Critical Reappraisal of Methods for Measuring Urine Saturation with Calcium Salts
Abstract
1. Introduction
2. Results and Discussion
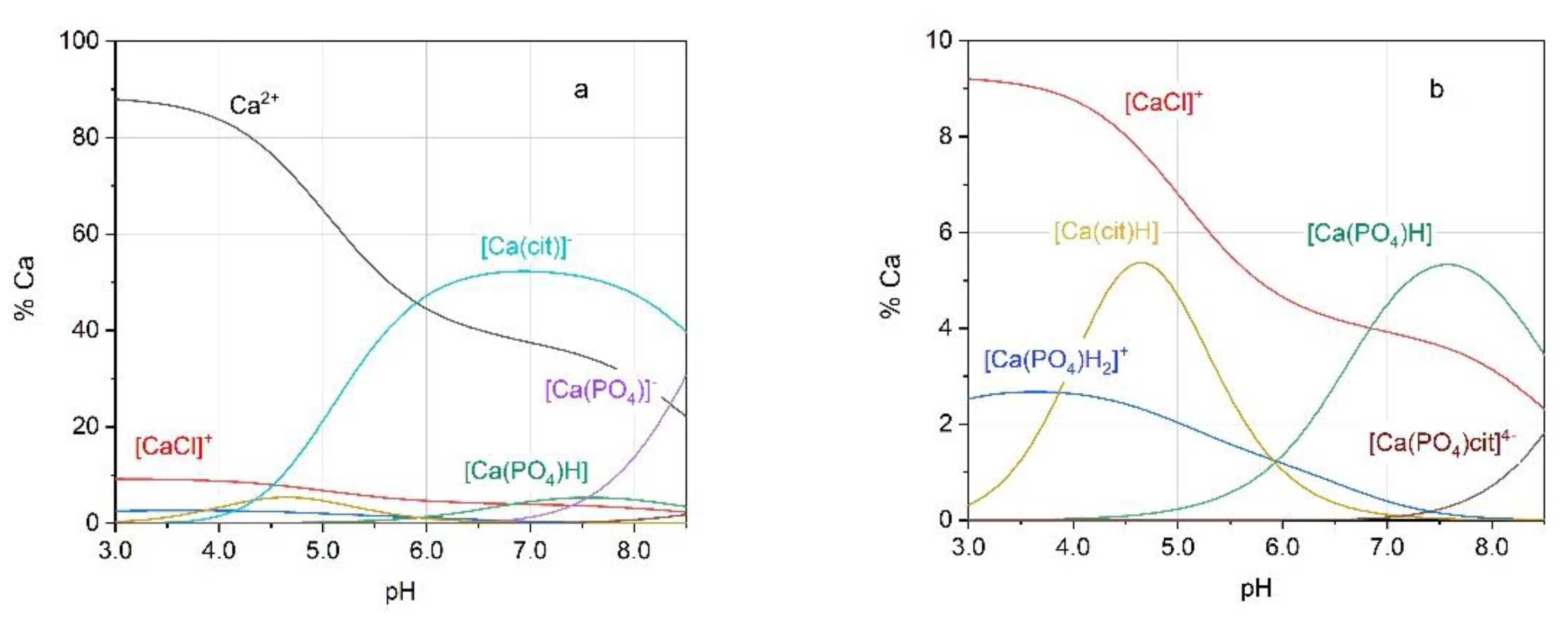
2.1. Stability of Calcium Mixed Complexes
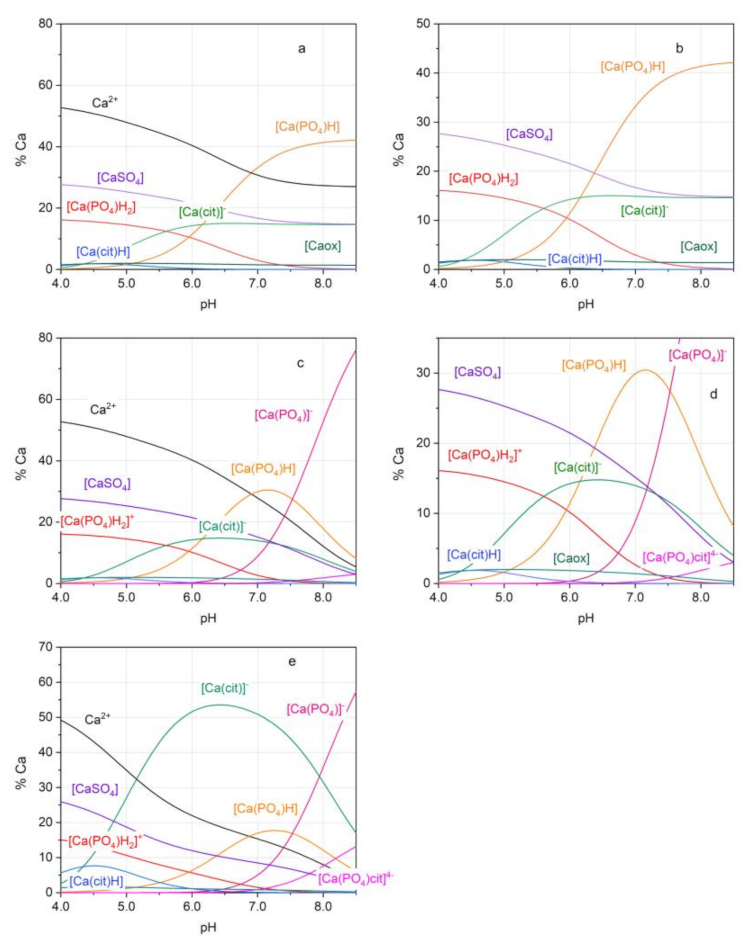
2.2. Calcium Complexes in Urinary Conditions
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Apparatuses
4.3. Calibration and Titration Procedures
4.4. Data Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearle, M.S.; Calhoun, E.A.; Curhan, G.C. Urologic Diseases in America project: Urolithiasis. J. Urol. 2005, 173, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C., Jr.; Gambaro, G.; Rodgers, A.; Asplin, J.; Bonny, O.; Costa-Bauzá, A.; Ferraro, P.M.; Fogazzi, G.; Fuster, D.G.; Goldfarb, D.S.; et al. Urine and stone analysis for the investigation of the renal stone former: A consensus conference. Urolithiasis 2021, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Robertson, W.G.; Peacock, M.; Marshall, R.W.; Marshall, D.H.; Nordin, B.E. Saturation-inhibition index as a measure of the risk of calcium oxalate stone formation in the urinary tract. N. Engl. J. Med. 1976, 294, 52–249. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.Y.; Hayashi, Y.; Finlayson, B.; Chu, S. Estimation of the state of saturation of brushite and calcium oxalate in urine: A comparison of three methods. J. Lab. Clin. Med. 1977, 89, 891–901. [Google Scholar] [PubMed]
- Robertson, W.G.; Peacock, M.; Nordin, B.E. Activity products in stone-forming and non-stone-forming urine. Clin. Sci. 1968, 34, 579–594. [Google Scholar] [PubMed]
- Werness, P.G.; Brown, C.M.; Smith, L.H.; Finlayson, B. EQUIL 2: A basic computer programme for the calculation of urinary saturation. J. Urol. 1985, 134, 1242–1244. [Google Scholar] [CrossRef]
- May, P.M.; Murray, K. JESS, a joint expert speciation system–1. Talanta 1991, 38, 1409–1417. [Google Scholar] [CrossRef]
- Marangella, M.; Petrarulo, M.; Vitale, C.; Daniele, P.; Sammartano, S. LITHORISK.COM: The novel version of a software for calculating and visualizing the risk of renal stone. Urolithiasis 2021, 49, 211–217. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Ticinesi, A.; Meschi, T.; Rodgers, A.; Di Maio, F.; Fulignati, P.; Borghi, L.; Gambaro, G. Short-term changes in urinary relative supersaturation predict recurrence of kidney stones: A tool to guide preventive measures in Urolithiasis. J. Urol. 2018, 200, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Marangella, M.; Daniele, P.G.; Ronzani, M.; Sonego, S.; Linari, F. Urine saturation with calcium salts in normal subjects and idiopathic calcium stone-formers estimated by an improved computer model system. Urol. Res. 1985, 13, 189–193. [Google Scholar] [CrossRef]
- Lentner, C. Geigy Scientific Tables, 8th ed.; CIBA-Geigy: Basel, Switzerland, 1983. [Google Scholar]
- Rodgers, A.; Allie-Hamdulay, S.; Jackson, G. Therapeutic action of citrate in urolithiasis explained by chemical speciation: Increase in pH is the determinant factor. Nephrol. Dial. Transplant. 2006, 21, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Amico, P.; Daniele, P.G.; Rigano, C.; Sammartano, S. Stability of calcium- and magnesium-citrate complexes in aqueous solution. Ann. Chim. 1982, 72, 1–24. [Google Scholar]
- Daniele, P.G.; Rigano, C.; Sammartano, S. Formation and stability of calcium- and magnesium-phosphate complexes in aqueous solution at 37 °C. A potentiometric investigation by glass and calcium ion-selective electrodes in the ionic strength range 0.03 <I <0.5. Ann. Chim. 1982, 72, 341–353. [Google Scholar]
- Zhang, J.; Ebrahimpour, A.; Nancollas, G.H. Ion associatopn in calcium phosphate solutions at 37 °C. J. Solut. Chem. 1991, 20, 455–465. [Google Scholar] [CrossRef]
- Sigel, H. Stability, structure, and reactivity of mixed ligand complexes in solution. In Proceedings of the Coordination Chemistry-XX: Invited Lectures Presented at the 20th International Conference on Coordination Chemistry, Calcutta, India, 10–14 December 1979; pp. 27–45. [Google Scholar] [CrossRef]
- Casale, A.; Daniele, P.G.; De Robertis, A.; Sammartano, S. Ionic strength dependence of formation constants. XI. An analysis of literature data on carboxylate ligand complexes. Ann. Chim. 1988, 78, 249–260. [Google Scholar]
- Daniele, P.G.; De Robertis, A.; De Stefano, C.; Gianguzza, A.; Sammartano, S. Salt effect on the protonation of ortho-phosphate between 10 and 50 °C in aqueous solution. A complex formation model. J. Solut. Chem. 1991, 20, 495–515. [Google Scholar] [CrossRef]
- Daniele, P.G.; Rigano, C.; Sammartano, S. The formation of proton and alkali-metal complexes with ligands of biological interest in aqueous solution. Potentiometric study of the H+-K+-citrate system at 37°C and 0.03 <I <1.0. Ann. Chim. 1980, 72, 119–130. [Google Scholar]
- Daniele, P.G.; De Robertis, A.; Sammartano, S. The effect of urea on the protonation of acetate, oxalate, malonate, citrate and sulfate in aqueous sodium, potassium, calcium chloride and tetraethylammonium iodide. Ann. Chim. 1992, 82, 503–516. [Google Scholar]
- Crea, F.; De Stefano, C.; Milea, D.; Pettignano, A.; Sammartano, S. SALMO and S3M: A saliva model and a single saliva salt model mfor equilibrium studies. Bioinorg. Chem. Appl. 2015, 267985. [Google Scholar] [CrossRef]
- Daniele, P.G.; De Stefano, C.; Marangella, M.; Rigano, C.; Sammartano, S. URSUS: A computer program for urine speciation. Clin. Biochem. 1989, 13, 507–510. [Google Scholar]
- Daniele, P.G.; Sonego, S.; Ronzani, M.; Marangella, M. Ionic strength dependence of formation constants. Part 8. Solubility of calcium oxalate monohydrate and calcium hydrogenphosphate dihydrate in aqueous solution, at 37 °C and different ionic strength. Ann. Chim. 1985, 75, 245–250. [Google Scholar]
- De Stefano, C.; Princi, P.; Rigano, C.; Sammartano, S. Computer-Analysis of Equilibrium Data in Solution Esab2M-An Improved Version of the Esab Program. Ann. Chim. 1987, 77, 643–675. [Google Scholar]
- De Stefano, C.; Mineo, P.; Rigano, C.; Sammartano, S. Ionic strength dependence of formation constants. XVII. The calculation of equilibrium concentrations and formation constants. Ann. Chim. 1993, 83, 243–277. [Google Scholar]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]

| Species | Formation Constants | ||||||
|---|---|---|---|---|---|---|---|
| I = 0.16 mol L−1, 25 °C | I = 0.16 mol L−1, 37 °C | I = 0.1 mol L−1, 37 °C a [17] | Reference | ||||
| logK | logβ | logK | logβ | logK | logβ | ||
| HPO42− | 11.79 | 11.79 | 11.64 b | 11.64 | 11.68 | 11.68 | [18] |
| H2PO4− | 6.84 | 18.63 | 6.83 b | 18.47 | 6.85 | 18.53 | [18] |
| H3PO4 | 1.98 | 20.61 | 2.03 b | 20.51 | 2.04 | 20.57 | [18] |
| Hcit2− c | 5.80 | 5.80 | 5.83 | 5.83 | [19] | ||
| H2cit− | 4.31 | 10.11 | 4.33 | 10.16 | [19] | ||
| H3cit | 2.86 | 12.97 | 2.87 | 13.03 | [19] | ||
| [KHPO4]− | 0.50 | 12.29 | 0.58 | 12.22 | 0.62 | 12.30 | [18] |
| [KPO4]2− | 0.81 | 0.81 | 0.85 | 0.85 | 0.88 | 0.88 | [18] |
| [Kcit]2− | 0.56 | 0.56 | 0.59 | 0.59 | [19] | ||
| [CaHcit] | 2.03 | 7.83 | 2.05 | 7.88 | [13] | ||
| [Cacit]− | 3.49 | 3.49 | 3.56 | 3.56 | [13] | ||
| I = 0 mol L−1, 37 °C | I = 0.1 mol L−1, 37 °C b | ||||||
| [CaCl]+ | −0.01 | −0.01 | 0.02 | 0.02 | [20] | ||
| [CaH2PO4]+ | 1.11 | 19.64 | [14] | ||||
| [CaHPO4] | 2.02 | 13.70 | [14] | ||||
| [CaPO4]− | 6.15 ± 0.02 | 6.15 ± 0.02 | This work | ||||
| [Ca(PO4)cit]4− d | 8.3 ± 0.8 | 8.3 ± 0.8 | This work | ||||
| Urine | pH 1 | Icalc | ||||||
|---|---|---|---|---|---|---|---|---|
| Model | 1 | 2 | 1 | 2 | 1 | 2 | ||
| A | 7.50 | 0.107 | 0.107 | 2.57 | 2.25 | 1.34 | 1.15 | |
| B | 7.24 | 0.148 | 0.148 | 6.76 | 5.74 | 5.71 | 4.73 | |
| C | 7.06 | 0.064 | 0.064 | 2.52 | 2.33 | 3.42 | 3.10 | |
| D | 6.84 | 0.148 | 0.146 | 4.67 | 4.52 | 2.37 | 2.27 | |
| E | 6.57 | 0.103 | 0.103 | 9.60 | 9.49 | 2.93 | 2.87 | |
| B* 3 | 7.27 | - | 0.155 | - | 3.69 | - | 2.93 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berto, S.; Marangella, M.; De Stefano, C.; Milea, D.; Daniele, P.G. Critical Reappraisal of Methods for Measuring Urine Saturation with Calcium Salts. Molecules 2021, 26, 3149. https://doi.org/10.3390/molecules26113149
Berto S, Marangella M, De Stefano C, Milea D, Daniele PG. Critical Reappraisal of Methods for Measuring Urine Saturation with Calcium Salts. Molecules. 2021; 26(11):3149. https://doi.org/10.3390/molecules26113149
Chicago/Turabian StyleBerto, Silvia, Martino Marangella, Concetta De Stefano, Demetrio Milea, and Pier Giuseppe Daniele. 2021. "Critical Reappraisal of Methods for Measuring Urine Saturation with Calcium Salts" Molecules 26, no. 11: 3149. https://doi.org/10.3390/molecules26113149
APA StyleBerto, S., Marangella, M., De Stefano, C., Milea, D., & Daniele, P. G. (2021). Critical Reappraisal of Methods for Measuring Urine Saturation with Calcium Salts. Molecules, 26(11), 3149. https://doi.org/10.3390/molecules26113149








