Roles of Nrf2 in Gastric Cancer: Targeting for Therapeutic Strategies
Abstract
1. Introduction
2. Treatment Modalities of Gastric Cancer
3. Nrf2 Inhibitors and Chemotherapy Drug Resistance
3.1. Anti-HER2 Drugs Resistance
3.2. 5-Fluorouracil Resistance
3.3. Oxaliplatin Resistance
3.4. Multidrug Resistance
4. Natural Therapeutic Agents for Gastric Cancer and Nrf2
5. Biomarkers of Gastric Cancer and Nrf2
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| AKR | Aldo-keto reductase |
| AREs | Antioxidant responsive elements |
| BCL2 | B-cell lymphoma 2 |
| BDH2 | 3-Hydroxybutyrate dehydrogenase 2 |
| DATS | Diallyl trisulfide |
| DHA | Dihydroartemisinin |
| DNA | Deoxyribonucleic acid |
| EBV | Epstein–Barr virus |
| EGFR | Anti-epidermal growth factor receptor |
| GC | Gastric cancer |
| GCL | Glutamate–cysteine ligase |
| GRIM | Retinoid-IFN-induced mortality |
| HER2 | Human epidermal growth factor receptor 2 |
| HIF-1a | Hypoxia-inducible factor-1a |
| HO-1 | Heme oxygenase-1 |
| IL | Interleukin |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LEF | Lymphoid enhancer factor |
| MAF | Musculoaponeurotic fibrosarcoma |
| MARE | MAF recognition elements |
| MDR | Multidrug resistance |
| ME | Malic enzyme |
| NAD | Nicotinamide adenine dinucleotide |
| NQO1 | Quinone oxidoreductase 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| P-gp | P-glycoprotein 1 |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| RPS6 | Ribosomal protein S6 |
| TCF | T cell factor |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Anti-vascular endothelial growth factor receptor |
References
- Wright, N.A.; Poulsom, R.; Stamp, G.; van Noorden, S.; Sarraf, C.; Elia, G.; Ahnen, D.; Jeffery, R.; Longcroft, J.; Pike, C.; et al. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology 1993, 104, 12–20. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Abadi, A.J.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Najafi, M.; Entezari, M.; Hushmandi, K.; Aref, A.R.; Khan, H.; Makvandi, P.; et al. The role of SOX family transcription factors in gastric cancer. Int. J. Biol. Macromol. 2021, 180, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Stock, M.; Otto, F. Gene deregulation in gastric cancer. Gene 2005, 360, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemipour, M.; Vosough, M.; Najafi, M.; Shahinozzaman, M.; Hushmandi, K.; Khan, H.; Mirzaei, H. Sensing the scent of death: Modulation of microRNAs by Curcumin in gastrointestinal cancers. Pharmacol. Res. 2020, 160, 105199. [Google Scholar] [PubMed]
- Ashrafizadeh, M.; Najafi, M.; Ang, H.; Moghadam, E.; Mahabady, M.; Zabolian, A.; Jafaripour, L.; Bejandi, A.; Hushmandi, K.; Saleki, H.; et al. PTEN, a Barrier for Proliferation and Metastasis of Gastric Cancer Cells: From Molecular Pathways to Targeting and Regulation. Biomedicines 2020, 8, 264. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Orouei, S.; Zarrin, V.; Rahmani Moghadam, E.; Zabolian, A.; Mohammadi, S.; Hushmandi, K.; Gharehaghajlou, Y.; Makvandi, P.; et al. STAT3 Pathway in Gastric Cancer: Signaling, Therapeutic Targeting and Future Prospects. Biology 2020, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, N.; Franceschi, S. Epidemiology of gastric cancer and perspectives for prevention. Salud Publica Mex. 1997, 39, 318–330. [Google Scholar] [CrossRef]
- Ladeiras-Lopes, R.; Pereira, A.K.; Nogueira, A.; Pinheiro-Torres, T.; Pinto, I.; Santos-Pereira, R.; Lunet, N. Smoking and gastric cancer: Systematic review and meta-analysis of cohort studies. Cancer Causes Control 2008, 19, 689–701. [Google Scholar] [CrossRef]
- Nishino, Y.; Inoue, M.; Tsuji, I.; Wakai, K.; Nagata, C.; Mizoue, T.; Tanaka, K.; Tsugane, S. Tobacco smoking and gastric cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2006, 36, 800–807. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Rafiei, H.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Wnt-regulating microRNAs role in gastric cancer malignancy. Life Sci. 2020, 250, 117547. [Google Scholar] [PubMed]
- Imai, S.; Koizumi, S.; Sugiura, M.; Tokunaga, M.; Uemura, Y.; Yamamoto, N.; Tanaka, S.; Sato, E.; Osato, T. Gastric carcinoma: Monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc. Natl. Acad. Sci. USA 1994, 91, 9131–9135. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, T.L.; Davis, S.; Kristal, A.; Thomas, D.B. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: Adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 1995, 4, 85–92. [Google Scholar]
- Hsing, A.W.; Hansson, L.E.; McLaughlin, J.K.; Nyren, O.; Blot, W.J.; Ekbom, A.; Fraumeni, J.F., Jr. Pernicious anemia and subsequent cancer. A population-based cohort study. Cancer 1993, 71, 745–750. [Google Scholar] [CrossRef]
- Aird, I.; Bentall, H.H.; Roberts, J.A. A relationship between cancer of stomach and the ABO blood groups. Br. Med. J. 1953, 1, 799–801. [Google Scholar] [CrossRef]
- Offerhaus, G.J.; Tersmette, A.C.; Huibregtse, K.; van de Stadt, J.; Tersmette, K.W.; Stijnen, T.; Hoedemaeker, P.J.; Vandenbroucke, J.P.; Tytgat, G.N. Mortality caused by stomach cancer after remote partial gastrectomy for benign conditions: 40 years of follow up of an Amsterdam cohort of 2633 postgastrectomy patients. Gut 1988, 29, 1588–1590. [Google Scholar] [CrossRef]
- Shen, J.; Rasmussen, M.; Dong, Q.R.; Tepel, M.; Scholze, A. Expression of the NRF2 Target Gene NQO1 is Enhanced in Mononuclear Cells in Human Chronic Kidney Disease. Oxid. Med. Cell. Longev. 2017, 2017, 9091879. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.-L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef]
- Zimta, A.A.; Cenariu, D.; Irimie, A.; Magdo, L.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. The Role of Nrf2 Activity in Cancer Development and Progression. Cancers 2019, 11, 1755. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Fekri, H.S.; Ahmadi, Z.; Farkhondeh, T.; Samarghandian, S. Therapeutic and biological activities of berberine: The involvement of Nrf2 signaling pathway. J. Cell. Biochem. 2020, 121, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin activates the Nrf2 pathway and induces cellular protection against oxidative injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. Pharmacotherapy. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Curr. Mol. Med. 2020, 127, 110234. [Google Scholar]
- Mirzaei, S.; Mohammadi, A.T.; Gholami, M.H.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Hushmandi, K.; Makvandi, P.; Samec, M.; Liskova, A.; et al. Nrf2 signaling pathway in cisplatin chemotherapy: Potential involvement in organ protection and chemoresistance. Pharmacol. Res. 2021, 167, 105575. [Google Scholar]
- Mirzaei, S.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Azami, N.; Hamzehlou, S.; Farahani, M.V.; Hushmandi, K.; Ashrafizadeh, M.; et al. Nrf2 Signaling Pathway in Chemoprotection and Doxorubicin Resistance: Potential Application in Drug Discovery. Antioxidants 2021, 10, 349. [Google Scholar] [CrossRef]
- Blagotinsek, K.; Rozman, D. Targeting Signalling Pathways in Hepatocellular Carcinoma. Curr. Pharm. Des. 2017, 23, 170–175. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fukutomi, T.; Sugawara, A.; Kawamura, H.; Takahashi, T.; Pi, J.; Uruno, A.; Yamamoto, M. Nrf2 protects pancreatic β-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes 2014, 63, 605–618. [Google Scholar] [CrossRef]
- Strom, J.; Xu, B.; Tian, X.; Chen, Q.M. Nrf2 protects mitochondrial decay by oxidative stress. FASEB J. 2016, 30, 66–80. [Google Scholar] [CrossRef]
- Noel, S.; Martina, M.N.; Bandapalle, S.; Racusen, L.C.; Potteti, H.R.; Hamad, A.R.; Reddy, S.P.; Rabb, H. T Lymphocyte-Specific Activation of Nrf2 Protects from AKI. J. Am. Soc. Nephrol. 2015, 26, 2989–3000. [Google Scholar] [CrossRef]
- Jiang, T.; Harder, B.; de la Vega, M.R.; Wong, P.K.; Chapman, E.; Zhang, D.D. p62 links autophagy and Nrf2 signaling. Free Radic. Biol. Med. 2015, 88, 199–204. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Farkhondeh, T.; Samarghandian, S. Back to nucleus: Combating with cadmium toxicity using Nrf2 signaling pathway as a promising therapeutic target. Biol. Trace Elem. Res. 2020, 197, 52–62. [Google Scholar]
- Ashrafizadeh, M.; Ahmadi, Z.; Samarghandian, S.; Mohammadinejad, R.; Yaribeygi, H.; Sathyapalan, T.; Sahebkar, A. MicroRNA-mediated regulation of Nrf2 signaling pathway: Implications in disease therapy and protection against oxidative stress. Life Sci. 2020, 244, 117329. [Google Scholar]
- Chen, W.; Sun, Z.; Wang, X.J.; Jiang, T.; Huang, Z.; Fang, D.; Zhang, D.D. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol. Cell 2009, 34, 663–673. [Google Scholar] [CrossRef]
- Moon, E.J.; Giaccia, A. Dual roles of NRF2 in tumor prevention and progression: Possible implications in cancer treatment. Free Radic. Biol. Med. 2015, 79, 292–299. [Google Scholar] [CrossRef]
- Na, H.K.; Surh, Y.J. Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free Radic. Biol. Med. 2014, 67, 353–365. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Li, R.; Jia, Z.; Zhu, H. Regulation of Nrf2 Signaling. React. Oxyg. Species 2019, 8, 312–322. [Google Scholar] [CrossRef]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef]
- Satoh, H.; Moriguchi, T.; Takai, J.; Ebina, M.; Yamamoto, M. Nrf2 Prevents Initiation but Accelerates Progression through the Kras Signaling Pathway during Lung Carcinogenesis. Cancer Res. 2013, 73, 4158–4168. [Google Scholar] [CrossRef]
- Tao, S.; de la Vega, M.R.; Chapman, E.; Ooi, A.; Zhang, D.D. The effects of NRF2 modulation on the initiation and progression of chemically and genetically induced lung cancer. Mol. Carcinog. 2018, 57, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Villeneuve, N.F.; Sun, Z.; Wong, P.K.; Zhang, D.D. Dual roles of Nrf2 in cancer. Pharmacol. Res. 2008, 58, 262–270. [Google Scholar] [CrossRef]
- Hayes, J.D.; McMahon, M. NRF2 and KEAP1 mutations: Permanent activation of an adaptive response in cancer. Trends Biochem. Sci. 2009, 34, 176–188. [Google Scholar]
- Singh, A.; Venkannagari, S.; Oh, K.H.; Zhang, Y.-Q.; Rohde, J.M.; Liu, L.; Nimmagadda, S.; Sudini, K.; Brimacombe, K.R.; Gajghate, S. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem. Biol. 2016, 11, 3214–3225. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Long, M.; Huang, Y.; Zhang, L.; Zhang, R.; Zheng, Y.; Liao, X.; Wang, Y.; Liao, Q.; et al. NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis. Sci. Transl. Med. 2016, 8, 334ra51. [Google Scholar] [CrossRef] [PubMed]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Johnston, F.M.; Beckman, M. Updates on management of gastric cancer. Curr. Oncol. Rep. 2019, 21, 67. [Google Scholar] [CrossRef]
- Huscher, C.G.; Mingoli, A.; Sgarzini, G.; Sansonetti, A.; di Paola, M.; Recher, A.; Ponzano, C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: Five-year results of a randomized prospective trial. Ann. Surg. 2005, 241, 232–237. [Google Scholar] [CrossRef]
- Orditura, M.; Galizia, G.; Sforza, V.; Gambardella, V.; Fabozzi, A.; Laterza, M.M.; Andreozzi, F.; Ventriglia, J.; Savastano, B.; Mabilia, A. Treatment of gastric cancer. World J. Gastroenterol. 2014, 20, 1635. [Google Scholar] [CrossRef]
- Zhao, S.L.; Fang, J.Y. The role of postoperative adjuvant chemotherapy following curative resection for gastric cancer: A meta-analysis. Cancer Investig. 2008, 26, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.S.; Wang, Y.; Chen, S.Y.; Sun, Y.H. An updated meta-analysis of adjuvant chemotherapy after curative resection for gastric cancer. Eur. J. Surg. Oncol. 2008, 34, 1208–1216. [Google Scholar] [CrossRef]
- Paoletti, X.; Oba, K.; Burzykowski, T.; Michiels, S.; Ohashi, Y.; Pignon, J.P.; Rougier, P.; Sakamoto, J.; Sargent, D.; Sasako, M.; et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: A meta-analysis. JAMA 2010, 303, 1729–1737. [Google Scholar] [CrossRef]
- Janunger, K.G.; Hafström, L.; Glimelius, B. Chemotherapy in gastric cancer: A review and updated meta-analysis. Eur. J. Surg. Acta Chir. 2002, 168, 597–608. [Google Scholar] [CrossRef]
- Schernberg, A.; del Campo, E.R.; Rousseau, B.; Matzinger, O.; Loi, M.; Maingon, P.; Huguet, F. Adjuvant chemoradiation for gastric carcinoma: State of the art and perspectives. Clin. Transl. Radiat. Oncol. 2018, 10, 13–22. [Google Scholar] [CrossRef]
- Moertel, C.; Reitemeier, R.; Childs, D.J.R.; Colby, M.J.R.; Holbrook, M. Combined 5-fluorouracil and Supervoltage Radiation Therapy of Locally Unresectable Gastrointestinal Cancer. Lancet 1969, 294, 865–867. [Google Scholar] [CrossRef]
- Gastrointestinal Tumor Study Group. A comparison of combination chemotherapy and combined modality therapy for locally advanced gastric carcinoma. Cancer 1982, 49, 1771–1777. [Google Scholar] [CrossRef]
- Smalley, S.R.; Benedetti, J.K.; Haller, D.G.; Hundahl, S.A.; Estes, N.C.; Ajani, J.A.; Gunderson, L.L.; Goldman, B.; Martenson, J.A.; Jessup, J.M.; et al. Updated Analysis of SWOG-Directed Intergroup Study 0116: A Phase III Trial of Adjuvant Radiochemotherapy Versus Observation After Curative Gastric Cancer Resection. J. Clin. Oncol. 2012, 30, 2327–2333. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Konecny, G.E.; Meng, Y.G.; Untch, M.; Wang, H.-J.; Bauerfeind, I.; Epstein, M.; Stieber, P.; Vernes, J.-M.; Gutierrez, J.; Hong, K. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin. Cancer Res. 2004, 10, 1706–1716. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.T.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 1263–1284. [Google Scholar]
- Lei, Y.-Y.; Huang, J.-Y.; Zhao, Q.-R.; Jiang, N.; Xu, H.-M.; Wang, Z.-N.; Li, H.-Q.; Zhang, S.-B.; Sun, Z. The clinicopathological parameters and prognostic significance of HER2 expression in gastric cancer patients: A meta-analysis of literature. World J. Surg. Oncol. 2017, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Satoh, T.; Lee, K.H.; Rha, S.Y.; Sasaki, Y.; Park, S.H.; Komatsu, Y.; Yasui, H.; Kim, T.-Y.; Yamaguchi, K.; Fuse, N.; et al. Randomized phase II trial of nimotuzumab plus irinotecan versus irinotecan alone as second-line therapy for patients with advanced gastric cancer. Gastric Cancer 2015, 18, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; van Cutsem, E.; Oh, S.-C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.-Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Arteaga, C.L.; Engelman, J.A. ERBB receptors: From oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 2014, 25, 282–303. [Google Scholar]
- Moasser, M.M. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar]
- Hecht, J.R.; Bang, Y.-J.; Qin, S.K.; Chung, H.C.; Xu, J.M.; Park, J.O.; Jeziorski, K.; Shparyk, Y.; Hoff, P.M.; Sobrero, A.; et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2–positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC—A randomized phase III trial. J. Clin. Oncol. 2016, 34, 443–451. [Google Scholar] [CrossRef]
- Tabernero, J.; Hoff, P.M.; Shen, L.; Ohtsu, A.; Shah, M.A.; Cheng, K.; Song, C.; Wu, H.; Eng-Wong, J.; Kim, K.; et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018, 19, 1372–1384. [Google Scholar] [CrossRef]
- Satoh, T.; Xu, R.-H.; Chung, H.C.; Sun, G.-P.; Doi, T.; Xu, J.-M.; Tsuji, A.; Omuro, Y.; Li, J.; Wang, J.-W.; et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN—A randomized, phase III study. J. Clin. Oncol. 2014, 32, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Thuss-Patience, P.C.; Shah, M.A.; Ohtsu, A.; van Cutsem, E.; Ajani, J.A.; Castro, H.; Mansoor, W.; Chung, H.C.; Bodoky, G.; Shitara, K.; et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017, 18, 640–653. [Google Scholar] [CrossRef]
- Gambardella, V.; Gimeno-Valiente, F.; Tarazona, N.; Ciarpaglini, C.M.; Roda, D.; Fleitas, T.; Tolosa, P.; Cejalvo, J.M.; Huerta, M.; Roselló, S.J.C.C.R. NRF2 through RPS6 activation is related to anti-HER2 drug resistance in HER2-amplified gastric cancer. Clin. Cancer Res. 2019, 25, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tian, Z.; Guo, R.; Ren, F. Nrf2 Inhibitor, Brusatol in Combination with Trastuzumab Exerts Synergistic Antitumor Activity in HER2-Positive Cancers by Inhibiting Nrf2/HO-1 and HER2-AKT/ERK1/2 Pathways. Oxid. Med. Cell. Longev. 2020, 2020, 9867595. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-F.; Yao, J.; Gao, S.-G.; Wang, X.-S.; Peng, X.-Q.; Yang, Y.-T.; Feng, X.-S. Nrf2 overexpression predicts prognosis and 5-FU resistance in gastric cancer. Asian Pac. J. Cancer Prev. 2013, 14, 5231–5235. [Google Scholar] [CrossRef]
- Pouremamali, F.; Jeddi, F.; Samadi, N. Nrf2-ME-1 axis is associated with 5-FU resistance in gastric cancer cell line. Process. Biochem. 2020. [Google Scholar] [CrossRef]
- Wen, D.; Liu, D.; Tang, J.; Dong, L.; Liu, Y.; Tao, Z.; Wan, J.; Gao, D.; Wang, L.; Sun, H.; et al. Malic enzyme 1 induces epithelial–mesenchymal transition and indicates poor prognosis in hepatocellular carcinoma. Tumor Biol. 2015, 36, 6211–6221. [Google Scholar] [CrossRef]
- Bécouarn, Y.; Agostini, C.; Trufflandier, N.; Boulanger, V. Oxaliplatin: Available data in non-colorectal gastrointestinal malignancies. Crit. Rev. Oncol. Hematol. 2001, 40, 265–272. [Google Scholar] [CrossRef]
- Gruenberger, B.; Schueller, J.; Heubrandtner, U.; Wrba, F.; Tamandl, D.; Kaczirek, K.; Roka, R.; Freimann-Pircher, S.; Gruenberger, T. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: A phase 2 study. Lancet Oncol. 2010, 11, 1142–1148. [Google Scholar] [CrossRef]
- Sun, W.; Sohal, D.; Haller, D.G.; Mykulowycz, K.; Rosen, M.; Soulen, M.C.; Caparro, M.; Teitelbaum, U.R.; Giantonio, B.; O’Dwyer, P.J.; et al. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer 2011, 117, 3187–3192. [Google Scholar] [CrossRef]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar]
- Mishima, M.; Samimi, G.; Kondo, A.; Lin, X.; Howell, S.B. The cellular pharmacology of oxaliplatin resistance. Eur. J. Cancer 2002, 38, 1405–1412. [Google Scholar] [CrossRef]
- Gourdier, I.; del Rio, M.; Crabbé, L.; Candeil, L.; Copois, V.; Ychou, M.; Auffray, C.; Martineau, P.; Mechti, N.; Pommier, Y.; et al. Drug specific resistance to oxaliplatin is associated with apoptosis defect in a cellular model of colon carcinoma. FEBS Lett. 2002, 529, 232–236. [Google Scholar] [CrossRef]
- Ding, Z.-B.; Hui, B.; Shi, Y.-H.; Zhou, J.; Peng, Y.-F.; Gu, C.-Y.; Yang, H.; Shi, G.-M.; Ke, A.-W.; Wang, X.-Y. Autophagy activation in hepatocellular carcinoma contributes to the tolerance of oxaliplatin via reactive oxygen species modulation. Clin. Cancer Res. 2011, 17, 6229–6238. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chen, L.T.; Tsou, T.C.; Pan, W.Y.; Kuo, C.C.; Liu, J.F.; Yeh, S.C.; Tsai, F.Y.; Hsieh, H.P.; Chang, J.Y. Combined modalities of resistance in an oxaliplatin-resistant human gastric cancer cell line with enhanced sensitivity to 5-fluorouracil. Br. J. Cancer 2007, 97, 334–344. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jeddi, F.; Soozangar, N.; Sadeghi, M.R.; Somi, M.H.; Shirmohamadi, M.; Eftekhar-Sadat, A.-T.; Samadi, N. Nrf2 overexpression is associated with P-glycoprotein upregulation in gastric cancer. Biomed. Pharmacother. 2018, 97, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Ma, Z.; Li, X.; Deng, Y.; Duan, G.; Zhao, L.; Xu, X.; Xiao, L.; Liu, H.; Zhu, Z. DJ-1 is involved in the multidrug resistance of SGC7901 gastric cancer cells through PTEN/PI3K/Akt/Nrf2 pathway. Acta Biochim. Biophys. Sin. 2020, 52, 1202–1214. [Google Scholar] [CrossRef]
- Zhu, Z.-M.; Li, Z.-R.; Huang, Y.; Yu, H.-H.; Huang, X.-S.; Yan, Y.-F.; Shao, J.-H.; Chen, H.-P. DJ-1 is involved in the peritoneal metastasis of gastric cancer through activation of the Akt signaling pathway. Oncol. Rep. 2014, 31, 1489–1497. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Duan, G.-L.; Xu, R.-Y.; Li, X.-R.; Xiao, L.; Zhao, L.; Ma, Z.-X.; Xu, X.-W.; Qiu, L.-J.; Zhu, Z.-M.; et al. DJ-1 overexpression confers the multidrug resistance phenotype to SGC7901 cells by upregulating P-gp and Bcl-2. Biochem. Biophys. Res. Commun. 2019, 519, 73–80. [Google Scholar] [CrossRef]
- Robinson, K.; Tiriveedhi, V. Perplexing role of P-glycoprotein in tumor microenvironment. Front. Oncol. 2020, 10, 265. [Google Scholar]
- Srivastava, R.K.; Sasaki, C.Y.; Hardwick, J.M.; Longo, D.L. Bcl-2–mediated drug resistance: Inhibition of apoptosis by blocking nuclear factor of activated T lymphocytes (NFAT)-induced Fas ligand transcription. J. Exp. Med. 1999, 190, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Clements, C.M.; McNally, R.S.; Conti, B.J.; Mak, T.W.; Ting, J.P.Y. DJ-1, a cancer-and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci. USA 2006, 103, 15091–15096. [Google Scholar] [CrossRef]
- Malhotra, D.; Thimmulappa, R.; Navas-Acien, A.; Sandford, A.; Elliott, M.; Singh, A.; Chen, L.; Zhuang, X.; Hogg, J.; Pare, P. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am. J. Respir. Crit. Care Med. 2008, 178, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and-independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef]
- Lee, J.-S.; Surh, Y.-J. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005, 224, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.H.; Peters, M.; Jang, Y.; Shi, W.; Pintilie, M.; Fletcher, G.C.; DeLuca, C.; Liepa, J.; Zhou, L.; Snow, B. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell 2005, 7, 263–273. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Kitaura, H.; Taira, T.; Iguchi-Ariga, S.M.M.; Ariga, H. Oxidation of DJ-1-dependent cell transformation through direct binding of DJ-1 to PTEN. Int. J. Oncol. 2009, 35, 1331–1341. [Google Scholar] [PubMed]
- Han, Z.; Hong, L.; Han, Y.; Wu, K.; Han, S.; Shen, H.; Li, C.; Yao, L.; Qiao, T.; Fan, D. Phospho Akt mediates multidrug resistance of gastric cancer cells through regulation of P-gp, Bcl-2 and Bax. J. Exp. Clin. Cancer Res. 2007, 26, 261–268. [Google Scholar]
- Michl, P.; Downward, J. Mechanisms of disease: PI3K/AKT signaling in gastrointestinal cancers. Z. Gastroenterol. 2005, 43, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.-H.; Hoek, T.L.V.; Qin, Y.; Becker, L.B.; Schumacker, P.T.; Li, C.-Q.; Dey, L.; Barth, E.; Halpern, H.; Rosen, G.M.; et al. Baicalein attenuates oxidant stress in cardiomyocytes. Am. J. Physiol. Circ. Physiol. 2002, 282, H999–H1006. [Google Scholar] [CrossRef] [PubMed]
- Bie, B.; Sun, J.; Guo, Y.; Li, J.; Jiang, W.; Yang, J.; Huang, C.; Li, Z. Baicalein: A review of its anti-cancer effects and mechanisms in Hepatocellular Carcinoma. Biomed. Pharmacother. 2017, 93, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Snyder, S.A.; Smith, J.N.; Chen, Y.C. Anticancer properties of baicalein: A review. Med. Chem. Res. 2016, 25, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hu, J.; Shi, B.; Tie, J. Baicalein enhanced cisplatin sensitivity of gastric cancer cells by inducing cell apoptosis and autophagy via Akt/mTOR and Nrf2/Keap 1 pathway. Biochem. Biophys. Res. Commun. 2020, 531, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Fleischauer, A.T.; Poole, C.; Arab, L. Garlic consumption and cancer prevention: Meta-analyses of colorectal and stomach cancers. Am. J. Clin. Nutr. 2000, 72, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Powolny, A.A.; Singh, S.V. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008, 269, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Chandra-Kuntal, K.; Lee, J.; Singh, S.V. Critical role for reactive oxygen species in apoptosis induction and cell migration inhibition by diallyl trisulfide, a cancer chemopreventive component of garlic. Breast Cancer Res. Treat. 2013, 138, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Antony, M.L.; Singh, S.V. Molecular mechanisms and targets of cancer chemoprevention by garlic-derived bioactive compound diallyl trisulfide. Indian J. Exp. Biol. 2011, 49, 805. [Google Scholar]
- Lai, K.C.; Hsu, S.C.; Yang, J.S.; Yu, C.C.; Lein, J.C.; Chung, J.G. Diallyl trisulfide inhibits migration, invasion and angiogenesis of human colon cancer HsT-29 cells and umbilical vein endothelial cells, and suppresses murine xenograft tumour growth. J. Cell. Mol. Med. 2015, 19, 474–484. [Google Scholar] [CrossRef]
- Jiang, X.-Y.; Zhu, X.-S.; Xu, H.-Y.; Zhao, Z.-X.; Li, S.-Y.; Li, S.-Z.; Cai, J.-H.; Cao, J.-M. Diallyl trisulfide suppresses tumor growth through the attenuation of Nrf2/Akt and activation of p38/JNK and potentiates cisplatin efficacy in gastric cancer treatment. Acta Pharmacol. Sin. 2017, 38, 1048–1058. [Google Scholar] [CrossRef]
- Waterman, M.L. Lymphoid enhancer factor/T cell factor expression in colorectal cancer. Cancer Metastasis Rev. 2004, 23, 41–52. [Google Scholar] [CrossRef]
- Murphy, M.; Chatterjee, S.S.; Jain, S.; Katari, M.; DasGupta, R. TCF7L1 modulates colorectal cancer growth by inhibiting expression of the tumor-suppressor gene EPHB3. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Slyper, M.; Shahar, A.; Bar-Ziv, A.; Granit, R.Z.; Hamburger, T.; Maly, B.; Peretz, T.; Ben-Porath, I. Control of Breast Cancer Growth and Initiation by the Stem Cell–Associated Transcription Factor TCF3. Cancer Res. 2012, 72, 5613–5624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, J.; Cai, Y.; Luo, M.; Wang, B.; Gu, Y. TCF7L1 indicates prognosis and promotes proliferation through activation of Keap1/NRF2 in gastric cancer. Acta Biochim. Biophys. Sin. 2019, 51, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Duan, Y.; Zhang, Y.; Zhao, D.; Wen, Y.; Yao, J.; Da, M. Expression of Nrf2 and NQO1 in human gastric cancer and their clinical significance. Int. J. Clin. Exp. Pathol. 2016, 9, 1635–1643. [Google Scholar]
- Yang, W.-C.; Tsai, W.-C.; Lin, P.-M.; Yang, M.-Y.; Liu, Y.-C.; Chang, C.-S.; Yu, W.-H.; Lin, S.-F. Human BDH2, an anti-apoptosis factor, is a novel poor prognostic factor for de novo cytogenetically normal acute myeloid leukemia. J. Biomed. Sci. 2013, 20, 58. [Google Scholar] [CrossRef]
- Guo, K.; Lukacik, P.; Papagrigoriou, E.; Meier, M.; Lee, W.H.; Adamski, J.; Oppermann, U. Characterization of Human DHRS6, an Orphan Short Chain Dehydrogenase/Reductase Enzyme a novel, cytosolic type 2 R-β-hydroxybutyrate dehydrogenase. J. Biol. Chem. 2006, 281, 10291–10297. [Google Scholar] [CrossRef]
- Zang, W.; Wang, T.; Wang, Y.; Chen, X.; Du, Y.; Sun, Q.; Li, M.; Dong, Z.; Zhao, G. Knockdown of long non-coding RNA TP73-AS1 inhibits cell proliferation and induces apoptosis in esophageal squamous cell carcinoma. Oncotarget 2016, 7, 19960. [Google Scholar] [CrossRef]

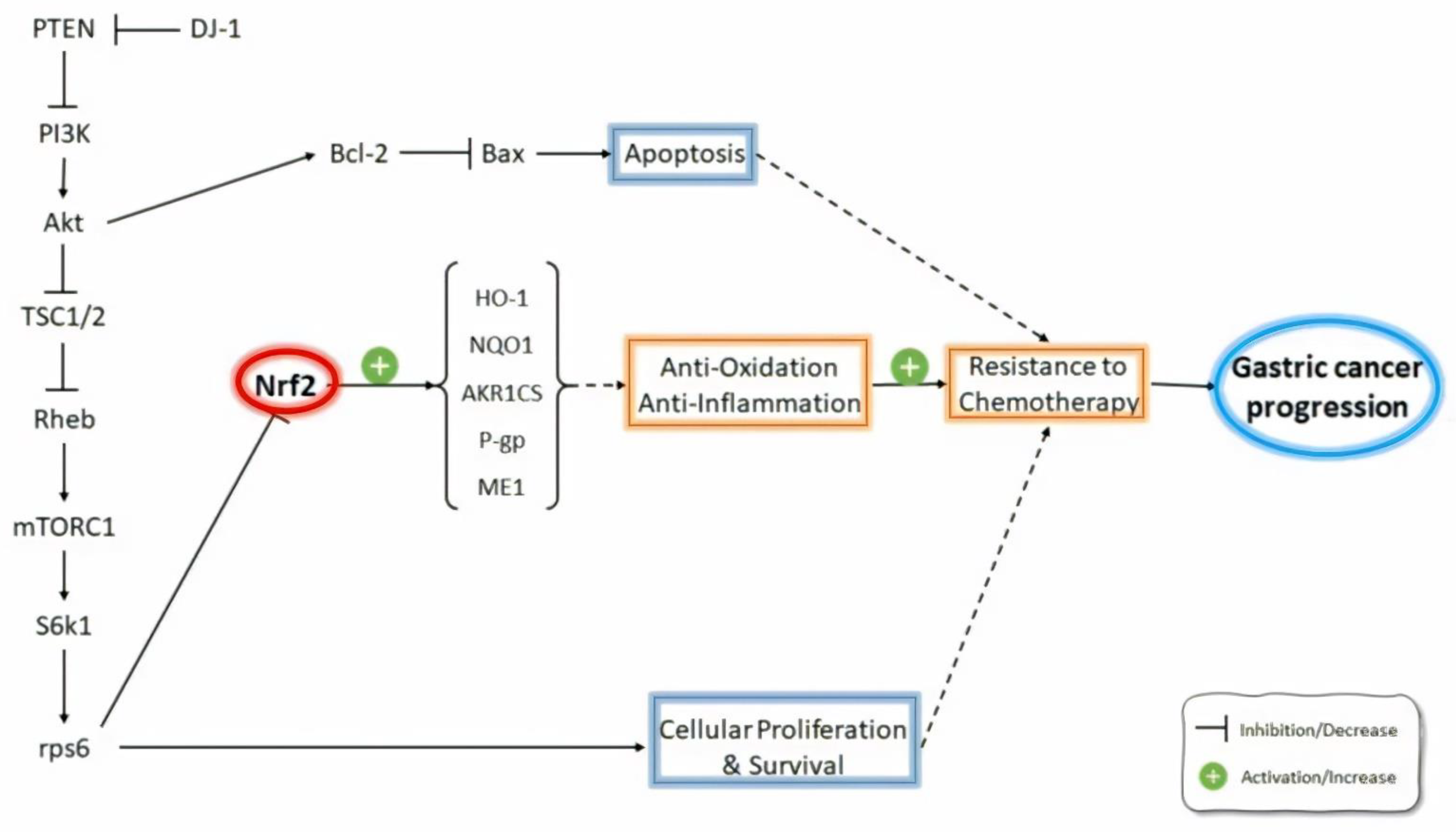
| Authors | Investigation Aims | Subjects | Possible Roles of Nrf2 |
|---|---|---|---|
| Gambardella et al. [73] | Mechanisms of primary and acquired resistance to the combination of trastuzumab and platinum-based chemotherapy | Lapatinib- and trastuzumab-resistant clones derived from two different HER2-amplified gastric cancer cell lines | NRF2 amplified the resistance to anti-HER2 drugs through the PI3K/AKT/mTOR/RPS6 pathway; RPS6 inhibition decreased NRF2 expression and restored sensitivity in HER2-amplified gastric cancer. |
| Yang et al. [74] | Efficiency of combination chemotherapy with trastuzumab and brusatol | HER2-positive SK-OV-3 and BT-474 cancer cells | Brusatol increased the antitumor activity of trastuzumab through the inhibition of the Nrf2/HO-1 and HER2-AKT/ERK1/2 signaling pathways. |
| Hu et al. [75] | Nrf2 protein expression in gastric cancer specimens + association between Nrf2 expression and 5-FU resistance | Samples from GC patients with gastrectomy and from GC patients who received first-line combination chemotherapy of either A) docetaxel, cisplatin, and 5-FU or B) S-1 plus cisplatin | Nrf2 was an independent prognostic factor in GC and caused resistance to the chemotherapeutic drug 5-FU in GC cells. |
| Pouremamali et al. [76] | The role of Nrf2 and its downstream target malic enzyme-1 (ME-1) in the resistance to 5-FU chemotherapy | Resistant MKN-45 (MKN-45/DR) cell line | In resistant cells, decreased Nrf2 and ME-1 expression levels increased MDR1 mRNA levels; the inhibition of Nrf2 with luteolin and brusatol increased the effects on 5-FU-induced cytotoxicity. |
| Jeddi et al. [75] | Association of Nrf2 and MDR1/P-gp in GC patients | Endoscopic biopsy samples from GC patients | Higher Nrf2 expression in GC patients compared with non-GC individuals; the induction of P-gp was associated with Nrf2 overexpression; there was correlation between Nrf2 overexpression and tumor size, histological grade, lymph node, and distant metastasis; P-gp up-regulation was associated with the histological grade and tumor size; the inhibition of Nrf2 expression improved the efficacy of chemotherapeutic agents for GC patients by the downregulation of P-gp expression. |
| Chen et al. [85] | Mechanisms of oxaliplatin resistance | Human gastric carcinoma cell line TSGH-S3 (S3) | The activation of Nrf2/AKR1C axis contributed to oxaliplatin resistance as the suppression of Nrf2 expression decreased the levels of AKR1C1, AKR1C2, and AKR1C3 and reversed oxaliplatin resistance in S3 cells. |
| Qiu et al. [86] | Mechanisms of DJ-1-induced multidrug resistance (MDR) | SGC7901 + MDR variant SGC7901/VCR gastric cancer cells | DJ-1 mediated the development of MDR in SGC7901 gastric cancer cells through inhibiting PTEN, activating the PI3k/Akt pathway and consequently resulting in Nrf2 activation, thereby inducing Nrf2-dependent P-glycoprotein (P-gp) and Bcl-2 expressions. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farkhondeh, T.; Pourbagher-Shahri, A.M.; Azimi-Nezhad, M.; Forouzanfar, F.; Brockmueller, A.; Ashrafizadeh, M.; Talebi, M.; Shakibaei, M.; Samarghandian, S. Roles of Nrf2 in Gastric Cancer: Targeting for Therapeutic Strategies. Molecules 2021, 26, 3157. https://doi.org/10.3390/molecules26113157
Farkhondeh T, Pourbagher-Shahri AM, Azimi-Nezhad M, Forouzanfar F, Brockmueller A, Ashrafizadeh M, Talebi M, Shakibaei M, Samarghandian S. Roles of Nrf2 in Gastric Cancer: Targeting for Therapeutic Strategies. Molecules. 2021; 26(11):3157. https://doi.org/10.3390/molecules26113157
Chicago/Turabian StyleFarkhondeh, Tahereh, Ali Mohammad Pourbagher-Shahri, Mohsen Azimi-Nezhad, Fatemeh Forouzanfar, Aranka Brockmueller, Milad Ashrafizadeh, Marjan Talebi, Mehdi Shakibaei, and Saeed Samarghandian. 2021. "Roles of Nrf2 in Gastric Cancer: Targeting for Therapeutic Strategies" Molecules 26, no. 11: 3157. https://doi.org/10.3390/molecules26113157
APA StyleFarkhondeh, T., Pourbagher-Shahri, A. M., Azimi-Nezhad, M., Forouzanfar, F., Brockmueller, A., Ashrafizadeh, M., Talebi, M., Shakibaei, M., & Samarghandian, S. (2021). Roles of Nrf2 in Gastric Cancer: Targeting for Therapeutic Strategies. Molecules, 26(11), 3157. https://doi.org/10.3390/molecules26113157








