Pigeon Pea Husk for Removal of Emerging Contaminants Trimethoprim and Atenolol from Water
Abstract
1. Introduction
2. Results and Discussions
2.1. Surface Morphology and Physical Characteristics of Pigeon Pea Husk (PPH)
2.2. Characterization of Surface Functionalities and Adsorption Process
2.3. Adsorption Isotherm and Saturation Limit
2.4. Adsorption Kinetics
2.5. Effect of Temperature and Adsorption Thermodynamics
2.6. Effect of pH on Adsorption Performance
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Pigeon Pea Husk (PPH) Adsorbent Material
3.3. Characterization of PPH and Functional Investigations
3.4. Batch Adsorption Design
3.5. Determination of Trimethoprim and Atenolol Concentration
3.6. Adsorption Isotherm Models
3.7. Isotherm Model Selection for Adsorption Equilibrium Description
3.8. External Mass Transfer Model (EMTM)
3.9. Adsorption Thermodynamics
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bhatnagar, A.; Sillanpää, M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Gani Khalid, M.; Kazmi Absar, A. Contamination of Emerging Contaminants in Indian Aquatic Sources: First Overview of the Situation. J. Hazard. ToxicRadioact. Waste 2017, 21, 04016026. [Google Scholar] [CrossRef]
- Grassi, M.; Kaykioglu, G.; Belgiorno, V.; Lofrano, G. Removal of Emerging Contaminants from Water and Wastewater by Adsorption Process. In Emerging Compounds Removal from Wastewater: Natural and Solar Based Treatments; Lofrano, G., Ed.; Springer: Dordrecht, The Netherland, 2012; pp. 15–37. [Google Scholar]
- Philip, J.M.; Aravind, U.K.; Aravindakumar, C.T. Emerging contaminants in Indian environmental matrices—A review. Chemosphere 2018, 190, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna, K.; Rath, A.; Praveenkumarreddy, Y.; Guruge, K.S.; Subedi, B. A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol. Environ. Saf. 2017, 137, 113–120. [Google Scholar] [CrossRef]
- Kraupner, N.; Ebmeyer, S.; Hutinel, M.; Fick, J.; Flach, C.F.; Larsson, D.G.J. Selective concentrations for trimethoprim resistance in aquatic environments. Environ. Int. 2020, 144, 106083. [Google Scholar] [CrossRef]
- Steinbach, C.; Burkina, V.; Fedorova, G.; Grabicova, K.; Stara, A.; Velisek, J.; Zlabek, V.; Schmidt-Posthaus, H.; Grabic, R.; Kocour Kroupova, H. The sub-lethal effects and tissue concentration of the human pharmaceutical atenolol in rainbow trout (Oncorhynchus mykiss). Sci. Total Environ. 2014, 497–498, 209–218. [Google Scholar] [CrossRef]
- Singh, K.P.; Rai, P.; Singh, A.K.; Verma, P.; Gupta, S. Occurrence of pharmaceuticals in urban wastewater of north Indian cities and risk assessment. Environ. Monit. Assess. 2014, 186, 6663–6682. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Parate, V.R.; Talib, M.I. Utilization of Pulse Processing Waste (Cajanus cajan Husk) for Developing Metal Adsorbent: A Value-added Exploitation of Food Industry Waste. Am. J. Food Sci. Technol. 2015, 3, 1–9. [Google Scholar]
- Hang, C.C.; Garnsey, E.; Ruan, Y. Opportunities for disruption. Technovation 2015, 39–40, 83–93. [Google Scholar] [CrossRef]
- Saxena, K.B.; Kumar, R.V.; Sultana, R. Quality nutrition through pigeonpea—A review. Health 2010, 2, 1335–1344. [Google Scholar] [CrossRef]
- Sarkar, S.; Panda, S.; Yadav, K.K.; Kandasamy, P. Pigeon pea (Cajanus cajan) an important food legume in Indian scenario—A review. Legume Res. Int. J. 2018, 43, 601–610. [Google Scholar] [CrossRef]
- Aravind, J.; Muthusamy, S.; Sunderraj, S.H.; Chandran, L.; Palanisamy, K. Pigeon pea (Cajanus cajan) pod as a novel eco-friendly biosorbent: A study on equilibrium and kinetics of Ni(II) biosorption. Int. J. Ind. Chem. 2013, 4, 25. [Google Scholar] [CrossRef]
- Venkata Ramana, D.K.; Harikishore Kumar Reddy, D.; Yu, J.S.; Seshaiah, K. Pigeon peas hulls waste as potential adsorbent for removal of Pb(II) and Ni(II) from water. Chem. Eng. J. 2012, 197, 24–33. [Google Scholar] [CrossRef]
- Aravind, J.; Lenin, C.; Nancyflavia, C.; Rashika, P.; Saravanan, S. Response surface methodology optimization of nickel (II) removal using pigeon pea pod biosorbent. Int. J. Environ. Sci. Technol. 2015, 12, 105–114. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Gupta, A.K. An insight into thermodynamics of adsorptive removal of fluoride by calcined Ca–Al–(NO3) layered double hydroxide. RSC Adv. 2015, 5, 105889–105900. [Google Scholar] [CrossRef]
- Spiess, A.-N.; Neumeyer, N. An evaluation of R2 as an inadequate measure for nonlinear models in pharmacological and biochemical research: A Monte Carlo approach. BMC Pharm. 2010, 10, 6. [Google Scholar] [CrossRef]
- Dotto, G.L.; Salau, N.P.G.; Piccin, J.S.; Cadaval, T.R.S.A.; de Pinto, L.A.A. Adsorption Kinetics in Liquid Phase: Modeling for Discontinuous and Continuous Systems. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D.I., Reynel-Ávila, H.E., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 53–76. [Google Scholar]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. Encycl. Anal. Chem. 2006. [Google Scholar] [CrossRef]
- Manrique, G.D.; Lajolo, F.M. FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Postharvest. Biol. Technol. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Tella, A.C.; Eke, U.B.; Owalude, S.O. Solvent-free mechanochemical synthesis and X-ray studies of Cu(II) and Ni(II) complexes of 5-(3,4,5-Trimethoxybenzyl)pyrimidine-2,4-diamine (Trimethoprim) in a ball-mill. J. Saudi Chem. Soc. 2016, 20, S376–S381. [Google Scholar] [CrossRef]
- Djellouli, F.; Dahmani, A.; Hassani, A. Characterization of the polymorph changes in Trimethoprim. J. Therm. Anal. Calorim. 2017, 130, 1585–1591. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. (Resumed) 1960, 786, 3973–3993. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Piccin, J.S.; Cadaval, T.R.S.A.; de Pinto, L.A.A.; Dotto, G.L. Adsorption Isotherms in Liquid Phase: Experimental, Modeling, and Interpretations. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D.I., Reynel-Ávila, H.E., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 19–51. [Google Scholar]
- Worch, E. Adsorption equilibrium I: General aspects and single-solute adsorption. In Adsorption Technology in Water Treatment, Fundamentals, Processes, and Modeling; De Gruyter: Berlin, Germany; Boston, FL, USA, 2012; pp. 41–76. [Google Scholar]
- Li, Y.; Taggart, M.A.; McKenzie, C.; Zhang, Z.; Lu, Y.; Pap, S.; Gibb, S. Utilizing low-cost natural waste for the removal of pharmaceuticals from water: Mechanisms, isotherms and kinetics at low concentrations. J. Clean. Prod. 2019, 227, 88–97. [Google Scholar] [CrossRef]
- Nielsen, L.; Bandosz, T.J. Analysis of sulfamethoxazole and trimethoprim adsorption on sewage sludge and fish waste derived adsorbents. Microporous Mesoporous Mater. 2016, 220, 58–72. [Google Scholar] [CrossRef]
- Bekçi, Z.; Seki, Y.; Yurdakoç, M.K. Equilibrium studies for trimethoprim adsorption on montmorillonite KSF. J. Hazard. Mater. 2006, 133, 233–242. [Google Scholar] [CrossRef]
- Hu, Y.; Fitzgerald, N.M.; Lv, G.; Xing, X.; Jiang, W.-T.; Li, Z. Adsorption of Atenolol on Kaolinite. Adv. Mater. Sci. Eng. 2015, 2015, 897870. [Google Scholar] [CrossRef]
- Haro, N.K.; Del Vecchio, P.; Marcilio, N.R.; Féris, L.A. Removal of atenolol by adsorption—Study of kinetics and equilibrium. J. Clean. Prod. 2017, 154, 214–219. [Google Scholar] [CrossRef]
- To, M.-H.; Hadi, P.; Hui, C.-W.; Lin, C.S.K.; McKay, G. Mechanistic study of atenolol, acebutolol and carbamazepine adsorption on waste biomass derived activated carbon. J. Mol. Liq. 2017, 241, 386–398. [Google Scholar] [CrossRef]
- Worch, E. Adsorption kinetics. In Adsorption Technology in Water Treatment, Fundamentals, Processes, and Modeling; De Gruyter: Berlin, Germany; Boston, FL, USA, 2012; pp. 123–168. [Google Scholar]
- Saha, P.; Chowdhury, S. Insight Into Adsorption Thermodynamics. In Thermodynamics; Tadashi, M., Ed.; IntechOpen: London, UK, 2011; pp. 349–364. [Google Scholar]
- Wang, P.; He, Y.-L.; Huang, C.-H. Oxidation of Antibiotic Agent Trimethoprim by Chlorine Dioxide: Reaction Kinetics and Pathways. J. Environ. Eng. 2012, 138, 360–366. [Google Scholar] [CrossRef]
- Martínez, V.; Maguregui, M.I.; Jiménez, R.M.; Alonso, R.M. Determination of the pKa values of β-blockers by automated potentiometric titrations. J. Pharm. Biomed. Anal. 2000, 23, 459–468. [Google Scholar] [CrossRef]
- Avvannavar, S.M.; Shrihari, S. Evaluation of water quality index for drinking purposes for river Netravathi, Mangalore, South India. Environ. Monit. Assess. 2008, 143, 279–290. [Google Scholar] [CrossRef]
- Bhardwaj, R.M. Water Quality Monitoring In India- Achievements and Constraints. Int. J. Inf. Comput. Technol. 2005, 3, 851–856. [Google Scholar]
- Dash, A.K. Characterization of Domestic Wastewater at Bhubaneswar, Odisha, India. Development 2013, 25, 27. [Google Scholar]
- Souza, P.R.; Dotto, G.L.; Salau, N.P.G. Detailed numerical solution of pore volume and surface diffusion model in adsorption systems. Chem. Eng. Res. Des. 2017, 122, 298–307. [Google Scholar] [CrossRef]
- Krisko, R.M.; McLaughlin, K.; Koenigbauer, M.J.; Lunte, C.E. Application of a column selection system and DryLab software for high-performance liquid chromatography method development. J. Chromatogr. A 2006, 1122, 186–193. [Google Scholar] [CrossRef][Green Version]
- Sugiura, N. Further analysts of the data by akaike’s information criterion and the finite corrections. Commun. Stat. Theory Methods 1978, 7, 13–26. [Google Scholar] [CrossRef]
- Furusawa, T.; Smith, J.M. Fluid-Particle and Intraparticle Mass Transport Rates in Slurries. Ind. Eng. Chem. Fundam. 1973, 12, 197–203. [Google Scholar] [CrossRef]
 ), ATN (
), ATN ( ), PPH (
), PPH ( ) and after adsorption of TMP (
) and after adsorption of TMP ( ) or ATN (
) or ATN ( ) on PPH.
) on PPH.
 ), ATN (
), ATN ( ), PPH (
), PPH ( ) and after adsorption of TMP (
) and after adsorption of TMP ( ) or ATN (
) or ATN ( ) on PPH.
) on PPH.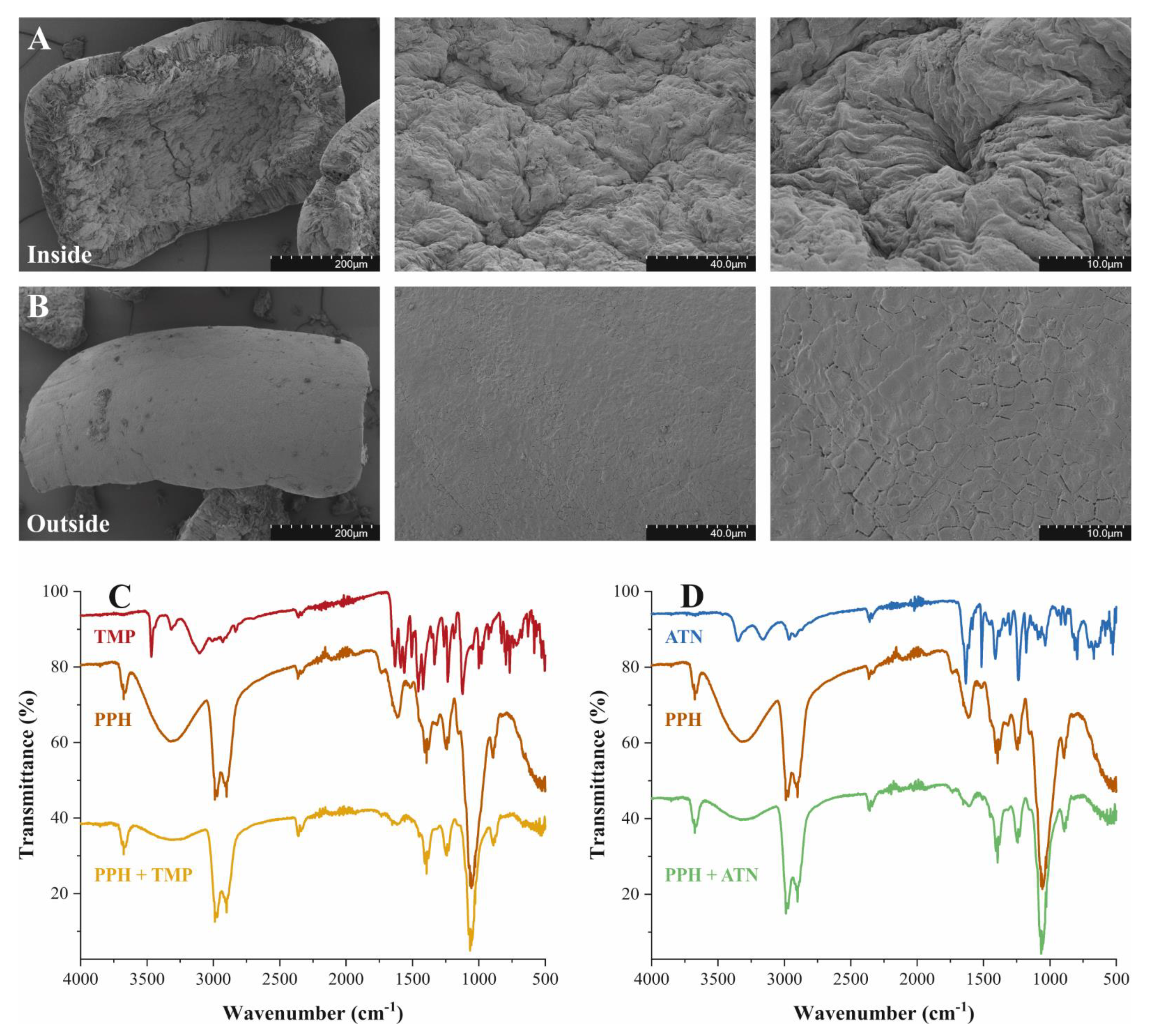
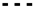 ) of the adsorption isotherms for (A) TMP (
) of the adsorption isotherms for (A) TMP ( ) and (B) ATN adsorption (
) and (B) ATN adsorption ( ) on PPH in the ppb concentration range. Experimental data (■) and Langmuir isotherm model (
) on PPH in the ppb concentration range. Experimental data (■) and Langmuir isotherm model (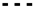 ) of the adsorption isotherms for (C) TMP (
) of the adsorption isotherms for (C) TMP ( ) and (D) ATN adsorption (
) and (D) ATN adsorption ( ) on PPH in the ppm concentration range. Different letters denote significant differences between isotherm points (p < 0.05, n = 3).
) on PPH in the ppm concentration range. Different letters denote significant differences between isotherm points (p < 0.05, n = 3).
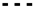 ) of the adsorption isotherms for (A) TMP (
) of the adsorption isotherms for (A) TMP ( ) and (B) ATN adsorption (
) and (B) ATN adsorption ( ) on PPH in the ppb concentration range. Experimental data (■) and Langmuir isotherm model (
) on PPH in the ppb concentration range. Experimental data (■) and Langmuir isotherm model (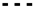 ) of the adsorption isotherms for (C) TMP (
) of the adsorption isotherms for (C) TMP ( ) and (D) ATN adsorption (
) and (D) ATN adsorption ( ) on PPH in the ppm concentration range. Different letters denote significant differences between isotherm points (p < 0.05, n = 3).
) on PPH in the ppm concentration range. Different letters denote significant differences between isotherm points (p < 0.05, n = 3).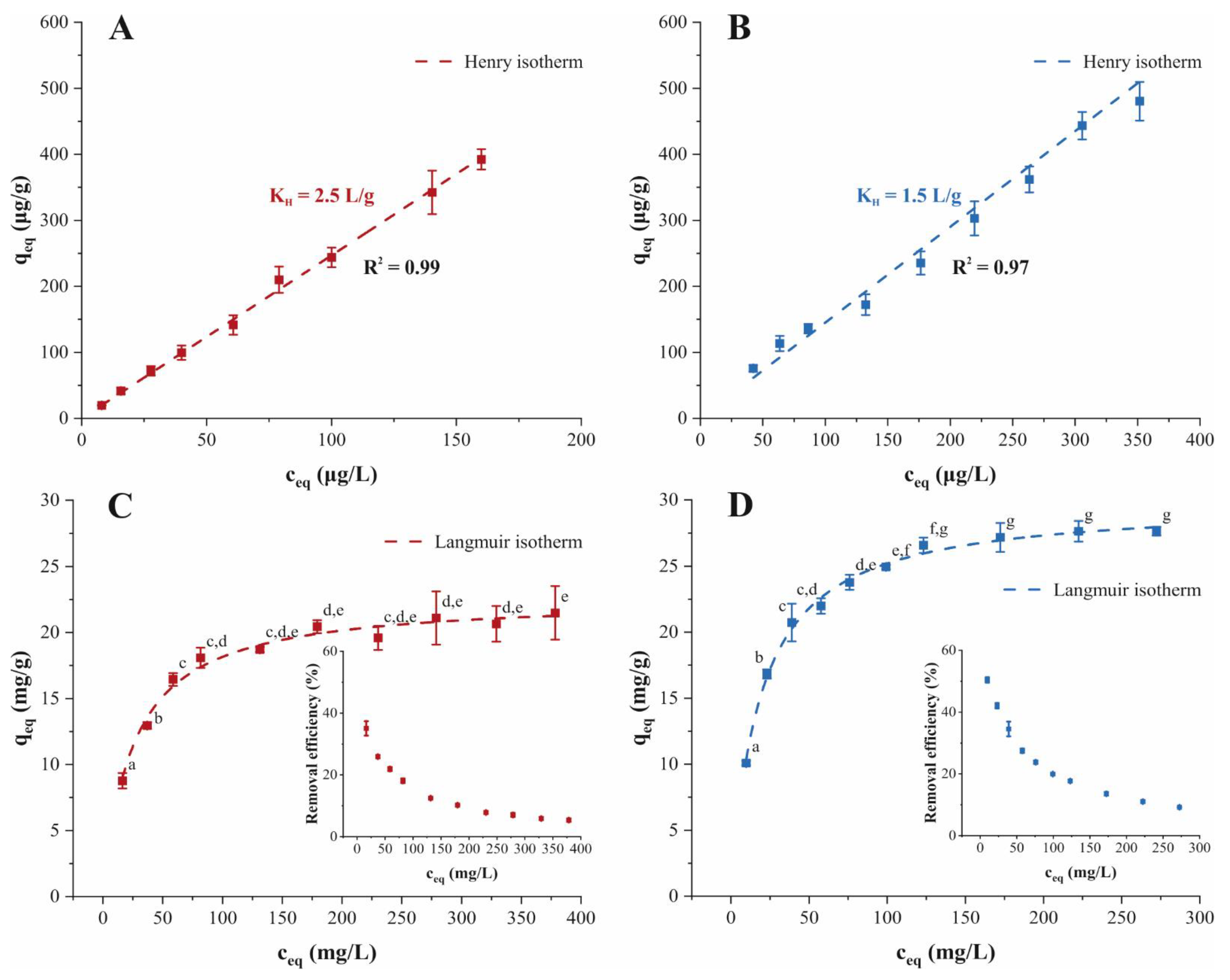
 ) and (B) ATN (
) and (B) ATN ( ) decay curves with the EMTM during adsorption on PPH at six initial concentrations, respectively.
) decay curves with the EMTM during adsorption on PPH at six initial concentrations, respectively.
 ) and (B) ATN (
) and (B) ATN ( ) decay curves with the EMTM during adsorption on PPH at six initial concentrations, respectively.
) decay curves with the EMTM during adsorption on PPH at six initial concentrations, respectively.
 ) of the effect of temperature on the adsorption performance of (A) TMP (
) of the effect of temperature on the adsorption performance of (A) TMP ( ) and (B) ATN (
) and (B) ATN ( ) on PPH. Van’t Hoff plot and regression line (
) on PPH. Van’t Hoff plot and regression line ( ) of (C) TMP (
) of (C) TMP ( ) and (D) ATN (
) and (D) ATN ( ) adsorption on PPH. Different letters denote significant differences between isotherm points (p < 0.05, n = 3).
) adsorption on PPH. Different letters denote significant differences between isotherm points (p < 0.05, n = 3).
 ) of the effect of temperature on the adsorption performance of (A) TMP (
) of the effect of temperature on the adsorption performance of (A) TMP ( ) and (B) ATN (
) and (B) ATN ( ) on PPH. Van’t Hoff plot and regression line (
) on PPH. Van’t Hoff plot and regression line ( ) of (C) TMP (
) of (C) TMP ( ) and (D) ATN (
) and (D) ATN ( ) adsorption on PPH. Different letters denote significant differences between isotherm points (p < 0.05, n = 3).
) adsorption on PPH. Different letters denote significant differences between isotherm points (p < 0.05, n = 3).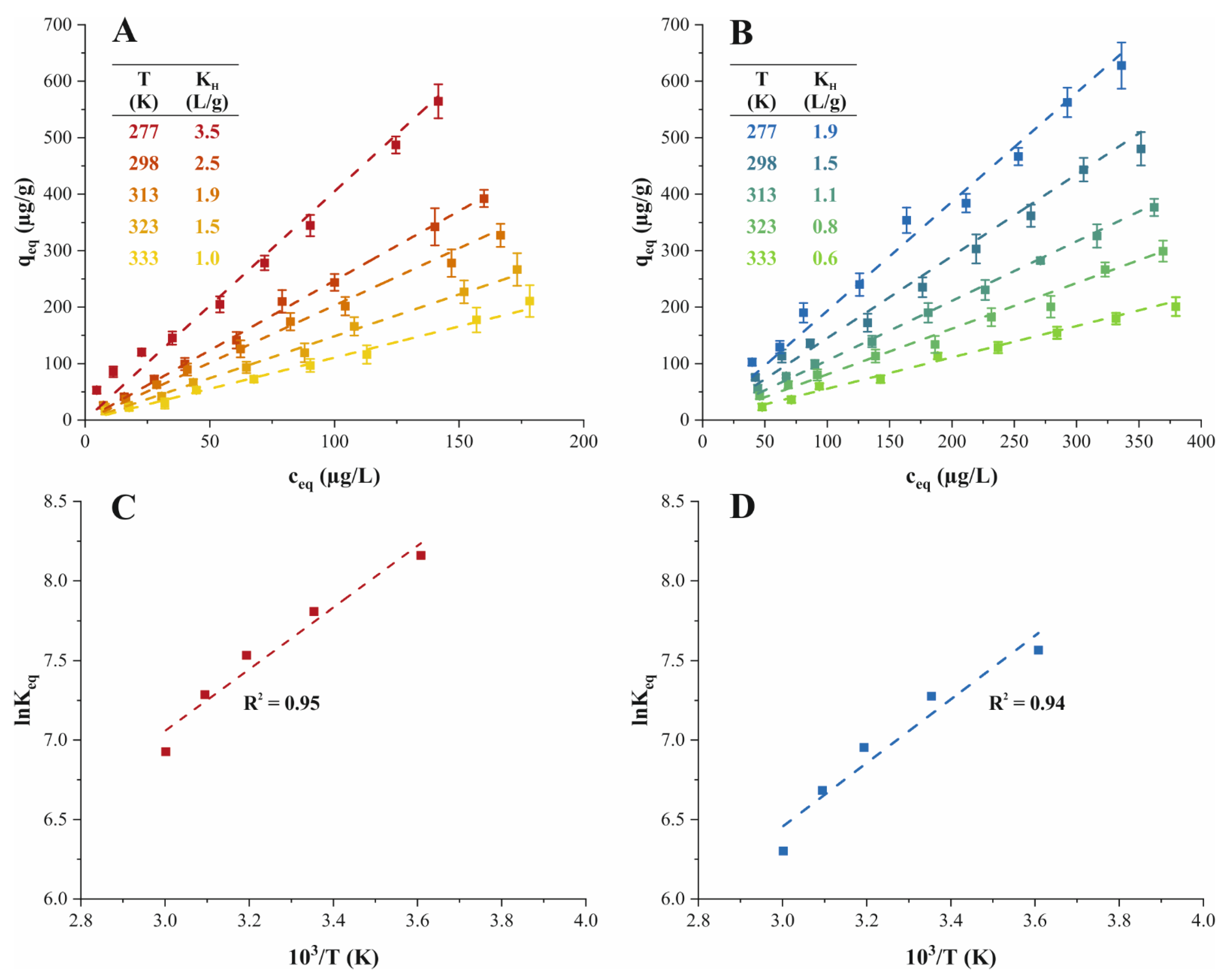

| Physical Properties | PPH |
|---|---|
| Specific surface area (m2/g) | 0.21 |
| Particle size (μm) | 74 |
| Apparent density (g/cm3) | 0.72 |
| Solid density (g/cm3) | 1.5 |
| Particle porosity (-) | 0.52 |
| Freundlich | Redlich-Peterson | Sips | Tóth | Langmuir | |||
|---|---|---|---|---|---|---|---|
| qmL (mg/g) | KL (L/mg) | RL | |||||
| TMP | 22.7 | 0.04 | 0.06–0.5 | ||||
| K | 2 | 3 | 3 | 3 | 2 | ||
| AIC | 33 | 9 | 9 | 9 | 7 | ||
| AICc | 34 | 10 | 10 | 10 | 8 | ||
| Δi | 26 | 2.5 | 1.8 | 1.7 | 0.0 | ||
| wi | 1.02 × 10−6 | 0.14 | 0.19 | 0.20 | 0.47 | ||
| ER | 461643 | 3 | 2 | 2 | 1 | ||
| qmL (mg/g) | KL (L/mg) | RL | |||||
| ATN | 29.8 | 0.054 | 0.06–0.48 | ||||
| K | 2 | 3 | 3 | 3 | 2 | ||
| AIC | 41 | −12 | −12 | −12 | −14 | ||
| AICc | 42 | −11 | −11 | −11 | −14 | ||
| Δi | 56 | 2.5 | 2.4 | 2.4 | 0.0 | ||
| wi | 4.4 × 10−13 | 0.15 | 0.16 | 0.16 | 0.53 | ||
| ER | 1.2 × 1012 | 3 | 3 | 3 | 1 | ||
| Adsorbent | Adsorbate | Isotherm Model | qmL (mg/g) | Reference |
|---|---|---|---|---|
| Wood chippings | TMP | Langmuir | 8.3 | [31] |
| Carbonized sewage sludge/fish waste | TMP | Langmuir | 90 | [32] |
| Montmorillonite KSF | TMP | Langmuir | 130 | [33] |
| PPH | TMP | Langmuir | 22.7 | This study |
| Kaolinite | ATN | Langmuir | 10.7 | [34] |
| Granular activated carbon | ATN | Langmuir | 18.8 | [35] |
| Activated palm kernel shell | ATN | Langmuir | 192 | [36] |
| PPH | ATN | Langmuir | 29.8 | This study |
| ΔH° (kJ/mol) | ΔS° (J/(mol × K)) | ΔG° (kJ/mol) | |||||
|---|---|---|---|---|---|---|---|
| 277 K | 298 K | 313 K | 323 K | 333 K | |||
| TMP | −17.3 | 7.1 | −19.1 | −19.4 | −19.8 | −19.6 | −19.4 |
| ATN | −16.7 | 3.7 | −17.4 | −18 | −18.1 | −18 | −17.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eder, S.; Torko, M.; Montalbetti, A.; Azzari, P.; Nyström, L. Pigeon Pea Husk for Removal of Emerging Contaminants Trimethoprim and Atenolol from Water. Molecules 2021, 26, 3158. https://doi.org/10.3390/molecules26113158
Eder S, Torko M, Montalbetti A, Azzari P, Nyström L. Pigeon Pea Husk for Removal of Emerging Contaminants Trimethoprim and Atenolol from Water. Molecules. 2021; 26(11):3158. https://doi.org/10.3390/molecules26113158
Chicago/Turabian StyleEder, Severin, Manuel Torko, Alessia Montalbetti, Paride Azzari, and Laura Nyström. 2021. "Pigeon Pea Husk for Removal of Emerging Contaminants Trimethoprim and Atenolol from Water" Molecules 26, no. 11: 3158. https://doi.org/10.3390/molecules26113158
APA StyleEder, S., Torko, M., Montalbetti, A., Azzari, P., & Nyström, L. (2021). Pigeon Pea Husk for Removal of Emerging Contaminants Trimethoprim and Atenolol from Water. Molecules, 26(11), 3158. https://doi.org/10.3390/molecules26113158






