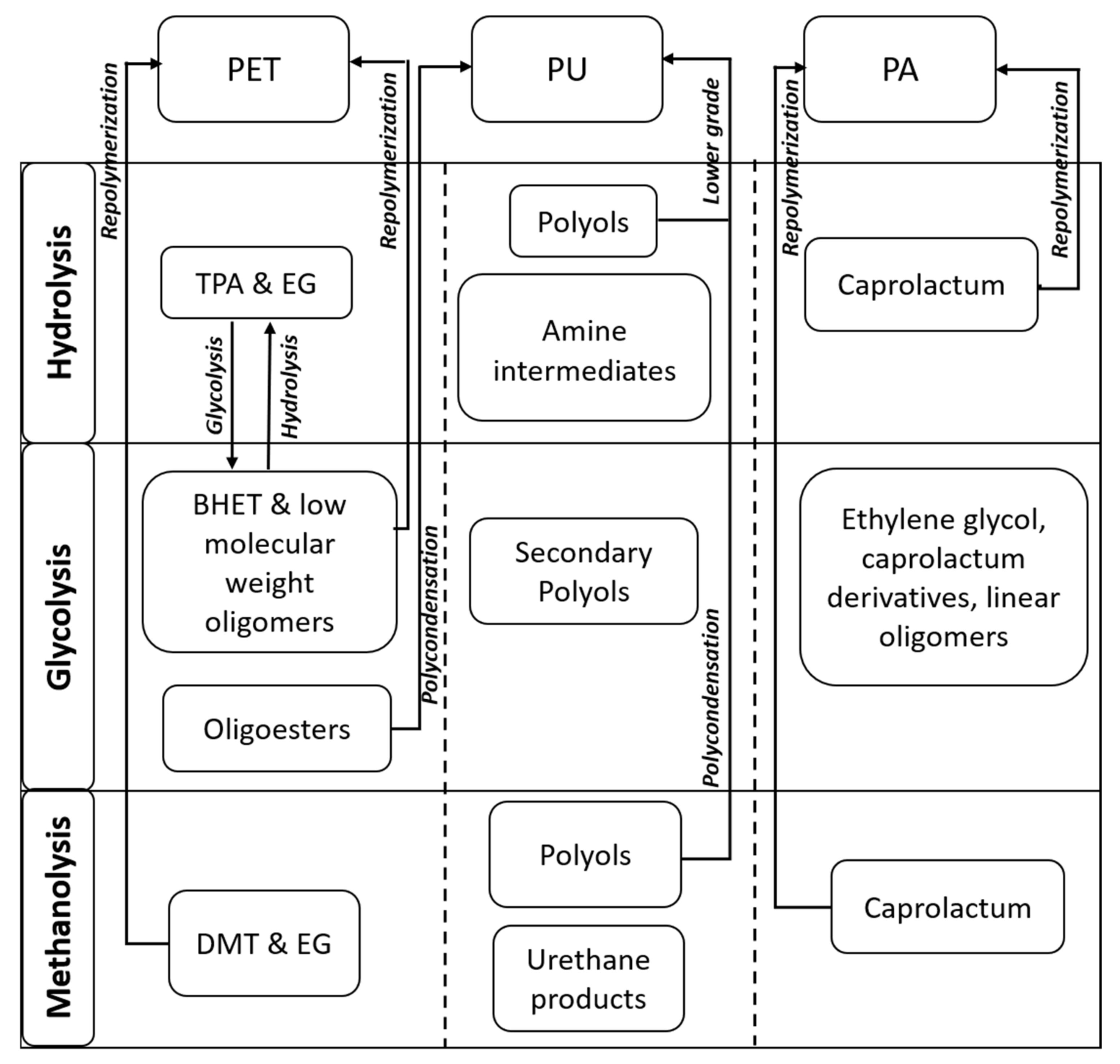
2.3. Polyethylene Terephthalate (PET)
2.4. Polyurethane (PU)
2.5. Polyamides (PA)
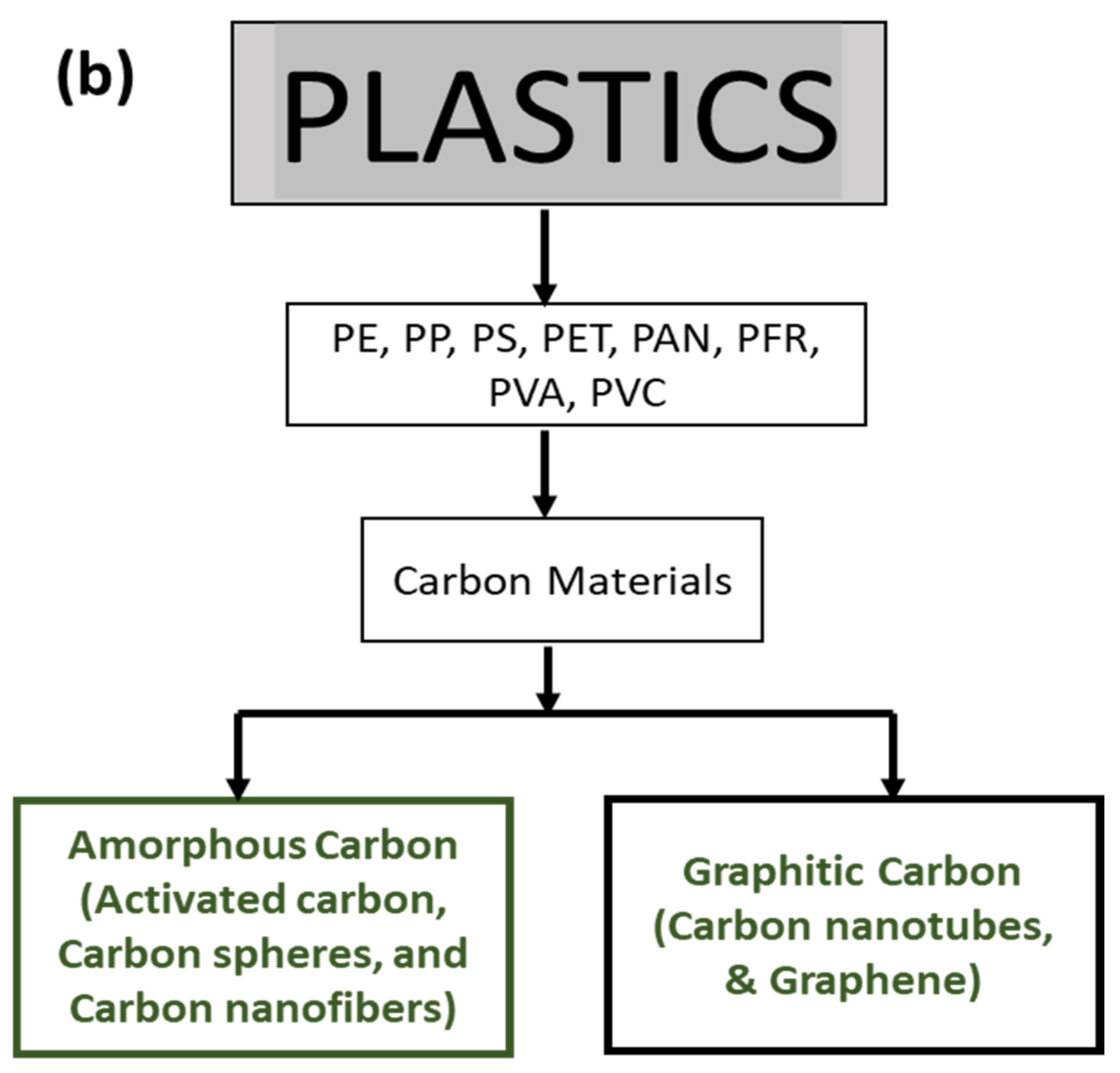
3. Production of Carbon Materials
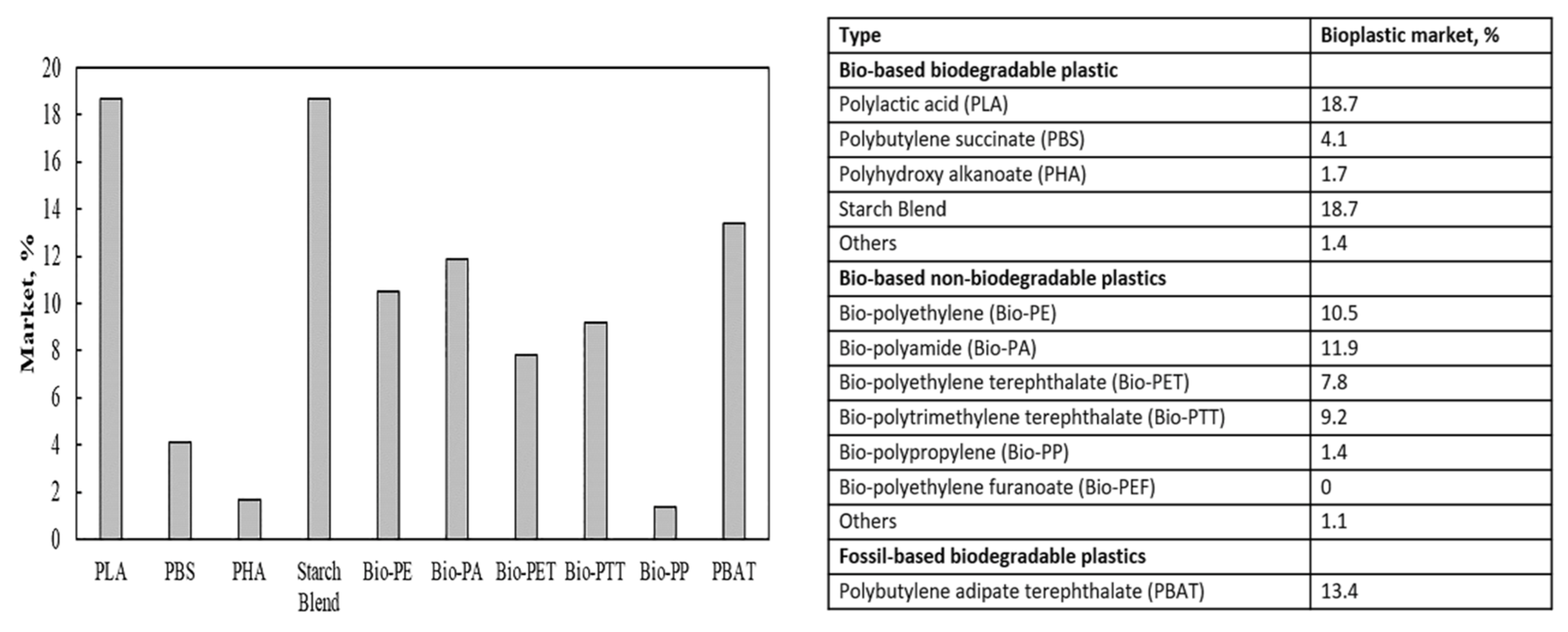
4. Biodegradable Plastics (BDP)
5. Research Challenges and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastic Market Size. Share & Trends Analysis Report by Product (PE, PP, PU, PVC, PET, Polystyrene, ABS, PBT, PPO, Epoxy Polymers, LCP, PC, Polyamide), by Application, by Region, and Segment Forecast, 2020–2027. Available online: https://www.researchandmarkets.com/reports/4751797/plastic-market-size-share-and-trends-analysis (accessed on 23 December 2020).
- Adyel, T.M. Accumulation of plastic waste during COVID-19. Science 2020, 369, 1314–1315. [Google Scholar]
- Leblanc, R. Plastic Recycling Facts and Figures. Available online: https://www.thebalancesmb.com/plastic-recycling-facts-and-figures-287788 (accessed on 21 January 2021).
- Hunertmark, T.; Mayer, M.; McNally, C.; Simons, T.J.; Witte, C. How Plastics Waste Recycling Could Transform the Chemical Industry. Available online: https://www.mckinsey.com/industries/chemicals/our-insights/how-plastics-waste-recycling-could-transform-the-chemical-industry (accessed on 16 November 2020).
- Acomb, J.C.; Wu, C.; Williams, P.T. Control of steam input to the pyrolysis-gasification of waste plastics for improved production of hydrogen or carbon nanotubes. Appl. Catal. B Environ. 2014, 147, 571–584. [Google Scholar] [CrossRef]
- Altway, R.T.A.; Susianto, S.R.J. Production of liquid fuel from plastic waste using integrated pyrolysis method with refinery distillation bubble cap plate column. Energy Rep. 2019, 5, 70–77. [Google Scholar]
- Sharma, B.K.; Moser, B.R.; Vermillion, K.E.; Doll, K.M.; Rajagopalan, N. Production, characterization and fuel properties of alternative diesel fuel from pyrolysis of waste plastic grocery bags. Fuel Process. Technol. 2014, 122, 79–90. [Google Scholar] [CrossRef]
- Karagöz, Y. Analysis of the impact of gasoline, biogas, and biogas + hydrogen fuels on emissions and vehicle performance in the WLTC and NEDC. Int. J. Hydrogen Energy 2019, 44, 31621–31632. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Current state and future prospects of plastic waste as source of fuel: A review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180. [Google Scholar] [CrossRef]
- Mohanraj, C.; Senthilkumar, T.; Chandradekar, M. A review on conversion techniques of liquid fuel from waste plastic materials. Int. J. Energy Res. 2017, 41, 1534–1552. [Google Scholar]
- Garforth, A.A.; Ali, S.; Martinez, J.H.; Akah, A. Feedstock recycling of polymer wastes. Curr. Opin. Solid State Mater. Sci. 2004, 8, 419–425. [Google Scholar] [CrossRef]
- Dufaud, V.; Basset, J.M. Catalytic hydrogenolysis at low temperature and pressure of polyethylene and polypropylene to diesels or lower alkanes by a zirconium hydride supported on silica alumina: A step toward polyolefin degradation by the microscope reverse of Ziegler−Natta polymerization. Angew. Chem. Int. Ed. 1998, 37, 806–810. [Google Scholar]
- Hibbitts, D.D.; Flaherty, D.W.; Iglesia, E. Effects of chain length on the mechanism and rates of metal-catalyzed hydrogenolysis of n-alkanes. J. Phys. Chem. C 2016, 120, 8125–8138. [Google Scholar] [CrossRef]
- Flaherty, D.W.; Uzun, A.; Iglesia, E. Catalytic ring opening of cycloalkanes on Ir clusters: Alkyl substitution effects on the structure and stability of C−C bond cleavage transition states. J. Phys. Chem. C 2015, 119, 2597–2613. [Google Scholar] [CrossRef]
- Flaherty, D.W.; Hibbitts, D.D.; Iglesia, E. Metal-catalyzed C−C bond cleavage in alkanes: Effects of methyl substitution on transition-state structures and stability. J. Am. Chem. Soc. 2014, 136, 9664–9676. [Google Scholar] [CrossRef]
- Escola, J.M.; Aguado, J.; Serrano, D.P.; Briones, L. Hydroreforming over Ni/H-beta of the thermal cracking products of LDPE, HDPE and PP for fuel production. J. Mater. Cycles Waste Manag. 2012, 14, 286–293. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M.I.; Navarro, R. Evolution of products generated during the dynamic pyrolysis of LDPE and HDPE over HZSM5. Energy Fuels 2008, 22, 2917–2924. [Google Scholar] [CrossRef]
- Bai, B.; Jin, H.; Fan, C.; Cao, C.; Wei, W.; Cao, W. Experimental investigation on liquefaction of plastic waste to oil in supercritical water. Waste Manag. 2019, 89, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.S.; Glasius, M.; Biller, P. Screening of common synthetic polymers for depolymerization by subcritical hydrothermal liquefaction. Process. Saf. Environ. Prot. 2020, 139, 371–379. [Google Scholar] [CrossRef]
- Murata, K.; Hirano, Y.; Sakata, Y.; Azhar Uddin, M. Basic study on a continuous flow reactor for thermal degradation of polymers. J. Anal. Appl. Pyrolysis 2002, 65, 71–90. [Google Scholar] [CrossRef]
- McCaffrey, W.C.; Kamal, M.R.; Cooper, D.G. Thermolysis of polyethylene. Polym. Degrad. Stab. 1995, 47, 133–139. [Google Scholar] [CrossRef]
- Demirbas, A. Recovery of chemicals and gasoline-range fuels from plastic wastes via pyrolysis. Energy Sources 2005, 27, 1313–1319. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Amutio, M.; Elordi, G.; Bilbao, J.; Olazar, M. Cracking of high-density polyethylene pyrolysis waxes on HZSM-5 catalysts of different acidity. Ind. Eng. Chem. Res. 2013, 52, 10637–10645. [Google Scholar] [CrossRef]
- Jung, S.H.; Cho, M.H.; Kang, B.S.; Kim, J.S. Pyrolysis of a fraction of waste polypropylene and polyethylene for recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010, 91, 277–284. [Google Scholar] [CrossRef]
- Zhang, F.; Zeng, M.; Yappert, R.D.; Sun, J.; Lee, Y.H.; LaPointe, A.M.; Peters, B.; Abu-Omar, M.M.; Scott, S.L. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 2020, 370, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.G.; Goff, M.; Donner, M.; Kaminsky, W.; O’Connor, K.E. A two-step chemo-biotechnological conversion of polystyrene to a biodegradable thermoplastic. Environ. Sci. Technol. 2006, 40, 2433–2437. [Google Scholar] [CrossRef] [PubMed]
- Karaduman, A. Pyrolysis of polystyrene plastic wastes with some organic compounds for enhancing styrene yield. Energy Sources 2002, 24, 667–674. [Google Scholar] [CrossRef]
- Zhang, Z.; Hirose, T.; Nishio, S.; Morioka, Y.; Azuma, N.; Ueno, A. Chemical recycling of waste polystyrene into styrene over solid acids and bases. Ind. Eng. Chem. Res. 1995, 34, 4514–4519. [Google Scholar] [CrossRef]
- Achilias, D.S.; Kanellopoulou, I.; Megalokonomous, P.; Antonakou, E.; Lappas, A.A. Chemical recycling of polystyrene by pyrolysis: Potential use of the liquid product for the reproduction of polymer. Macromol. Mater. Eng. 2007, 292, 923–934. [Google Scholar] [CrossRef]
- Park, K.B.; Jeong, Y.S.; Guzelciftci, B.; Kim, J.S. Two-stage pyrolysis of polystyrene: Pyrolysis oil as a source of fuels or benzene, toluene, ethylbenzene, and xylenes. Appl. Energy 2020, 259, 114240. [Google Scholar] [CrossRef]
- Yue, Q.F.; Xiao, L.F.; Zhang, M.L.; Bai, X.F. The glycolysis of poly(ethylene terephthalate) waste: Lewis acidic ionic liquids as high efficient catalysts. Polymers 2013, 5, 1258–1271. [Google Scholar] [CrossRef]
- Sinha, V.; Patel, M.R.; Patel, J.V. PET waste management by chemical recycling: A review. J. Polym. Envrion. 2010, 18, 8–25. [Google Scholar] [CrossRef]
- Kao, C.Y.; Wan, B.Z.; Cheng, W.H. Kinetics of hydrolytic depolymerization of melt poly(ethyleneterephthalate). Ind. Eng. Chem. Res. 1998, 37, 1228–1234. [Google Scholar] [CrossRef]
- Zahn, H.; Pfeifer, H. Aminolysis of polyethylene terephthalate. Polymer 1963, 4, 429–432. [Google Scholar] [CrossRef]
- Jia, H.; Ben, H.; Luo, Y.; Wang, R. Catalytic fast pyrolysis of poly(ethylene terephthalate) (PET) with zeolite and nickel chloride. Polymers 2020, 12, 705. [Google Scholar] [CrossRef]
- Dimitrov, N.; Kratofil Krehula, L.; Pticek Sirocic, A.; Hrnjak Murgic, Z. Analysis of recycled PET bottles products by pyrolysis-gas chromatography. Polym. Degread. Stab. 2013, 98, 972–979. [Google Scholar] [CrossRef]
- Sheratte, M.B. Process for Converting the Decomposition Products of Polyurethane and Novel Compositions Thereby Obtained. U.S. Patent 4,110,266, 29 August 1978. [Google Scholar]
- Duch, M.W.; Allgeier, A.M. Deactivation of nitrile hydrogenation catalysts: New mechanistic insight from a nylon recycle process. Appl. Catal. A 2007, 318, 190–198. [Google Scholar] [CrossRef]
- Cesarek, U.; Pahovnik, D.; Zagar, E. Chemical recycling of aliphatic polyamides by microwave-assisted hydrolysis for efficient monomer recovery. ACS Sustain. Chem. Eng. 2020, 8, 16274–16282. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Garcia, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 46. [Google Scholar] [CrossRef]
- De la Puente, G.; Sedran, U.A. Recycling polystyrene into fuels by means of FCC: Performance of various acidic catalysts. Appl. Catal. B Environ. 1998, 19, 305–311. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, K.M.; Basheer, N.; Hussain, K. Co-liquefaction of Makarwal coal and waste polystyrene by microwave-metal interaction pyrolysis in copper coil reactor. J. Anal. Appl. Pyrolysis 2011, 90, 53–55. [Google Scholar] [CrossRef]
- Bartolome, L.; Cho, B.; Do, H.; Imran, M.; Al-Masry, W. Recent Developments in the Chemical Recycling of PET; INTECH Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Global Polyethylene Terephthalate Production 2014–2020, Statista Research Department, 10 December 2015. Available online: https://www.statista.com/statistics/650191/global-polyethylene-terephthalate-production-outlook/ (accessed on 23 December 2020).
- Polyurethane Market by Raw Material (MDI, TDI, Polyols), Products (Coatings, Adhesives, and Sealants, Flexible & Rigid Foams, Elastomers), End-User (Building & Construction, Automotive & Transportation, Bedding & Furniture)-Global Forecast to 2021. Available online: https://www.marketsandmarkets.com/Market-Reports/polyurethane-market-151784541.html (accessed on 23 December 2020).
- Cit, I.; Smag, A.; Yumak, T.; Ucar, S.; Misirhogiu, Z.; Canel, M. Comparative pyrolysis of polyolefins (PP and LDPE) and PET. Polym. Bull. 2009, 64, 817–834. [Google Scholar] [CrossRef]
- Loong, K.C. Simulation: Optimize the Production of Benzoic Acid by Using Benzene and Acetic Anhydride. Ph.D. Thesis, Universiti Tunku Abdul Rahman, Petaling Jaya, Malaysia, 2011. [Google Scholar]
- Benzoic Acid Market Size, Industry Analysis Report, Regional Outlook (U.S. Germany, UK, Italy, Russia, China, India, Japan, South Korea, Brazil, Mexico, Saudi Arabia, UAE South Africa), Application Development Price Trend, Competitive Market Share and Forecast, 2016–2023. Available online: https://www.gminsights.com/industry-analysis/benzoic-acid-market (accessed on 21 January 2021).
- Rahman, W.M.N.W.A.; Wahab, A.F.A. Green pavement using recycled polyethylene terephthalate (PET) as partial fine aggregate replacement in modified asphalt. Procedia Eng. 2013, 53, 124–128. [Google Scholar] [CrossRef]
- Taherkhani, H.; Arshadi, M.R. Investigating the mechanical properties of asphalt concrete containing waste polyethylene terephthalate. Road Mater. Pavement Des. 2019, 20, 381–398. [Google Scholar] [CrossRef]
- Merkel, D.R.; Kuang, W.; Malhotra, D.; Petrossian, G.; Zhong, L.; Simmons, K.L.; Zhang, J.; Cosimbescu, L. Waste PET chemical processing to terephthalic amides and their effect on asphalt performance. ACS Sustain. Chem. Eng. 2020, 8, 5615–5625. [Google Scholar] [CrossRef]
- Arnold, T.S. What in Your Asphalt. Fed. Highw. Adm. Res. Technol. 2017, 81, 2. [Google Scholar]
- Simon, D.; Borreguero, A.M.; de Lucas, A.; Rodriguez, J.F. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef]
- Zia, K.M.; Bhatti, H.N.; Bhatti, I.A. Methods for polyurethane and polyurethane composites, recycling, and recovery: A review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Alavi Nikje, M.M.; Nikrah, M.; Mohammadi, F.H.A. Microwave-assisted polyurethane bond cleavage via hydrogenolysis process at atmospheric pressure. J. Cell Plast. 2008, 44, 367–380. [Google Scholar] [CrossRef]
- Grancharov, G.; Mitova, V.; Stoitchov, S.; Antonya, T.; Gitsov, I.; Troev, K. Smart polymer recycling: Synthesis of novel rigid polyurethanes using phosphorous-containing oligomers formed by controlled degradation of microporous polyurethane elastomer. J. Appl. Polym. Sci. 2007, 105, 302–308. [Google Scholar] [CrossRef]
- Herzog, B.; Kohan, M.I.; Mestemacher, S.A.; Pagilagan, R.U.; Redmond, K. Polyamides. Ullmann’s Encyclopedia of Industrial Chemistry; Barbara, E., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 697–732. [Google Scholar]
- Chen, J.; Liu, G.; Jin, L.; Ni, P.; Li, Z.; He, H.; Xu, Y.; Zhang, J.; Dong, J. Catalytic hydrolysis of waste Nylon 6 to produce caprolactam in subcritical waste. J. Anal. Appl. Pyrolysis 2010, 87, 50–55. [Google Scholar] [CrossRef]
- Datta, J.; Błażek, K.; Włoch, M.; Bukowski, R. A new approach to chemical recycling of polyamide 6.6 and synthesis of polyurethanes with recovered intermediates. J. Polym. Environ. 2018, 26, 4415–4429. [Google Scholar] [CrossRef]
- Kamimura, A.; Ikeda, K.; Suzuki, S.; Kato, K.; Akinari, Y.; Sugimoto, T.; Kashiwagi, K.; Kaiso, K.; Matsumoto, H.; Yoshimoto, M. Efficient conversion of polyamides to ω-hydroxyalkanoic acids: A new method for chemical recycling of waste plastics. ChemSusChem 2014, 7, 2473–2477. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Akinari, Y.; Kaiso, K.; Kamimura, A. Efficient depolymerization and chemical conversion of Polyamide 66 to 1,6- hexanediol. J. Mater. Cycles Waste Manag. 2017, 19, 326–331. [Google Scholar] [CrossRef]
- Samieadel, A.; Fini, E.H. Interplay between wax and polyphosphoric acid and its effect on bitumen thermochemical properties. Constr. Build. Mater. 2020, 243, 118194. [Google Scholar] [CrossRef]
- Samieadel, A.; Hogsaa, B.; Fini, E.H. Examining the implications of wax-based additives on the sustainability of construction practices: Multiscale characterization of wax-doped aged asphalt binder. ACS Sustain. Chem. Eng. 2019, 7, 2943–2954. [Google Scholar] [CrossRef]
- Jixing, L.; Shuyuan, W.; Xuan, L.; Xiang, Y. Study on the conversion technology of waste polyethylene plastic to polyethylene wax. Energy Sources 2003, 25, 77–82. [Google Scholar] [CrossRef]
- Khan, H.U.; Sahai, M.; Kumar, S.; Kumar, A.; Thakre, G.D.; Kaul Nanoti, S.M.; Shukla, B.M.; Garg, M.O. Process for the conversion of low polymer wax to paraffin wax, microcrystalline wax, lube, and grease base stocks using organic peroxides or hydroperoxide and metal oxides. U.S. Patent 9,714,385, 25 July 2017. [Google Scholar]
- Celik, G.; Kennedy, R.M.; Hackler, R.A.; Ferrandon, M.; Tennakoon, A.; Patnaik, S.; LaPointe, A.M.; Ammal, S.C.; Heyden, A.; Perras, F.A.; et al. Upcycling single-use polyethylene into high-quality liquid products. ACS Cent. Sci. 2019, 5, 1795–1803. [Google Scholar] [CrossRef]
- Miller, S.J. Conversion of waste plastic to lubricating base oil. Energy Fuels 2005, 19, 1580–1586. [Google Scholar] [CrossRef]
- Bazargan, A.; McKay, G. A review-synthesis of carbon nanotubes from plastic wastes. Chem. Eng. J. 2012, 195, 377–391. [Google Scholar] [CrossRef]
- Zhou, C.W.; Levendis, Y.A. Upcycling waste plastics into carbon nanomaterials: A review. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Z.; Jiang, S.; Hou, H. Carbonization: A feasible route for reutilization of plastic wastes. Sci. Total Environ. 2020, 710, 136250. [Google Scholar] [CrossRef]
- Wong, H.W.; Peck, J.; Bonomi, R.E.; Assif, J.; Panerai, F.; Reinisch, G. Quantitative determination of species production from phenol-formaldehyde resin pyrolysis. Polym. Degrad. Stab. 2015, 112, 122–131. [Google Scholar] [CrossRef]
- Castelo-Quiben, J.; Pastrana-Martinez, L.M.; Carrasco-Marin, F.; Perez-Cadenas, A.F. From polyethylene to highly graphitic and magnetic carbon spheres nanocomposites: Carbonization under pressure. Nanomaterials 2019, 9, 606. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Kil, H.S.; Lee, S. Fabrication of low-cost carbon fibers using economical precursors and advanced processing technologies. Carbon 2019, 142, 610–649. [Google Scholar] [CrossRef]
- Behr, M.J.; Landes, B.G.; Barton, B.E.; Bernius, M.T.; Billovits, G.F.; Hukkanen, E.J. Structure-property model for polyethylene-derived carbon fiber. Carbon 2016, 107, 525–535. [Google Scholar] [CrossRef]
- Gou, X.; Zhao, D.; Wu, C. Catalytic conversion of hard plastics to valuable carbon nanotubes. J. Anal. Appl. Pyrolysis 2020, 145, 104748. [Google Scholar] [CrossRef]
- Yao, D.; Yang, H.; Hu, Q.; Chen, Y.; Chen, H.; Williams, P.T. Carbon nanotubes from post-consumer waste plastics: Investigations into catalyst metal and support material characteristics. Appl. Catal. B Environ. 2021, 280, 119413. [Google Scholar] [CrossRef]
- Yao, D.; Li, H.; Dai, Y.; Wang, C.H. Impact of temperature on the activity of Fe-Ni catalysts for pyrolysis and decomposition processing of plastic waste. Chem. Eng. J. 2021, 408, 127268. [Google Scholar] [CrossRef]
- He, S.; Xu, Y.; Zhang, Y.; Bell, S.; Wu, C. Waste plastics recycling for producing high-value carbon nanotubes: Investigation of the influence of manganese content in Fe-based catalysts. J. Hazard. Mater. 2021, 402, 123726. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Park, K.C.; Endo, M. Carbonization under pressure. N. Carbon Mater. 2010, 25, 409–420. [Google Scholar] [CrossRef]
- Adolfsson, K.H.; Lin, C.F.; Hakkarainen, M. Microwave-assisted hydrothermal carbonization and solid-state post modification of carbonized polypropylene. ACS Sustain. Chem. Eng. 2018, 6, 11105–11114. [Google Scholar] [CrossRef]
- Jie, X.; Li, W.; Slocombe, D.; Gao, Y.; Banerjee, I.; Gonzalez-Cortes, S.; Yao, B.; AlMegren, H.; Alshihri, S.; Dilworth, J.; et al. Microwave-initiated catalytic deconstruction of plastic waste into hydrogen and high-value carbon. Nat. Catal. 2020, 3, 902–912. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumari, M.; Chauhan, P.; Chaudhary, G.R. Upcycling of plastic waste into fluorescent carbon dots: An environmentally viable transformation to biocompatible C-dots with potential prospective in analytical applications. Waste Manag. 2021, 120, 675–686. [Google Scholar] [CrossRef]
- Hu, Y.; Li, M.; Gao, Z.; Wang, L.; Zhang, J. Waste polyethylene terephthalate derived carbon dots for separable production of 5-hydroxymethylfurfural at low temperature. Catal. Lett. 2021. [Google Scholar] [CrossRef]
- Lauria, A.; Lizundia, E. Luminescent carbon dots obtained from polymeric waste. J. Clean. Prod. 2020, 262, 121288. [Google Scholar] [CrossRef]
- Liu, X.; Ma, C.; Wen, Y.; Chen, X.; Zhao, X.; Tang, T.; Holze, R.; Mijowska, E. Highly efficient conversion of waste plastic into thin carbon nanosheets for superior capacitive energy storage. Carbon 2021, 171, 819–829. [Google Scholar] [CrossRef]
- Pandey, S.; Karakoti, M.; Surana, K.; Dhapola, P.S.; SanthiBhushan, B.; Ganguly, S.; Singh, P.K.; Abbas, A.; Srivastava, A.; Sahoo, N.G. Graphene nanosheets derived from plastic waste for the application of DSSCs and supercapacitors. Sci. Rep. 2021, 11, 3916. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Li, Y.; Liu, X.; Ma, C.; Jiang, H.; Zhu, J.; Chen, X.; Tang, T.; Mijowska, E. Controllable carbonization of plastic waste into three-dimensional porous carbon nanosheets by combined catalyst for high performance capacitor. Nanomaterials 2020, 10, 1097. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Qi, X.; Ren, Y.; Wang, X. New advances in the biodegradation of poly(lactic acid). Int. Biodeterior. Biodegrad. 2017, 117, 215–223. [Google Scholar] [CrossRef]
- Narayan, R. Carbon footprint of bioplastics using biocarbon content analysis and life-cycle assessment. J. MRS Bull. 2011, 36, 716–721. [Google Scholar] [CrossRef]
- De Vargas Mores, G.; Finocchio, C.P.S.; Barichello, R.; Pedrozo, E.A. Sustainability and innovation in the Brazilian supply chain of green plastic. J. Clean. Prod. 2018, 177, 12–18. [Google Scholar] [CrossRef]
- European Bioplastic Association. Bioplastic Facts and Figure; European Bioplastic Association: Berlin, Germany, 2019. [Google Scholar]
- Rahman, M.H.; Bhoi, P.R. An overview of non-biodegradable bioplastics. J. Clean. Prod. 2021, 294, 126218. [Google Scholar] [CrossRef]
- Aeschelmann, F.; Michael Carus, M. Biobased Building Blocks and Polymers in the World: Capacities, Production, and Applications–Status quo and Trends towards 2020; Technical Report; nova-Institut GmbH: Schelt, Germany, 2015. [Google Scholar]
- Kalendova, A.; Smotek, J.; Stloukal, P.; Kracalik, M.; Slouf, M.; Laske, S. Transport properties of polylactic acid/clay nanocomposites. Polym. Eng. Sci. 2019, 59, 2498. [Google Scholar] [CrossRef]
- Ostrowska, J.; Sadurski, W.; Paluch, M.; Tynski, P.; Bogusz, J. The effect of poly(butylene succinate) content on the structure and thermal and mechanical properties of its blends with polylactide. Polym. Int. 2019, 68, 1271. [Google Scholar] [CrossRef]
- Taniguchi, I.; Yoshida, S.; Hiraga, K.; Miyamoto, K.; Kimura, Y.; Oda, K. Biodegradation of PET: Current status and application aspects. ACS Catal. 2019, 9, 4089–4105. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, W.; Wu, Q.; Ren, J. Preparation, mechanical, and thermal properties of biodegradable polyesters/polylactic acid blends. J. Nanomater. 2010, 2010, 8. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Wan, G.; Li, B.; Zhao, G. Glass fibre reinforced PLA composites with enhanced mechanical properties, thermal behavior, and foaming ability. Polymer 2019, 181, 121803. [Google Scholar] [CrossRef]
- Khalid, S.; Yu, L.; Meng, L.; Liu, H.; Ali, A.; Chen, L. Poly(lactic acid)/starch composites: Effect of microstructure and morphology of starch granules on performance. J. Appl. Polym. Sci. 2017, 134, 45504. [Google Scholar] [CrossRef]
- Avérous, L.; Pollet, E. Nanobiocomposites based on plasticized starch. In Starch Polymers; Halley, P.J., Avérous, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; p. 211. [Google Scholar]
- Hajiali, F.; Tajbakhsh, S.; Shojaei, A. Fabrication properties of polycarprolactone composites containing calcium phosphate-based ceramics and bioactive glasses in bone tissue engineering: A review. Polym. Rev. 2018, 58, 164. [Google Scholar] [CrossRef]
- Pei, E.; Lanzotti, A.; Grasso, M.; Staiano, G.; Martorelli, M. The impact of process parameters on mechanical properties of parts fabricated in PLA with an open-source 3-D printer. Rapid Prototyp. J. 2015, 21, 5. [Google Scholar]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. Synthesis and production of poly(lactic acid). Pdl. Handb. Ser. 2013, 71–107. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of polylactic acid. J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Batori, V.; Akesson, D.; Zamani, A.; Taherzadeh, M.J.; Sarvari Horvath, I. Anaerobic degradation of bioplastic: A review. Waste Manag. 2018, 80, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Pantani, R. Hydrolysis and biodegradation of poly(lactic acid). Adv. Polym. Sci. 2017, 279, 119–151. [Google Scholar]
- Iniguez-Franco, F.; Auras, R.; Rubino, M.; Dolan, K.; Soto-Valdez, H.; Selke, S. Effect of nanoparticles on the hydrolytic degradation of PLA-nanocomposites by water-ethanol solutions. Polym. Degrad. Stab. 2017, 146, 287. [Google Scholar] [CrossRef]
- Luo, Y.B.; Wang, X.L.; Wang, Y.Z. Effect of TiO2 nanoparticles on the long-term hydrolytic degradation behavior of PLA. Polym. Degrad. Stab. 2012, 97, 721. [Google Scholar] [CrossRef]
- Ndazi, B.S.; Karlsson, S. Characterization of hydrolytic degradation of polylactic acid/rice hulls composites in water at different temperatures. Express Polym. Lett. 2011, 5, 119. [Google Scholar] [CrossRef]
- Ozdemir, E.; Lekesiz, T.; Hacaloglu, J. Polylactide/organically modified montmorillonite composites; effects of organic modifier on thermal characteristics. Polym. Degrad. Stab. 2016, 134, 87–96. [Google Scholar] [CrossRef]
- Limsukon, W.; Auras, R.; Selke, S. Hydrolytic degradation and lifetime prediction of poly(lactic acid) modified with a multifunctional epoxy-based chain extender. Polym. Test. 2019, 80, 106108. [Google Scholar] [CrossRef]
- Shirahase, T.; Komatsu, Y.; Marubayashi, H.; Tominaga, Y.; Asai, S.; Sumita, M. Miscibility and hydrolytic degradation in alkaline solution of poly(1-lactide) and poly(p-vinyl phenol) blends. Polym. Degrad. Stab. 2007, 92, 1626–1631. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, R.N.; Kumar, G.; Kim, D.S.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, L.; Wang, Y. Development of PLA-PHB-based biodegradable active packaging and its application to salmon. Packag. Technol. Sci. 2018, 31, 739–746. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Castro-Lopez, M.D.M.; Rayon, E.; Barral-Losada, L.F.; Lopez-Vilarino, J.M.; Lopez, J.; Gonzalez-Rodriguez, M.V. Plasticized poly(lactic acid)-poly(hydroxybutyrate) blends incorporated with catechin intended for active food-packaging applications. J. Agric. Food Chem. 2014, 62, 10170–10180. [Google Scholar] [CrossRef] [PubMed]
- Amini, F.; Semnani, D.; Karbasi, S.; Banitaba, S.N. A novel bilayer drug-loaded wound dressing of PVDF and PHB/Chitosan nanofibers applicable for post-surgical ulcers. Int. J. Polym. Mater. 2019, 68, 772–777. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, Z.R.; Gong, T.; Chen, G.Q.; Sun, X. A rapid-acting, long-acting insulin formulation based on a phospholipid complex loaded PHBHHx nanoparticles. Biomaterials 2012, 33, 1583–1588. [Google Scholar] [CrossRef]
- Sabarinathan, D.; Chandrika, S.P.; Venkatraman, P.; Easwaran, M.; Sureka, C.S.; Preethi, K. Production of polyhydroxybutyrate (PHB) from Pseudomonas plecoglossicida and its application towards cancer detection. Inf. Med. Unlocked 2018, 11, 61–67. [Google Scholar] [CrossRef]
- Zaharia, C.; Vasile, E.; Galateanu, B.; Bunea, M.C.; Casarica, A.; Stanescu, P.O. Bacterial cellulose-polyhydroxyalkanoates composites synthesis, physico-chemical characterization and biological evaluation for tissue engineering. Mater. Plast. 2014, 51, 1–5. [Google Scholar]
- Li, S.Y.; Dong, C.L.; Wang, S.Y.; Ye, H.M.; Chen, G.Q. Microbial production of polyhydroxyalkanoate block copolymer by recombinant Pseudomonas putida. Appl. Microbiol. Biotechnol. 2011, 90, 659–669. [Google Scholar] [CrossRef] [PubMed]
| Polymer | Low-Temperature Products | High-Temperature Products |
|---|---|---|
| Polyethylene (PE) | Waxes, paraffin oil, alpha-olefins | Gases and light oils |
| Polypropylene (PP) | Vaseline, olefins | Gases and light oils |
| Polystyrene (PS) | Styrene and its oligomers | Styrene and its oligomers |
| Polymethyl methacrylate (PMMA) | Methylmethacrylate (monomer) | Low methyl methacrylate, more decomposition products |
| Polyethylene terephthalate (PET) | Benzoic acid (BA), Vinyl terephthalate | |
| Polyamide 6 (PA6) | ε-caprolactum (CPL) |
| S. No. | Plastics | Method | Conditions | Product Yields | Key Findings | Source |
|---|---|---|---|---|---|---|
| 1. | PE | Noncatalytic pyrolysis | T = 602 °C | Paraffins 45%; Olefins 32%; Naphthalenes 17%;Aromatics 6%; | A whole spectrum of HCs, including paraffins, olefins, naphthalenes, and aromatics. | [24] |
| PP | T = 602 °C | Paraffins 27%; Olefins 36%; Aromatics 11%; | ||||
| PS | T = 477 °C | Styrene 63% | ||||
| 2. | HDPE | Thermal-catalytic two-step pyrolysis | T = 500 °C | Light olefin 59% | Higher efficiency of the two-step reaction system compared to the in situ catalytic pyrolysis (single-step) for production of 10 wt.% ethylene, 32 wt.% propylene, and 17 wt.% butenes. | [25] |
| 3. | PP and PE | Fluidized bed reactor | T = 650–750 °C | BTX 32–53% | Higher feed rates and gaseous fluidizing medium have a positive effect on liquid oil production. | [26] |
| 4. | PE | Mini-autoclave reactor (unstirred) | T = 280 °C, t = 24 h, Pt/Al2O3 | Liquid product 80% | Tandem catalytic conversion produces a high yield of low-molecular-weight liquid/wax products. | [27] |
| 5. | PS | Fluidized bed reactor | T = 520 °C | Styrene 83% | Complete conversion of PS to styrene oil was reported, with only traces of aliphatic compounds | [28] |
| 6. | PS + organic compounds | Autoclave reactor | T = 400 °C, t = 1 h | Liquid 91%; Residue < 4% | Maximum styrene yield in the liquid was obtained with naphthalene as an organic compound with PS | [29] |
| 7. | PS | Flow reactor | T = 350 °C, t = 3 h, Fe2O3 | Liquid 83.6%; Residue 4.8% Styrene 74.3% (in liquid oil) | Barium oxide powder was found to be most effective catallyst for chemical recyling of PS waste | [30] |
| T = 350 °C, t = 3 h, BaO | Liquid 93.4%; Residue 3.2% Styrene 76.4% (in liquid oil) | |||||
| T = 350 °C, t = 3 h, HSM5 | Liquid 78.2%; Residue 8.5% Styrene 64.4% (in liquid oil) | |||||
| 8. | PS | Fixed-bed reactor | T = 510 °C thermal | Liquid 91.8%; Residue 5.7% | Other aromatic compounds can behave like a chain transfer agent and reduce the Tg of product polymer. | [31] |
| T = 510 °C BaO (cat.) | Liquid 91.2%; Residue 8.1% | |||||
| T = 510 °C FCC cat. | Liquid 90.7%; Residue 7.1% | |||||
| 9. | PS | Two-stage auger and fluidized bed reactor | T = 780 °C | BTEX 26.3% | Product yields depend on the reaction temperatures and fluidizing mediums used. | [32] |
| 10. | PET | Glycolysis | T = 190 °C; atm pressure | BHET 100% conversion, 84% yields | Lewis acidic ionic liquids [Bmim]ZnCl3 catalyst was found to be effective. | [33] |
| Hydrolysis | T = 200–250 °C; P = 1.4–2 MPa | TPA, EG | [34] | |||
| Methanolysis | T = 200 °C | DMT 64%; EG 63% | The product yields depend on the solubility of PET. | [35] | ||
| Aminolysis | T = 70–110 °C | Diamides of TPA 66–89% | The bifunctional 1,5,7-triazabicyclo [4.4.0]dec-5-ene activates the carbonyl group and catalyzes the reaction. | [36] | ||
| Pyrolysis | T = 450–600 °C ZSM-5 zeolite and NiCl2 used as catalyst | Aromatic hydroxyl groups increased by 22% | ZSM-5 facilitated the decomposition of carboxyl, aliphatic groups, and ether bonds in the primary products produced from the PET pyrolysis. | [37] | ||
| Pyrolysis | T = 400–700 °C | Phenyl carboxylic acid 44–79% | Pd loaded on activated carbon used as a catalyst and produced more environmentally friendly products | [37] | ||
| 11. | PET | Py-GCMS, EGA-MS, and TGA | T = 600 °C | 4(vinyl oxy carbonyl) BA 27%; BA 10% | Wide range of liquid products obtained by different pyrolysis mechanisms. | [38] |
| 12. | PU | Glycolysis | T = 200–210 °C; t = 2 h | Acetone-soluble products 80.8%; Residue 19%; Amines in total acetone soluble products 58.3 mgKOH/g | Polyol products produced from the process and used as initiators to produce oxy-alkylated polyols. | [39] |
| 13. | PA 6 and PA66 | Aminolysis | T = 100 °C; P = 3.5 MPa; t = 5.6 h; Raney® Co 2724 | ACN = 2; HMD = 32%; CPL = 46.2%; Other components = 13.6% | Raney® Co provided a better catalytic activity along with long catalyst life | [40] |
| T = 100 °C; P = 3.5 MPa; t = 5.6 h; Raney® Ni 2400 | ACN = 19.6; HMD = 15%; CPL = 46.5%; Other components = 14.7% | |||||
| 14. | PA66 | Microwave irradiation | T = 200 °C; t = 0.16 h; HCl:PA66 = 1:0.25 | AA 90%; HMDA 86%; with 100% purity | The rate of PA hydrolysis depended on the PA type and HCl/amide molar ratio. With microwave treatment, high-purity and high-quality products were formed. | [41] |
| Process | Operating Conditions | Plastics | Products |
|---|---|---|---|
| Anoxic pyrolysis Carbonization | |||
| Without stabilization | T= 500–1000 °C in an inert atmosphere or in molten salt; | PET, PFR | Amorphous carbon products without metal impurities such as activated carbon, mesoporous carbon, and carbon fibers |
| Oxidation stabilization | Oxidation at T = 200–350 °C in the air; carbonization at T = 500–1000 °C in an inert atmosphere | PAN, LDPE, PVC, | |
| Chemical stabilization | Sulfonation or Friedel–Crafts reaction | PE, PS | |
| Catalytic Carbonization | |||
| Catalytic carbonization | T = 400–900 °C in an inert atmosphere; with metal catalysts | PE, PP, PS, PVC, PTFE, PVA, PET, PFR | Graphitic carbons contain trace metals such as carbon nanotubes, carbon nanosheets, graphene, carbon foam |
| Catalytic pressure carbonization | T = 600–850 °C; with metal catalysts (in sealed reactor) | PP, PE, PS | Graphitic carbons |
| Pressure Carbonization | |||
| Pressurized atmosphere | T = 600–850 °C (in sealed reactor) | PP, PE, PS, PVC PVC | Amorphous carbon such as carbon spheres, activated carbon, and carbon dots without metal impurities |
| Hydrothermal carbonization | T = 150–300 °C in the presence of water (in sealed reactor) | ||
| Plastics | Process | Catalyst | Reaction Conditions | Product Yields, wt.% | Source |
|---|---|---|---|---|---|
| Post-consumer plastic waste | Pyrolysis– catalysis | Ni-Fe/ZSM5 | Catalysis T = 800 °C; Pyrolysis T = 500 °C for 15 min. | Carbon deposition = 50; Oil = 17; Gas yield = 37.80; H2 yield * = 35.80 | [78] |
| Ni-Fe/MCM41 | Carbon deposition = 55.60; Oil = 16.30; Gas yield = 30.80; H2 yield * = 38.10 | ||||
| Ni-Fe/NKF5 | Carbon deposition = 36.60; Oil = 27.40; Gas yield = 34; H2 yield * = 22.40 | ||||
| Ni-Fe/Beta | Carbon deposition = 47; Oil = 15.10; Gas yield = 32.10; H2 yield * = 32.80 | ||||
| PP | Pyrolysis–catalysis | Ni-Fe | Catalysis T = 800 °C; | Gas = 48.5; Liquid = 20; Solid = 36 Carbon deposition = 360 mg/g feed | [79] |
| PP | - | 10Mn-9Fe/Al2O3 | T = 800 °C | Filamentous C = 32.89; Amorphous C = 8.69 | [80] |
| PVC + PP | Pressure carbonization | T = 650 °C; P = 30 MPa | Carbon yield = 45 | [81] | |
| PP | Microwave assisted hydrothermal treatment | - | T = 250 °C; t =60 min | Amorphous carbon = 69 | [82] |
| Plastic waste mixture | Microwave-assisted catalytic pyrolysis | FeAlOx | 1000 W microwave power; t = 3-5 min | Carbon production = 1560 mgC/g plastic/g catalyst with 92% multiwalled CNTs | [83] |
| Waste Plastic (used cups, bottles, and PE bags) | Hydrothermal carbonization | - | T = 400 °C; t = 2 h | C-dot | [84] |
| PET | Air oxidation and acid treatment | - | Air Oxidation (T = 300 °C, time = 2 h); acid treatment (T = 120 °C, t = 6 h) | C-dots of diameters 1–6 nm | [85] |
| PLA | Hydrothermal carbonization | T = 180–240 °C; t = 4 h | C-dots of diameter 3 nm | [86] | |
| PP waste | Ferrocene and Sulfur | T = 700 °C; t = 1.5 h | CNS = 62.8 | [87] | |
| Plastic waste (PP, PE, and PET) | Two-stage pyrolysis | Bentonite nanoclay | T1 = 450 °C; T2 = 945 °C | Graphene nanosheet | [88] |
| PET | Catalytic carbonization | MgO/Co (acac)3 | PET:catalyst massratio = 1:2; T = 700 °C | CNS = 36 | [89] |
| BDP | Feedstock | Properties | Limitations | Source |
|---|---|---|---|---|
| Polyhydroxyalkanoates (PHAs) | Microorganisms | UV stable, good humidity, and moisture resistance | Expensive and low thermal stability | [97] |
| Polybutylene succinate (PBS) | Succinic acid and 1,4-butanediol | Compostable, high flexibility, and outstanding thermal stability | Insufficient melt viscosity and stiffness | [98] |
| Lignin-based polymer composites such as polyethylene terephthalate (PET) | Lignin | Light weight, antimicrobial and environmentally friendly | High agglomeration | [99] |
| Polybutylene adipate terephthalate (PBAT) | 1,4-butanediol, adipic acid, and dimethyl terephthalate (DMT) with 1,4-butanediol | Flexible and resilient | Low crystallization degree | [100] |
| Polylactic acid (PLA) | Starch | Compostable | Brittle and nonbiodegradable | [101] |
| Thermoplastic starch blend, for example, PLA/starch | Starch and plasticizers | Low cost | Brittle and low biodegradability | [102] |
| Cellulose bioplastic | Cellulose | Trouble-free processing | More costly | [103] |
| Polycaprolactones (PCL) | Petrochemical products | Very low degradation rate | Resistance to solvents | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prajapati, R.; Kohli, K.; Maity, S.K.; Sharma, B.K. Potential Chemicals from Plastic Wastes. Molecules 2021, 26, 3175. https://doi.org/10.3390/molecules26113175
Prajapati R, Kohli K, Maity SK, Sharma BK. Potential Chemicals from Plastic Wastes. Molecules. 2021; 26(11):3175. https://doi.org/10.3390/molecules26113175
Chicago/Turabian StylePrajapati, Ravindra, Kirtika Kohli, Samir K. Maity, and Brajendra K. Sharma. 2021. "Potential Chemicals from Plastic Wastes" Molecules 26, no. 11: 3175. https://doi.org/10.3390/molecules26113175
APA StylePrajapati, R., Kohli, K., Maity, S. K., & Sharma, B. K. (2021). Potential Chemicals from Plastic Wastes. Molecules, 26(11), 3175. https://doi.org/10.3390/molecules26113175






