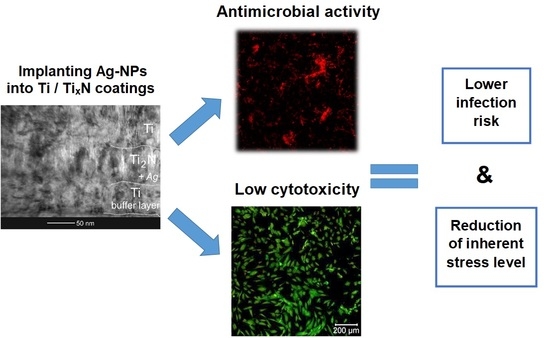
Antibacterial Optimization of Highly Deformed Titanium Alloys for Spinal Implants
Abstract
1. Introduction
2. Results
2.1. Fatigue Properties of the Strengthened Substrate
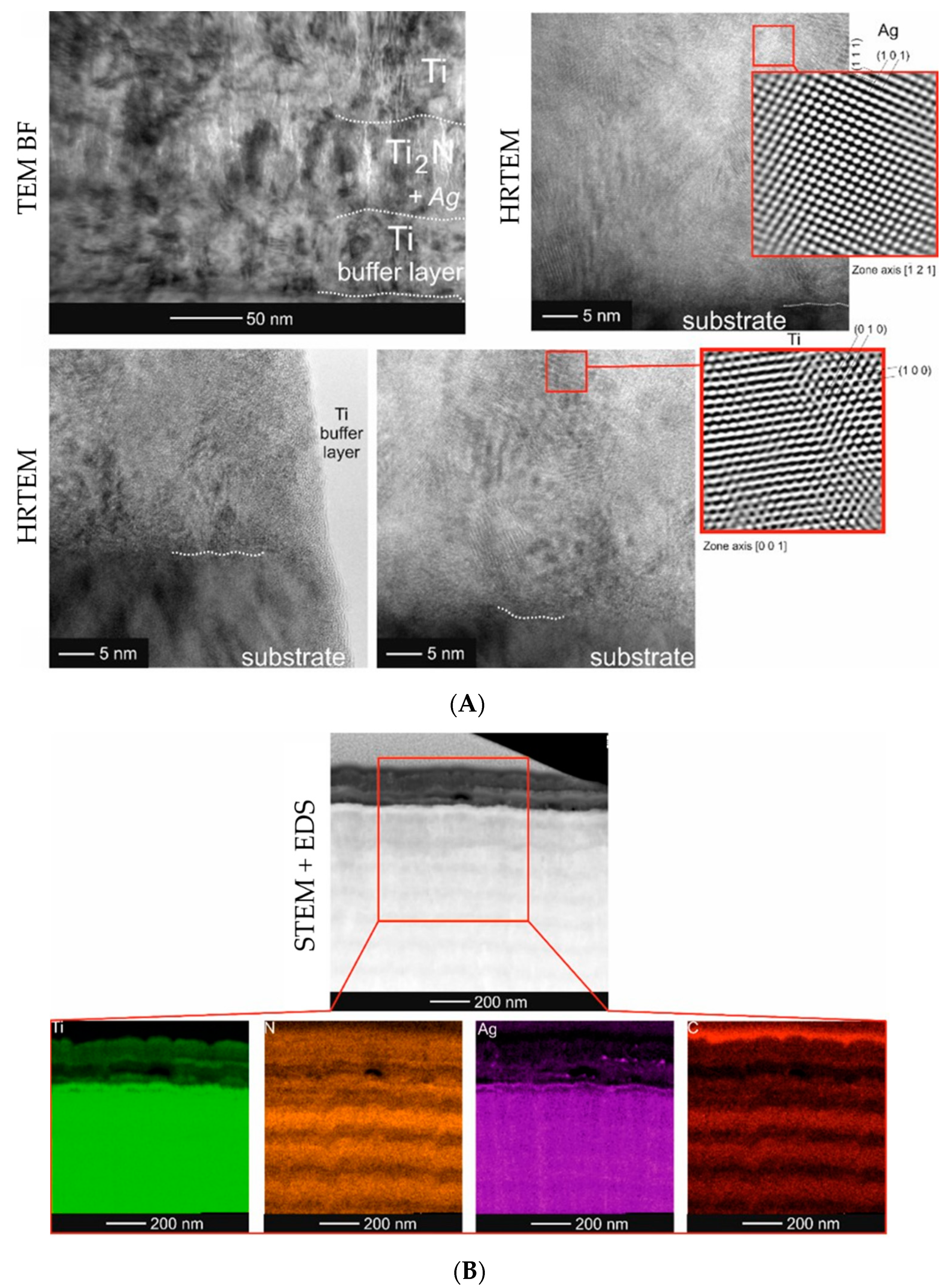
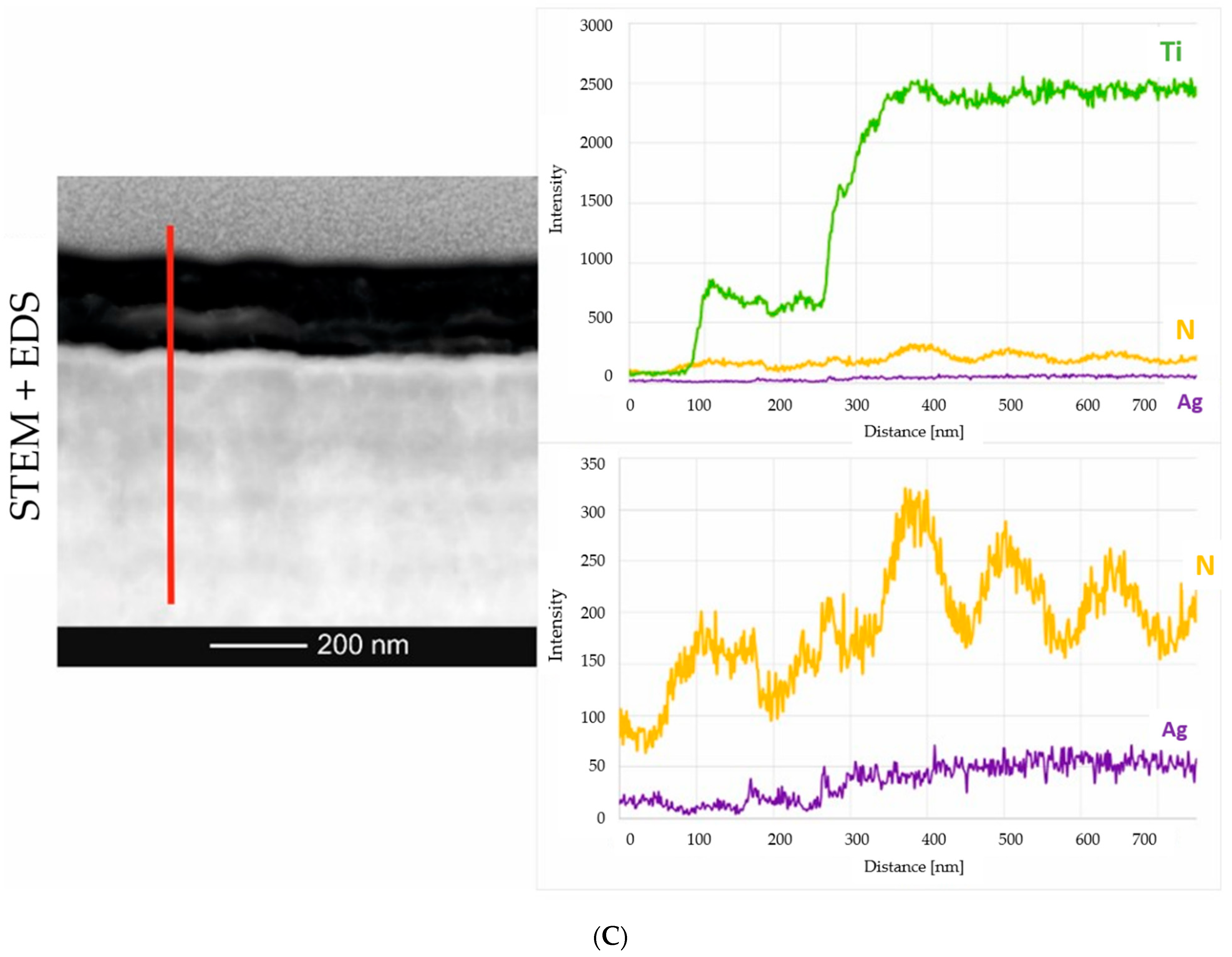
2.2. Microstructure Analysis of the Smart Bioactive Coating
2.3. Mechanical Properties
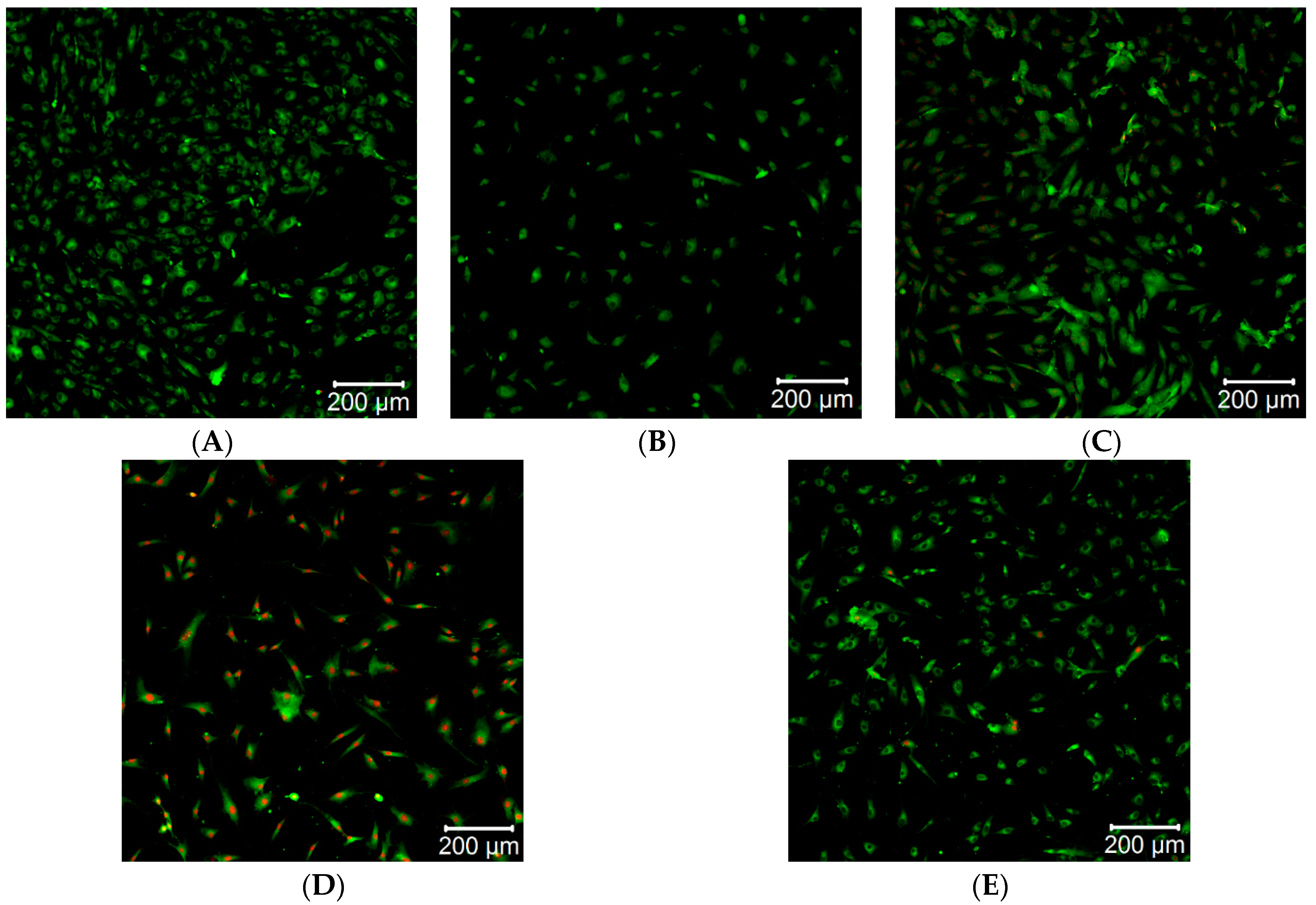
2.4. Direct Cytotoxicity Analysis
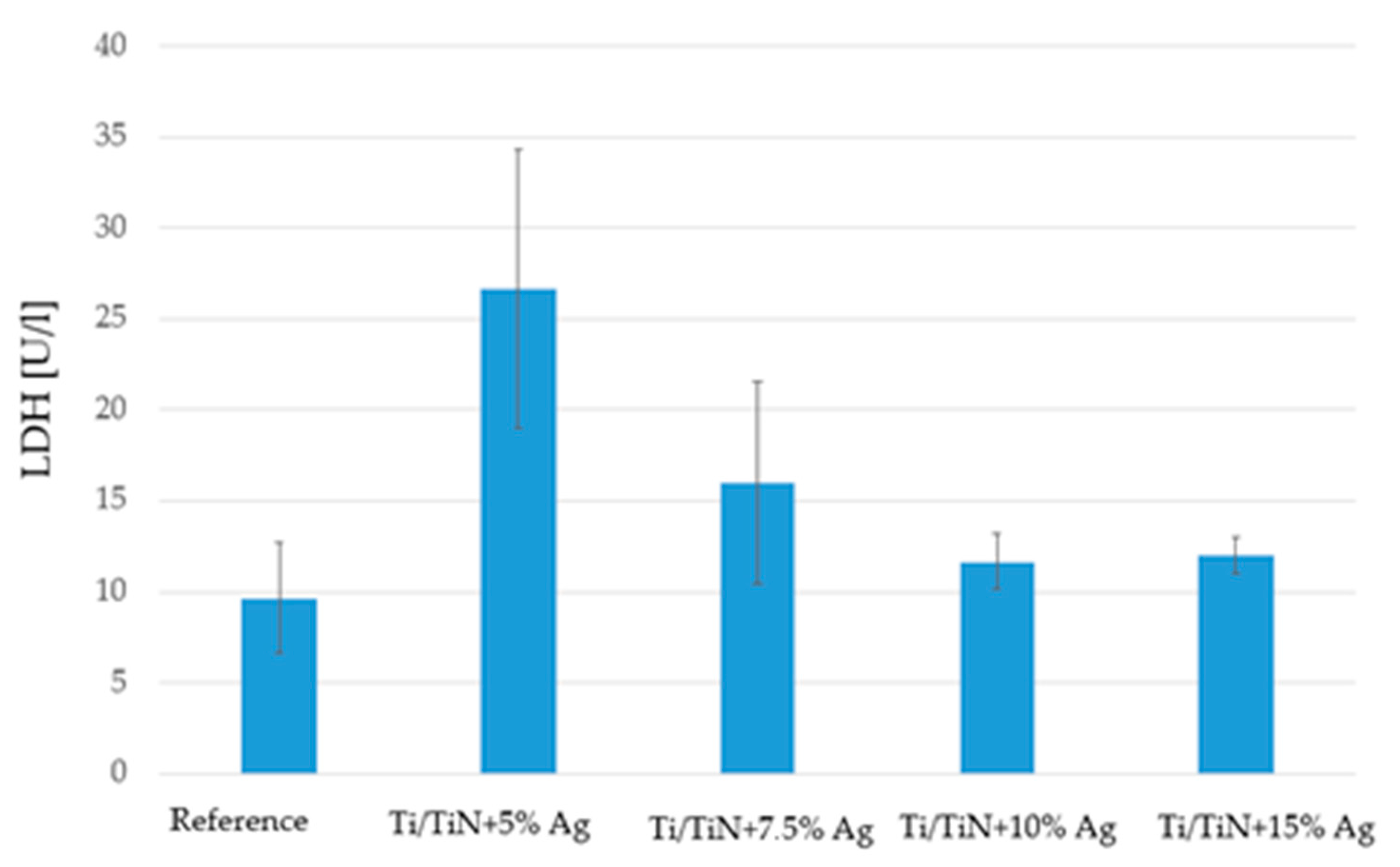
2.5. Lactate Dehydrogenase
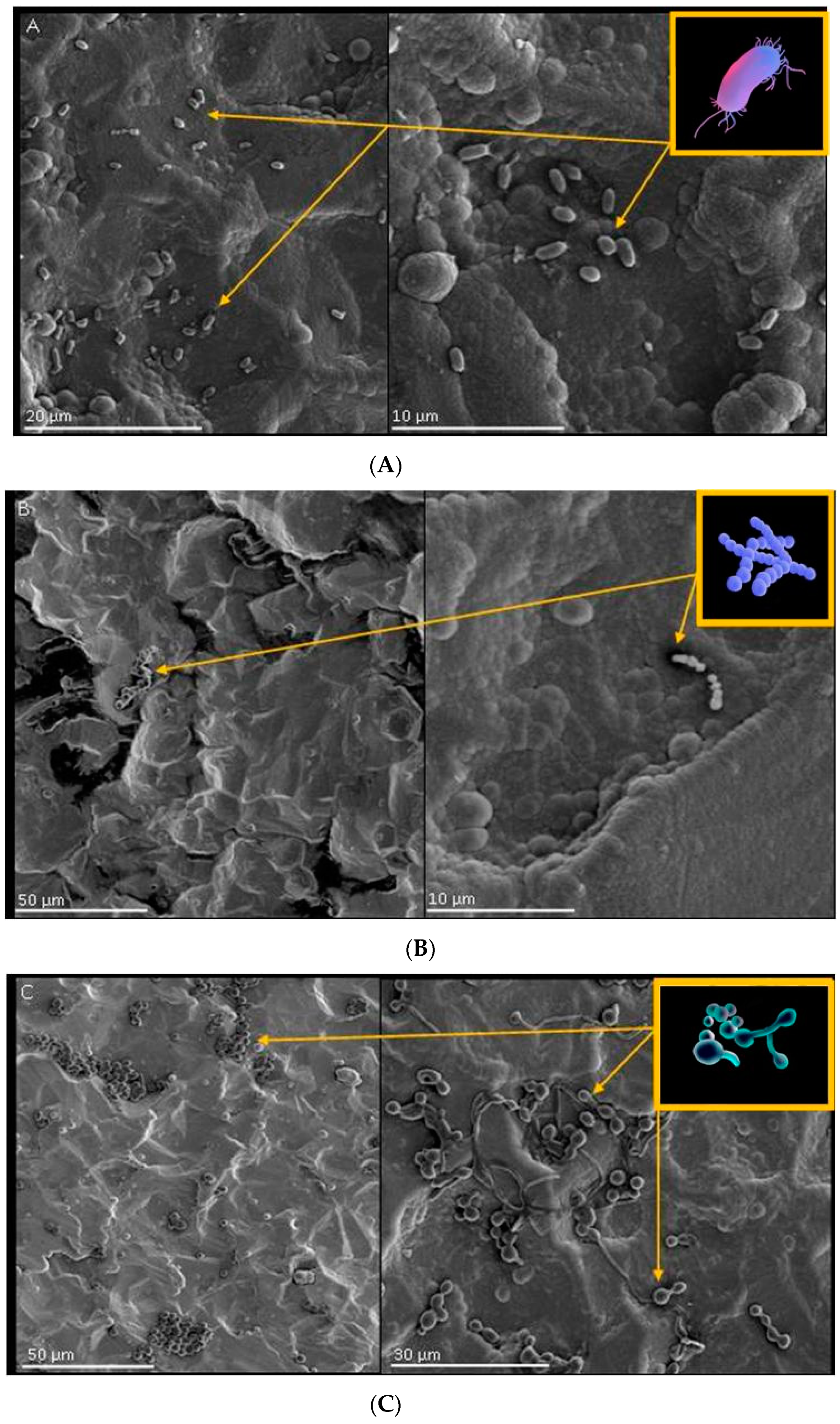
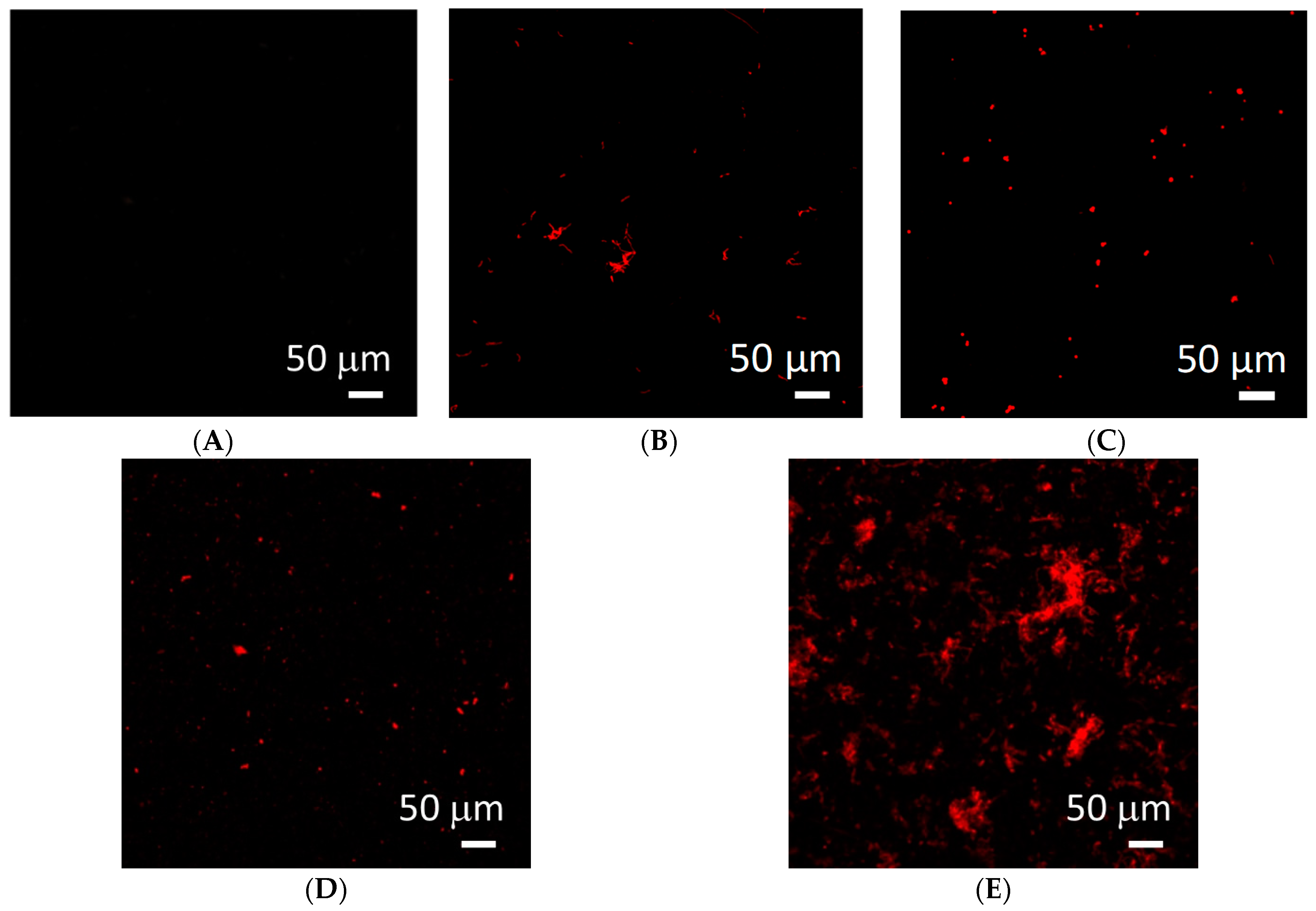
2.6. Surface Interaction of Coatings with Selected Strains of Bacteria and Fungi
2.6.1. Growth, Adhesion and Bacterial Biofilm Formation on the Surface of Biomaterials
2.6.2. Tests of Viability of Microorganisms on the Tested Biomaterials
2.6.3. Test of Antimicrobial Activity of Biomaterials According to ISO 22196
3. Discussion
4. Materials and Methods
4.1. Substrate Preparation
4.2. Smart Bioactive Coatings to Control Biological Interaction
4.2.1. Microstructure
4.2.2. Mechanical Properties of the Thin Films
4.2.3. Cytotoxicity
4.2.4. Lactate Dehydrogenase
4.2.5. Analysis of the Surface Interaction of Coatings with Selected Strains of Bacteria and Fungi
- Pseudomonas aeruginosa (ATCC® 27,853 ™): TSA medium, cultivation at 37 °C; composition of the substrate: TSA 40 g per 1000 mL MP water; sterilization: autoclave 1 h, 121 °C.
- Streptococcus pyogenes Rosenbach (ATCC® 19,615 ™): TSA + blood mutant, culture at 37 °C; composition of the substrate: TSA 40 g per 1000 mL MP water, sheep blood 50 mL per 1000 mL medium (enriched the medium and allowed hemolytic reactions). This was the composition of media dedicated to the culture of streptococci (identified as alpha- or beta-hemolytic, depending on the appearance of the colony on this medium); added after sterilization (autoclave 1 h, 121 °C).
- Candida albicans: medium, broth + sugar; composition of the medium: ordinary broth—13 g per 1000 mL of MP water; glucose—20 g per 1000 mL of medium; sterilization: autoclave 1 h, 121 °C.
4.2.6. Test for Antimicrobial Activity of Biomaterials According to ISO 22,196 (JIS Z 2801 Test for Antimicrobial Activity of Plastics)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 1, 157. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G. The physics of biofilms-an introduction. J. Phys. D 2016, 49, 203001. [Google Scholar] [CrossRef]
- Souza, J.G.; Bertolini, M.M.; Costa, R.C.; Nagay, B.E.; Dongari-Bagtzoglou, A.; Barão, V.A. Targeting implant-associated infections: Titanium surface loaded with antimicrobial. iScience 2020, 24, 102008. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.; Rudkin, J.K.; Black, N.S.; Gallagher, L.; O’Neill, E.; O’Gara, J.P. Methicillin resistance and the biofilm phenotype in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2015, 5, 1. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni Aranya, A.; Pushalkar, S.; Zhao, M.; LeGeros, R.Z.; Zhang, Y.; Saxena, D. Antibacterial and bioactive coatings on titanium implant surfaces. J. Biomed. Mater. Res. Part A 2017, 105, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Auñón, Á.; Esteban, J.; Doadrio, A.L.; Boiza-Sánchez, M.; Mediero, A.; Eguibar-Blázquez, D.; Cordero-Ampuero, J.; Conde, A.; Arenas, M.Á.; de-Damborenea, J.J.; et al. Staphylococcus aureus Prosthetic Joint Infection Is Prevented by a Fluorine- and Phosphorus-Doped Nanostructured Ti-6Al-4V Alloy Loaded with Gentamicin and Vancomycin. J. Orthop. Res. 2020, 38, 588–597. [Google Scholar] [CrossRef]
- Mondal, S.; Dorozhkin, S.V.; Pal, U. Recent progress on fabrication and drug delivery applications of nanostructured hydroxyapatite. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Braun, B.M.; Skelly, J.D.; Ayers, D.C.; Song, J. Significant Suppression of Staphylococcus aureus Colonization on Intramedullary Ti6Al4V Implants Surface-Grafted with Vancomycin-Bearing Polymer Brushes. ACS Appl. Mater. Interfaces 2019, 11, 28641–28647. [Google Scholar] [CrossRef]
- Silverman, S.M.; Moses, J.E.; Sharpless, K.B. Reengineering Antibiotics to Combat Bacterial Resistance: Click Chemistry [1,2,3]-Triazole Vancomycin Dimers with Potent Activity against MRSA and VRE. Chemistry 2017, 23, 79–83. [Google Scholar] [CrossRef]
- Nichol, T.; Callaghan, J.; Townsend, R.; Stockley, I.; Hatton, P.V.; Le Maitre, C.; Smith, T.J.; Akid, R. The antimicrobial activity and biocompatibility of a controlled gentamicin-releasing single-layer sol-gel coating on hydroxyapatite-coated titanium. Bone Jt. J. 2021, 103-B, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [PubMed]
- Sista, S.; Nouri, A.; Li, Y.; Wen, C.; Hodgson, P.D.; Pande, G. Cell biological responses of osteoblasts on anodized nanotubular surface of a titanium-zirconium alloy. J. Biomed. Mater. Res. Part A 2013, 101, 3416–3430. [Google Scholar] [CrossRef] [PubMed]
- Vörös, P.; Dobrindt, O.; Perka, C.; Windisch, C.; Matziolis, G.; Röhner, E. Human osteoblast damage after antiseptic treatment. Int. Orthop. 2014, 38, 177–182. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Yang, Y.; Li, W.; Wu, Z.; Li, J.; Xu, K.; Zhang, W.; Zheng, X.; Chen, J. Study of the Relationship Between Chlorhexidine-Grafted Amount and Biological Performances of Micro/Nanoporous Titanium Surfaces. ACS Omega 2019, 4, 18370–18380. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Bhattacharjee, C.; Curcio, S. Studies on adsorption, reaction mechanisms and kinetics for photocatalytic degradation of CHD, a pharmaceutical waste. Ecotoxicol. Environ. Saf. 2015, 121, 154–163. [Google Scholar] [CrossRef]
- Liu, L.; Wu, H.; Riduan, S.N.; Ying, J.Y.; Zhang, Y. Short imidazolium chains effectively clear fungal biofilm in keratitis treatment. Biomaterials 2013, 34, 1018–1023. [Google Scholar] [CrossRef]
- Li, P.; Poon, Y.F.; Li, W.; Zhu, H.Y.; Yeap, S.H.; Cao, Y.; Qi, X.; Zhou, C.; Lamrani, M.; Beuerman, R.W.; et al. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 2011, 10, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Nederberg, F.; Zhang, Y.; Tan, J.P.; Xu, K.; Wang, H.; Yang, C.; Gao, S.; Guo, X.D.; Fukushima, K.; Li, L.; et al. Biodegradable nanostructures with selective lysis of microbial membranes. Nat. Chem. 2011, 3, 409–414. [Google Scholar] [CrossRef]
- Xu, Z.; Kim, S.K.; Yoon, J. Revisit to imidazolium receptors for the recognition of anions: Highlighted research during 2006–2009. Chem. Soc. Rev. 2010, 39, 1457–1466. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- O’Sullivan, C.; O’Neill, L.; O’Leary, N.D.; O’Gara, J.P.; Crean, A.M.; Ryan, K.B. Osteointegration, antimicrobial and antibiofilm activity of orthopaedic titanium surfaces coated with silver and strontium-doped hydroxyapatite using a novel blasting process. Drug Deliv. Transl. Res. 2021, 11, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Lovati, A.B.; Lopa, S.; Bottagisio, M.; Talò, G.; Canciani, E.; Dellavia, C.; Alessandrino, A.; Biagiotti, M.; Freddi, G.; Segatti, F.; et al. Peptide-Enriched Silk Fibroin Sponge and Trabecular Titanium Composites to Enhance Bone Ingrowth of Prosthetic Implants in an Ovine Model of Bone Gaps. Front. Bioeng. Biotechnol. 2020, 8, 563203. [Google Scholar] [CrossRef]
- Slate, A.J.; Wickens, D.J.; El Mohtadi, M.; Dempsey-Hibbert, N.; West, G.; Banks, C.E.; Whitehead, K.A. Antimicrobial activity of Ti-ZrN/Ag coatings for use in biomaterial applications. Sci. Rep. 2018, 8, 1497. [Google Scholar] [CrossRef]
- Sevencan, A.; Kartal Doyuk, E.; Köse, N. Silver ion doped hydroxyapatite-coated titanium pins prevent bacterial colonization. Jt. Dis. Relat. Surg. 2021, 32, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.G.; Toccaceli, C.; Gillett, A.R.; Ng, C.H.; Hodgson, S.D.; Lawrence, J. Surface Treatments to Modulate Bioadhesion: A Critical Review. Rev. Adhes. Adhes. 2016, 4, 69–103. [Google Scholar] [CrossRef]
- Hussmann, B.; Johann, I.; Kauther, M.D.; Landgraeber, S.; Jäger, M.; Lendemans, S. Measurement of the silver ion concentration in wound fluids after implantation of silver-coated megaprostheses: Correlation with the clinical outcome. BioMed Res. Int. 2013, 2013, 763096. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Li, H.; Kelly, P.J.; Verran, J. The Antimicrobial Properties of Titanium Nitride/Silver Nanocomposite Coatings. J. Adhes. Sci. Technol. 2011, 25, 2299–2315. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Mazur, M.; Kaczmarek, D.; Mazur, P.; Szponar, B.; Domaradzki, J.; Kepinski, L. Influence of the surface properties on bactericidal and fungicidal activity of magnetron sputtered Ti-Ag and Nb-Ag thin films. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 86–95. [Google Scholar] [CrossRef]
- Huang, H.L.; Chang, Y.Y.; Lai, M.C.; Lin, C.R.; Lai, C.H.; Shieh, T.M. Antibacterial TaN-Ag coatings on titanium dental implants. Surf. Coat. Technol. 2010, 205, 1636–1641. [Google Scholar] [CrossRef]
- Hsieh, J.H.; Yeh, T.H.; Li, C.; Chiu, C.H.; Huang, C.T. Antibacterial properties of TaN-(Ag,Cu) nanocomposite thin films. Vaccum 2013, 87, 160–163. [Google Scholar] [CrossRef]
- Marciano, F.R.; Bonetti, L.F.; Mangolin, J.F.; Da-Silva, N.S.; Corat, E.J.; Trava-Airoldi, V.J. Investigation into the antibacterial property and bacterial adhesion of diamond-like carbon films. Vaccum 2011, 85, 662–666. [Google Scholar] [CrossRef]
- Lackner, J.M.; Waldhauser, W. Inorganic PVD and CVD Coatings in Medicine—A Review of Protein and Cell Adhesion on Coated Surfaces. J. Adhes. Sci. Technol. 2010, 24, 925–961. [Google Scholar] [CrossRef]
- Inal, K.; Lackner, J.M.; Waldhauser, W.; Gümüş, S.; Polat, S.; Dalçik, H. Antibacterial Osseoconductive Thin Film for Implant. Turkish Patent EP3220969A1, 17 November 2014. [Google Scholar]
- Syromotina, D.S.; Surmeneva, M.A.; Gorodzha, S.N.; Pichugin, V.F.; Ivanova, A.A.; Grubova, I.Y.; Kravchuk, K.S.; Gogolinskii, K.V.; Prymak, O.; Epple, M.; et al. Physical-Mechanical Characteristics of RF Magnetron Sputter-Deposited Coatings Based on Silver-Doped Hydroxyapatite. Russ. Phys. J. 2014, 56, 1198–1205. [Google Scholar] [CrossRef]
- Liou, J.W.; Chang, H.H. Bactericidal effects and mechanisms of visible light-responsive titanium dioxide photocatalysts on pathogenic bacteria. Arch. Immunol. Ther. Exp. 2012, 60, 267–275. [Google Scholar] [CrossRef]
- Yue, C.; Kuijer, R.; Kaper, H.J.; van der Mei, H.C.; Busscher, H.J. Simultaneous interaction of bacteria and tissue cells with photocatalytically activated, anodized titanium surfaces. Biomaterials 2014, 35, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.C.; Siedlecki, C.A. Protein adsorption, platelet adhesion, and bacterial adhesion to polyethylene-glycol-textured polyurethane biomaterial surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Lih, E.; Choi, S.G.; Ahn, D.J.; Joung, Y.K.; Han, D.K. Optimal conjugation of catechol group onto hyaluronic acid in coronary stent substrate coating for the prevention of restenosis. J. Tissue Eng. 2016, 7, 2041731416683745. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, Y.; Xu, D.; Cai, K. Surface functionalization of titanium substrates with chitosan-lauric acid conjugate to enhance osteoblasts functions and inhibit bacteria adhesion. Colloids Surf. B Biointerfaces 2014, 119, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Aveyard, J.; Fleming, G.; Curran, J.M.; McBride, F.; Raval, R.; D’Sa, R.A. Nitric Oxide Releasing Titanium Surfaces for Antimicrobial Bone-Integrating Orthopedic Implants. ACS Appl. Mater. Interfaces 2020, 12, 22433–22443. [Google Scholar] [CrossRef]
- Peng, Z.; Ao, H.; Wang, L.; Guo, S.; Tang, T. Quaternised chitosan coating on titanium provides a self-protective surface that prevents bacterial colonisation and implant-associated infections. RSC Adv. 2015, 5, 54304–54311. [Google Scholar] [CrossRef]
- Arciola, C.R.; Visai, L.; Testoni, F.; Arciola, S.; Campoccia, D.; Speziale, P.; Montanaro, L. Concise survey of Staphylococcus aureus virulence factors that promote adhesion and damage to peri-implant tissues. Int. J. Artif. Organs 2011, 34, 771–780. [Google Scholar] [CrossRef]
- Feuillie, C.; Vitry, P.; McAleer, M.A.; Kezic, S.; Irvine, A.D.; Geoghegan, J.A.; Dufrêne, Y.F. Adhesion of Staphylococcus aureus to Corneocytes from Atopic Dermatitis Patients Is Controlled by Natural Moisturizing Factor Levels. Mbio 2018, 9, e01184-17. [Google Scholar] [CrossRef]
- Foster, T.J. The remarkably multifunctional fibronectin binding proteins of Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1923–1931. [Google Scholar] [CrossRef]
- Eick, S.; Kindblom, C.; Mizgalska, D.; Magdoń, A.; Jurczyk, K.; Sculean, A.; Stavropoulos, A. Adhesion of Porphyromonas gingivalis and Tannerella forsythia to dentin and titanium with sandblasted and acid etched surface coated with serum and serum proteins—An in vitro study. Arch. Oral Biol. 2017, 75, 81–88. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L. Polysaccharide intercellular adhesin in biofilm: Structural and regulatory aspects. Front. Cell. Infect. Microbiol. 2015, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Kaba, S.I.; Egorova, E.M. In vitro studies of the toxic effects of silver nanoparticles on HeLa and U937 cells. Nanotechnol. Sci. Appl. 2015, 8, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.C.; Bayles, K.W. Molecular Control of Bacterial Death and Lysis. Microbiol. Mol. Biol. Rev. 2008, 72, 85–109. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Elbehiry, A.; Al-Dubaib, M.; Marzouk, E.; Moussa, I. Antibacterial effects and resistance induction of silver and gold nanoparticles against Staphylococcus aureus -induced mastitis and the potential toxicity in rats. MicrobiologyOpen 2019, 8, e00698. [Google Scholar] [CrossRef] [PubMed]
- Hosny, A.E.; Rasmy, S.A.; Aboul-Magd, D.S.; Kashef, M.T.; El-Bazza, Z.E. The increasing threat of silver-resistance in clinical isolates from wounds and burns. Infect. Drug Resist. 2019, 12, 1985–2001. [Google Scholar] [CrossRef] [PubMed]
- Major, R.; Kowalczyk, P.; Surmiak, M.; Łojszczyk, I.; Podgórski, R.; Trzaskowska, P.; Ciach, T.; Russmueller, G.; Kasperkiewicz, K.; Major, Ł.; et al. Patient specific implants for jawbone reconstruction after tumor resection. Colloids Surf. B Biointerfaces 2020, 193, 111056. [Google Scholar] [CrossRef] [PubMed]
- Trembecka-Wójciga, K.; Kopernik, M.; Surmiak, M.; Major, R.; Gawlikowski, M.; Bruckert, F.; Kot, M.; Lackner, J.M. Effect of the mechanical properties of carbon-based coatings on the mechanics of cell-material interactions. Colloids Surf. B Biointerfaces 2021, 197, 111359. [Google Scholar] [CrossRef] [PubMed]
- Kopernik, M.; Milenin, A.; Major, R.; Lackner, J.M. Identification of material model TiN using numerical simulation of nanoindentation test. Mater. Sci. Technol. 2011, 27, 604–616. [Google Scholar] [CrossRef]
- Prideaux, M.; Wijenayaka, A.R.; Kumarasinghe, D.D.; Ormsby, R.T.; Evdokiou, A.; Findlay, D.M.; Atkins, G.J. SaOS2 osteosarcoma cells as an in vitro model for studying the transition of human osteoblasts to osteocytes. Calcif. Tissue Int. 2014, 95, 183–193. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Wang, H.R.; Huo, K.F.; Cui, L.Y.; Zhang, W.R.; Ni, H.W.; Zhang, Y.M.; Wu, Z.F.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef]
- Wróblewski, F.; Ladue, J.S. Lactic Dehydrogenase Activity in Blood. Exp. Biol. Med. 1955, 90, 210–213. [Google Scholar] [CrossRef]


| Layer | Wear Rate F = 0.5 N N = 2000c × 10−6 | Friction Coefficient |
|---|---|---|
| Ti/TiN + 5% Ag | 251.7 | 0.428 |
| Ti/TiN + 7.5% Ag | 564.8 | 0.494 |
| Ti/TiN + 10% Ag | 574.1 | 0.478 |
| Ti/TiN + 15% Ag | 835.7 | 0.404 |
| L.P. | Layer | Thickness [µm] |
|---|---|---|
| 1 | Ti/TiN 1:1 doped 5% Ag | 3.40 |
| 2 | Ti/TiN 1:1 doped 7.5% Ag | 3.40 |
| 3 | Ti/TiN 1:1 doped 10% Ag | 2.70 |
| 4 | Ti/TiN 1:1 doped 15% Ag | 3.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasperkiewicz, K.; Major, R.; Sypien, A.; Kot, M.; Dyner, M.; Major, Ł.; Byrski, A.; Kopernik, M.; Lackner, J.M. Antibacterial Optimization of Highly Deformed Titanium Alloys for Spinal Implants. Molecules 2021, 26, 3145. https://doi.org/10.3390/molecules26113145
Kasperkiewicz K, Major R, Sypien A, Kot M, Dyner M, Major Ł, Byrski A, Kopernik M, Lackner JM. Antibacterial Optimization of Highly Deformed Titanium Alloys for Spinal Implants. Molecules. 2021; 26(11):3145. https://doi.org/10.3390/molecules26113145
Chicago/Turabian StyleKasperkiewicz, Katarzyna, Roman Major, Anna Sypien, Marcin Kot, Marcin Dyner, Łukasz Major, Adam Byrski, Magdalena Kopernik, and Juergen M. Lackner. 2021. "Antibacterial Optimization of Highly Deformed Titanium Alloys for Spinal Implants" Molecules 26, no. 11: 3145. https://doi.org/10.3390/molecules26113145
APA StyleKasperkiewicz, K., Major, R., Sypien, A., Kot, M., Dyner, M., Major, Ł., Byrski, A., Kopernik, M., & Lackner, J. M. (2021). Antibacterial Optimization of Highly Deformed Titanium Alloys for Spinal Implants. Molecules, 26(11), 3145. https://doi.org/10.3390/molecules26113145