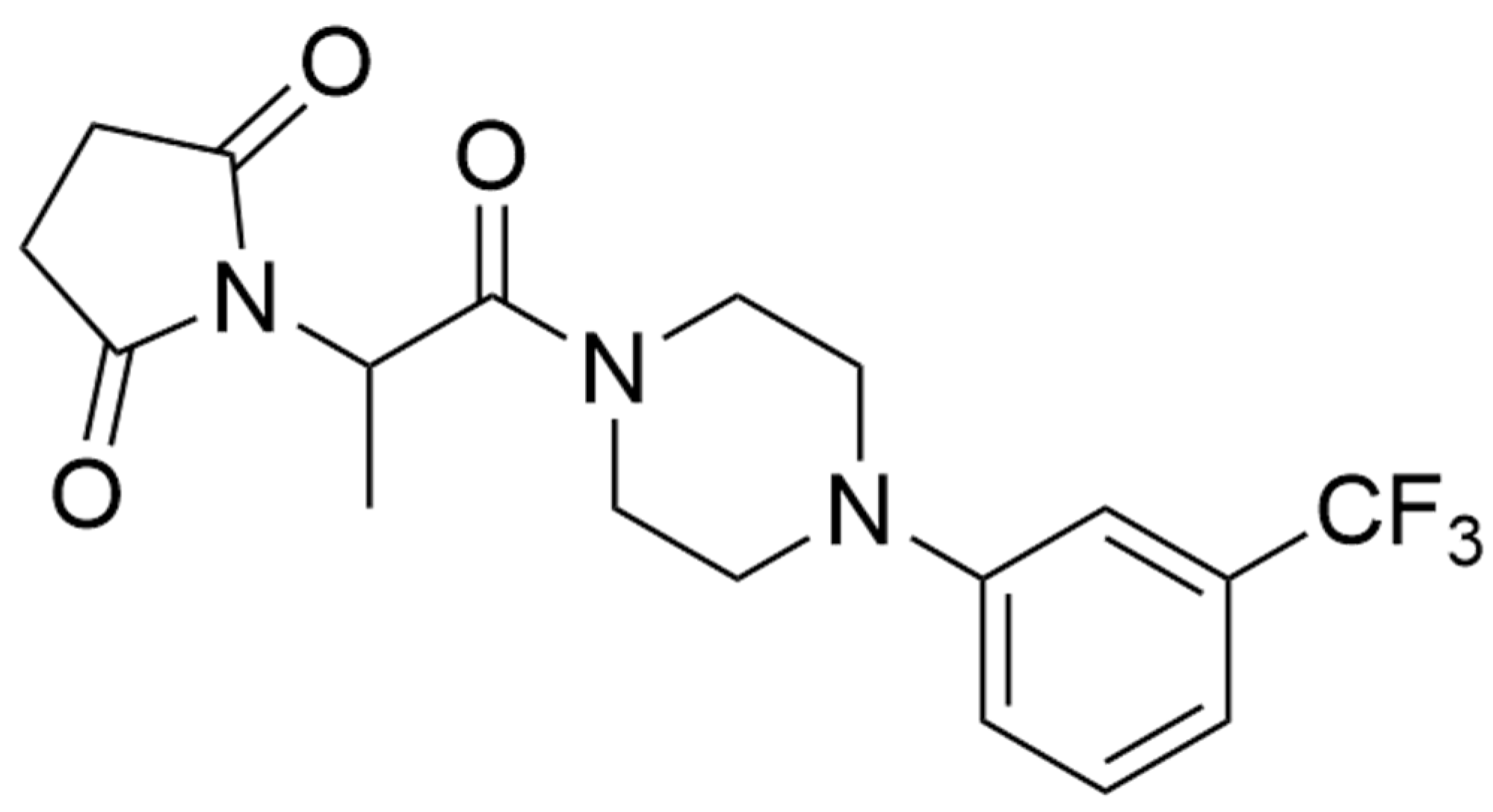
C-11, a New Antiepileptic Drug Candidate: Evaluation of the Physicochemical Properties and Impact on the Protective Action of Selected Antiepileptic Drugs in the Mouse Maximal Electroshock-Induced Seizure Model
Abstract
1. Introduction
2. Results
2.1. Effect of C-11 on the Anticonvulsant Activity of Various AEDs in the MES Model in Mice
2.2. Effects of C-11 Alone and in Combination with Studied Aeds on Muscular Strength, Motor Coordination, and Long-Term Memory in Mice
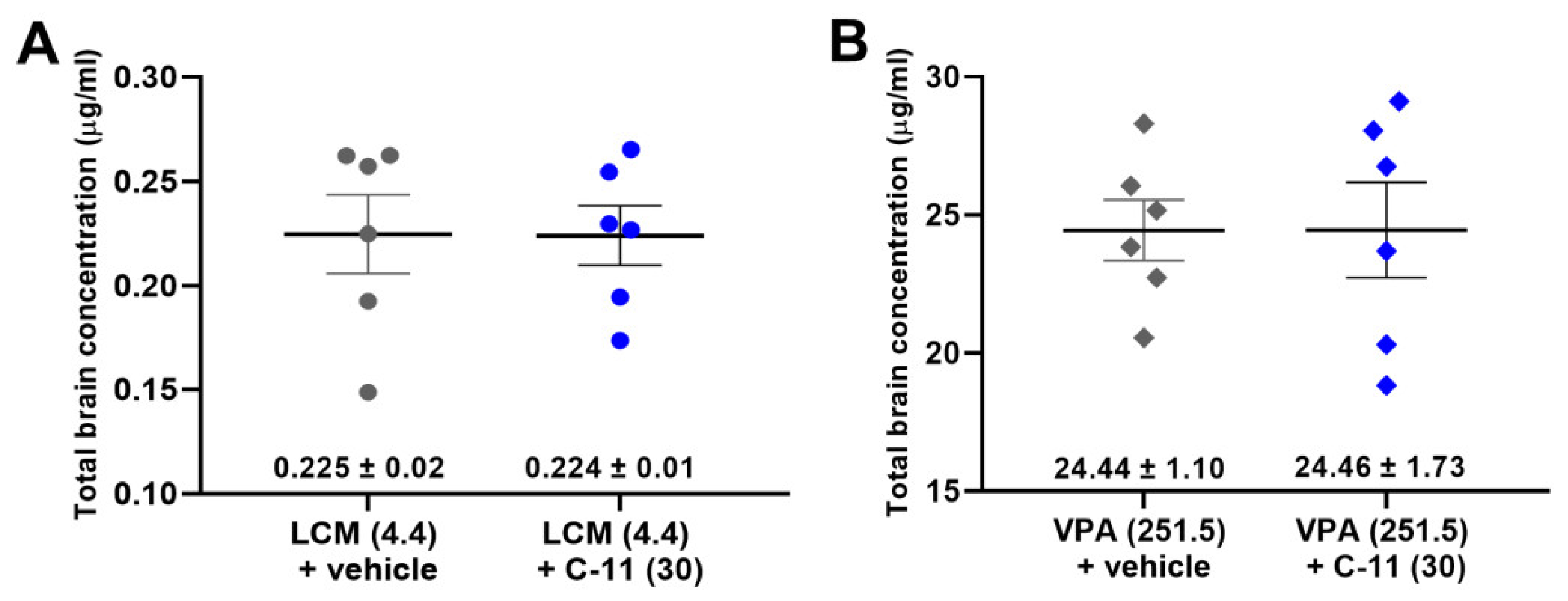
2.3. Effect of C-11 on Total Brain AED Concentrations
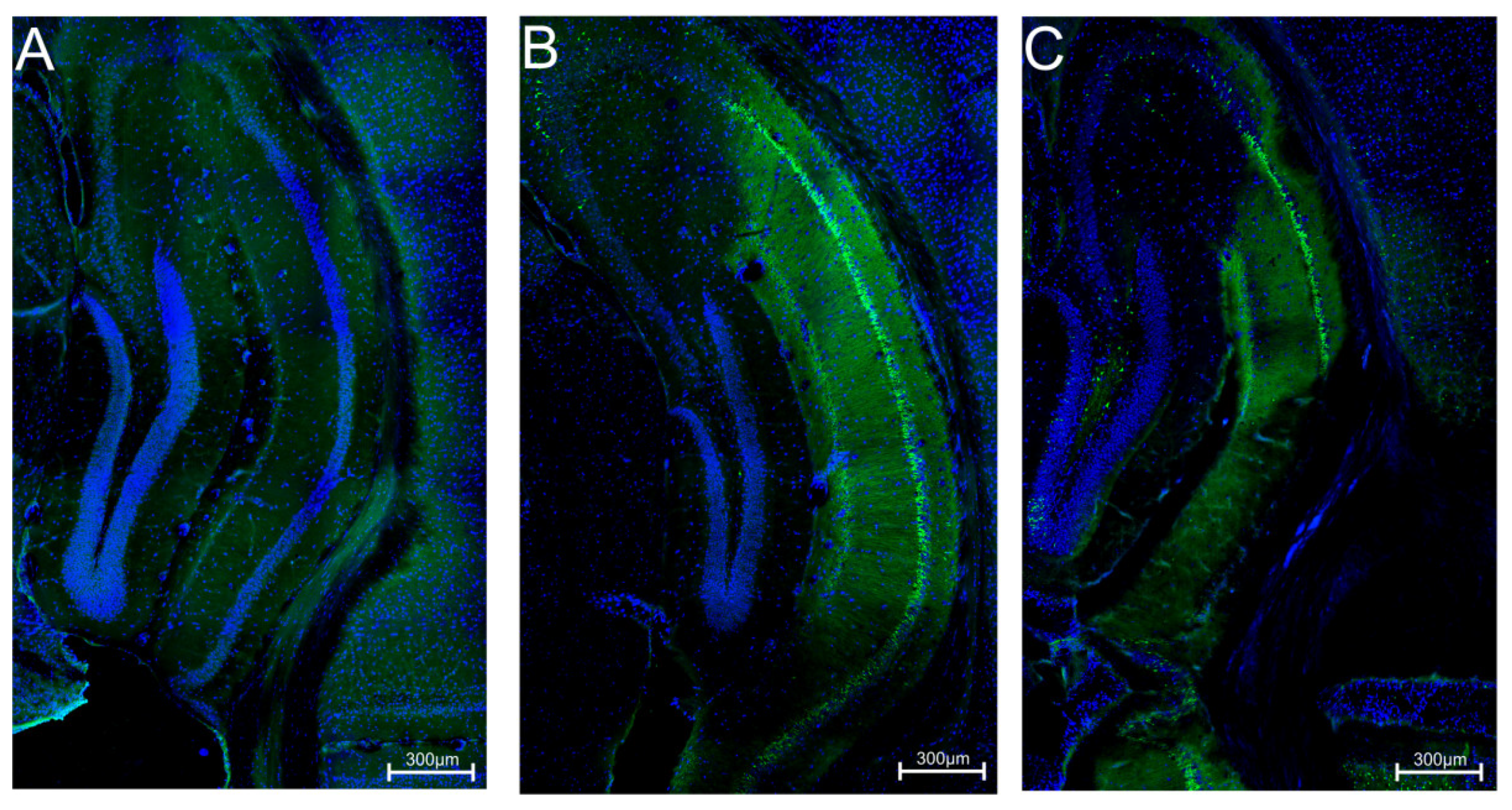
2.4. Influence of C-11 on Neuroprotection in Pilocarpine Convulsion in Mice
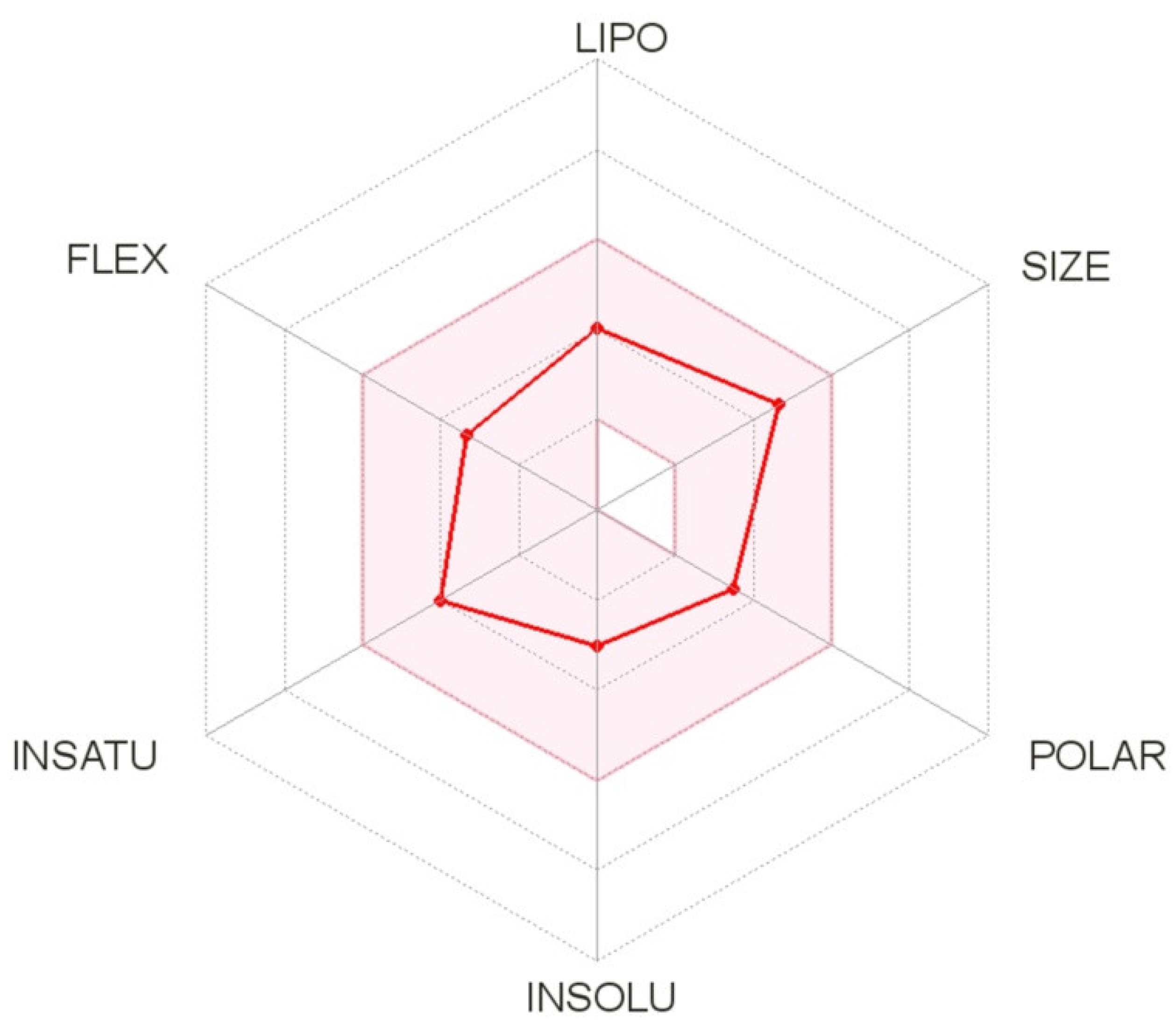
2.5. In Silico Physicochemical Descriptors Determination of C-11
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Maximal Electroshock Seizure Test
4.4. Behavioral Tests
4.4.1. Chimney Test
4.4.2. Grip-Strength Test
4.4.3. Passive Avoidance Task
4.5. Measurement of Total Brain Antiepileptic Drug Concentrations
4.6. Neuroprotection of C-11
4.6.1. Pilocarpine-Induced Convulsion in Mice
4.6.2. Brain Slice Preparation
4.6.3. Fluoro-Jade B Staining
4.7. In Silico Physicochemical Descriptors Determination
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brodie, M.J.; Barry, S.J.; Bamagous, G.A.; Norrie, J.D.; Kwan, P. Patterns of treatment response in newly diagnosed epilepsy. Neurology 2012, 78, 1548–1554. [Google Scholar] [CrossRef]
- Perucca, P.; Gilliam, F.G. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012, 11, 792–802. [Google Scholar] [CrossRef]
- Santulli, L.; Coppola, A.; Balestrini, S.; Striano, S. The challenges of treating epilepsy with 25 antiepileptic drugs. Pharmacol. Res. 2016, 107, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, R.; Brodie, M.J. Early predictors of outcome in newly diagnosed epilepsy. Seizure 2013, 22, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Schachter, S.C.; Brodie, M.J. Drug-resistant epilepsy. N. Engl. J. Med. 2011, 365, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Stephen, L.J.; Brodie, M.J. Antiepileptic drug monotherapy versus polytherapy: Pursuing seizure freedom and tolerability in adults. Curr. Opin. Neurol. 2012, 25, 164–172. [Google Scholar] [CrossRef]
- Andres-Mach, M.; Dudra-Jastrzebska, M.; Haratym-Maj, A.; Czerwonka, A.; Raszewski, G.; Luszczki, J.J. No anticonvulsant effect of the water extract of scutellariae radix on the anticonvulsant action of valproate, tiagabine and topiramate in two animal models of epilepsy. J. Pre-Clin. Clin. Res. 2009, 3, 95–98. [Google Scholar]
- Andres-Mach, M.; Haratym-Maj, A.; Zagaja, M.; Rola, R.; Maj, M.; Chrościńska-Krawczyk, M.; Luszczki, J.J. ACEA (a highly selective cannabinoid CB1 receptor agonist) stimulates hippocampal neurogenesis in mice treated with antiepileptic drugs. Brain Res. 2015, 1624, 86–94. [Google Scholar] [CrossRef]
- Luszczki, J.J.; Czuczwar, P.; Cioczek-Czuczwar, A.; Czuczwar, S.J. Arachidonyl-2′- chloroethylamide, a highly selective cannabinoid CB1 receptor agonist, enhances the anticonvulsant action of valproate in the mouse maximal electroshock-induced seizure model. Eur. J. Pharmacol. 2006, 547, 65–74. [Google Scholar] [CrossRef]
- Luszczki, J.J.; Czuczwar, P.; Cioczek-Czuczwar, A.; Dudra-Jastrzebska, M.; Andres-Mach, M.; Czuczwar, S.J. Effect of arachidonyl-2′-chloroethylamide, a selective cannabinoid CB1 receptor agonist on the protective action of the various antiepileptic drugs in the mouse maximal electroshock-induced seizure model. Prog. Neuro-Psychopharmacol. 2010, 34, 18–25. [Google Scholar] [CrossRef]
- Luszczki, J.J.; Misiuta-Krzeminska, M.; Florek, M.; Tutka, P.; Czuczwar, S.J. Syntetic cannabinoid WIN 55,212-2 mesylate enhances the protective action of four classical antiepileptic drugs against maximal electroshock-induced seizures in mice. Pharamcol. Biochem. Behav. 2011, 98, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Zagaja, M.; Pyrka, D.; Skalicka-Woźniak, K.; Glowniak, K.; Florek-Luszczki, M.; Glensk, M.; Luszczki, J.J. Effect of xanthotoxin (8-methoxypsoralen) on the anticonvulsant activity of classical antiepileptic drugs against maximal electroshock-induced seizures in mice. Fitoterapia 2015, 105, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zagaja, M.; Andres-Mach, M.; Skalicka-Woźniak, K.; Rękas, A.R.; Kondrat-Wróbel, M.W.; Gleńsk, M.; Łuszczki, J.J. Assessment of the combined treatment with umbelliferone and four classical antiepileptic drugs against maximal electroshock-induced seizures in mice. Pharmacology 2015, 96, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Zagaja, M.; Andres-Mach, M.; Patrzylas, P.; Pyrka, D.; Szpringer, M.; Florek-Łuszczki, M.; Żółkowska, D.; Skalicka-Woźniak, K.; Łuszczki, J.J. Influence of xanthotoxin (8-methoxypsoralen) on the anticonvulsant activity of various novel antiepileptic drugs against maximal electroshock-induced seizures in mice. Fitoterapia 2016, 115, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, K.; Zagaja, M.; Łuszczki, J.J.; Rapacz, A.; Andres-Mach, M.; Latacz, G.; Kieć- Kononowicz, K. Design, synthesis, and anticonvulsant activity of new hybrid compounds derived from 2-(2,5-dioxopyrrolidin-1-yl) propanamides and 2-(2,5 dioxopyrrolidin- 1-yl) butanamides. J. Med. Chem. 2015, 58, 5274–5286. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Mogilski, S.; Pieróg, M.; Nieoczym, D.; Abram, M.; Szulczyk, B.; Lubelska, A.; Latacz, G.; Doboszewska, U.; Wlaź, P.; et al. KA-11, a novel pyrrolidine- 2,5-dione derived broad-spectrum anticonvulsant: Its antiepileptogenic, antinociceptive properties and in vitro characterization. ACS Chem. Neurosci. 2019, 10, 636–648. [Google Scholar] [CrossRef]
- Andres-Mach, M.; Szewczyk, A.; Zagaja, M.; Luszczki, J.; Maj, M.; Rola, R.; Abram, M.; Kaminski, K. Evaluation of the impact of compound C11 a new anticonvulsant candidate on cognitive functions and hippocampal neurogenesis in mouse brain. Neuropharmacology 2020, 163, 107849. [Google Scholar] [CrossRef]
- Andres-Mach, M.; Szewczyk, A.; Zagaja, M.; Szala-Rycaj, J.; Lemieszek, M.; Maj, M.; Abram, M.; Kaminski, K. Preclinical assessment of a new hybrid compound C11 efficacy on neurogenesis and cognitive functions after pilocarpine induced status epilepticus in mice. Int. J. Mol. Sci. 2021, 22, 3240. [Google Scholar] [CrossRef]
- Łuszczki, J.J.; Plech, T.; Wujec, M. Effect of 4-(4-bromophenyl)-5-(3-chlorophenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione on the anticonvulsant action of different classical antiepileptic drugs in the mouse maximal electroshock-induced seizure model. Eur. J. Pharm. 2012, 690, 99–106. [Google Scholar] [CrossRef]
- Löscher, W.; Fassbender, C.P.; Nolting, B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. II. Maximal electroshock seizure models. Epilepsy Res. 1991, 8, 79–94. [Google Scholar] [CrossRef]
- Curia, G.; Longo, D.; Biagini, G.; Jones, S.G.R.; Avoli, M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Kow, R.L.; Jiang, K.; Naydenov, A.V.; Le, J.H.; Stella, N.; Nathanson, N.M. Modulation of pilocarpine-induced seizures by cannabinoid receptor 1. PLoS ONE 2014, 9, e95922. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andres-Mach, M.; Zagaja, M.; Haratym-Maj, A.; Rola, R.; Maj, M.; Haratym, J.; Dudra-Jastrzębska, M.; Łuszczki, J.J. A long-term treatment with arachidonyl-2’-chloroethylamide combined with valproate increases neurogenesis in a mouse pilocarpine model of epilepsy. Int. J. Mol. Sci. 2017, 18, 900. [Google Scholar] [CrossRef] [PubMed]
- Zagaja, M.; Szewczyk, A.; Haratym-Maj, A.; Rola, R.; Maj, M.; Łuszczki, J.J.; Andres-Mach, M. Increased neurogenesis after ACEA and levetiracetam treatment in mouse pilocarpine model of epilepsy. J. Pre-Clin. Clin. Res. 2017, 11, 136–141. [Google Scholar] [CrossRef]
- Juvale, I.I.A.; Has, A.T.C. The evolution of the pilocarpine animal model of status epilepticus. Heliyon 2020, 6, e045572. [Google Scholar]
- SwissADME Website. Available online: http://www.swissadme.ch/ (accessed on 10 January 2021).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Shank, R.P.; Gardocki, J.F.; Streeter, A.J.; Maryanoff, B.E. An overview of the preclinical aspects of Topiramate: Pharmacology, pharmacokinetics, and mechanism of action. Epilepsia 2000, 41, S3–S9. [Google Scholar] [CrossRef]
- Russo, E.; Constanti, A.; Ferreri, G.; Citraro, R.; De Sarro, G. Nifedipine affects the anticonvulsant activity of Topiramate in various animal models of epilepsy. Neuropharmacology 2004, 46, 865–878. [Google Scholar] [CrossRef]
- Errington, A.C.; Stöhr, T.; Heers, C.; Lees, G. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Mol. Pharmacol. 2008, 73, 157–169. [Google Scholar] [CrossRef]
- Rogawski, M.A.; Tofighy, A.; White, H.S.; Matagne, A.; Wolff, C. Current understanding of the mechanism of action of the antiepileptic drug lacosamide. Epilepsy Res. 2015, 110, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Doty, P.; Hebert, D.; Mathy, F.X.; Byrnes, W.; Zackheim, J.; Simontacchi, K. Development of lacosamide for the treatment of partial-onset seizures. Ann. N. Y. Acad. Sci. 2013, 1291, 56–68. [Google Scholar] [CrossRef]
- Wilson, S.M.; Khanna, R. Specific binding of lacosamide to collapsin response mediator protein 2 (CRMP2) and direct impairment of its canonical function: Implications for the therapeutic potential of lacosamide. Mol. Neurobiol. 2015, 51, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Basic pharmacology of valproate: A review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs 2002, 16, 669–694. [Google Scholar] [CrossRef] [PubMed]
- Rogawski, M.A.; Cavazos, J.E. Mechanisms of action of antiepileptic drugs. In Wyllie’s Treatment of Epilepsy: Principles and Practice, 6th ed.; Wyllie, E., Ed.; Professional Communications Inc.: New York, NY, USA, 2015; pp. 522–529. [Google Scholar]
- Englund, M.; Hyllienmark, L.; Brismar, T. Effect of valproate, lamotrigine and levetiracetam on excitability and firing properties of CA1 neurons in rat brain slices. Cell Mol. Neurobiol. 2011, 31, 645–652. [Google Scholar] [CrossRef]
- Lason, W.; Dudra-Jastrzebska, M.; Rejdak, K.; Czuczwar, S.J. Basic mechanisms of antiepileptic drugs and their pharmacokinetic/pharmacodynamic interactions: An update. Pharmacol. Rep. 2011, 63, 271–292. [Google Scholar] [CrossRef]
- Grunze, H.; von Wegerer, J.; Greene, R.W.; Walden, J. Modulation of calcium and potassium currents by lamotrigine. Neuropsychobiology 1998, 38, 131–138. [Google Scholar] [CrossRef]
- Ketter, T.A.; Manji, H.K.; Post, R.M. Potential mechanisms of action of lamotrigine in the treatment of bipolar disorders. J. Clin. Psychopharmacol. 2003, 23, 484–495. [Google Scholar] [CrossRef]
- Beyreuther, B.K.; Freitag, J.; Heers, C.; Krebsfanger, N.; Scharfenecker, U.; Stohr, T. Lacosamide: A review of preclinical properties. CNS Drug Rev. 2007, 13, 21–42. [Google Scholar] [CrossRef]
- Cawello, W.; Boekens, H.; Bonn, R. Absorption, disposition, metabolic fate and elimination of the anti-epileptic drug lacosamide in humans: Mass balance following intravenous and oral administration. Eur. J. Drug. Metab. Pharmacokinet. 2012, 37, 241–248. [Google Scholar] [CrossRef]
- Argikar, U.A.; Remmel, R.P. Effect of aging on glucuronidation of valproic acid in human liver microsomes and the role of UDP-glucuronosyltransferase UGT1A4, UGT1A8, and UGT1A10. Drug Metab. Dispos. 2009, 37, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yu, J.T.; Sun, Y.P.; Ou, J.R.; Song, J.H.; Yu, Y. The influence of cytochrome oxidase CYP2A6, CYP2B6, and CYP2C9 polymorphisms on the plasma concentrations of valproic acid in epileptic patients. Clin. Neurol. Neurosurg. 2010, 112, 320–323. [Google Scholar] [CrossRef] [PubMed]
- The Food and Drug Administration (FDA). A Draft Guidance for Industry: Drug Interaction Studies-Study Design, Data Analysis, and Implications for Dosing and Labeling; Government Printing Office: Washington, DC, USA, 2006.
- Pitkänen, A.; Kubova, H. Antiepileptic drugs in neuroprotection. Expert Opin. Pharmacother. 2004, 5, 777–798. [Google Scholar] [CrossRef] [PubMed]
- Trojnar, M.K.; Małek, R.; Chroscinska, M.; Nowak, S.; Błaszczyk, B.; Czuczwar, S.J. Neuroprotective effects of antiepileptic drugs. Pol. J. Pharmacol. 2002, 54, 557–566. [Google Scholar]
- Ahn, J.Y.; Yan, B.C.; Park, J.H.; Ahn, J.H.; Lee, D.H.; Kim, I.H.; Cho, J.-H.; Chen, B.H.; Cho, Y.S.; Shin, M.C.; et al. Novel antiepileptic drug lacosamide exerts neuroprotective effects by decreasing glial activation in the hippocampus of a gerbil model of ischemic stroke. Exp. Ther. Med. 2015, 10, 2007–2014. [Google Scholar] [CrossRef]
- Nirwan, N.; Siraj, F.; Vohora, D. Inverted-U response of lacosamide on pilocarpine-induced status epilepticus and oxidative stress in C57BL/6 mice is independent of hippocampal collapsin response mediator protein-2. Epilepsy Res. 2018, 145, 93–101. [Google Scholar] [CrossRef]
- Kanai, H.; Sawa, A.; Chen, R.W.; Leeds, P.; Chuang, D.M. Valproic acid inhibits histone deacetylase activity and suppresses excitotoxicity-induced GAPDH nuclear accumulation and apoptotic death in neurons. Pharm. J. 2004, 4, 336–344. [Google Scholar] [CrossRef]
- Rekling, J.C. Neuroprotective effects of anticonvulsants in rat hippocampal slice cultures exposed to oxygen/glucose deprivation. Neurosci. Lett. 2003, 335, 167–170. [Google Scholar] [CrossRef]
- Sinn, D.I.; Kim, S.J.; Chu, K. Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation. Neurobiol Dis. 2007, 26, 464–472. [Google Scholar] [CrossRef]
- Kim, H.J.; Rowe, M.; Ren, M.; Hong, J.S.; Chen, P.S.; Chuang, D.M. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: Multiple mechanisms of action. J. Pharmacol. Exp. Ther. 2007, 321, 892–901. [Google Scholar] [CrossRef]
- Hansson, E.; Rönnbäck, L. Glial neuronal signaling in the central nervous system. FASEB J. 2003, 17, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Pöyhönen, S.; Er, S.; Domanskyi, A.; Airavaara, M. Effects of neurotrophic factors in glial cells in the central nervous system: Expression and properties in neurodegeneration and injury. Front. Physiol. 2019, 10, 486. [Google Scholar] [CrossRef]
- Okada, M. Can rodent models manifest pathomechanisms of genetic epilepsy? Br. J. Pharmacol. 2021, 1–20. [Google Scholar] [CrossRef]
- Harte-Hargrove, L.C.; Galanopoulou, A.S.; French, J.A.; Pitkanen, A.; Whittemore, V.; Scharfman, H.E. Common data elements (CDEs) for preclinical epilepsy research: Introduction to CDEs and description of core CDEs. A TASK3 report of the ILAE/AES joint translational TASK force. Epilepsia Open 2018, 3, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Mazarati, A.; Jones, N.C.; Galanopoulou, A.S.; Harte-Hargrove, L.C.; Kalynchuk, L.E.; Lenck-Santini, P.P.; Medel-Matus, J.S.; Nehlig, A.; de la Prida, L.M.; Sarkisova, K.; et al. A companion to the preclinical common data elements on neurobehavioral comorbidities of epilepsy: A report of the TASK3 behavior working group of the ILAE/AES Joint Translational Task Force. Epilepsia Open 2018, 3, 24–52. [Google Scholar] [CrossRef] [PubMed]
- Łuszczki, J.J.; Lepiech, J.; Zagaja, M.; Wróblewska-Łuczka, P.; Florek-Łuszczki, M.; Bojar, H.; Walczak, A.; Plech, T. Anticonvulsant and neurotoxic effects of a novel 1,2,4-triazole-3-thione derivative (TPF-34) and its isobolographic interaction profile with classical antiepileptic drugs in mice. Pharmacol. Rep. 2020, 72, 87–95. [Google Scholar] [CrossRef]
- Litchfield, J.T., Jr.; Wilcoxon, F. A simplified method of evaluating dose–effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Boissier, J.R.; Tardy, J.; Diverres, J.C. Une nouvelle methode simple pour explorer l’action tranquilisante: Le test de la cheminee. Med. Exp. 1960, 3, 81–84. [Google Scholar]
- Venault, P.; Chapouthier, G.; de Carvalho, L.P.; Simiand, J.; Morre, M.; Dodd, R.H.; Rossier, J. Benzodiazepine impairs and beta-carboline enhances performance in learning and memory tasks. Nature 1986, 321, 864–866. [Google Scholar] [CrossRef]
- Racine, R.J.; Gartner, J.G.; Burnham, W.M. Epileptiform activity and neural plasticity in limbic structures. Brain Res. 1972, 47, 262–268. [Google Scholar] [CrossRef]
- Schmued, L.C.; Hopkins, K.J. Fluoro-Jade B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000, 874, 123–130. [Google Scholar] [CrossRef]


| Pretreatment Time (min) | ED50 MES (mg/kg) | ED50 PTZ (mg/kg) | ED50 6Hz (mg/kg) | TD50 (mg/kg) | PI |
|---|---|---|---|---|---|
| 30 | 88.4 ± 8.5 | 59.9 ± 4.0 | 21.0 ± 6.6 | >1500 | >16.97 (MES) >25.04 (PTZ) >71.43 (6 Hz) |
| 60 | 85.1 ± 5.5 | 88.7 ± 3.1 | 35.0 ± 8.2 | 823.6 ± 107.9 | 9.68 (MES) 9.28 (PTZ) 23.53 (6 Hz) |
| Treatment (mg/kg) | (1) Retention Time (s) | (2) Grip Strength (gf) | (3) Motor Coordination Impairment (%) |
|---|---|---|---|
| Vehicle | 180 (173; 180) | 98.51 ± 5.37 | 0 |
| C-11 (30) | 151 (25; 180) | 94.89 ± 4.09 | 25 |
| VPA (251,5) | 137 (35; 180) | 89.63 ± 6.15 | 0 |
| VPA (251,5)+ C-11 (30) | 156 (107; 180) | 80.26 ± 4.94 | 12.5 |
| CBZ (15.0) | 180 (74; 180) | 113.5 ± 4.25 | 0 |
| CBZ (15.0) + C-11 (30) | 180 (135; 180) | 102.2 ± 4.73 | 0 |
| LCM (4.4) | 180 (130; 180) | 94.80 ± 5.61 | 12.5 |
| LCM (4.4)+ C-11 (30) | 180 (29; 180) | 98.26 ± 6.30 | 25 |
| LTG (4.4) | 180 (151; 180) | 104.8 ± 6.41 | 0 |
| LTG (4.4) + C-11 (30) | 180 (161; 180) | 97.94 ± 4.82 | 37.5 |
| Groups | Number of Animals | Percentage Convulsion (%) | Percentage SE (%) | Percentage of Survival (%) |
|---|---|---|---|---|
| Control | 10 | 0 | 0 | 0 |
| PILO | 10 | 100 | 100 | 50 (5/10) |
| C-11 | 10 | 100 | 100 | 60 (6/10) |
| Compound | Lipinski Rule | Veber Rule | ||||
|---|---|---|---|---|---|---|
| MW ≤500 | LogP ≤5 | NHD a ≤5 | NHA b ≤10 | NBR c ≤10 | TPS d ≤140 | |
| C-11 | 383.37 | 1.98 | 0 | 6 | 5 | 60.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagaja, M.; Szewczyk, A.; Szala-Rycaj, J.; Raszewski, G.; Chrościńska-Krawczyk, M.; Abram, M.; Kamiński, K.; Andres-Mach, M. C-11, a New Antiepileptic Drug Candidate: Evaluation of the Physicochemical Properties and Impact on the Protective Action of Selected Antiepileptic Drugs in the Mouse Maximal Electroshock-Induced Seizure Model. Molecules 2021, 26, 3144. https://doi.org/10.3390/molecules26113144
Zagaja M, Szewczyk A, Szala-Rycaj J, Raszewski G, Chrościńska-Krawczyk M, Abram M, Kamiński K, Andres-Mach M. C-11, a New Antiepileptic Drug Candidate: Evaluation of the Physicochemical Properties and Impact on the Protective Action of Selected Antiepileptic Drugs in the Mouse Maximal Electroshock-Induced Seizure Model. Molecules. 2021; 26(11):3144. https://doi.org/10.3390/molecules26113144
Chicago/Turabian StyleZagaja, Mirosław, Aleksandra Szewczyk, Joanna Szala-Rycaj, Grzegorz Raszewski, Magdalena Chrościńska-Krawczyk, Michał Abram, Krzysztof Kamiński, and Marta Andres-Mach. 2021. "C-11, a New Antiepileptic Drug Candidate: Evaluation of the Physicochemical Properties and Impact on the Protective Action of Selected Antiepileptic Drugs in the Mouse Maximal Electroshock-Induced Seizure Model" Molecules 26, no. 11: 3144. https://doi.org/10.3390/molecules26113144
APA StyleZagaja, M., Szewczyk, A., Szala-Rycaj, J., Raszewski, G., Chrościńska-Krawczyk, M., Abram, M., Kamiński, K., & Andres-Mach, M. (2021). C-11, a New Antiepileptic Drug Candidate: Evaluation of the Physicochemical Properties and Impact on the Protective Action of Selected Antiepileptic Drugs in the Mouse Maximal Electroshock-Induced Seizure Model. Molecules, 26(11), 3144. https://doi.org/10.3390/molecules26113144






