Chemical Profiling and Antimicrobial Properties of Honey Bee (Apis mellifera L.) Venom
Abstract
1. Introduction
2. Results and Discussion
2.1. Honey Bee Venom Composition
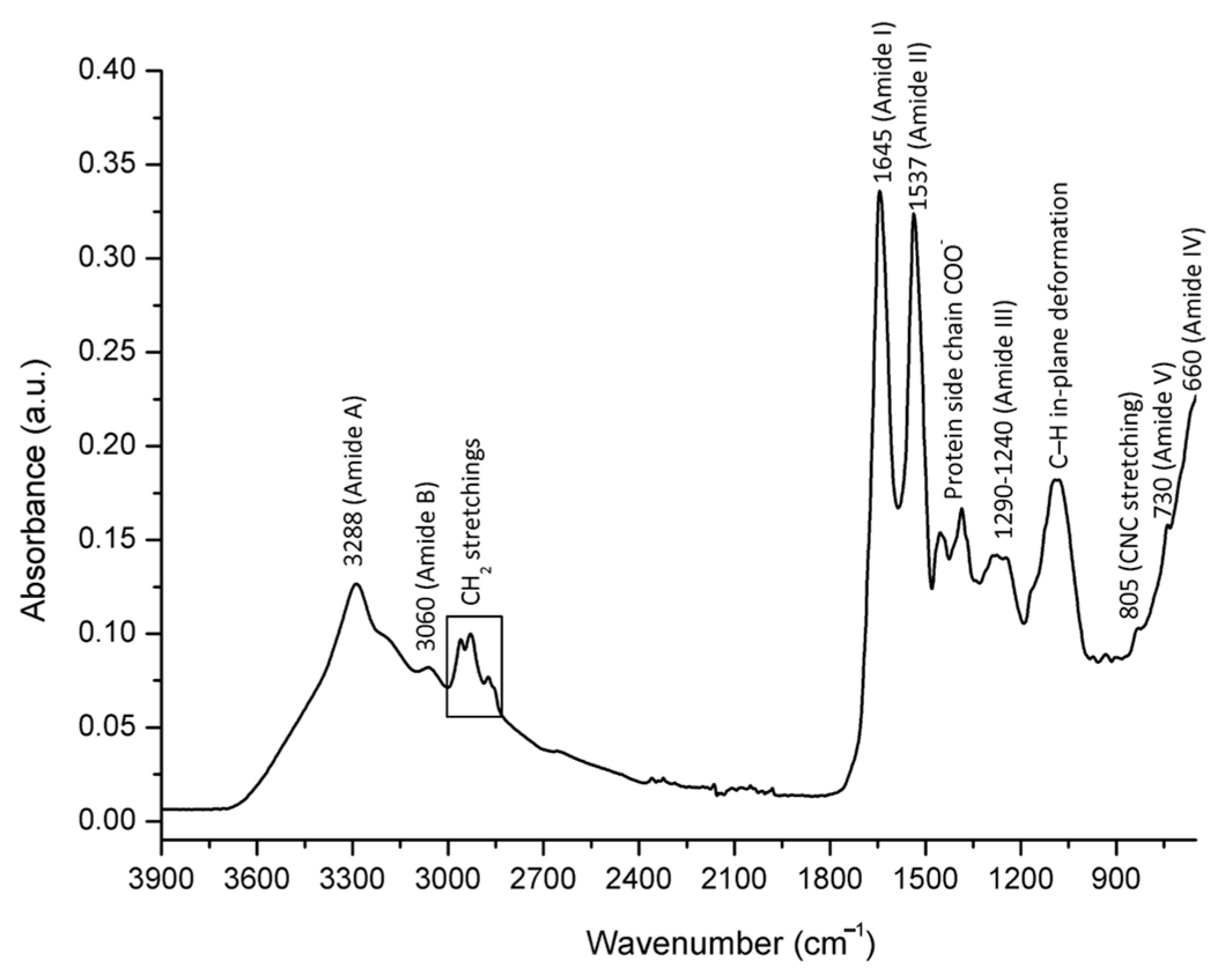
2.2. Spectral Assignment of Molecular Vibrations Observed in Bee Venom Powder/FTIR-ATR Spectral Profiling
2.3. Antibacterial Activity of Honey Bee Venom (HBV)
2.4. Comparison of Antibacterial Activity of HBV and Conventional Antibiotics
3. Materials and Methods
3.1. Honey Bee Venom Collection
3.2. Chemical Characterization of Honey Bee Venom
3.2.1. Determination of Melittin in Honey Bee Venom Using High-Performance Liquid Chromatography (HPLC)
3.2.2. Determination of Total Proteins in Honey Bee Venom
3.2.3. Chemical Fingerprinting by FTIR-ATR Spectroscopy
3.3. Evaluation of Bee Venoms’ Antibacterial Activity
3.3.1. Bacterial Strains
3.3.2. Determination of Minimum Inhibitory (MIC) and Bactericidal (MBC) Concentrations
3.3.3. Antibacterial Assay of Bee Venom and Conventional Antibiotics
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Haldar, J. Membrane-active small molecules: Designs inspired by antimicrobial peptides. Chem. Med. Chem. 2015, 10, 1606–1624. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, H.; Hamill, P.; Hancock, R.E.W. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef]
- Perumal Samy, R.; Stiles, B.G.; Franco, O.L.; Sethi, G.; Lim, L.H.K. Animal venoms as antimicrobial agents. Biochem. Pharmacol. 2017, 134, 127–138. [Google Scholar] [CrossRef]
- Yacoub, T.; Rima, M.; Karam, M.; Sabatier, J.M.; Fajloun, Z. Antimicrobials from venomous animals: An overview. Molecules 2020, 25, 2402. [Google Scholar] [CrossRef]
- De Castro Figueiredo Bordon, K.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From animal poisons and venoms to medicines: Achievements, challenges and perspectives in drug discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Simal-Gandara, J. Bee venom: An updating review of its bioactive molecules and its health applications. Nutrients 2020, 12, 3360. [Google Scholar] [CrossRef]
- Liu, X.; Chen, D.; Xie, L.; Zhang, R. Effect of honey bee venom on proliferation of K1735M2 mouse melanoma cells in-vitro and growth of murine B16 melanomas in-vivo. J. Pharm. Pharmacol. 2002, 54, 1083–1089. [Google Scholar] [CrossRef]
- Oršolić, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef]
- Hegazi, A.; Abdou, A.M.; El-Moez, S.I.; Allah, F.A. Evaluation of the antibacterial activity of bee venom from different sources. World Appl. Sci. J. 2014, 30, 266–270. [Google Scholar]
- Ko, S.J.; Park, E.; Asandei, A.; Choi, J.Y.; Lee, S.C.; Seo, C.H.; Luchian, T.; Park, Y. Bee venom-derived antimicrobial peptide melectin has broad-spectrum potency, cell selectivity, and salt-resistant properties. Sci. Rep. 2020, 10, 10145. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, K.H.; Kim, S.J.; Lee, W.R.; Lee, K.G.; Park, K.K. Bee venom protects hepatocytes from tumor necrosis factor-α and actinomycin D. Arch. Pharm. Res. 2010, 33, 215–223. [Google Scholar] [CrossRef]
- Liu, C.C.; Yang, H.; Zhang, L.L.; Zhang, Q.; Chen, B.; Wang, Y. Biotoxins for cancer therapy. Asian Pac. J. Cancer Prev. 2014, 15, 4753–4758. [Google Scholar] [CrossRef]
- Bogdanov, S. Bee venom: Production, composition, quality. In The Bee Venom Book; e-book Bee Product Science: Muehlethurnen, Switzerland, 2016; pp. 1–8. [Google Scholar]
- Pascoal, A.; Estevinho, M.M.; Choupina, A.B.; Sousa-Pimenta, M.; Estevinho, L.M. An overview of the bioactive compounds, therapeutic properties and toxic effects of apitoxin. Food Chem. Toxicol. 2019, 134, 110864. [Google Scholar] [CrossRef]
- Abd El-Wahed, A.A.; Khalifa, S.A.M.; Sheikh, B.Y.; Farag, M.A.; Saeed, A.; Larik, F.A.; Koca-Caliskan, U.; AlAjmi, M.F.; Hassan, M.; Wahabi, H.A.; et al. Bee venom composition: From chemistry to biological activity. Stud. Nat. Prod. Chem. 2018, 60, 459–484. [Google Scholar]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Bee venom: Overview of main compounds and bioactivities for therapeutic interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ying, B.; Huang, S.; Ma, S.; Long, P.; Tu, X.; Yang, W.; Wu, Z.; Chen, W.; Miao, X. High-performance liquid chromatography combined with intrinsic fluorescence detection to analyse melittin in individual honeybee (Apis mellifera) venom sac. J. Chromatogr. B 2015, 1002, 139–143. [Google Scholar] [CrossRef]
- Fennell, J.F.; Shipman, W.H.; Cole, L.J. Antibacterial Action of a Bee Venom Fraction (Melittin) against a Penicillin-Resistant Staphylococcus and Other Microorganisms; Research and Development Technical Report; United States Naval Radiological Defense Laboratory: San Francisco, CA, USA, 1967; pp. 1–25. [Google Scholar]
- Raghuraman, H.; Chattopadhyay, A. Melittin: A membrane-active peptide with diverse functions. Biosci. Rep. 2007, 27, 189–223. [Google Scholar] [CrossRef]
- Han, S.M.; Kim, J.M.; Hong, I.P.; Woo, S.O.; Kim, S.G.; Jang, H.R.; Pak, S.C. Antibacterial activity and antibiotic-enhancing effects of honeybee venom against methicillin-resistant staphylococcus aureus. Molecules 2016, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.W.; Vinks, A.A. Relationship between minimum inhibitory concentration and stationary concentration revisited. Clin. Pharmacokinet. 2005, 44, 767–768. [Google Scholar] [CrossRef] [PubMed]
- Udekwu, K.I.; Parrish, N.; Ankomah, P.; Baquero, F.; Levin, B.R. Functional relationship between bacterial cell density and the efficacy of antibiotics. J. Antimicrob. Chemother. 2009, 63, 745–757. [Google Scholar] [CrossRef]
- Clinical & Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; CLSI Document M100-S25; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- EUCAST. Disk Diffusion Method for Antimicrobial Susceptibility Testing, v 9.0. EUCAST. 2021. Available online: https://eucast.org (accessed on 12 May 2021).
- Chen, J.; Guan, S.M.; Sun, W.; Fu, H. Melittin, the major pain-producing substance of bee venom. Neurosci. Bull. 2016, 32, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Pucca, M.B.; Cerni, F.A.; Oliveira, I.S.; Jenkins, T.P.; Argemí, L.; Sørensen, C.V.; Ahmadi, S.; Barbosa, J.E.; Laustsen, A.H. Bee updated: Current knowledge on bee venom and bee envenoming therapy. Front. Immunol. 2019, 10, 2090. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, A.F.; da Rocha, T.C.; de Lima, A.B.S.; Cavalcanti, M.T. Honeybee venom: Influence of collection on quality and cytotoxicity. Ciência Rural 2017, 47, e20160486. [Google Scholar] [CrossRef][Green Version]
- Costa, H.; Boni-Mitake, M.; Souza, C.F.; Rogero, J.R. Effects of gamma radiation on bee venom: Preliminary studies. VII Gen. Congr. Nucl. Energy 1999, 1–4. [Google Scholar]
- Darwish, D.A.; Masoud, H.M.M.; Abdel-Monsef, M.M.; Helmy, M.S.; Zidan, H.A.; Ibrahim, M.A. Phospholipase A2 enzyme from the venom of Egyptian honey bee Apis mellifera lamarckii with anti-platelet aggregation and anti-coagulation activities. J. Genet. Eng. Biotechnol. 2021, 19, 1–8. [Google Scholar] [CrossRef]
- Han, S.; Yeo, J.; Baek, H.; Lin, S.M.; Meyer, S.; Molan, P. Postantibiotic effect of purified melittin from honeybee (Apis mellifera) venom against Escherichia coli and Staphylococcus aureus. J. Asian Nat. Prod. Res. 2009, 11, 796–804. [Google Scholar] [CrossRef]
- Park, D.; Jung, J.W.; Lee, M.O.; Lee, S.Y.; Kim, B.; Jin, H.J.; Kim, J.; Ahn, Y.J.; Lee, K.W.; Song, Y.S.; et al. Functional characterization of naturally occurring melittin peptide isoforms in two honey bee species, Apis mellifera and Apis cerana. Peptides 2014, 53, 185–193. [Google Scholar] [CrossRef]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 2015, 22, 245–255. [Google Scholar] [CrossRef]
- Picoli, T.; Peter, C.M.; Zani, J.L.; Waller, S.B.; Lopes, M.G.; Boesche, K.N.; Vargas, G.D.; de Oliveira Hübner, S.; Fischer, G. Melittin and its potential in the destruction and inhibition of the biofilm formation by Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa isolated from bovine milk. Microb. Pathog. 2017, 112, 57–62. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, D.C.; Brochetto Braga, M.R.; de Abreu, R.M.M.; Blank, S.; Bridts, C.H.; De Clerck, L.S.; Devreese, B.; Ebo, D.G.; Ferris, T.J.; Hagendorens, M.M.; et al. Standard methods for Apis mellifera venom research. J. Apic. Res. 2020, 1–31. [Google Scholar] [CrossRef]
- Banks, B.E.C.; Shipolini, R.A. Chemistry and Pharmacology of Honey-bee Venom. In Venoms of the Hymenoptera: Biochemical, Pharmacological and Behavioural Aspects; Piek, A., Ed.; Academic Press: Orlando, FL, USA, 1986; pp. 329–416. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: New York, NY, USA, 2004. [Google Scholar]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. (Shanghai) 2007, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Jun, H.S.; Jeon, J.W.; Park, J.K.; Lee, B.J.; Suh, G.H.; Park, J.S.; Cho, C.W. Preparation and characterization of bee venom-loaded PLGA particles for sustained release. Pharm. Dev. Technol. 2018, 23, 857–864. [Google Scholar] [CrossRef]
- Oren, Z.; Shai, Y. Selective lysis of bacteria but not mammalian cells by diastereomers of melittin: Structure-function study. Biochemistry 1997, 36, 1826–1835. [Google Scholar] [CrossRef]
- Ebbensgaard, A.; Mordhorst, H.; Overgaard, M.T.; Nielsen, C.G.; Aarestrup, F.M.; Hansen, E.B. Comparative evaluation of the antimicrobial activity of different antimicrobial peptides against a range of pathogenic Bacteria. PLoS ONE 2015, 10, e0144611. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Lee, B.H.; Cole, S.; Badel-Berchoux, S.; Guillier, L.; Felix, B.; Krezdorn, N.; Hébraud, M.; Bernardi, T.; Sultan, I.; Piveteau, P. Biofilm formation of listeria monocytogenes strains under food processing environments and pan-genome-wide association study. Front. Microbiol. 2019, 10, 2698. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Christiansson, A.; Bertilsson, J.; Svensson, B. Bacillus cereus spores in raw milk: Factors affecting the contamination of milk during the grazing period. J. Dairy Sci. 1999, 82, 305–314. [Google Scholar] [CrossRef]
- Čujová, S.; Bednárová, L.; Slaninová, J.; Straka, J.; Čeřovský, V. Interaction of a novel antimicrobial peptide isolated from the venom of solitary bee Colletes daviesanus with phospholipid vesicles and Escherichia coli cells. J. Pept. Sci. 2014, 20, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Zolfagharian, H.; Mohajeri, M.; Babaie, M. Bee venom (Apis mellifera) an effective potential alternative to gentamicin for specific bacteria strains-Bee venom an effective potential for bacteria. J. Pharmacopuncture 2016, 19, 225–230. [Google Scholar] [CrossRef]
- Navarre, W.W.; Schneewind, O. Surface Proteins of Gram-Positive Bacteria and Mechanisms of Their Targeting to the Cell Wall Envelope. Microbiol. Mol. Biol. Rev. 1999, 63, 174–229. [Google Scholar] [PubMed]
- Baker, C.N.; Stocker, S.A.; Culver, D.H.; Thornsberry, C. Comparison of the E test to agar dilution, broth microdilution, and agar diffusion susceptibility testing techniques by using a special challenge set of bacteria. J. Clin. Microbiol. 1991, 29, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Yount, N.Y. Mode of Action and Resistance Mechanisms of Antimicrobial Macrolides. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Rybak-Chmielewska, H.; Szczêsna, T. HPLC study of chemical composition of honeybee (Apis mellifera L.) venom. J. Apic. Sci. 2004, 48, 103–109. [Google Scholar]
- ISO 1871:2009. Food and Feed Products-General Guidelines for the Determination of Nitrogen by the Kjeldahl Method. Available online: https://www.iso.org/standard/41320.html (accessed on 12 February 2021).
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard, 10th ed.; CLSI Document M07-A10; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.M.; Tuck, M. Antibiotic susceptibility testing by a standardize disc diffusion method. Am. J. Clin. Patholagy 1966, 45, 49. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard, 11th ed.; CLSI Document M02-A11; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]

| Bacterial Strains | MIC/MBC [µg/mL] | ||
|---|---|---|---|
| 3 log CFU/mL | 6 log CFU/mL | 8 log CFU/mL | |
| S. aureus subsp. aureus | 12.5/12.5 | 25.0/50.0 | 200.0/400.0 |
| B. cereus | 12.5/50.0 | 12.5/50.0 | 25.0/50.0 |
| L. innocua | 12.5/25.0 | 12.5/50.0 | 25.0/100.0 |
| E. coli | 12.5/25.0 | 25.0/25.0 | 200.0/400.0 |
| S. enterica subsp. enterica | 12.5/25.0 | 25.0/50.0 | 200.0/200.0 |
| Bacterial Strains | HBV (100 µg) | HBV (200 µg) | HBV (300 µg) | HBV (400 µg) | TE (30 µg) | E (15 µg) | CIP (5 µg) | SAM (20 µg) |
|---|---|---|---|---|---|---|---|---|
| S. aureus subsp. aureus | 8.5 ± 0.7 afgh | 9.6 ± 0.1 b | 9.6 ± 0.1 bg | 9.6 ± 0.1 bg | 33.0 ± 0.0 | 28.8 ± 0.4 | n.d. | n.d. |
| B. cereus | 6.0 ± 0.0 d | 7.0 ± 0.0 e | 8.0 ± 0.0 af | 8.8 ± 0.4 afgh | 31.5 ± 2.1 | 16.8 ± 0.4 | n.d. | n.d. |
| L. innocua | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 8.3 ± 0.4 afgh | 8.5 ± 0.1 afgh | 41.5 ± 2.1 | 38.5 ± 2.1 | n.d. | n.d. |
| E. coli | 6.5 ± 0.0 de | 8.0 ± 0.0 f | 9.0 ± 0.0 g | 9.0 ± 0.0 abgh | n.d. | n.d. | 42.5 ± 0.7 | 26.0 ± 0.0 |
| S. enterica subsp. enterica | 6.3 ± 0.4 de | 6.0 ± 0.0 d | 8.5 ± 0.0 h | 9.0 ± 0.0 bh | n.d. | n.d. | 45.5 ± 0.3 | 27.8 ± 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanuwidjaja, I.; Svečnjak, L.; Gugić, D.; Levanić, M.; Jurić, S.; Vinceković, M.; Mrkonjić Fuka, M. Chemical Profiling and Antimicrobial Properties of Honey Bee (Apis mellifera L.) Venom. Molecules 2021, 26, 3049. https://doi.org/10.3390/molecules26103049
Tanuwidjaja I, Svečnjak L, Gugić D, Levanić M, Jurić S, Vinceković M, Mrkonjić Fuka M. Chemical Profiling and Antimicrobial Properties of Honey Bee (Apis mellifera L.) Venom. Molecules. 2021; 26(10):3049. https://doi.org/10.3390/molecules26103049
Chicago/Turabian StyleTanuwidjaja, Irina, Lidija Svečnjak, Domenika Gugić, Marko Levanić, Slaven Jurić, Marko Vinceković, and Mirna Mrkonjić Fuka. 2021. "Chemical Profiling and Antimicrobial Properties of Honey Bee (Apis mellifera L.) Venom" Molecules 26, no. 10: 3049. https://doi.org/10.3390/molecules26103049
APA StyleTanuwidjaja, I., Svečnjak, L., Gugić, D., Levanić, M., Jurić, S., Vinceković, M., & Mrkonjić Fuka, M. (2021). Chemical Profiling and Antimicrobial Properties of Honey Bee (Apis mellifera L.) Venom. Molecules, 26(10), 3049. https://doi.org/10.3390/molecules26103049









